Abstract
Objectives
Environmental surfaces have been suggested as likely contributors in the transmission of COVID-19. This study assessed the infectivity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) contaminating surfaces and objects in two hospital isolation units and a quarantine hotel.
Methods
SARS-CoV-2 virus stability and infectivity on non-porous surfaces was tested under controlled laboratory conditions. Surface and air sampling were conducted at two COVID-19 isolation units and in a quarantine hotel. Viral RNA was detected by RT-PCR and infectivity was assessed by VERO E6 CPE test.
Results
In laboratory-controlled conditions, SARS-CoV-2 gradually lost its infectivity completely by day 4 at ambient temperature, and the decay rate of viral viability on surfaces directly correlated with increase in temperature. Viral RNA was detected in 29/55 surface samples (52.7%) and 16/42 surface samples (38%) from the surroundings of symptomatic COVID-19 patients in isolation units of two hospitals and in a quarantine hotel for asymptomatic and very mild COVID-19 patients. None of the surface and air samples from the three sites (0/97) were found to contain infectious titres of SARS-Cov-2 on tissue culture assay.
Conclusions
Despite prolonged viability of SARS-CoV-2 under laboratory-controlled conditions, uncultivable viral contamination of inanimate surfaces might suggest low feasibility for indirect fomite transmission.
Keywords: Contamination, Coronavirus, COVID-19, SARS-CoV-2, Surface, Viability
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resulting coronavirus disease 2019 (COVID-19) pandemic is transmitted by respiratory droplets and direct contact with a contagious individual, but its transmissibility by indirect contact through contaminated inanimate surfaces remains undetermined [1,2]. Coronaviruses, including SARS-CoV-2, can remain viable and infective on various surfaces for prolonged periods of time under controlled laboratory conditions [[3], [4], [5]]. Furthermore, environmental studies have demonstrated contamination of surfaces by SARS-CoV-2 RNA in hospital settings and in outpatient settings such as hotel quarantine rooms [[6], [7], [8], [9]]. Nevertheless, positive viral RNA PCR tests do not prove viral viability or infectivity. Herein we have investigated the stability of SARS-CoV-2 on smooth, non-porous surfaces, as well as environmental contamination, viability and infectivity of SARS-CoV-2 in the surroundings of COVID-19 patients in two hospital isolation units and a quarantine hotel.
Methods
SARS-CoV-2 surface stability testing
SARS-CoV-2 strain MUC-IMB-1 was propagated and titrated on VERO E6 (ATCC-CRL-1586) as described previously [10]. All handling of SARS-CoV-2 was conducted under biosafety level 3 (BSL-3) conditions in accordance with the biosafety guidelines of the IIBR (Israel Institute for Biological Research). Plastic and metal coupons were inoculated with 1 × 106 plaque-forming units (PFUs) of SARS-CoV-2. Coupons were kept under controlled temperature and 50% relative humidity for 6 h to 14 days at 22°C or 10–180 min at 40–70°C, and then resuspended in 1 mL minimum essential medium (MEM) without serum. Viable virus titres were determined by applying 200 μL from tenfold serial sample dilutions upon VERO E6 cell cultures in 24-well plates. After 1 h, wells were overlaid with 1 mL of MEM medium supplemented with 2% foetal calf serum (FCS), MEM non-essential amino acids, 2 mM L-glutamine, 100 units/mL penicillin, 0.1% streptomycin, 12.5 units/mL nystatin and 0.15% sodium bicarbonate. Cells were incubated for 5 days (37°C, 5% CO2), and cytopathic effects (CPEs) were observed after fixation with crystal violet solution.
Description of sampled environments
Sampling was performed in COVID-19 isolation units in two hospitals and one quarantine facility. The isolation unit in Hospital A is a 28-bed ward comprising secluded rooms occupied by one to three patients each. Staff routinely used gowns, masks, shoe covers, face shields, and disposable surgical caps. The standard operating procedure (SOP) was the use of one pair of gloves at a time, exchanging when leaving each patient's bed. At the time of sampling the unit contained patients with mild to moderate disease, with no ventilated patients. A few patients were using high-flow oxygen cannulas. The isolation unit in Hospital B is a 40-bed ward comprising secluded rooms occupied by one to six patients each. Staff used coveralls, masks, shoe covers and face shields. SOP was the use of two pairs of gloves at a time. The outer pair was not consistently changed during patient care. At the time of sampling, the unit contained patients with mild to severe disease, including seven ventilated patients. In both hospitals, patients were free to ambulate in the unit if they were fit to walk. In addition, routine cleaning and decontamination were done twice a day using 1000 ppm bleach solution in both wards. The quarantine facility was a hotel repurposed for the isolation of patients with asymptomatic to mild disease until they become negative for virus by PCR of nasopharyngeal samples. Sampling was conducted in main public areas and in hotel rooms. Patients stayed in private rooms either alone or as a family, but were free to move around the hotel and socialize in public spaces. Routine cleaning and decontamination were done once daily at best only in communal areas.
SARS-CoV-2 environmental sampling
The study was approved by the ethics committees of Assuta Ashdod and Laniado hospitals and IIBR's IBC. Surfaces were swabbed with sterile 6-inch cotton-tipped applicators in an area of 20 × 20 cm. Smaller objects were swabbed entirely. Two wet swabs plus one dry swab were used and pooled into one 15-mL tube containing 2 mL transfer medium (MEM medium supplemented with 2% FCS and 200 units/mL penicillin, 0.2% streptomycin, 25 units/mL nystatin). Air sampling was performed using an MD8 air sampler (Sartorius, Göttingen, Germany) equipped with gelatine membranes (3.0 μm filtration cut-off) at 50 L/min sampling rate for 20 min. Cold-chain transport of samples was maintained (4–8°C), and sample processing was performed within 2–3 h from sampling.
RNA extraction, RT-PCR tests and virus infectivity testing
Samples were vigorously vortexed, and from each elution 200 μL swab extraction were processed using RNAdvance Viral kit (Beckman Coulter) on the Biomek i7 Automated Workstation (Beckman Coulter), according to the manufacturer's protocol. Each sample was eluted in 50 μL of RNase-free water. Real-time RT-PCR assays, targeting the SARS-CoV-2 E gene, were performed using the SensiFAST Probe Lo-ROX One-Step kit (Bioline). The final concentration of primers was 600 nM and the probe concentration was 300 nM. Primers and probe for the E gene assay were taken from the Berlin protocol published in the WHO recommendation for the detection of SARS-CoV-2. Thermal cycling was performed at 48°C for 20 min for reverse transcription, followed by 95°C for 2 min, and then 45 cycles of 94°C for 15 s, 60°C for 35 s. Virus infectivity was tested by seeding quadruplets of 200 μL on VERO E6 cells for CPE assay as described above. The limit of detection of the CPE assay has been determined to be 10 pfu/mL.
Statistical analysis
The significance of the differences in positive RT-PCR samples from different sites was determined by χ-square with Fisher's exact test using Prism 6 software (Graphpad, USA).
Results
SARS-CoV-2 surface stability testing
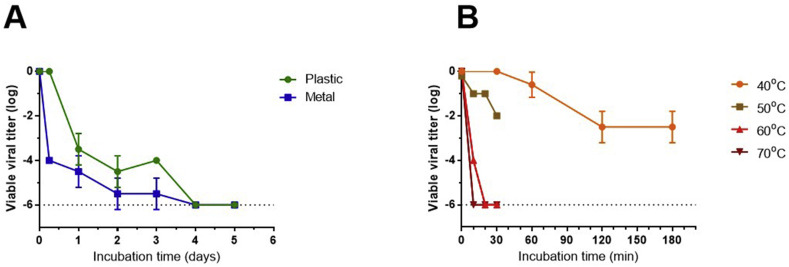
In this section we demonstrate the stability of viable SARS-CoV-2 on non-porous surfaces under controlled conditions using a sensitive CPE viability assay (Fig. 1 A). On SARS-CoV-2-contaminated plastic coupons, titres of viable virus remained unchanged after 6 h of incubation. Viral titres were decreased by 3.5 orders of magnitude after 24 h, and <100 virus particles were retrieved at days 2 and 3. On metal coupons a faster reduction of 4 orders of magnitude was observed after 6 h of incubation, and similar levels of viable virus were detected at 24 h. A further decrease in viability on metal surfaces was detected at days 2 and 3. No viable virus was recovered from plastic or metal coupons after 4–14 days of incubation.
Fig. 1.
Stability of SARS-CoV-2 on non-porous surfaces under controlled laboratory conditions. Plastic and metal coupons (1 cm2) inoculated with 1 × 106 PFU SARS-CoV-2 (by applying 10 μl of 1 × 108 PFU/ml) were incubated at room temperature (A) or at 40-70 °C (B). At selected time points the coupons were extracted as described and residual viable virus titers on each sampled surface was determine by CPE assay on VERO E6 cells. Results are presented as mean ± std of viral titer log reduction index from 3 independent experiments.
We next determined the viral stability on surfaces at increased temperatures. As shown in Fig. 1B, the viability of SARS-CoV-2 on plastic coupons was unaffected for at least 30 min at 40°C, while a minor reduction of one order of magnitude in viral viability was obtained after 1 h. An additional one order of magnitude reduction in virus titres was observed after 2–3 h of incubation. After incubation at 50°C of 10–20 min, viral titres were further reduced by one order of magnitude, and an additional order of magnitude was achieved by extending the incubation time to 30 min (Fig. 1B). After 20 min of incubation at 60°C and 10–30 min at 70°C no viable particles were detected.
Viral SARS-CoV-2 RNA and viable shedding in environmental sampling
We next tested viral shedding in environment samples collected from patients' rooms in the two hospital isolation units; sampling results are presented in Table 1 . In non-ventilated patients' rooms, viral RNA was recovered from 9/21 of sampled surfaces (43%), mostly on floors, bedside tables, faucet handles and patients' personal belongings (e.g. eyeglasses and a walker). A higher proportion of contaminated surfaces was detected in mechanically ventilated patients' rooms (13/18, 72%), although this trend did not reach statistical significance (p 0.0652). Contaminated surfaces included mainly bed rails, ventilator touch screens, staff computer accessories and faucet handles. Viral RNA was detected on only one of seven mobile phones. Using an air sampler, we detected viral RNA in one of one sample in a ventilated patients' room, compared to none of three samples taken in non-ventilated patients' rooms, and one out of two samples was positive for RNA at the nursing station and in the doffing area. There were differences between the levels of contamination between the two hospitals. Only six out of 23 samples (26%) in Hospital A were positive, with an average cycle threshold (CT) value of 37.9, contrasting with Hospital B which had 23 out of 32 samples positive (72%) and an average CT value of 34 (p 0.00079). Although viral RNA could be detected in 29/55 environmental samples (51%), we couldn't propagate any viable virus from any of the samples in Vero-E6 cultured cells.
Table 1.
Environmental sampling results from COVID-19 isolation units of Hospitals A and B
| Sampled area | Sampled objects | No. of RNA-positive samples/total no. of samples (%) | RT-PCR Ct value range of positive samples (average) |
|---|---|---|---|
| Patient rooms (1–3 patients in mild condition) | Floor | 2/3 (67%) | 33.9–38 (36) |
| Bed rail | 1/3 (33%) | 39 (39) | |
| Bedside table | 2/4 (50%) | 35.4–39.8 (37.6) | |
| Faucet handle | 2/2 (100%) | 30.3–32.1 (31.2) | |
| Patient's mobile phone | 0/4 (0%) | NA | |
| Patient's eyeglasses | 1/1 (100%) | 35.2 (35.2) | |
| Patient's walker | 1/1 (100%) | 34.4 (34.4) | |
| Air sampling filter | 0/3 (0%) | NA | |
| Total | 9/21 (43%) | 30.3–39.8 (35.3) | |
| Ventilated patients' rooms (invasive and non-invasive ventilation) | Bed rails | 4/5 (80%) | 30–36.4 (33.9) |
| Faucet handle | 2/2 (100%) | 33.1–33.6 (33.3) | |
| Ventilator (touch screen) | 2/2 (100%) | 33.9–35.1 (34.5) | |
| Staff computer mouse | 2/3 (67%) | 33.4–36.6 (35) | |
| Staff mobile phone | 1/2 (50%) | 35.1 (35.1) | |
| Bedside table | 1/1 (100%) | 33.7 (33.7) | |
| Trash bin top | 0/1 (0%) | NA | |
| Bench top | 0/1 (0%) | NA | |
| Air sampling filter | 1/1 (100%) | 34.1 (34.1) | |
| Total | 13/18 (72%) | 30–36.6 (34.2) | |
| Patient's toilets | Toilet seat | 1/1 (100%) | 33.2 (33.2) |
| Handle grip | 1/2 (50%) | 36.3 (36.3) | |
| Door handle | 1/1 (100%) | 34.5 (34.5) | |
| Total | 3/4 (75%) | 33.2–36.3 (34.7) | |
| Nurse station | Floor | 0/1 (0%) | NA |
| Bench top | 1/1 (100%) | 31.7 (31.7) | |
| Computer mouse | 0/1 (0%) | NA | |
| Staff mobile phone | 0/1 (0%) | NA | |
| Glucometer | 0/1 (0%) | NA | |
| Electric thermometer | 1/1 (100%) | 35.4 (35.4) | |
| Blood pressure cuff | 0/1 (0%) | NA | |
| Air sampling filter | 1/1 (100%) | 38.8 (38.8) | |
| Total | 3/8 (38%) | 31.7–38.8 (35.3) | |
| Doffing area | Floor | 0/1 (0%) | NA |
| Door handle | 1/1 (100%) | 38.1 (38.1) | |
| Trash bin top | 0/1 (0%) | NA | |
| Air sampling filter | 0/1 (0%) | NA | |
| Total | 1/4 (25%) | 38.1 (38.1) | |
| All areas | Total | 29/55 (51%) | 30–39.8 (37.4) |
Finally, we mapped the contamination in a quarantine hotel for asymptomatic patients (those found to be infected during contact testing but had no symptoms) and patients with very mild COVID-19. Sampling was performed in the hotel communal areas and in three hotel rooms that had recently been occupied (2–4 days) by newly diagnosed patients. We found that 16/42 samples (38%) were positive for viral RNA (Table 2 ). At the communal areas we found viral RNA contamination on a water cooler, chairs, most elevator buttons, a kettle, and a used cup. Contaminated samples in patients' rooms included toilet seats, closet doors, a kettle, used cups and one air sample from a room occupied by three patients. In agreement with our findings in COVID-19 isolation units, only one of four personal mobile phones was found to be positive. Again, none of the samples was culturable.
Table 2.
Environmental sampling results from quarantine hotel for asymptomatic and mild COVID-19 patients
| Sampled area | Sampled objects | No. of RNA-positive samples/total no. of samples (%) | RT-PCR Ct value range of positive samples (average) |
|---|---|---|---|
| Hotel rooms | Door handle | 0/1 (0%) | NA |
| Closet door | 1/2 (50%) | 35 (35) | |
| Faucet handle | 0/2 (0%) | NA | |
| Sink | 0/1 (0%) | NA | |
| Toilet seat | 1/1 (100%) | 34.9 (34.9) | |
| Mobile phone | 1/4 (25%) | 32.7 (32.7) | |
| Electric kettle | 1/1 (100%) | 34.9 | |
| Prayer beads | 0/2 (0%) | NA | |
| Glass cup | 2/2 (100%) | 29.3–35 (32.1) | |
| Bedside table | 0/1 (0%) | NA | |
| Eyeglasses | 0/1 (0%) | NA | |
| TV remote controller | 0/1 (0%) | NA | |
| Drinking bottle | 0/1 (0%) | NA | |
| Air sampling filter | 1/1 (100%) | 35 (35) | |
| Total | 7/21 (33%) | 29.3–35 (33.5) | |
| Public spaces | Microwave oven handle | 0/1 (0%) | NA |
| Cold water bar | 1/1 (100%) | 35.2 (35.2) | |
| Hot water bar | 0/1 (0%) | NA | |
| Door handle | 0/1 (0%) | NA | |
| Staircase railing | 0/1 (0%) | NA | |
| Coffee table | 0/2 (0%) | NA | |
| Sitting chair handle | 0/2 (0%) | NA | |
| Synagogue chair handle | 2/2 (100%) | 34.97–35.1 (35) | |
| Prayer book | 0/2 (0%) | NA | |
| Electric kettle | 1/1 (100%) | 34.9 (34.9) | |
| Used plastic cup | 1/1 (100%) | 35 (35) | |
| Elevator button panels | 4/5 (80%) | 30.6–35 (33) | |
| Air sampling filter | 0/1 (0%) | NA | |
| Total | 9/21 (43%) | 30.6–35.1 (34.1) | |
| All areas | Total | 16/42 (38%) | 29.3–35.1 (33.9) |
Discussion
Using cell cultures, we tested the viability of SARS-CoV-2 on non-porous surfaces under controlled laboratory conditions and found it to remain viable for up to 3 days at room temperature. The decay of viral viability on surfaces directly correlated with increasing temperature. Next, we showed the presence of SARS-CoV-2 RNA in 45 out of 97 environmental samples (46.4%) taken in the vicinity of COVID-19 patients in both hospital and quarantine hotel settings. Contaminated surfaces included patients' personal belongings and fomites frequently touched by patients and staff. Eight of these samples were air samples, of which three (37.5%) were positive by PCR. Despite the presence of viral RNA in these samples, no viable virus was cultured from any of the 97 surface and air samples taken.
Since the beginning of the COVID-19 pandemic, substantial efforts have been directed towards a better understanding of how SARS-CoV-2 is transmitted. Although droplet transmission and transmission by direct contact are the primary transmission routes, the rapid spread of the disease led to hypotheses concerning indirect-contact-based and aerosol-based transmission [11]. Both SARS-CoV and MERS-CoV have been shown to be viable for prolonged durations in many experimental settings [3,5]. Initial results with SARS-CoV-2 showed high stability in transport medium (14 days, 4°C) and significant surface stability on non-porous materials at room temperature [4]. In agreement with these findings, we also demonstrate that SARS-CoV-2 remains viable and culturable under controlled laboratory conditions for up to 3 days, and that viability depends on environmental temperature.
Contamination with SARS-CoV-2 RNA has been found in multiple environmental sampling studies in hospitals, including contaminated surfaces and objects commonly used by staff (including footwear) [6,7,9]. Environmental contamination was also shown in 8/22 samples from a room of a pre-symptomatic patient [8]. Inability to detect contamination in isolation units [12,13] might demonstrate the success of extensive and frequent disinfection routines instituted in those hospitals. For transmission to occur through contact with inanimate surfaces, viable virions at sufficient titres must be carried by contact from the surface onto susceptible mucous membranes. Environmental sampling studies performed during the SARS outbreak in 2003 could not demonstrate infectious viruses in PCR-positive samples [14,15]. Although indirect contact is an established transmission route for several pathogens—such as Staphylococcus aureus, Acinetobacter baumanii, Clostidioides difficile, and non-enveloped viruses such as norovirus—fomite transmission is still controversial for coronaviruses, and epidemiological proof for indirect contact transmission of SARS-CoV-2 has yet to be reported [16]. One recent study could not find viable virus in the vicinity of COVID-19 patients, with only two samples that were reported positive for SARS-CoV-2 RNA [17].
In this study, viral RNA contamination was found in 46% of the surface and air samples. However, we could not isolate viable SARS-CoV-2 from any environmental sample. This finding may be explained by the relatively high CT values (>34) in our samples. A similar pattern was seen in a recent study in which culturable virus could not be isolated from nasopharyngeal samples with Ct values > 34 [18]. Recently, two small-scale studies reported similarly low levels of RNA without culturable virus [17,19]. While we used a well-defined sampling method—including three vigorous swabbings of the entire area of each surface [20]—it is possible that remnants of cleaning materials and disinfectants on surfaces could rapidly inactivate SARS-CoV-2, as shown in laboratory experiments with common disinfectants [4]. Sunlight and other forms of illumination may rapidly inactivate the virus as well [21]. Furthermore, antimicrobial compounds in sweat, saliva and other human excretions [22,23] could slowly inactivate naturally shed viruses on handled surfaces, which may explain the absence of viable virus on personal objects that are not routinely disinfected, such as eyeglasses. While three out of eight air samples were positive by PCR, no viable virus was cultured even from samples taken from the rooms of ventilated patients. While this may demonstrate efficient air treatment in the hospital setting, these findings indicate a low likelihood of aerosol transmission playing a role in the hospital setting if appropriate personal protective equipment (PPE) is used.
Our study has some limitations. There was a delay between onset of symptoms and the actual sampling in patients' rooms. Therefore, at the time of sampling, these patients might not have shed viable virus, as suggested by studies that showed culturable viruses in respiratory samples up to the 8th or 9th day of illness [18,24]. For that reason, we have noted new patients with recent disease onset in Hospital A and the quarantine hotel and sampled around them. The CPE assay has a 10 pfu/mL limit of detection that is comparable to a CT value of 34, therefore a very low level of viability cannot be ruled out. Unforeseen technical issues could have compromised viability of the virus after sampling. We addressed this limitation by collecting nearly 100 samples in three separate campaigns and maintaining strict cold storage conditions after sampling and during transport. Moreover, re-culturing of all the negative cultures was preformed to overcome any problems of culture adaptation of freshly isolated virus.
In summary, despite prolonged viability of SARS-CoV-2 in controlled conditions, aerosol or indirect transmission from inanimate surfaces around hospitalized or quarantined COVID-19 patients is not supported by the data presented in this study. In healthcare settings, infection control and prevention should focus mainly on prevention of direct face-to-face transmission and droplet protection. Fomite transmission may still be a possibility with heavily contaminated surfaces around patients during their most contagious stages of infection, and in closed and crowded environments.
Author contributions
AB-S and TB-N are equal first authors and contributed equally to this study. Environmental sampling: TB-N, IG, RC and SW. SARS-CoV-2 stability experiments: AB-S, IG, EB-D, AS, RP, SY, HL and SW. Viral propagation and infectivity experiments: AB-S, HA, HT, YY-R, BP, SM, EV, LC, NP and TI. RNA extraction and RT-PCR tests: OI and AB-D. Writing—original draft: TBS, AB-S, IG and SW. Writing—review and editing: NP, SW, AB-S and HL. All authors have read and approved the final manuscript.
Transparency declaration
The authors declare that they have no conflicts of interest. No external funding was received.
Acknowledgements
SARS-CoV-2 strain MUC-IMB-1 was kindly provided by Dr R. Ehmann and Dr G. Dobbler from the Bundeswehr Institute of Microbiology, Germany.
Editor: M. Cevik
References
- 1.WHO Coronavirus disease (COVID-19) Situation Report 120: WHO. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200519-covid-19-sitrep-120.pdf?sfvrsn=515cabfb_4 Available from:
- 2.CDC . 2020. How COVID-19 Spreads: CDC.https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html Available from: [Google Scholar]
- 3.Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol. 2010;76:2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye G., Lin H., Chen S., Wang S., Zeng Z., Wang W. Environmental contamination of SARS-CoV-2 in healthcare premises. J Infect. 2020;81:e1–e5. doi: 10.1016/j.jinf.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang F.C., Jiang X.L., Wang Z.G., Meng Z.H., Shao S.F., Anderson B.D. Detection of severe acute respiratory syndrome coronavirus 2 RNA on surfaces in quarantine rooms. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2609.201435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yung C.F., Kam K.Q., Wong M.S.Y., Maiwald M., Tan Y.K., Tan B.H. Environment and personal protective equipment tests for SARS-CoV-2 in the isolation room of an infant with infection. Ann Intern Med. 2020;173:240–242. doi: 10.7326/M20-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yahalom-Ronen Y., Tamir H., Melamed S., Politi B., Shifman O., Achdout H. A single dose of recombinant VSV-ΔG-spike vaccine provides protection against SARS-CoV-2 challenge. bioRxiv. 2020;2020 doi: 10.1038/s41467-020-20228-7. 06.18.160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren S.-Y., Wang W.-B., Hao Y.-G., Zhang H.-R., Wang Z.-C., Chen Y.-L. Stability and infectivity of coronaviruses in inanimate environments. World J Clin Cases. 2020;8:1391–1399. doi: 10.12998/wjcc.v8.i8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colaneri M., Seminari E., Piralla A., Zuccaro V., Filippo A.D., Baldanti F. Lack of SARS-CoV-2 RNA environmental contamination in a tertiary referral hospital for infectious diseases in Northern Italy. J Hosp Infect. 2020;105:474–476. doi: 10.1016/j.jhin.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int J Infect Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth T.F., Kournikakis B., Bastien N., Ho J., Kobasa D., Stadnyk L. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis. 2005;191:1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowell S.F., Simmerman J.M., Erdman D.D., Wu J.S., Chaovavanich A., Javadi M. Severe acute respiratory syndrome coronavirus on hospital surfaces. Clin Infect Dis. 2004;39:652–657. doi: 10.1086/422652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutter J.S., Spronken M.I., Fraaij P.L., Fouchier R.A., Herfst S. Transmission routes of respiratory viruses among humans. Curr Opin Virol. 2018;28:142–151. doi: 10.1016/j.coviro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colaneri M., Seminari E., Novati S., Asperges E., Biscarini S., Piralla A. Severe acute respiratory syndrome coronavirus 2 RNA contamination of inanimate surfaces and virus viability in a health care emergency unit. Clin Microbiol Infect. 2020;26:1094e1–1094e5. doi: 10.1016/j.cmi.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 19.Döhla M., Wilbring G., Schulte B., Kümmerer B.M., Diegmann C., Sib E. SARS-CoV-2 in environmental samples of quarantined households. medRxiv. 2020 doi: 10.3390/v14051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julian T.R., Tamayo F.J., Leckie J.O., Boehm A.B. Comparison of surface sampling methods for virus recovery from fomites. Appl Environ Microbiol. 2011;77:6918–6925. doi: 10.1128/AEM.05709-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratnesar-Shumate S., Williams G., Green B., Krause M., Holland B., Wood S. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J Infect Dis. 2020;222:214–222. doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barlow P.G., Svoboda P., Mackellar A., Nash A.A., York I.A., Pohl J. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vila T., Rizk A.M., Sultan A.S., Jabra-Rizk M.A. The power of saliva: antimicrobial and beyond. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1008058. e1008058-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kujawski S.A., WK, Collins J.P., Epstein L., Killerby M.E., Midgley C.M. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med. 2020;26:861–868. doi: 10.1038/s41591-020-0877-5. [DOI] [PubMed] [Google Scholar]