Abstract
Febrile neutropenia (FN) is one of the most concerning complications of chemotherapy, and its prediction remains difficult. This study aimed to reveal the risk factors for and build the prediction models of FN using machine learning algorithms. Medical records of hospitalized patients who underwent chemotherapy after surgery for breast cancer between May 2002 and September 2018 were selectively reviewed for development of models. Demographic, clinical, pathological, and therapeutic data were analyzed to identify risk factors for FN. Using machine learning algorithms, prediction models were developed and evaluated for performance. Of 933 selected inpatients with a mean age of 51.8 ± 10.7 years, FN developed in 409 (43.8%) patients. There was a significant difference in FN incidence according to age, staging, taxane-based regimen, and blood count 5 days after chemotherapy. The area under the curve (AUC) built based on these findings was 0.870 on the basis of logistic regression. The AUC improved by machine learning was 0.908. Machine learning improves the prediction of FN in patients undergoing chemotherapy for breast cancer compared to the conventional statistical model. In these high-risk patients, primary prophylaxis with granulocyte colony-stimulating factor could be considered.
Subject terms: Cancer, Computational biology and bioinformatics, Oncology, Risk factors
Introduction
Chemotherapy-induced febrile neutropenia (FN) is one of the most concerning complications in patients with breast cancer undergoing chemotherapy1. Neutropenia is a principal dose-limiting toxicity of myelosuppressive chemotherapy that predisposes patients to grave infections2. Moreover, infection in patients with neutropenia is the direct consequence of chemotherapy-induced neutropenia3. Chemotherapy-induced neutropenia is a principal risk factor for infection-related morbidity4. Further, mortality rates related with FN vary from 2 to 21%5,6.
Chemotherapy-induced FN commonly occurs during the initial cycle of cytotoxic therapy and increases in frequency with both duration and depth of the neutropenia3. In addition to an influence on quality of life, chemotherapy-induced FN exposes patients with cancer to life-threatening infections. Considering the severity of FN, the most patients who develop FN are hospitalized for evaluation and injected with broad-spectrum antibiotics. Along with infections, chemotherapy-induced FN frequently results in dose reductions and treatment delays which have been known to compromise treatment1,7. The risk of developing FN appears to depend on diverse factors, including patient-related factors, tumor burden, and chemotherapy regimen6.
Thus, primary prevention, through the administration of granulocyte colony-stimulating factor (G-CSF), is recommended by guidelines when a significant risk of FN exists4,8,9. G-CSF stimulates the maturation, proliferation, and release of neutrophils, leading to a dose-dependent increase in circulating neutrophils10. Primary prophylaxis with G-CSF decreased the risk of FN by 50% in patients with solid tumors without altering tumor response, overall survival, or infection-related mortality8. Currently, the criteria for the use of G-CSF and other means to reduce the risk of FN are based on low-quality evidence11.
Trials to prevent FN events during chemotherapy administration require an evaluation of risk factors related with the development of critical neutropenia12. However, this evaluation remains inaccurate3. Until now, no available prediction model has gained general acceptance12. A particularly important field of uncertainty that is emphasized by these findings is the absence of risk prediction models that estimate the risk of FN in patients reliably8.
Machine learning techniques have been widely adopted for the investigation of biomedical big data over the past years13. Recently, machine learning frameworks known as deep learning, which are based on artificial neural networks, have attracted more attention because of its notable success in predicting clinical outcomes of interest14. In this study, we aimed to unravel the predictive factors for and improve the prediction of FN by machine learning.
Results
Of the 933 patients, the mean age was 51.8 ± 10.7 years. 611 (65.5%) patients underwent breast-conserving surgery. Regarding staging, 737 (79.0%) patients were staged as I/II, while 196 (21.0%) patients were staged as III/IV. The median length of follow-up was 4.9 ± 2.9 years. FN developed in 409 (43.8%) patients, and the period until the development of FN was 10.2 ± 2.8 days.
In the training dataset, 843 patients were grouped according to the presence of FN. Patients with and without FN are compared in Table 1. There was a significant difference in the incidence of FN according to age, staging, and taxane-based regimen. The group with FN was older, had advanced disease, and received taxane-based regimens more frequently. Differences between the FN and non-FN groups were also found in complete blood count/differential blood count 5 days after chemotherapy. Lymphocyte count was significantly lower in the group with FN. We calculated and validated this predictive model using the testing dataset. The demographic characteristic of the 90 patients in the testing dataset are presented in Table 2. The highest AUC value was 0.870 on the basis of logistic regression.
Table 1.
Clinical demographic characteristics of patients with and without febrile neutropenia in the training dataset.
| Parameters | FN group (n = 366) | non-FN group (n = 477) | p value |
|---|---|---|---|
| Age (years), means ± SD | 0.004 | ||
| ≤ 50 | 157 (42.9) | 276 (57.9) | < 0.001 |
| > 50 | 209 (57.1) | 201 (42.1) | |
| Body surface area (m2), means ± SD | 1.58 ± 0.14 | 1.57 ± 0.13 | 0.498 |
| Hypertension, n (%) | 112 (30.6) | 113 (23.7) | 0.028 |
| Diabetes mellitus, n (%) | 42 (11.5) | 44 (9.2) | 0.303 |
| Tuberculosis, n (%) | 9 (2.5) | 14 (2.9) | 0.832 |
| Breast-conserving surgery, n (%) | 201 (55.1) | 345 (72.3) | < 0.001 |
| Tumor size (cm), mean ± SD | 2.7 ± 2.0 | 2.3 ± 1.4 | 0.002 |
| Positive lymph node, means ± SD | 2.6 ± 5.2 | 1.2 ± 3.4 | < 0.001 |
| ER, n (%) | 268 (74.0) | 312 (66.0) | 0.012 |
| PR, n (%) | 225 (62.2) | 280 (59.2) | 0.392 |
| Her-2, n (%) | 108 (29.5) | 133 (27.9) | 0.645 |
| CA 15–3, means ± SD | 56.3 ± 202.0 | 24.0 ± 136.5 | 0.009 |
| TNM staging, n (%) | < 0.001 | ||
| I/II | 248 (67.8) | 410 (86.0) | |
| III/IV | 118 (32.2) | 67 (14.0) | |
| Taxane-based regimen, n (%) | 245 (66.9) | 184 (38.6) | < 0.001 |
| CBC (before chemotherapy), means ± SD | |||
| Hemoglobin (g/dL) | 12.9 ± 1.2 | 13.0 ± 1.4 | 0.426 |
| Platelet (× 103/µL) | 262 ± 63 | 269 ± 65 | 0.168 |
| Neutrophil (× 103/µL) | 3.725 ± 1.550 | 3.815 ± 1.420 | 0.383 |
| Lymphocyte (× 103/µL) | 1.905 ± 0.603 | 1.971 ± 0.621 | 0.119 |
| CBC (5 days after chemotherapy), means ± SD | |||
| Hemoglobin (g/dL) | 10.9 ± 1.7 | 11.5 ± 1.1 | < 0.001 |
| Platelet (× 103/µL) | 221 ± 82 | 226 ± 62 | 0.378 |
| Neutrophil (× 103/µL) | 3.329 ± 2.278 | 3.067 ± 1.343 | 0.052 |
| Lymphocyte (× 103/µL) | 0.867 ± 0.374 | 1.561 ± 0.593 | < 0.001 |
| SERM, n (%) | 139 (38.0) | 221 (46.3) | 0.017 |
| LHRH, n (%) | 112 (30.6) | 190 (39.8) | 0.006 |
| Aromatase inhibitor, n (%) | 148 (40.4) | 121 (25.4) | < 0.001 |
| Radiation treatment, n (%) | 292 (79.8) | 401 (84.1) | 0.122 |
| Herceptin, n (%) | 101 (27.6) | 130 (27.3) | 0.938 |
FN febrile neutropenia, SD standard deviation, BSA body surface area, ER estrogen receptor, PR progesterone receptor, Her-2 human epidermal growth factor receptor 2, CA cancer antigen, CBC complete blood count, SERM selective estrogen receptor modulator, LHRH luteinizing hormone-releasing hormone, F/U follow-up.
Table 2.
Clinical demographic characteristics of patients with and without febrile neutropenia in the testing dataset.
| Parameters | FN group (n = 43) | non-FN group (n = 47) | p value |
|---|---|---|---|
| Age (years), means ± SD | 0.050 | ||
| ≤ 50 | 13 (30.2) | 24 (51.1) | |
| > 50 | 30 (69.8) | 23 (48.9) | |
| Body surface area (m2), means ± SD | 1.61 ± 0.14 | 1.63 ± 0.14 | 0.383 |
| Hypertension, n (%) | 12 (27.9) | 11 (23.4) | 0.638 |
| Diabetes mellitus, n (%) | 5 (11.6) | 5 (10.6) | 1.000 |
| Tuberculosis, n (%) | 0 (0) | 0 (0) | 1.000 |
| Breast-conserving surgery, n (%) | 28 (65.1) | 37 (78.7) | 0.166 |
| Tumor size (cm), mean ± SD | 2.7 ± 1.3 | 2.5 ± 1.2 | 0.490 |
| Positive lymph node, means ± SD | 1.7 ± 4.0 | 0.3 ± 1.4 | 0.038 |
| ER, n (%) | 34 (79.1) | 26 (55.3) | 0.046 |
| PR, n (%) | 26 (60.5) | 18 (38.3) | 0.057 |
| Her-2, n (%) | 12 (27.9) | 7 (14.9) | 0.196 |
| CA 15–3, means ± SD | 15.2 ± 12.0 | 11.7 ± 7.8 | 0.140 |
| TNM staging, n (%) | 0.003 | ||
| I/II | 33 (76.7) | 46 (97.9) | |
| III/IV | 10 (23.3) | 1 (2.1) | |
| Taxane-based regimen, n (%) | 23 (53.5) | 14 (29.8) | 0.032 |
| CBC (before chemotherapy), means ± SD | |||
| Hemoglobin (g/dL) | 13.0 ± 1.6 | 13.1 ± 1.2 | 0.940 |
| Platelet (× 103/µL) | 263 ± 56 | 265 ± 77 | 0.590 |
| Neutrophil (× 103/µL) | 3.661 ± 1.574 | 4.006 ± 1.683 | 0.550 |
| Lymphocyte (× 103/µL) | 2.024 ± 0.575 | 2.193 ± 0.640 | 0.250 |
| CBC (5 days after chemotherapy), means ± SD | |||
| Hemoglobin (g/dL) | 10.7 ± 1.1 | 5.314 ± 1.593 | 0.017 |
| Platelet (× 103/µL) | 219 ± 99 | 11.3 ± 1.0 | 0.170 |
| Neutrophil (× 103/µL) | 3.977 ± 3.468 | 3.348 ± 1.208 | 0.220 |
| Lymphocyte (× 103/µL) | 0.838 ± 0.339 | 1.703 ± 0.683 | < 0.001 |
| SERM, n (%) | 13 (30.2) | 23 (48.9) | 0.087 |
| LHRH, n (%) | 11 (25.6) | 20 (42.6) | 0.121 |
| Aromatase inhibitor, n (%) | 22 (51.2) | 14 (29.8) | 0.053 |
| Radiation treatment, n (%) | 35 (81.4) | 41 (87.2) | 0.564 |
| Herceptin, n (%) | 13 (30.2) | 10 (21.3) | 0.346 |
FN febrile neutropenia, SD standard deviation, ER estrogen receptor, PR progesterone receptor, Her-2 human epidermal growth factor receptor 2, CA cancer antigen, CBC complete blood count, SERM selective estrogen receptor modulator, AI aromatase inhibitor, LHRH luteinizing hormone-releasing hormone, F/U follow-up.
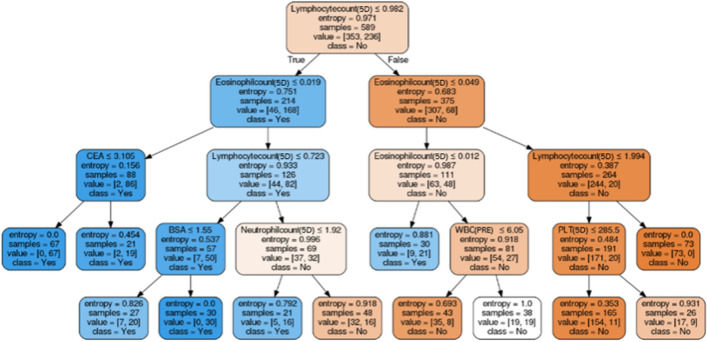
Factors associated with FN were selected by machine learning algorithms. The performances of prediction models in the testing dataset are presented in Table 3. XGboosting showed the best performance with an AUC of 0.908. The AUC of each algorithm is presented in Fig. 1. Data collected for hierarchical levels were used as input data for the decision tree model. The root node of the decision tree was lymphocyte count 5 days after chemotherapy, and the cut-off was 0.982 (× 103/µL) (Fig. 2).
Table 3.
Performance of machine learning algorithms for the prediction of febrile neutropenia.
| LR | DT | XGboosting | LASSO | SVM | ANN | |
|---|---|---|---|---|---|---|
| AUC | 0.870 | 0.855 | 0.908 | 0.862 | 0.880 | 0.865 |
| Accuracy | 0.781 | 0.759 | 0.816 | 0.805 | 0.782 | 0.782 |
| Sensitivity | 0.878 | 0.707 | 0.829 | 0.805 | 0.829 | 0.854 |
| Specificity | 0.696 | 0.804 | 0.804 | 0.804 | 0.739 | 0.717 |
| PPV | 0.720 | 0.763 | 0.791 | 0.786 | 0.739 | 0.729 |
| NPV | 0.865 | 0.755 | 0.841 | 0.822 | 0.829 | 0.846 |
LR logistic regression, DT decision tree, LASSO least absolute shrinkage and selection operator, SVM support vector machine, ANN artificial neural network, AUC area under the curve, PPV positive predictive value, NPV negative predictive value.
Figure 1.

The AUC of each algorithm shown using colored lines. The image was drawn in Python 3.6. AUC area under the curve, ROC receiver operating characteristic, TPR true positive rate, LASSO least absolute shrinkage and selection operator regression, SVM support vector machine, ANN artificial neutral network.
Figure 2.
Detailed cut-off values displayed in a decision tree model. The image was drawn in Python 3.6. 5D 5 days after chemotherapy, CEA carcinoembryonic antigen, BSA body surface area, WBC white blood cell, PRE pretreatment, PLT platelet.
Discussion
In the present study, multivariate analysis demonstrated predictive factors for FN, including age, staging, and taxane-based regimen. The lymphocyte count 5 days after chemotherapy was also a strong predictive factor for FN. Based on these findings, logistic regression showed an AUC of 0.870 for validation. Even in machine learning, the lymphocyte count 5 days after chemotherapy was the strongest predictive factor for FN. The AUC improved by machine learning was 0.908, although with a slight difference.
Chemotherapy regimen is one of the main determinants of the risk of FN as shown in the present study. In practice, some regimens are more myelotoxic than others4. Taxane- and anthracycline-based regimens were previously reported as regimens with a high risk of FN when used for the treatment of breast cancer4. CMF is less toxic than AC or FA(E)C3. Because the rates of FN for these and similar regimens vary considerably, it is difficult to determine the actual risk15. In addition to the regimen-specific risks, evaluating the individual risk factors in each patient can be valuable in determining appropriate treatment16.
The cycle number of the current round of chemotherapy is an important factor for FN, although only the first cycle was investigated in the present study11. Previous studies have demonstrated that the first cycle of chemotherapy is related with a greater risk for the development of FN than subsequent cycles2,17. The decreased risk of FN after subsequent cycles may be the result of clinicians’ understanding of the nadir of blood counts and clinical features of patients during the first round of chemotherapy. The history of FN in a patient is a generally recognized risk factor for the development of FN11,18. Guidelines recommend the use of G-CSF as secondary prophylaxis in patients who develop FN during the equitoxic chemotherapy regimen, considering the patient’s prior tolerance to chemotherapy11.
Blood counts may indicate comorbid conditions, the extent of disease, or individual response to cytotoxic chemotherapy. Even in the present study, the lymphocyte count 5 days after chemotherapy was the strongest predictive factor for FN. The slow decrease of the nadir of the lymphocyte count is apparently protective against FN19. Higher lymphocyte counts 5 days after chemotherapy may reflect higher resistance to infection, as these patients may have the potential to activate their cellular or humoral immunity rapidly19–21. However, the explicit role of lymphocytes in the development of FN remains to be elucidated.
Previous studies have reported that prophylactically administered G-CSF is significantly related with a lower risk of FN10,22. Primary prophylaxis with G-CSF can decrease the need for dose delay or reduction, antibiotics, and hospital admission4,18. Moreover, prophylactic G-CSF reduces early death, including infection-related mortality10,22. Currently, guidelines recommend prophylaxis with G-CSF when the FN risk is high (> 20%) on the basis of either chemotherapy regimen alone (high-risk regimen) or the combination of chemotherapy regimen (intermediate-risk regimen with 10–20% FN risk) and personal risk factors4,9.
In this study, some machine learning algorithms outperformed logistic regression. This phenomenon has been observed in many prediction models using machine learning13,14. Logistic regression models are an extension of linear models using logit function as a link. Therefore, a non-linear interaction between associated factors and the outcome may not be fitted optimally. Using non-linear functions, machine learning recognizes the patterns present in the medical data and predicts the outcomes by minimizing the error23.
Our machine learning algorithm can be implemented in a clinical workflow to bridge the gap between research and practice. Considering that the period until the development of FN was 10.2 ± 2.8 days in our study, clinicians may use parameters including the complete blood count/differential blood count 5 days after chemotherapy to decide whether to use prophylactic G-CSF. Therefore, we envision a software tool for the prediction of FN after chemotherapy in patients with breast cancer (Supplementary Fig. S1). The software provides the predicted probability of FN if parameters regarding FN are entered using a user-friendly interface.
To the best of our knowledge, this study is the first to improve the prediction of FN after chemotherapy in patients with breast cancer by machine learning. Our predictive model defines the risk of FN after chemotherapy. The current model represents progress in predicting FN and optimizing protection against its development. This machine learning model has the potential to become a routine tool in daily clinical practice to guide the use of prophylactic G-CSF.
The present study has some limitations. First, our data showed the high rate of FN, considering that the relevant literature reported the incidence of FN as 10–50%8,9,24–28. In the current study, inpatients were purely selected because they had more lucid serial data. However, hospitalized patients usually have more severe status rather than outpatients, which can cause a selection bias. Moreover, according to the criteria of our national health insurance coverage G-CSF should be given at less than 500 of neutrophils or at less than 1,000 of neutrophils if patients have fever. Therefore, generalization should be avoided. Second, only the first cycle of each regimen was investigated. Subsequent cycles were not regarded as independent since FN may be affected by the accumulation of drugs during previous cycles. Thus, a more customized model needs to be developed for the subsequent cycles. Lastly, the decision to use G-CSF was not analyzed. Regarding the use of G-CSF, cost and national insurance coverage should be considered jointly.
In conclusion, machine learning improved the prediction of FN in patients undergoing chemotherapy for breast cancer. In these high-risk patients, primary prophylaxis with G-CSF could be considered. With this strategy, patient safety could be ensured during chemotherapy in patients with breast cancer.
Methods
Study design
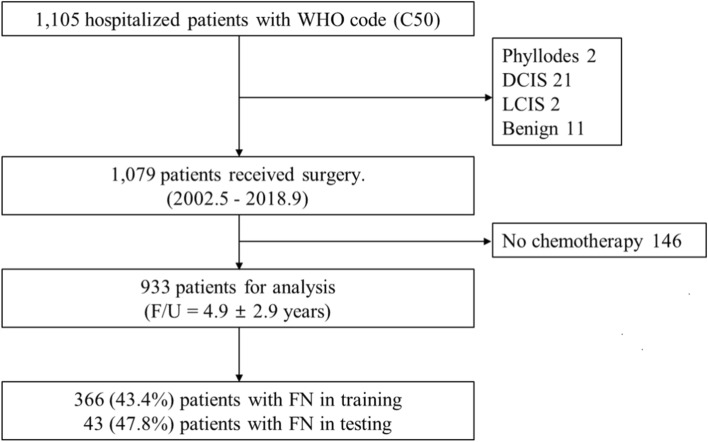
Medical records of 1,105 hospitalized patients diagnosed with breast cancer between May 2002 and September 2018 in the Department of Breast and Endocrine surgery, Hallym University Sacred Heart Hospital were selectively reviewed for inclusion. Among them, 1,079 patients underwent surgery and were confirmed pathologically as having breast cancer. Finally, of the 1,079 patients, 933 who received chemotherapy after surgery were included in this study (Fig. 3). All patients received the first cycle of full-dose chemotherapy in the hospital, and biometric data were recorded during the treatment period. This study was approved by the Institutional Review Board of Hallym University Sacred Heart Hospital (No. 2018-04-018) and adhered to the tenets of the Helsinki Declaration. The requirement for written informed consent was waived by the Institutional Review Board.
Figure 3.
Flow diagram depicting the study design. The image was drawn in Microsoft PowerPoint 2016. WHO World Health Organization, DCIS ductal carcinoma in situ, LCIS lobular carcinoma in situ, F/U follow-up, FN febrile neutropenia.
Datasets
The entire cohort was divided into a training and testing dataset which were mutually exclusive. The training dataset was built with 843 patients treated between May 2002 and January 2018. The testing dataset consisted of 90 patients treated between February 2018 and September 2018 and was used to validate the performance of machine learning models. In both datasets, patients who had any missing data for clinical, pathological, or therapeutic variables of interest were excluded from the analyses.
Assessments
Demographic, clinical, pathological, and therapeutic information were obtained from the medical records of study participants. Tumors were staged according to the 8th edition of the American Joint Committee on Cancer staging system. FN was defined as the incidence of fever of 38.3 °C or 38.0 °C for over 1 h orally, and neutrophil count < 500 or 500–999/mm3 with predicted drop to < 500/mm3 over next 48 h9. During the first cycle of chemotherapy, each patient was monitored carefully for the development of FN.
Analysis
To extract the factors associated with FN, classical and recent machine learning algorithms were applied. Least absolute shrinkage and selection operator regression, ridge regression, support vector machine, decision tree, XGboosting, and artificial neural network were used for machine learning algorithms. Conventional stepwise logistic regression was used as a reference method. Factors associated with FN were selected from the dataset using the recursive feature elimination method29. The p value used to select and remove the factor in the forward stepwise process was 0.05. Factor selection and model construction were done on the platform with scikit-learn 0.20 in Python 3.6 (Python Software Foundation, Wilmington, DE). Prediction models were constructed for each machine learning algorithm with the training dataset using the optimal feature subset for each machine-learning algorithm. Five-fold cross-validation was used for evaluation. The performance of the prediction models was evaluated in the testing dataset. The area under the curve (AUC) was used as the main measurement.
Supplementary information
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF), and the Korean government fund (MSIT) (No. NRF-2017M3A9E8033207, 2019R1G1A1004679).
Author contributions
B.C. contributed to data analysis, manuscript preparation, and Figs. (2 and 3). K.K. contributed to data analysis and manuscript review. S.B. contributed to data collection and manuscript review. Y.S. contributed to study design, data analysis, manuscript preparation, and Figs. (1 and S1). All authors read and approved the final manuscript.
Data availability
All the data supporting the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-71927-6.
References
- 1.de Naurois J, et al. Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2010;21:v252–v256. doi: 10.1093/annonc/mdq196. [DOI] [PubMed] [Google Scholar]
- 2.Hosmer W, Malin J, Wong M. Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal, and prostate cancer. Support. Care Cancer. 2011;19:333–341. doi: 10.1007/s00520-010-0821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100:228–237. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- 4.Aapro MS, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur. J. Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–2266. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 6.Lyman GH, et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer. 2011;117:1917–1927. doi: 10.1002/cncr.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang J. Chemotherapy dose reduction and delay in clinical practice. Evaluating the risk to patient outcome in adjuvant chemotherapy for breast cancer. Eur. J. Cancer. 2000;36:S11–S14. doi: 10.1016/S0959-8049(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 8.Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colony-stimulating factors for febrile neutropenia during cancer therapy. N. Engl. J. Med. 2013;368:1131–1139. doi: 10.1056/NEJMct1210890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford J, et al. Myeloid growth factors, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2017;15:1520–1541. doi: 10.6004/jnccn.2017.0175. [DOI] [PubMed] [Google Scholar]
- 10.Herbst C, et al. Prophylactic antibiotics or G-CSF for the prevention of infections and improvement of survival in cancer patients undergoing chemotherapy. Cochrane Database Syst. Rev. 2009;1:CD007107. doi: 10.1002/14651858.CD007107.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Bozcuk H, et al. A prospectively validated nomogram for predicting the risk of chemotherapy-induced febrile neutropenia: a multicenter study. Support. Care Cancer. 2015;23:1759–1767. doi: 10.1007/s00520-014-2531-6. [DOI] [PubMed] [Google Scholar]
- 12.Lyman GH, Lyman CH, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005;10:427–437. doi: 10.1634/theoncologist.10-6-427. [DOI] [PubMed] [Google Scholar]
- 13.Mirza B, et al. Machine learning and integrative analysis of biomedical big data. Genes. 2019;10:87. doi: 10.3390/genes10020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low SK, Zembutsu H, Nakamura Y. Breast cancer: The translation of big genomic data to cancer precision medicine. Cancer Sci. 2018;109:497–506. doi: 10.1111/cas.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale DC, McCarter GC, Crawford J, Lyman GH. Myelotoxicity and dose intensity of chemotherapy: reporting practices from randomized clinical trials. J. Natl. Compr. Canc. Netw. 2003;1:440–454. doi: 10.6004/jnccn.2003.0038. [DOI] [PubMed] [Google Scholar]
- 16.Dranitsaris G, et al. Identifying patients at high risk for neutropenic complications during chemotherapy for metastatic breast cancer with doxorubicin or pegylated liposomal doxorubicin: the development of a prediction model. Am. J. Clin. Oncol. 2008;31:369–374. doi: 10.1097/COC.0b013e318165c01d. [DOI] [PubMed] [Google Scholar]
- 17.Crawford J, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J. Natl. Compr. Canc. Netw. 2008;6:109–118. doi: 10.6004/jnccn.2008.0012. [DOI] [PubMed] [Google Scholar]
- 18.Kelly S, Wheatley D. Prevention of febrile neutropenia: use of granulocyte colony-stimulating factors. Br. J. Cancer. 2009;101:S6–S10. doi: 10.1038/sj.bjc.6605269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi CW, et al. Early lymphopenia as a risk factor for chemotherapy-induced febrile neutropenia. Am. J. Hematol. 2003;73:263–266. doi: 10.1002/ajh.10363. [DOI] [PubMed] [Google Scholar]
- 20.Blay JY, et al. Early lymphopenia after cytotoxic chemotherapy as a risk factor for febrile neutropenia. J. Clin. Oncol. 1996;14:636–643. doi: 10.1200/JCO.1996.14.2.636. [DOI] [PubMed] [Google Scholar]
- 21.Ray-Coquard I, et al. Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy. Br. J. Cancer. 2003;88:181–186. doi: 10.1038/sj.bjc.6600724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J. Clin. Oncol. 2007;25:3158–3167. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 23.Dreiseitl S, Ohno-Machado L. Logistic regression and artificial neural network classification models: a methodology review. J. Biomed. Inform. 2002;35:352–359. doi: 10.1016/S1532-0464(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 24.White L, Ybarra M. Neutropenic Fever. Hematol. Oncol. Clin. North. Am. 2017;31:981–993. doi: 10.1016/j.hoc.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Engert A, et al. Incidence of febrile neutropenia and myelotoxicity of chemotherapy: a meta-analysis of biosimilar G-CSF studies in breast cancer, lung cancer, and non-Hodgkin's lymphoma. Onkologie. 2009;32:599–604. doi: 10.1159/000232580. [DOI] [PubMed] [Google Scholar]
- 26.Aapro MS, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur. J. Cancer. 2006;42:2433–2453. doi: 10.1016/j.ejca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Lyman GH, Kuderer NM, Djulbegovic B. Prophylactic granulocyte colony-stimulating factor in patients receiving dose-intensive cancer chemotherapy: a meta-analysis. Am. J. Med. 2002;112:406–411. doi: 10.1016/S0002-9343(02)01036-7. [DOI] [PubMed] [Google Scholar]
- 28.Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer. 2005;103:1916–1924. doi: 10.1002/cncr.20983. [DOI] [PubMed] [Google Scholar]
- 29.Romero E, Sopena JM. Performing feature selection with multilayer perceptrons. IEEE Trans. Neural Netw. 2008;19:431–441. doi: 10.1109/TNN.2007.909535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data supporting the findings of this study are available from the corresponding author upon reasonable request.