Abstract
Currently, there is no effective vaccine for tackling the ongoing COVID-19 pandemic caused by SARS-CoV-2 with the occurrence of repeat waves of infection frequently stretching hospital resources beyond capacity. Disease countermeasures rely upon preventing person-to-person transmission of SARS-CoV2 so as to protect front-line healthcare workers (HCWs). COVID-19 brings enormous challenges in terms of sustaining the supply chain for single-use-plastic personal and protective equipment (PPE). Post-COVID-19, the changes in medical practice will drive high demand for PPE. Important countermeasures for preventing COVID-19 transmission include mitigating potential high risk aerosol transmission in healthcare setting using medical PPE (such as filtering facepiece respirators (FFRs)) and the appropriate use of face coverings by the general public that carries a lower transmission risk. PPE reuse is a potential short term solution during COVID-19 pandemic where there is increased evidence for effective deployment of reprocessing methods such as vaporized hydrogen peroxide (30 to 35% VH2O2) used alone or combined with ozone, ultraviolet light at 254 nm (2000 mJ/cm2) and moist heat (60 °C at high humidity for 60 min). Barriers to PPE reuse include potentially trust and acceptance by HCWs. Efficacy of face coverings are influenced by the appropriate wearing to cover the nose and mouth, type of material used, number of layers, duration of wearing, and potentially superior use of ties over ear loops. Insertion of a nose clip into cloth coverings may help with maintaining fit. Use of 60 °C for 60 min (such as, use of domestic washing machine and spin dryer) has been advocated for face covering decontamination. Risk of virus infiltration in improvised face coverings is potentially increased by duration of wearing due to humidity, liquid diffusion and virus retention. Future sustained use of PPE will be influenced by the availability of recyclable PPE and by innovative biomedical waste management.
Keywords: COVID-19, PPE, Face coverings, Reuse, Sustainability, Waste management
Graphical abstract
1. Introduction
Since first reported as a cause of serious human pneumonia in Wuhan, Hubei, China in December 2019, the novel coronavirus COVID-19 has spread worldwide with devastating consequences. At the time of writing (29th August 2020), there has been 25.1 million cases of COVID-19 reported (in accordance with the applied case definitions and testing strategies in the affected countries) including 845,343 deaths (European Centre for Disease Control and Prevention, 2020). There is evidence of resurgence of the SARS-CoV-2 globally with the emergence of second waves of infection in many countries (European Centre for Disease Control and Prevention, 2020). Hong Kong is addressing its third wave of COVID-19 infections, where Australia is battling a second wave of infection having previously reduced viral transmission cases close to zero. COVID-19 has also emerged strongly in developing low-resource countries that already have significant healthcare challenges, such as across the African continent that is also challenged with Acquired Immunodeficiency Syndrome (AIDS) and Mycobacterium tuberculosis as co-morbidities (African Centre for Disease Control and Prevention, 2020).
Currently, there is still no effective vaccine or anti-viral therapy for COVID-19 with reliance upon the prevention of transmission by way of imposing a lockdown, cocooning, social distancing, and wearing of face masks in order to protect vulnerable groups and to safeguard frontline healthcare professionals (Murphy et al., 2020) Epidemiological studies show that social distancing prevents person-to-person transmission of SARS-CoV-2, which is relevant given that there is growing recognition that asymptomatic carriers may also contribute to this transmission (Li et al., 2020). There is evidence to suggest that COVID-19 is a super-spreader of infectious airborne viral particles where several people can be infected at the same time (Li et al., 2020). Clusters of COVID-19 infection reflect vulnerabilities, such as nursing homes catering for the elderly or meat packing industry (Carswell, 2020), where there are challenges in practicing appropriate safe social distancing. However, recent epidemiology studies for past 14 days from the Health Service Executive in the Republic of Ireland shows that 55% of COVID-19 occurred in people aged below 35 years of age that would not have been considered to be a highly susceptible group earlier, thus highlighting the challenges of reopening countries too quickly. Global studies have revealed that there is insufficient herd immunity to COVID-19 due to low exposure levels typically at ca. 5% of total population (Carswell, 2020). Many countries have introduced innovative approaches to flattening the curve of infection, such as, in the Republic of Ireland where a mobile phone COVID-19 tracker alerts users as to the status of SARS-CoV-2 in the community.
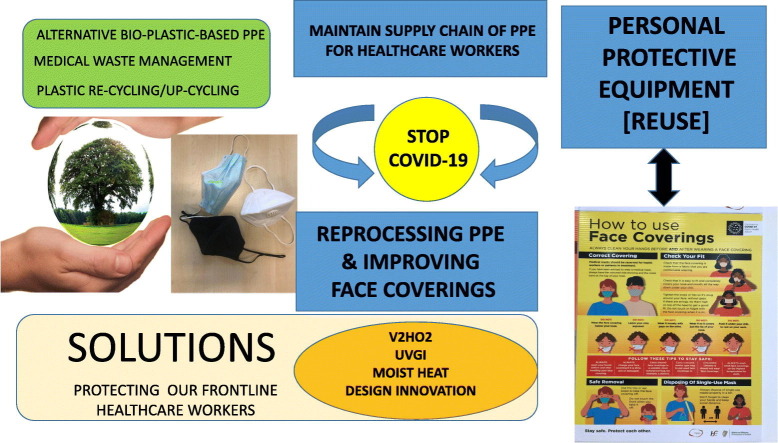
In pandemic situations, such as the ongoing COVID-19 pandemic, hospital resources are frequently stretched beyond capacity (Derraik et al., 2020). There is a pressing need to sustain the supply chain of disposable personal and protective equipment (PPE) in order to prevent the spread of COVID-19 to and from healthcare workers (HCWs) and patients. The availability and effective use of PPE are essential that includes masks, eye protection, gloves, gowns, and, for aerosol generating procedures in particular, N95 and KN95 filtering facepiece respirators (FFRs) or equivalent (Derraik et al., 2020; Rubio-Romero et al., 2020). This interest in PPE reuse, arising from the ongoing COVID-19 pandemic, is also attested by the 52 journal citations that used best-available information provided in our initial opinion article on this topic that was published on 4th April 2020 (Rowan and Laffey, 2020).
Subsequently, there has been a pressing need to provide safe and simple solutions to disinfect and reuse improvised or homemade (non-certified) cloth face coverings in order to prevent person-to-person transmission in the community and workplace. Wearing a face covering reduces the spread of coronavirus in the community as it helps to reduce the spread of respiratory droplets from people infected with SARS-CoV-2. Use of face coverings helps to stop people who are not aware they have the virus from spreading (HSE, 2020). Face coverings are not recommended for children under the age of 13 unless attending a healthcare setting, as young children may not follow the advice about wearing a face covering correctly such as not touching it. Silva et al. (2020) noted that over 50 countries have mandatory wearing of facemasks or coverings for the general public. Medical face masks are typically worn by healthcare workers and by people in self-isolation who cannot keep a distance of 2 m between themselves and other people in their household (HSE, 2020). For those who find it difficult to wear a cloth face covering, it's appropriate to wear a full face visor or face shield instead. However, use of face shields are not as good as wearing a face covering, but they provide some level of protection (CDC, 2020). The visor should wrap around the sides of your face (ear to ear) and extend to below the chin. Reusable visors should be cleaned after each use and then stored in a clean place until needed. Coordinating the supply chain for PPE in the midst of a pandemic with many closed borders and limited freight compounds the challenge (Derraik et al., 2020). Derraik et al. (2020) also noted that people need to have trust in systems set up to support supply chain for PPE in the workplace. Arising from the unprecedented surge in single-use plastic PPE usage, there also appears to be a need to consider effective waste management and recycling strategies to limit SARS-CoV-2 cross-transmission.
Therefore, given the aforementioned, there is a pressing need to understand the efficacy of disinfection methods and for reuse of medical PPE in high risk healthcare environment where there is a supply chain shortage. Also, to define simple solutions for reuse of non-certified cloth or fabric face coverings used by general public in low risk settings, which the focus of this study. Consequently, we carried out a rapid review to summarize the literature with two inter-related aims – first, to examine the current knowledge about survival of SARS-Cov-2 on surfaces and to provide an update on studies conducted in the Republic of Ireland (Rowan and Laffey), − second, to examine current knowledge on the efficacy and potential barriers for implementing key PPE disinfection methods against SAR-CoV-2; and third, to examine biomedical waste management strategies to meet surge in PPE usage across the globe. Given the very recent discovery of SARS-CoV-2, our study also encompassed appropriate review articles and primary data sources that focused on SARS-CoV-1 as this is sister virus from the same species (Gorbalenya et al., 2020; Derraik et al., 2020).
2. Methods
We carried out a rapid review of the literature to inform the research questions that addressed a search strategy involved Google Scholar, Scopus, Web of Science, and PubMed as per approached described recently by Tiedeken et al. (2017). Searches were restricted to publication from 1 January 2003 (as the first recorded human infection of SARS-1 occurred in November 2002 (as per Derraik et al. (2020)) and 8 August 2020. Titles and/or abstracts were screened by the first author and where appropriate, full text of individual research studies, opinion pieces and reviews were consulted. Key words used were PPE; reuse; reprocessing; disinfection; decontamination; N95; COVID-19; SARS-CoV-1; SARS-CoV-2; UV; hydrogen peroxide vapour (VH2O2); ozone; waste management; recycling. Data extraction and rapid analysis was supplemented by conducting a short observation study where the first author noted the types of facemasks and face coverings worn by the public on entering a large shopping centre in the Republic of Ireland on 14th and 15th August 2020.
3. Knowledge that informed PPE use and potential reuse from a Republic of Ireland perspective
At the time of initial writing (3rd April, 2020) (Rowan and Laffey et al., 2020), the number of confirmed COVID-19 cases had reached 1 million, including 51,515 deaths, which highlights that a 25-fold increase in the prevalence of SARS-Cov-2 has occurred in only 4 months (European Centre for Disease Prevention and Control, 2020). Rowan and Laffey (2020) had predicted an unprecedented high demand for PPE across the globe and therefore, it was prudent to consider PPE reuse as a potential option to meet the critical shortage in the supply chain for frontline HCWs. Rowan and Laffey (2020) intimated that the structure of SARS-Cov-2 is such that is sensitive to harsh environmental stresses. Moreover, the structure of SARS-CoV-2, and related coronaviruses, includes a RNA genome, a protein capsid, and an outer envelope. Viral inactivation is linked to the alteration of one of these structural elements by an environmental stress, such as, heating, ultraviolet light, and biocides (Bentley et al., 2016; Pinon and Vialette, 2018; Gorbalenya et al., 2020). The proteins and lipids of the envelope may be disrupted more easily than the other parts of the virus (Howie et al., 2008; Pinon and Vialette, 2018). Thus, naked viruses are generally more resistant than enveloped viruses (such as SARS-Cov-2 and other coronaviruses) to similar levels of the same or different adverse environmental conditions (Fitzgibbon and Sagripanti, 2008; Pinon and Vialette, 2018). The enveloped structure of SARS-Cov-2 (Meo et al., 2020), and Influenza (cited in Li, 2016), is such that these viruses are more likely to be sensitive to disinfection technologies (Pinon and Vialette, 2018; Rowan and Laffey, 2020). Kampf et al. (2020) had also analysed 22 studies of different human coronaviruses where SARS, MERS, HCoV (but not including COVID-19) were efficiently inactivated by disinfection on variety of contact surface using 62 to 71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypocholorite within 1 min of exposure, but survived on untreated surfaces for up to 9 days. van Doremalen et al. (2020) also conducted tests that showed that SARS-CoV-1 remains on plastic, stainless steel, copper and cardboard for up to 72 h. These and other studies (Zhao et al., 2020) have informed selection of many current disinfection procedures to address SARS-CoV-2 pandemic, including PPE reuse.
Given that disposable, plastic-based, PPE (gowns, eye protection, gloves, face masks, filtering facepiece respirators (FFRs)) are heat sensitive, existing healthcare technologies were considered to be either not available, unsuitable or not configured for reprocessing of PPE in healthcare for emergency use (Rowan and Laffey, 2020). However, potential solutions for effective reprocessing of PPE that considered virus inactivation, material compatibility and device functionality (filtration efficacy, penetration, fit test and so forth) post processing included use of low temperature hydrogen peroxide vapour (VH2O2), ultraviolet germicidal light (UVGI), moist heat, and use of weak bleach for liquid decontamination (Rowan and Laffey, 2020; CDC, 2020). McEvoy and Rowan (2019) had published a comprehensive review on the background and efficacy of VH2O2 for terminal sterilization of medical devices that was used to provide supportive technical information in choice of procedures. This information was supported by prior findings of Bentley et al. (2016) who reported on 4 log10 viral titre reductions for the recalcitrant naked Norovirus in a variety of hospital settings (stainless steel, glass, vinyl flooring, ceramic tile, PVC plastic cornering) using 30% w/w hydrogen peroxide vapour. Rowan (2019) had also reviewed potential microbial mechanistic information underpinning UV disinfection that also provided supportive foundation knowledge for the potential use of pulsed light technology for PPE.
Information underpinning these candidate technology solutions included best-published information of efficacy of these approaches to surface disinfection cornoavirus (COVID-19) or related viruses and surrogate biological indicator organisms on different surface materials (Kampf et al., 2020).
The FDA had authorized use of VH2O2 technology, under emergency use authorization (EUA), for the reprocessing of critical N95 face masks in the United States in order to help address COVID-19 transmission. This was informed by Columbus-based Battelle process studies (Battelle, 2016). Given exceptional circumstances, original equipment manufacturers (OEMs) of PPE had also suggested possible appropriate reprocessing strategies, but they also reiterated that their products had been manufactured with the sole intention of single use. The contingency plan to be adopted in hospitals on the west of Ireland was to procure, install and seek approval from competent authority for the deployment of VH2O2 (Bioquell BQ50 system) for filtering face-piece respirators (FFRs) and surgical gowns, UV technologies (NanoClave low-pressure UVGI system and Claranor Pulsed Light system) for simple PPE such as face shields, and use of mild sodium hypochlorite (4000 ppm) for liquid decontamination of critical Starmed hoods. The VH202, UVGI and mild liquid disinfection strategies have been set up, but there remains a requirement to gain trust and confidence by HCWs for PPE reuse post treatments.
4. Currents status and challenges for the reprocessing of PPE for COVID-19
Coronavirus SARS-CoV-2 transmission has become a significant global challenge where the number of confirmed cases has increased twenty five times in just over 4 months to ca. 25 million cases (Rowan and Laffey, 2020) highlighting the high demand for PPE (European Centre for Prevention and Disease Control, 2020). There has been increasing concerns about the potential exposure to frontline healthcare workers from SARS-CoV-2, and the commensurate need to deploy effective PPE disinfection procedures to ameliorate the threat of cross-transmission and infection (Barceló, 2020; Faridi, 2020). Silva et al. (2020) have reported that over 50 countries across the globe have mandated the use of PPE by the general public to help prevent person-to-person transmission of SARS-CoV2.
Several authors have reported on the viability of SARS-CoV1 and SARS-CoV-2 on various contact surface such as printed paper, printed tissue, cloth, wood, glass, banknotes, plastic, stainless steel, surgical mask layers over different environmental temperatures, relative humidity and durations (Li et al., 2003; Lai et al., 2005; Pagat et al., 2007; Chan et al., 2020; Chin et al., 2020; Fischer et al., 2020; Kasloff et al., 2020; Behzadinasab et al., 2020; Biryukov et al., 2020). In general, lower environmental temperatures support the longer survival of SARS-CoV-2 on materials as reported by Chin et al. (2020) where only a 0.7 log10 reduction was observed for SAR-CoV-2 at 4 °C after 14 days compared with ≥4.5 log10 reduction at 22 °C (room temperature) after 14 days and ≥ 4.5 log reduction at 37 °C after just 2 days. Similarly, Chan et al. (2020) also noted only a 2 log10 reduction of SARS-CoV-1 at 4 °C after 14 days when the virus was inoculated onto glass surfaces. The longer survival of SARS-CoV-2 at colder temperatures may have future implications for viral persistence on contaminated face coverings as we are approaching the winter flu season.
However, public health practices that have been put in place to mitigate the spread of SARS-CoV-2 are likely to have a positive impact on the occurrence of influenza cases given that these viruses share similar modes of transmission to cause illness. Derraik et al. (2020) comprehensively reported on the viability of SARS-CoV-1 and SARS-CoV-2 on different contact surfaces, without and with UV or heat treatments, and noted the importance of virus load and inoculum size on inactivation performance. Lai et al. (2005), who looked specifically at PPE, highlighted the variability in SARS-CoV-1 viability of 2 days on a disposable polypropylene gown and 24 h on a cotton gown for same 6 log10 reduction. Akin to studies reported by Derraik et al. (2020), we also observed that the majority of researchers used medium tissue culture infective dose (TCID50) to report inactivation of SARS-CoV-1 and SARS-CoV-2 on various surfaces. Kasloff et al. (2020) simulated typical infectious body fluids of infective patients and showed that to achieve a 5 log10 reduction in SARS-CoV2 at 20 °C (room temperature), it took ca 14 days on nitrile gloves and as much as 21 days on plastic face shields, N100 respirators and polyethylene overalls, with some residual infectivity evident on N95 respirators after 3 weeks. This highlights the importance of safely discarding soiled PPE, which are not fit for reuse.
Surface disinfection studies against SARS-CoV-2, such as use of sodium hypochlorite, hydrogen peroxide or alcohol (Kampf et al., 2020; van Doremalen et al., 2020), have informed the suitability of different technologies for PPE reuse (Table 1 ). In is notable that Yang et al. (2020) used a chlorine-containing disinfectant spray (2000 mg/L) for treating a variety of contaminated areas in hospitals in Wuhan city, China during SARS-CoV-2 pandemic. All healthcare workers donned significant layers of PPE in the following sequence: white coats, N95 respirator, surgical masks, surgical hat, protective goggles, shoe coverings, isolation gowns, gloves, protective suits, another pair of gloves, protective hoods, and boot coverings where hand disinfection and spraying of 75% ethanol is applied to PPE again on entering the hospital. This highlights the emphasis placed on implementing rigorous infection control strategies to remain safe from SARS-CoV2 and the enormous quantities of PPE used to prevent SARS-CoV-2 transmission.
Table 1.
Frequently cited publications for PPE reuse, decontamination, waste management and recycling.a
Powered-air purifying respirators (PARPs); Filtering Facepiece Respirators (FFRs); Relative Humidity (RH).
The U.S. Food and Drug Administration (FDA) led the way in the strategic authorization of PPE and related medical devices processing under Emergency Use Authorization (EUA) where a number of established sterilization companies have been issued authority to meet these critical supply chain needs arising from this COVID-19 pandemic (FDA, 2020). The common reprocessing technology across these sterilization industries is use of hydrogen peroxide in vapour state (VH2O2) for PPE treatment, where Battelle demonstrated it's potential early in pandemic (Rowan and Laffey, 2020). Stryker STERIZONE VP4 Sterilizer, was approved for N95 face mask reprocessing by the FDA under this EUA, which also incorporates the use of ozone combined with VH2O2 for this purpose. As part of this EUA, the FDA reviews the totality of scientific evidence available, including testing data that was submitted within previous applications supporting device clearance for other uses that considers different types of polymer materials, including materials, consistent with those found in compatible N95 respirators. The FDA also reviews performance data such as sporicidal test, residual analysis, bioburden reduction validation demonstrating logarithmic reductions of a non-enveloped virus challenge; testing regarding material compatibility, functionality and filtration performance of compatible N95 respirators after multiple decontamination cycles; and testing regarding VH2O2 residuals after decontamination of compatible N95 respirators. Typically, reprocessed PPE are discarded after ≤10 treatments as per respective factsheet for facilities and personnel furnished to FDA. It is appreciated, that EUA reprocessing of PPE in healthcare settings will reflect supply and demand, if there is sufficient supply of PPE, then the use of reprocessing technologies will not be required. Many countries recommend VH2O2 for N95 respirator decontamination, leaving the decision to health service managers (Kobayashi et al., 2020).
Preparations in the Republic of Ireland for PPE reprocessing are still ongoing with a trajectory towards use of VH2O2 (Bioquell BQ50, UV germicidal irradiation (UVGI, NanoClave) and sodium hypochlorite liquid decontamination by trained healthcare staff within the hospital setting. Challenges observed include unexpected delay in the delivery of reprocessing technologies that took several months, where the low-pressure UVGI system is turn-key innovation for ease and reliability of operation. Standard operating procedures (SOPs) were generated for safe use to meet expected PPE reprocessing needs. It is likely that deployment of the technologies will be met by use of emergency authorization issued by the hospital through infection control and crisis management committee. This would infer that PPE reprocessing technologies, if authorized by this committee, can only be deployed within that specific hospital for fixed purposes under emergency use. Kobayashi et al. (2020) described extended use of N95 respirators, defined as respirator was used, removed, stored and used again at least 1 more time. The maximum duration of extended use of N95 respirators ranged from 4 h (France, New Zealand, and Sweden) to 40 h (Mexico), and the maximum number of cycles of decontamination ranged from 2 (Germany) to 5 (United States).
At the time of writing, there is still no consensus in the Republic of Ireland as to the strategic deployment of these technologies for PPE reuse, such as, for extended community needs where there has been clusters of infections. However, there has been significant progress made on important guidance documents (NSAI, 2020) that also includes Health Products Regulatory Authority (HPRA) regulatory derogation pathways. The PPE that were observed to be in short supply were surgical gowns and Starmed Hoods for use in ICU, with less pressure on supply of face mask and FFRs. Surgical gowns have similar material consistency to that of surgical wrapping used for reprocessing of endoscopes, but the chamber size of in house plasma-generated VH2O2 is too small to cater for large throughput of PPE for user needs. A limiting factor for bespoke in-house reprocessing of PPE is the ability to test viruses along with biological indicator to confirm efficacy of decontamination along with expertise and capacity to demonstrate functionality (Rowan and Laffey, 2020). There is also a pressing need to explore attitudes, perceptions and possible barriers for use of reprocessed PPE by frontline clinicians and nurses that would entail conducting a social marketing study so as to inform overall acceptance and to overcome behaviour change factors for PPE reuse.
Increased use of face masks by people in communities in Irish society is aligned with similar recommendations in other countries across the globe (Rubio-Romero et al., 2020; Holland et al., 2020; Government of Ireland, 2020). WHO (2020) also advocate that “use of face masks alone are insufficient to provide adequate level of protection, and other measures should also be adopted”. WHO (2020) also advises for each country to apply a risk-based approach that considers benefits (such as reduction of potential risk of exposure), along with potential risk (such as self-contamination, false sense of security, impact of PPE shortage) when deciding to use facemasks by general population. The Centre for Disease Control and Prevention (2020) and Health Service Executive (HSE) in the Republic of Ireland (2020) recommends the use of cloth face coverings to help slow down the spread of COVID-19. Face cloths can be decontaminated and reused after putting through a washing machine and dryer where the combination of elevated temperature and use of detergent the virus inactivates SARS-Cov-2 (Zhao et al., 2020).
The WHO (2020b) estimates that approximately 89 million medical masks will be required each month to respond to COVID-19 reflecting general use of these across both community and healthcare. Given that approximately 230,000 new cases of SARS-CoV-2 have been reported daily (European Centre for Disease Prevention and Control, 2020), there has been global interest in gaining an understanding of appropriate procedures for safe PPE reuse (Table 1). It is notable that the term “N95” refers to the US National Institute or Occupational Safety and Health (NOISH) certification (Derraik et al., 2020). N95 filtering facepiece respirators (FFRs) are defined as respirators no resistant to oils, but with a particle filtration efficiency ≥95% when challenged with sodium chloride particles of a median diameter of 0.075 μm at a flow rate of 85 L/min (Derraik et al., 2020). The equivalent Conformité Européen (CE) certifications are FFP2 and FFP3 respirators that have minimum required particle filtration efficiencies of 94% and 995 respective. Thus, we have referred to this generic group as FFRs.
Disposable PPE are regulated by Regulation (EU) 2016/425 of the European Parliament and repealing Council Directive 89/686/EEC (European Parliament and the Council of the European Union, 2016, which obliges the manufacturer to apply the aforementioned CE marking and to follow the procedure for evaluating and complying with the requirements for that marking. The full suite of European standards and certifications for manufacturer respiratory protective devices are described by Rubio-Romero et al. (2020). However, the European Commission also published specific Commission Recommendations (EU) 2020/43 of March 13, 2020 on conformity assessment and market surveillance procedures with the context of COVID-19 threat to allow for commercialization of PPE or medical devices that comply with non-European standards, even if they do not have CE marking in the event of shortage of supplies, but an adequate level of protection must be guaranteed and the corresponding authority informed (European Commission, 2020). Similarly, the United States government published authorizations to import Non-NIOSH-approved filtering facepiece (FFP) respirators from other countries (Food and Drug Administration, 2020). The situation of widespread shortages of PPE has led civic society making different kinds of improvised facemasks using a variety of materials without any guarantee of certification internationally (Rubio-Romero et al., 2020). In order to help standardize this practice, some standard organization released reference documents such as French Association for Standardization published AFNOR SPEC S-76-001 that addresses mass manufacturer of homemade masks (AFNOR, 2020). Effectiveness of disposable masks varies depending on the type and certification standards, which focuses on the leakage of all particles in the interior at 22%, 8% and 2% for FFP1, FFP2 and FFP3 respectively (Rubio-Romero et al., 2020).
4.1. Translating knowledge from use of medical face masks to informing efficacy of commercial or homemade cloth face coverings
Sickbert-Bennett et al. (2020) recently reported on the aerosol filtration efficiency for FFR alternatives that have been used during the COVID-19 pandemic. Surgical masks with ties were shown to have filtration efficiency (FFE) of 71.5%(±5.5), while procedural masks with ear loops had lower FFE at only 38.1% (±11.4%). The FFE of 3 M's N95 Respirator (Model 1830) was reported to be high at 98.5% (±0.4) (Sickbert-Bennett et al., 2020). Quality improvement studies of 29 fitted face mask alternatives, expired N95 respirator with elastic bands subjected to ethylene oxide or hydrogen peroxide vapour had unchanged fitted filtration efficiency (FFE) of more than 95%, while performance of N95 respirators in wrong size resulted in decreased FFEs between 90 and 95%. As a group, surgical and procedural masks had lower FFEs related to N95 respirators with masks secured with elastic ear loops showing lowest performance. Clinicians and HCWs have voiced concerns about discomfort arising with ear loops from wearing face masks for prolonged period. Thus, there appears merit in use of head ties, instead of ear loops, for securing face masks or face coverings to protect against infectious aerosols harbouring SARS-CoV2.
A short observational study of the types of face masks and face covering used by 1043 shoppers as they entered a large retail centre was conducted in the Irish midlands on 14th and 15th August, 2020. Findings revealed that 461 wore coverings with ear loops, 320 wore procedural masks with elastic ear loops, 140 wore KN95/N95 respirators, 38 wore face shields, 5 wore bandanas, 3 wore scarfs, and 56 shoppers did not wear face coverings. There was no evidence of anyone using surgical masks secured with ties. It was observed that 64 appeared to be wearing face masks or coverings over their mouth only, or below their chin, or were improperly fitting such that these did not cover the nose or mouth. Some shoppers removed their face masks, or raised their face shields to the top of their head, in order to have conversations, which indicated a lack of understanding of their purpose and function. Recnet evidence from Fischer et al. (2020) with FFRs suggests that face masks and face coverings should consider use of adjustable cloth ties, as this design potentially offers better filtration efficacy of the virus compared to using a face coverings that have elasticated ear loops. Creativity in the design of cloth coverings was observed including insertion of a clear panel to facilitate lip reading. Face cloths are likely to be disinfected through use of domestic washing machine for re-use where combination of moist heat above 60 °C and detergent will kill COVID-19 (Zhao et al., 2020; Rubio-Romero et al., 2020). CDC (2020b) report that it is not known if face shields provide any benefit as source control to protect others from the spray of respiratory particles. CDC does not recommend use of face shields for normal everyday activities or as a substitute for cloth face coverings. If face shields are used without a mask, they should wrap around the sides of the wearer's face and extend to below the chin.
5. Reprocessing of PPE during COVID-19 to meet critical shortages in supply chain using different technologies and approaches
Albeit limited, published studies have demonstrated efficacy of various chemical biocides at mild concentrations against several viruses (including SARS, MERS, HCoV) on different inanimate surfaces (Kampf et al., 2020; van Doremalen et al., 2020). This informed PPE re-use studies, such as in the US, where HCWs were provided with 5 FFP respirators to a factor in one usage per day where at the end of the working day, used FFP respirator must be kept in a breathable paper bag and stored by order of usage (Centre for Disease Control and Prevention, 2020). If the workers store and use FFP respirators in order of each day, which would infer that 5 days would have occurred between initial usage of each respirator (Rubio-Romero et al., 2020. The focus of this review is to describe decontamination procedures that are readily scalable for safe PPE reuse based upon best published information.
Procedures that have been reported in the literature for the reuse of PPE in healthcare focus on applying mild treatments that seeks to achieve a balance between exploiting the sensitivity of SARS-CoV-2 with that of ensuring viral bioburden reduction and functionality of PPE post processing. The majority of reported approaches have adopted use of hydrogen peroxide in vapour state (VH2O2) that are authorized by competent authorities (such as FDA), with one company reporting combining VH2O2 with ozone. The authorized use of other processes include moderate heat with steam, low-pressure UV technologies and mild liquid disinfection (CDC, 2020). Physical technologies (such as gamma irradiation) are not been pursued as they affect material functionality of treated PPE (Rowan and Laffey, 2020). The majority of studies have reported on reduction in surrogate microorganisms, but there has been limited publications addressing holistic component aspects such as complex functionality testing such as filtration efficacy of FFRs (Table 1). Several studies have used SARS-CoV-2 as test organism for viability and for reprocessing of PPE with an emphasis on cell culture determinations (Table 1). Others studies have used a range of virial surrogates at different concentrations, such as T1, T7, phi-6 and MS2 bacteriophages, Influenza A H1NI, N5NI, H7N9, MERS-CoV, SARS-CoV, or established bacterial endospore indicators such as Bacillus atrophaeus or G. stearothermophilus (CDC, 2020; Rowan and Laffey, 2020).
5.1. Reprocessing of PPE using dry and moist heat
Some studies have been reported on the use of different regimes of heating for PPE processing. Heating causes irreversible structural damage in virus proteins that prevents binding to host cells (Derraik et al., 2020); the challenge is for thermal procedures is to eliminate SARS-CoV-2 with damaging PPE. The guiding principle, similar to the concept of pasteurization for use with heat sensitive foods, is that one can achieve a similar one-log reduction in viral load by reducing exposure time with increasing temperature. For example use 72 °C for 15 s provides similar level of lethality to that of using a holding temperature of 60 °C for 30 min. In general, heat treatment at 60 °C for ≥30 min would lead to ca 4.6 to 7 log10 reduction in SARS-CoV-2 (Table 1). However, doubling exposure duration at 60 °C to 60 min would be prudent given the variability in heat inactivation studies reported for SARS-CoV-1 and SARS-CoV-2 (Derraik et al., 2020). For example, Darnell et al. (2004) reported on residual infectivity after exposure of SARS-CoV-1 to heating at 65 °C for 90 min. Also, there is considerable variability in the manner by which the viruses have been tested by researchers that includes use of artificial solutions, surfaces and materials, with and without soiling, where the lack of harmonized procedures makes it challenging to appreciate significance of findings and relevance to practice, such as PPE (Table 1). Variable factors influencing the efficacy of heat inactivation procedures for SARS-CoV-1 and SARS-CoV-2 include number of viruses present (viral load), presence of organic matter (soiling), temperature, humidity and duration of treatment (Table 1).
Song et al. (2020) reported on the use of heating of face masks in an oven at 56 °C for 30 min combined with hot air from a hair dryer for 30 min to inactivate influenza virus without observing efficacy in filtering capacity. Rubio-Romero et al. (2020) noted that findings from this particular study was used by the International Medical Center of Beijing (2020) and the Spanish Ministry of Labour and Social Economy as basis for indicating that FFRs maintain their filtration efficiency after decontamination at 70 °C for 30 min, although fit and deformation testing is not reported. Price and Chu (2020) and Spanish Society of Preventive Medicine, Public Health and Hygiene (2020) recommend use of dry heat at 70 °C for 30 min in a convection oven to ensure constant and uniform temperature maintenance. However, there is a general lack of information on the effect of dry heat on filtration, fit-test or deformity over several decontamination cycles (N95DECON, 2020a; Rubio-Romero et al., 2020; Derraik et al., 2020).
The CDC (2020) stated that, based on limited research available as of April 2020, moist heat has shown promise as a potential method to decontaminate FFRs. The CDC's National Institute for Occupational Safety and Health (2020) reiterated that before using any decontamination method, it should be evaluated for its ability to retain 1) filtration performance, 2) fit characteristics achieved prior to decontamination, and 3) safety of the FFR for the wearer (e.g. by inactivating SARS-CoV-2). Moist heat, consisting of 60 °C and 80% relative humidity (RH) caused degradation in the filtration and fit performance of tested FFRs (Bergman et al., 2010; Bergman et al., 2011; Viscusi et al., 2011). Heimbuch et al. (2011) disinfected FFRs contaminated with HINI influenza using moist heat of 65 °C and 85% RH that achieved a minimum of 99.99% reduction in the test virus. CDC (2020) noted that one limitation of the most heat method is the uncertainty of disinfection efficacy for various pathogens. This is particularly relevant as there could be more than one respiratory virus or pathogen on contaminated FFRs in healthcare environment and during COVID-19 pandemic.
5.2. Reprocessing of PPE using ozone
Ozone can disrupt lipids and proteins in the cell envelope of viruses exposing vital genetic material, thus causing oxidative inactivation (Rowan, 2019). Zhang et al. (2004) had previously reported on decontamination of FFP respirators using ozone where SARS-CoV1 was inactivated using different concentrations of ozone solution disinfection with efficacy at 27.73 mg/L for 4 min exposure. Toon (2020) also described the efficacy of ozone for decontaminating PPE where the relative humidity needed to be maintained above 50%, Dennis et al. (2020) reported virucidal potential of ozone where they implemented a simple disinfection-box system for treating FFRs. The authors recommended ozone concentrations at 10 to 20 ppm combined with an exposure of at least 10 min. Dennis et al. (2020) note advantages of ozone that include rapid virucidal action that is effective for fibrous material, which included addressing crevices and shading. However, Dennis et al. (2020) also stated that ozone is a lung irritant and can be dangerous to humans, animals, and plants. There is very limited published information on ozone for broader PPE and medical device treatment due possibly to the risk associated with its volatility and lung health implications.
5.3. Reprocessing of PPE using hydrogen peroxide vapour
The majority of authorized approaches advocated by competent bodies deploy hydrogen peroxide vaporization (VH2O2) for emergency reprocessing of PPE where there is critical shortage (Table 1). Jatta et al. (2020) reported on effective filtration efficiency and fit testing of N95 respirators (3 M 8211FF and 9210FF) after 5 and 10 cycles of VH2O2 by V-PRO®maX Low Temperature Sterilization System at higher concentration of 59% VH2O2 to prolong the supply of respirators. Battelle and Duke University had validated hospital protocols for decontaminating respirators using 30 to 35%. VH2O2 (Jatta et al., 2020; Rowan and Laffey, 2020). The background and benefits of using VH2O2 as a reprocessing agent or sterilising modality for medical device application have been comprehensively reviewed by McEvoy and Rowan, 2019. However, VH2O2 compatibility with cellulose-based materials in PPE needs consideration (Zhao et al., 2020).
Grossmann et al. (2020) noted that several VH2O2 sterilization systems are currently approved for use under Emergency Use Authorization (EUA), but these technologies can be difficult to obtain due to the significant demand around the world. Grossmann et al. (2020) described the VH2O2 process (closed and sealed off room using Bioquell Z-2 disinfection cycle) for N95 respirators. These FFRs had been placed in Tyvck pouches where the process includes conditioning, gassing, dwell, and aeration of the VH2O2. Grossmann et al. (2020) demonstrated a reproducible and scalable process for decontaminating N95 respirator within a large academic hospital and healthcare system.
The CDC (2020) reviewed all relevant publications on VHO2H decontamination of FFRs in order to provide evidence of minimal effects to filtration and fit, while demonstrating 99.9999% efficiency in killing bacterial spores. VH2O2 did not reduce the filtration performance of ten N95 FFR models tested while showing a 6-log reduction in Geobacillus stearothermophilus spores (Viscusi et al., 2009; Bergman et al., 2010; Battelle et al., 2016; van Doremalen et al., 2020). In a report prepared by Battelle Memorial Institute, the 3 M 1860 FFR was shown to maintain filtration performance for 50 treatment cycles of V2HO2, using the Clarus® R HPV generator form Bioquell (utilizing 30% H2O2). Additionally, FFR fit was shown to be unaffected for up to 20 VH2O2 treatments cycles using NPPTL's Static Advanced Headform (Bergman et al., 2014; Battelle et al., 2016). Strap degradation occurred after 20 treatment cycles. Kenney et al. (2020) contaminated 3 M 1870 FFRs with three bacteriophages, T1, T7, and Phi 6, and decontaminated the FFRs using VHP generated from the Bioquell's BQ-50 system. The V2HO2 treatment was shown to inactivate >99.999% of all phages which was below the limit of detection. Viscusi et al. (2009) found that 9 FFR models (three particulate N95, three surgical N95 FFRs and three P100) exposed to one cycle of VH2O2 treatment using the STERRAD 100S H2O2 Gas Plasma Sterilizer (Advanced Sterilization Products, Irvine, CA) had filter aerosol penetration and filter airflow resistance levels similar to untreated models; however, Bergman et al. (2010) found that three cycles of gas plasma treatment using the STERRAD 100S H2O2 Gas Plasma Sterilizer negatively affected filtration performance. Table 1 lists the most frequently published papers on the decontamination of reuse of PPE using VH2O2.
5.4. Reprocessing of PPE using ethylene oxide (EO)
The CDC (2020) reported that ethylene oxide (EO) is not recommended as a decontamination method for FFRs as it is carcinogenic and teratogenic and may be harmful to the wearer, even at very low concentrations. NIOSH set a low exposure limit due to residual cancer risk below the quantitative limits of detection, i.e., preferring lowest feasible exposure (CDC, 2020). The CDC reviewed several studies where EO was shown to not harm filtration performance for the 9 tested FFR models. All tests were conducted for one hour at 55 °C with EO gas concentration ranging from 725 to 833 mg/L (Viscusi et al., 2007a; Viscusi et al., 2009; Bergman et al., 2010). Also, six models that were exposed to three cycles of 736 mg/L EtO all passed the filtration performance assessment (Bergman et al., 2010).
5.5. Reprocessing of PPE using ultraviolet light (UV)
Ultraviolet (UV) irradiation causes inactivation of viruses by damaging RNA or DNA via a photo-dimerization process (Darnell et al., 2004). UV decontamination exploits different wavelength bands where UVC (200–280 nm) is superior to UVB (280–300 nm) and UVA (320–400 nm). Optimum irreversible molecular damage occurs around the 254 nm wavelength, hence the reason why this fixed wavelength has been successfully exploited by the water industry for ca. 50 decades (Rowan, 2019). Microbial pigments have evolved for peak absorbance to match 254 nm for to protection, however, viruses (including SARS-CoV-2) do not produce pigments or other defence mechanisms against UV and are not capable of independent lift. Hence, they require a host such as people for replication. Thus, the majority of PPE decontamination studies have focused on exploiting UVC, rather than UVB or UVA (Table 1) Next-generation, pulsed UV light technology exploits an ultra-short, intensive broad light spectrum (ca 200 nm to 1100), and has been previously shown to be superior to conventional low-pressure fixed wavelength UVC methods. However, PUV is an emerging disruptive technology (Rowan, 2019) that is used commercially by the food industry, such as for decontamination of packaging. Only one study, Jinadatha et al. (2015), reported on the disinfection of PPE materials prior to doffing by a pulsed xenon light source that was artificially contaminated with Ebola viral surrogate with reduction in viral load.
There is growing interest in the use of UV technologies for treating COVID-19 with varied findings (Torres et al., 2020; Rubio-Romero et al., 2020; CDC, 2020; Derraik et al., 2020). The CDC (2020) also noted that ultraviolet germicidal irradiation (UVGI) is a promising method for PPE reuse, but stated that not all UV lamps provide the same intensity, thus treatment times would have to be adjusted accordingly (Table 2 ). Moreover, UVGI is unlikely to inactivate all the viruses and bacteria on an FFR due to shadow effects produced by the multiple layers of the FFR's construction. The CDC (2020) noted that acceptable filtration performance was recorded for eleven FFR models exposed to various UV doses ranging from roughly 0.5–950 J/cm2 and UVGI was shown to have minimal effect on fit. Heimbuch and Harnish (2019) tested filtration and fit of 15 FFRs and found no adverse effects to FFR performance. An approximate inactivation of 99.9% of bacteriophage MS2, a non-enveloped virus, and H1N1 influenza A/PR/8/34 on FFPs were achieved with approximately UV dose of 1 J/cm2 (Fisher and Shaffer, 2011; Heimbuch et al., 2011). Heimbuch and Harnish (2019) also tested the performance of 1 J/cm2 of UVGI against Influenza A (H1N1), Avian influenza A virus (H5N1), Influenza A (H7N9) A/Anhui/1/2013, Influenza A (H7N9) A/Shanghai/1/2013, MERS-CoV, and SARS-CoV and reported virus inactivation from 99.9% to greater than 99.999% (Fisher and Shaffer, 2011). Bodell et al. (2016) also reported on the use of UV technology to destroy HINI influenza viruses where ≥3 log reduction was achieved after 60 to 70 s at irradiance of 17 mW/cm2. However, these authors reported on significant variably in efficacy depending upon the prototype and operating conditions used. Lindsley et al. (2015) reported treatment of FFP respiratory N95 with low pressure UV light at 950 J/cm2 produced greater particle penetration (up to 1.25%) and had little effect on flow resistance. However, higher fluence levels (such as 2360 J/cm2) reduced the strength of filtered layers and the breaking strength of straps. O'Hearn et al. (2020a) conducted systematic review of 13 papers focusing on UV-disinfection of N95 respirators where they recommended a cumulative UV-dose or fluence of 40,000 J/m2 in future validation studies including filtration, fit and deformation testing.
Table 2.
Recommended information to be reported in studies on microbial/virucidal (SARS-CoV-2) inactivation by UV technologies for harmonization of PPE reuse and for scalability.
| Main factors | Recommended information for reporting |
|---|---|
| Microorganism, recovery and enumeration | Genus, species and strain of microorganism *Provide appropriate culture collection reference number and/or include type strain for test microorganism(s) in studies *Include Bacillus atrophaeus and/or Geobacillus stearothermophilus endospore along with test organisms *Confirmation of identify of test microorganisms by biochemical, physiological, morphological, immunological and/or molecular means (provide name of supplier for rapid test kits) *Method of storing cultures (cryoprotectant) and frequency of sub-culturing (using fresh microbial slope every month kept at 4 °C where bacterial indicators used) Initial inoculum * Description of procedures for microbial cultivation including name of supplier company for media (to include in vitro analysis) * Growth medium composition, growth temperature, pH, incubation time, and growth phase (exponential or stationary) * Growth achieved under static or orbital cultivation (rpm) * Confirm purity by identifying 3 randomly selected isolates Recovery conditions and enumeration methods for test strains * Composition of media used for recovery to include basal media or physiological saline as diluent * Time and storage conditions between treatment and microbiological analysis * Description of procedure for enumerating viral test strains post treatments, such as use of in vitro tissue culture procedures |
| UV treatment medium properties and conditions | For commercial: description of power unit used for generating pulses to include equipment name of the supplier company and model For prototype: adequate description of components including treatment chamber, electrical configurations and specifications Auxiliary devices – ∗ Temperature probe ∗ Thermophile power detector and software for total broad-spectrum dose received by sample ∗ Transmissivity sensor to monitor %UV transmittance Ensure microbial population density is ≤5-log orders to mitigate against influence of protective shading effects Include description of media composition, pH, aw Composition of menstruum used as diluent for treated samples Sufficient number of treatment trials and replications to provide statistical confidence of findings at 95% level; description of statistic test and version of software package (such as Minitab or SPSS) Description of method used to generate bacterial endospores (natural aged for 7 days or incorporation of manganese sulphate to expedite conversion of vegetative cells to spores on agar surfaces) Include native microflora along with artificially seeded test microorganisms due to variability in resistance profile to PL Consider occurrence of cavities in plant surface microstructures that may protect microorganisms from incident light due to shading |
| As part of EUA, the FDA (2020) reviews the totality of scientific evidence for PPE reprocessing including specialist testing | *testing submitted within previous applications supporting device clearance for other uses that considers different types of polymer materials, such as materials consistent with those found in compatible N95 respirators. *performance data such as sporicidal test, residual analysis, bioburden reduction validation demonstrating >3 log reduction of a non-enveloped virus challenge; testing regarding material compatibility, functionality and filtration performance of compatible N95 respirators after multiple decontamination cycles *testing regarding residuals after decontamination of compatible N95 respirators. *Typically, reprocessed PPE are discarded after 10 treatments as per respective factsheet for facilities and personnel furnished to FDA |
Card et al. (2020) reported on the potential efficacy of FFP respirator decontamination using UVGI using biosafety cabinets that describes irradiation for 15–20 min per side with a fluence of 100 μW/cm2. Lowe et al. (2020) reported that use of UVGI effectively inactivates a range of complex human pathogens including coronaviruses with a focus on FFP respirators (N95) disinfection. This online Nebrasca Medicine also reported on fit and functionality efficacy of UVGI-treated N95 were not affected at different levels of fluence (Lowe et al., 2020; N95DECON, 2020a). The Spanish Society of Preventative Medicine recommends decontamination of FFP respirators using UVGI with double lamps at 36 W for 148 s exposure. However, Rowan and Laffey (2020) and Rowan (2019) reported on technical challenges of using UV irradiance for disinfecting complex devices that includes variance in UV fluence and complex shading affects such as presented in filter mesh. The International Medical center of Beijing (2020) does not recommend UV disinfection for FFP respirators as the efficacy of disinfection it produces for COVID-19 is to be determined. The Centres for Disease Control and Prevention (2020) also advocates against use of UV disinfection of filtering facepiece respirators due to “shadowing effects produced by the multiple layers of the filtering respirators construction”. Rubio-Romero et al. (2020) noted that the advantages of UV could be that ≥ J/cm2 of UV-C inactivates viruses similar to SARS-CoV2 on N95s that maintain fit and filtering performance after 10–20 cycles but shadowing may affect disinfection efficacy (N95DECON, 2020b). Straps also become degraded after multiple cycles of UV (Mills et al., 2018).
Zhao et al. (2020) reported on the treatment of N95-rated masks and nonrated surgical masks, where they demonstrated that neither 254 nor 265 nm UV-C irradiation at 1 and 10 J/cm2 had adverse effects on the masks' ability to remove aerosolized virus-sized particles. The authors noted that additional testing showed no change in polymer structure, morphology, or surface hydrophobicity for multiple layers in the masks and no change in pressure drop or tensile strength of the mask materials. Inagaki et al. (2020) recently reported (via a non-peer reviewed paper), that a deep ultraviolet light-emitting diode (DUV-LED) instrument generating around 250–300 nm wavelength (fluence 3.75 mW/cm2) showed potential for inactivating (in vitro) a strain of SARS-CoV-2 that had been isolated from a patient who developed COVID-19 in the cruise ship Diamond Princess in Japan in February 2020. This strain was obtained from the Kanagawa Prefectural Institute of Public Health (SARS-CoV-2/Hu/DP/Kng/19–027, LC528233). Proper precautions are required to avoid UVGI exposure to skin or the eyes, as UVGI is harmful. Rowan (2019) described a reliable protocol for harmonizing the UV dose of fluence generated from different technologies in order to enable repeatability. Generally, the consensus for use of UV technologies, is that one needs to apply extended doses of UV light for PPE material decontamination to at least a UV dose of 2000 mJ/cm2 for efficacy. However, fixed UV lights sources produce significant heat over extended treatments and it is not clear from many of the published studies that focused on UV decontamination of SARS-CoV-1 or SARS-CoV-2 how temperature was monitored and controlled (Table 2). UV technologies are only effective when treating 2D surfaces as they need to irradiate the target; thus, a virus that is trapped in crevices or hidden behind a mesh such as found in the layers of material in FFRs will not be inactivated as it will not have received treatment (CDC, 2020; Rowan, 2019).
5.6. Use of bleach for PPE reuse
Rowan and Laffey (2020) reported on the use of bleach (sodium hypochlorite at ≤4000 ppm), along with a counter water immersion phase to remove residuals, for testing disinfection performance of Starmed Hoods for ICU. While 3 M stated that Viscusi et al. (2009) measured the filtration performance of two FFR models submerged into a range of sodium hypochlorite solutions (0.525% - 5.25% sodium hypochlorite) and noted some degradation in filtration performance, but not below acceptable levels. Viscusi et al. (2009) and Bergman et al. (2010) also examined the performance of multiple FFR models submerged into 6% sodium hypochlorite and found filtration performance not to be affected. However, residual bleach odours and chlorine off-gassing was noticed and Viscusi et al. (2009) concluded that bleach decontamination of FFRs should be further evaluated using lower concentrations of sodium hypochlorite and to consider chemical methods for neutralizing residuals. Based upon published information, use of liquid bleach (sodium hypochlorite) should only be considered for simple PPE configurations, such as visors or starmed hoods, which must have a water rinsing post-process step to ensure residuals are removed in a vented environment so as to remove chlorine vapour. A comparison of different potential approaches for decontaminating PPE is presented in Table 3 .
Table 3.
Properties of different decontamination approaches considered for PPE reprocessing and reuse.a
| Hydrogen Peroxide Vapour (VH2O2) | Ethylene Oxide (EO) | Ultraviolet Germicidal Light | Moist Heat | Chemical Liquid Disinfectants | Gamma Irradiation | |
|---|---|---|---|---|---|---|
| Methodology | Penetration of sterilant gas | Penetration of sterilant gas | Surface irradiation | Penetration by heat (such as 60 °C for 30 min delivers 4 log reduction) | Surface disinfection | Irradiation of product using photons from radioisotope |
| Efficacy of process | Process efficacy confirmed by biological indicators and/or process monitoring | Process efficacy confirmed by biological indicators and/or process monitoring | Variable, but process efficacy confirmed by biological indicators or monitoring UV dose | Process efficacy confirmed by biological indicators and/or process monitoring | Process efficacy confirmed by international standards on biocide testing | Process parameter confirmed using dosimetry |
| Penetration (such as use of packaging) | Limited penetration Requires gas permeable packaging and product design | Requires gas permeable packaging and product design | Not suitable for packaged PPE | Suitable for treatment of packaged PPE – but depends upon specific sensitivity of materials | Not applied or suitable for packaged PPE but could be used for surface disinfection | Good penetration complete even at high densities (>0.4 g/cc) |
| Material Compatibility | Good material compatibility but not with cellulose-based materials as degrades VH2O2 | Very few material compatibility concerns | Broad material compatibility – longer exposures affects brittleness of PVC, straps of FFRs | Very broad compatibility | Variable depending upon biocide – but sodium hypochlorite or hydrogen peroxide (≤ 5% compatible with PPE | Compatible with most materials: plastics need to be evaluated. Avoid acetals, PTFE (Teflon), unstable polypropylene |
| Turnaround Time | All in one day processing | Days: conventional = 9–10 days. All-in-one processing = one day | Relatively short – typically ≤1 h but depends on UV dose | Relatively short, typically ≤1 h | Relatively short (generally ≤30 min) | Hours: time varies based on dose requirement |
| Process | Complex process that introduces VH2O2 under vacuum, treatment, aeration | Complex process: variables include time, temperature, humidity, and EO concentration | Simple rapid process: delivery of UV dose (J/cm2) in enclosed chamber | Simple rapid process – duration depends on combination of temp, RH and time | Simple rapid process – but affected by bioburden, pH, temperature | Simple process – variables include time in the cell and isotope load |
| Mechanisms of destruction | Potent oxidizer of proteins – but mechanism still not fully understood | Alkylation of proteins, enzymes (targeting sulfhydryl groups), DNA, and RNA. | Irreversible RNA damage affecting replication / infection in host | Thermal aggregation of SARS-CoV-2 nucleo-capsid and membrane proteins | Varied depending on biocide - targets cell envelope / capsid protein via coagulation | Physically breaks down viral RNA |
| Limitation | Not compatible with cellulose-based materials – complex process requiring monitoring and control | Concerns over residuals left on material that are toxic (carcinogenic and teratogenic) | Operator safety due to UV exposure – shading issues with filters of FFRs– need to turn item, but not with PUV | Limited by thermal-sensitivity of materials used in PPE | Certain disinfectants, sanitizers | Adversely affects material |
| Suitability for PPE Reuse | Yes – | No | Yes – but limited to eye protection | Yes | Yes – limited to Eye Protection; Starmed hood | No |
Hydrogen peroxide in vapour (VH2O2); Filtering facepiece respirators (FFRs); ethylene oxide (EO); Relative Humidity (RH). Adapted from McEvoy and Rowan (2019).
6. Enhanced production of PPE that encompasses improvisation for COVID-19 crisis
There has been a staggering increase in the production of ventilators and supply of single-use PPE to meet unprecedented demands globally (Health Products Regulatory Authority, 2020; Global News Wire, 2020). Cocking (2020) report on the innovative activities of Irish researchers in production of bespoke hoods for ICU and to support decontamination of PPE in healthcare setting. Flanagan and Ballard (2020) also reported that healthcare workers have been improvising and rationing: sometime outside the lines of CDC and FDA guidelines (Ranney et al., 2020). For example, Flanagan and Ballard (2020) have noted that that healthcare workers have worn refuse/bin bags or rain ponchos due to shortage of PPE as healthcare have distributed policies regarding reuse and rationing for frontline staff. In response to FDA not objecting to the distribution of improvised face shields as long as they create no ‘undue risks’ and to support attempts to foster greater availability of PPE for betterment of public health, Flanagan and Ballard (2020) reported on range of innovative activities leading to increased production of face shields including sharing open source face shield designs allowing everyone with a 3D printer to download free design. There has also been an increased surge in the production and wearing of community-made bespoke cloth face masks, where Zhao et al. (2020) advocated decontamination of these through use of domestic washing machines and dryer for their reuse. There is a pressing need for sharing of information globally for harmonization of appropriate best-approaches using open access platforms that will meet need for accelerate rate of usage so as to ensure no undue risks aligned with bringing together multi-actors, particularly competent authorities/regulators. There is a commensurate need for an understanding of the appropriateness and impact of different reprocessing modalities on materials when considering future reprocessing of PPE and medical devices (Rowan and Laffey, 2020).
7. Waste management, resource utilization and environmental impact of existing PPE usage to address COVID-19 with green opportunities for innovative change
There is an unprecedented surge in plastic-based PPE usage, arising a s consequence from the ongoing COVID-19 pandemic, which constitutes a new form of single-use-plastic (SUP) waste that will to plague our oceans posing a threat to our marine ecosystems (Euronews, 2020). Shorelines have been littered with discarded PPE, such as masks and visors, with the gullets of birds stuffed with latex gloves, along with crabs tangled in face masks. Marine conservation organization OceansAsia highlighted the growing number of single-use face masks being discovered during its plastic pollution research in the Soko Islands near Hong Kong (Clark, 2020). To provide context, Republic of Ireland is a small country with a population of ca 4.5 million, yet it's HCWs require 9 million face masks per week at a cost to the exchequer of €1billion a year (Farsaci, 2020). Nzediaegwu and Chang (2020) reported that the number of PPE used daily in Africa is estimated to reach seven hundred million, as several African states with confirmed COVID-19 cases have mandated compulsory facemask use for their citizens. For example, and estimated 171,506,138 facemasks to be used per day in Nigeria with a population of 206 million. These authors noted while developed countries have green and sustainable waste management strategies capable of addressing COVID-19, the risks are much higher in developing countries that have poor waste management.
In developing countries, solid waste are dumped in the open and in poorly managed landfills where waste pickers, without wearing proper PPE, would be exposed to COVID-19 as they scavenge for recyclable materials. Such landfills serve as ‘food banks’ for livestock and dogs that can roam about (Word Bank, 2019) that increase chances of exposure to diseases. Rhee (2020) mentioned that PPE used during COVID-19 pandemic is classified as isolation medical waste under South Korea Waste Control Act and is disposed follow principles of sustainability, transparency and safety; this entailed discarding used PPE to containers, thereafter transportation by vehicle for incineration or landfill on the same day as discard. Elhadi et al. (2020) noted that developing countries are struggling to meet PPE needs for their healthcare workers in Libya where they revealed that 56.7% hospitals lacked PPE and 53% of healthcare workers reported that they did not receive proper PPE training. In addition, 70% reported that they were buying the PPE themselves as hospitals did not provide them.
7.1. PPE has added to single-use-plastic global challenges for our environment
Fossil fuel and plastic production are currently integrated where about 80% of manufactured plastic accumulates as waste in landfills and natural environments, presenting an increasing hazard (Karan et al., 2019). Dangaville et al. (2020) need for global engineering and research to exploit innovative polymer degradation and stability fields for PPE to address shortages in supply arising due to COVID-19 pandemic. There is a pressing requirement for access to large scale recycling facilities, effective waste management, and to designate individual usage to match user (Singh et al., 2020). The World Health Organization (2020) projected that supplies of PPE must increase 40% monthly to deal effectively with COVID-19 pandemic. Essential PPE includes an estimated 89 million medical masks, 76 million pairs of medical gloves and 1.6 million pairs of goggles. The increased demand for PPE is expected to be sustained beyond COVID-19 with an estimated compound annual growth of 20% in facial and surgical masks supply from 2020 to 2025 (Singh et al., 2020). It is noteworthy that China produced 240 tons of medical waste daily during peak of pandemic in Wuhan (Singh et al., 2020). Horton and Barnes (2020) reported that microplastics have now been found in the most remote places on earth, far away from human activities. In addition with climate-induced stress, microplastics may lead to enhanced multi-stress impacts, potentially affecting the health and resilience of species and ecosystems. The impact on PPE contamination on the marine environment has yet to be determined where there is significant gaps in knowledge.
Silva et al. (2020) recently reported that single use, plastic-based PPE (such as masks and nitrile gloves) is now adding to anthropogenic pollution threatening environmental sustainability where the COVID-19 precautionary measures are reversing some plastic waste measures. Singh et al. (2020) also note substantial environmental challenges for enormous quantities of used PPE used during COVID-19 pandemic globally. Silva et al. (2020) also noted that the sudden increase in plastic waste and composition (that includes PPE) due to the COVID-19 pandemic undermines the critical need to reinforce plastic reduction policies, to scale up innovation for sustainable and green plastic solutions, along to develop dynamic and responsive waste management systems immediately. This further emphasizes the importance of decoupling plastic production from fossil-fuel resources and to exploit alternative innovation means of meeting pressing need to replace single-use plastic (SUP) that encompasses a holistic community ecosystem approach including Citizen-science. The reader is directed towards the review of Silva et al. (2020) for a comprehensive review on the single-use plastics, plastic waste directives and challenges for the environment arising from COVID-19 including waste management. They noted that without improvements to current system, an estimated 12 billion Mt. of plastic litter will end up in landfills and in the natural environment by 2050, along with green-house gas (GHG) emissions from the entire plastic lifecycle contributing to 15% of the total global carbon budget (Zheng and Suh, 2019). Indiscriminate use and inappropriate disposal or mismanagement SUPs that have low biodegradation have led to accumulation of plastic debris in terrestrial and aquatic ecosystems globally (Singh et al., 2020; Silva et al., 2020). This will affect natural biota, agriculture, fisheries along with threatening human and animal health (Jambeck et al., 2015).
Despite recent progress made in plastic sustainability and waste management, Silva et al. (2020) have noted widespread drawbacks in the use and management of plastics in the fight against COVID-19 pandemic that area associated with government imposed partial and total lockdown of cities/regions/municipalities that has promoted greater use of SUPs, including PPE, by the general public and healthcare workers (Tobías, 2020). There has also been a shift towards mandatory use of PPE by the general public, along with frontline healthcare workers where Silva et al. (2020) noted that over 50 countries are mandated to wear masks in public places. There is also a commensurate need for increased production of PPE globally. World Health Organisation (2020) had expressed concerns about use of masks by general public due to lack of correct handling, and disposal, and the shortage of this material in healthcare materials. Silva et al. (2020) noted that surgical masks should not be worn longer than a few hours (such as 3 h) and should be appropriately discarded to avoid cross-contamination (i.e., in a sealed plastic bag). However, incorrect disposal of PPE is widespread and has been found in several public places and natural environments (Prata et al., 2020; NGO Oceans Asia, 2020). Prata et al. (2020) observed that masks are likely to degraded into smaller microplastic pieces as are made from nonwoven materials (e.g., spunbond and meltdown spunbond) often incorporating polypropylene and polyethylene. These authors also noted that significant enhancement in the usage of PPE and other SUPs is likely to result in an overload increase in waste generation that would disrupt viable options for effective waste management. Many countries have classified all such hospital and household waste potentially contaminated with SARS-CoV2 as infectious that should be incinerated under high temperature (ensuring sterilization), followed by landfilling of residual ash (European Commission, 2020; Silva et al., 2020; Ilyas et al., 2020). Ilyas et al. (2020) reviewed, and reported on the merit, of developing different disinfection technologies for handling COVID-19-generated waste from separate collection to using various physical and chemical steps with view to reducing health and environmental risks.
There is also a significant void in communication channels to general public about appropriate disposal of used face-masks and gloves during COVID-19 that may require user behavioural change, such as exploiting health belief model through social marketing approaches (Suanda et al., 2013; Suanda et al., 2017). However, Silva et al. (2020) noted that not all countries are capable of managing such waste appropriately and are been forced to use direct landfills or open burning as alternative strategies. There is also commensurate concerns about the short, and more longer term, impact of burning considerable amount of plastic that may increase environmental footprint due to release of GHGs and undesirable hazardous compounds (Prata et al., 2020). As some items of PPE are lightweight, there is potential for them to be blown by wind to pollute natural environments including threatening terrestrial and aquatic biota, such as by entanglement. Silva et al. (2020) noted that up 40,000 kg of masks may find their way inappropriately into the natural environment arising from WWF (2020) reporting of inappropriate disposal of only 1% for over 10 million masks introduced to the environment monthly. In order to allay environmental problems arising from COVID-19 due to high demand on SUPs and PPE that produces increased medical waste, Silva et al. (2020) advocated (1) redesigning plastics and decoupling them from fuel-based resources, (2) reduce plastic waste by reducing SUPs and PPE, and (3) optimize plastic waste management. Horton and Barnes (2020) noted that PPE (non-COVID-19 related) have already polluted Antarctica, which are made from synthetic polymer-based fibres, often treated with water repellents such as per/polyfluorinated compounds and flame retardants such as polybrominated diphenyl ethers where their occurrence as contaminations for their toxicity.
7.2. Bio-based plastics as potential alternative sustainable materials for PPE for COVID-19 and future viral pandemics
In the short term, it is important to maintain the PPE supply chain in order to the ensure health and safety of our citizens and our frontline HCWs. However, we now need to look at contingency planning in order to future proof against the potentiale environmental impact of increased single-use plastic (SUP) PPE waste using sustainable solutions. Opportunities will arise to address this challenge through seamlessly connecting research and entrepreneurial ecosystems that will generate a new pipe-line of potentially usable bioplastic products. This could be accelerated through multi-actor innovation hubs linked to healthcare, industry and academia (Rowan and Galanakis, 2020). Silva et al. (2020) noted that the replacement of plastic value chain from fuel-based raw materials and energy has been priorities, which features in many international agreements addressing a green and circular economy. Silva et al. (2020) also noted that bio-based plastics supports are emerging, but at an early stage capturing a market share of ca. 2% due mostly to low-cost of fossil-based plastics, the intense requirement for land use and related financial investment, and undeveloped recycling and/or disposal routes.
Hutti-Kaul et al. (2020) described screening for microbial strains for enhanced hydrolytic and biodegradation abilities for direct conversion of biomass (such as microalgae), extraction of value-added products, and synthesis (polymerisation) process. However, such potentially high-performance bio-based polymers, similar to physical properties of fossil fuel-counterparts (such as low degradability, high durability) (Silva et al., 2020), would need to be characterized and tested for suitability to match design specifications of future PPE including tolerance to thermal processing and potential re-use. OEMs of PPE, academia and regulators should play as strong role in informing the efficacy of bio-based reusable polymers for next-generation products that considers suitability from design, safety and life cycle assessment perspectives. End-of-life strategies need to be consider for waste management and recycling of PPE during COVID-19 used by general public without compromising on safety, where landfill and waste-to-energy should be a last resort option (Silva et al., 2020).
The rapid accumulation of plastic waste is driving international demand for renewable plastics with superior qualities (e.g., full biodegradability to CO2 without harmful by-products), as part of an expanding circular Bioeconomy (Karan et al., 2019). There has been increasing interest in the identification of alternatives to petroleum-based plastics for various industrial applications where desirable bio-based material properties would include ease of biodegradation and renewability (Emadian et al., 2016; Thakuv et al., 2018).
Bioplastics partly or wholly made from biological materials, and not crude oil, represent an effective way of keeping the huge advantages of conventional plastics but mitigating their disadvantages (Carbon Commentary, 2020). A bioplastic is a plastic that is made partly or wholly from polymers derived from biological sources such as sugar cane, potato starch or the cellulose from trees, straw and cotton (Thakuv et al., 2018). Some bioplastics degrade in the open air, others are made so that they compost in an industrial composting plant, aided by fungi, bacteria and enzymes. Others mimic the robustness and durability of conventional plastics such as polyethylene or PET. Bioplastics can generally be directly substituted for their oil-based equivalent. Bioplastics can generally be made to be chemically identical to the standard industrial plastics (Carbon Commentary, 2020). Bioplastics can be distinguished as two different types in terms of usage (1) items that has the potential to eventually litter such as food where alternative bioplastic could be produced to degrade either in industrial composting units or in the open air or in water, and (2) permanent bioplastics, such as polythene manufactured from sugar cane, can provide a near-perfect substitute for oil-based equivalents in products where durability and robustness is vital. Plastics made from biological materials generally need far smaller amounts of energy to manufacture but are equally recyclable. They use fewer pollutants during the manufacturing process. Per tonne of finished products, the global warming impact of the manufacture of bioplastics is less, and often very substantially less, than conventional plastics (Carbon Commentary, 2020; Emadian et al., 2016). Other emerging sources of bioplastics include seaweed (Thiruchelvi et al. (2020) and microalgae (Rizman et al., 2018). Thiruchelvi et al. (2020) recently reported that seaweed-based bioplastics were found to durable, less brittle and more resistant to microwave radiation. These authors also reviewed the role of seaweed in bioplastic production. Higher plants, microalgae, and cyanobacteria can drive solar-driven processes for the production of feedstocks that can be used to produce a wide variety of biodegradable plastics, as well as bioplastic-based infrastructure that can act as a long-term carbon sink. Karan et al. (2019) noted that well-crafted legislated standards on plastic biodegradability and environmental and animal/human health impacts could fast-track and optimize industry transition. The diversity of bio-based feedstocks opens up the opportunity to produce an expanding range of renewable plastics. However, biodegradable plastics should ideally fully degrade to CO2 and water without harmful byproducts. Durable bioplastics can act as carbon sinks if well integrated into large-scale long-term infrastructure.
Biorefinery and GMO strategies can support viable business development and the emerging circular Bioeconomy (Karan et al., 2019). Emadian et al. (2016) also reviewed sources of bioplastics for biodegradation in different environment and noted that Actinomyces, bacteria and fungi are key actors responsible for this important activity. Also, the type of bioplastic and environment in which bioplastics are located influences their biodegradation potential. Aeschelmann and Carus (2020) also reviewed bio-based building blocks and biopolymer for capabilities and applications highlighting advances over plastics. However, there is a gap in current information that could be met potentially by conducting comprehensive life cycle assessment (LCA) evaluation of new biopolymers to meet sustainability needs that would include ecotoxicology and ameliorating carbon footprint that follows pathway along biodegradation to CO2 (Ruiz-Salmón et al., 2020).
There presents an opportunity to exploit the 9 stages of technology readiness developed by NASA (Straub, 2015) to evaluate the sustainability and maturity of emerging innovations for COVID-19 that also addresses environmental friendliness as well as functionality. This strategy is particularly relevant as it address potentially sustainable products from conceptualisation to commercial deployment at higher technology readiness levels: this is particularly relevant given that industry would be familiar with this concept and would allow ease of transitioning for environmental impact. This evaluation of new bioplastics could include life cycle (Ruiz-Salmón et al., 2020) and ecotoxicological (Garvey et al., 2015) assessments of different trophic levels reflecting impact on biodiversity that connects academia with industry partners and policy makers. O'Neill et al. (2020) described development of freshwater aquaculture on cutaway peatlands using organic principles where vast quantities of microalgae, used as natural means of water quality waste remediation, could be used as test system for advancing bioplastic-based PPE innovation and recycling for circular economy developments. Future green innovative research could be extended to new biopolymer-based wrapping and packaging (including for adjacent food industry) to investigate non-thermal treatments that encompass both complex viruses and parasites (Gerard et al., 2019; Franssen et al., 2019). A limiting factor in the production of alternative biomaterials for alternative to single-use PPE relates to thermal stability of materials for fabrication and potential for deformation due to thermal processes. Skrzypczak et al. (2020) recently reported on a new 3D printing approach for meeting such a need where they described an affordable, self-replicating, rapid prototyper that would also make this approach more accessible to home-based 3D printing activities. Chen et al. (2019) also demonstrated potential for exploiting different forms of polymer processing (such as 3D printing and injection moulding) after novel vapour hydrogen peroxide and electron beam treatments that could be advance next-generation PPE and medical device technologies.
7.3. Medical waste management – a global challenge for PPE
In response to meeting threats of COVID-19, there is substantially increased volumes of medical waste produced that also contains PPE, which presents unprecedented challenges for meeting effective waste management strategies globally with significant potential for overload of systems (Singh et al., 2020; Wang et al., 2020; Silva et al., 2020). Singh et al. (2020) have noted that the unprecedented demand has also impacted other industries reliant upon PPE including manufacturing, construction, oil and gas energy, transportation, firefighting and food production. Singh et al. (2020) also noted that this pandemic has substantially impacted upon how solid-waste management activities are performed as prior to COVID-19 resource recycling and waste management were not regarded as essential services and were placed in lockdown. However, the strategically important disease mitigation role of waste management has been recognised given the need to properly dispose and handle SARS-CoV2 contaminated waste to avoid transmission (Reuters, 2020; Price et al., 2020). The United Nation's Basel Convention on the Trans-boundary Movement of Hazardous Wastes and their Disposal has urged countries to treat waste management amid COVID-19 as urgent and essential public service. These authors noted that PPE includes plastics as major constitutes representing ca 25% by weight, which if not recycled or their disposal may contribute substantially to hazardous environmental pollutants, such as dioxins or toxic metals. Polypropylene is a common constituent of PPEs, such as found in N95 masks, Tyvek protective suits, gloves, and medical face shields. Singh et al. (2020) also noted that the potential for recovery of polymers from mixed healthcare waste including PPE is challenging. This would be further influenced by the low-level of recycling worldwide and lack of government policies. Singh et al. (2020) noted that single-use PPE is not a sustainable practice, however development of safe and sustainable PPE management beyond the healthcare settings under pandemic emergency conditions is nebulous as there are no clear understanding of best practices, monitoring, and enforcement of policy and regulations.
Wang et al., 2020 reviewed efficacy of disinfection technologies and approaches for hospital waste and wastewater that considered use of chlorine and/or incineration for such infectious but negated to mention PPE as particular waste for treatments. These authors observed that hospitals in China are potential sources of environmental pollutants resulting from diagnostic, laboratory and research activities and therefore ensuring effective treatment of waste is important, particularly under the COVID-19 pandemic context. Wang et al. (2020) reviewed different types of technologies for the treatment of hospital wastes and waste water disinfection in China where incineration, chemical and physical disinfection are commonly used for hospital waste disinfection. Typical composition of healthcare waste is approximately 85% general non-infectious, 10% infectious/hazardous, and 5% chemical/radioactive. Factors considered for treating infectious healthcare waste included amount of waste, costs, maintenance of technologies and types of waste. Incineration technologies can be adapted for amount of waste, but if scale of hospital waste is small and investment is limited, then chemical disinfection and high temperature steam disinfection that are easier to maintain are preferred in China. Incineration is widely deployed as is deemed to be safe, simple and effective (Ghodrat et al., 2017) where extreme high temperatures completely kill microorganisms along with converting organic matter into inorganic dust. However, hospitals vary in type of incineration approach depending upon waste preparation and flue gas purification that includes pyrolysis vaporization incinerator where organic components of waste are converted to flammable gases to avoid dust at temperatures above 850 °C that reduces particle emission to air.
Wang et al. (2020) report that these high temperatures is conducive to complete destruction of toxic and hazardous components, thereby reducing production of toxic pollutants such as dioxins due to low temperature combustion (Zhu et al., 2008). In addition, rotary kiln incinerators are deployed that generate temperature as high as 1200 °C or more, where there are advantages that include wide range of applications, good adaptability, handling a different variety of wastes, good gas and solid contact, and uniform reaction, but are not small or medium scale usage with disposal capacity below 8 t/d that investment costs are high and dust content exhaust is higher than pyrolysis incinerator. Plasma incineration technology is novel waste disposal technology that transfers energy through plasma where waste can be rapidly decomposed into small molecules where most of the gases produced are flammable that are sent to secondary combustion chamber and purification, thereafter discharged to atmosphere. Plasma incinerators have higher energy efficiency compared to other incinerators (Messerele et al., 2018).
Wang et al. (2020) described chemical disinfection technologies for treatment of hospital waste that is typically used in combination with mechanical and crushing treatments in China. Generally, crushed hospital waste are mixed with chemical disinfections such as sodium hypochlorite, calcium hypocholorite, chlorine dioxide for fixed contact times during which organic wastes are decomposed and microbial threats inactivated. Chemical disinfection have desirable attributes including low effective concentrations, rapid action, stable performance and broad sterilization efficacy for different types of microorganisms. These chemical disinfectants are generally used as are non-corrosive, safe, easily soluble in water but not easily affected by chemical or physical factors with low toxicity and reported to have no residual hazard post disinfection (Chen and Yang, 2016). Wang et al. (2020) suggest that chemical disinfection technology could be considered when amount of waste is small.
Wang et al. (2020) also reported on use of microwave disinfection as a means of energy saving, low action temperature, slow heat loss, light damage and low environmental pollutions with no residues or toxic wastes after disinfection, but requires strict control by special microwave devices. Wang et al. (2020) stated that microwave technology only used at present for treatment of biohazardous wastes, but the technology is been promoted as effective supplementation technology for incineration to enable diversification of hospital wastes in China. Wang et al. (2020) also reported that microwave technology can achieve logarithmic value for killing complex pathogens such as parasites and viruses at >6 log along with killing of Bacillus subtilis endospores at >4 spores. Wang et al. (2020) also reported on high temperature steam disinfection (saturated water vapour with temperatures greater than 100C) to kill microorganisms. In China, a log kill of thermophilic lipobacillus endospores at >5 logs is required. However, this approach has a low volume reduction rate and easily generates toxic volatile organic compounds during disinfection. From perspective of investment and operation costs, as well as economic and social benefit, high temperature incineration is still most popular approach to hospital waste disinfection in China. Thus, there are pressing needs to define effective decontamination strategies for medical waste through appropriate management strategies will also contribute to global collective effort in reducing SARS-COVID-19 transmission along with future safeguarding our environment.
8. Conclusions
There is extraordinary pressure to meet shortages in single-use PPE supply for our frontline clinicians and healthcare workers. PPE treatment is challenging as the constituent material, including single-use plastics (SUPs), are sensitive to harsh decontamination processes. There has been an unprecedented surge in the production of commercial and homemade cloth and fabric face coverings to offset this challenge and to help with preventing person-to-person transmission in the community setting. Many countries across the globe are extending, decontaminating and reusing PPE where there is critical shortage for frontline healthcare workers (HCWs), but under emergency use only. This unprecedented need will continue given the absence of a vaccine and occurrence of successive waves of SARS-CoV-2 globally; and, the likely high demand for PPE by the medical and nursing profession beyond COVID-19. Table 4 highlights succinctly the main developments surrounding decontamination and reuse of PPE that includes pressure on waste management and recycling. Table 1 also provides a list of key papers that have been published on this topic.
Table 4.
New developments in PPE decontamination, reuse and waste management.
| Trending information on PPE and face coverings reuse, and waste management | Reference |
|---|---|
| PPE is designed for single-use for medical/nursing staff, but supply chain has been insufficient to meet global needs with many countries adopting reuse practices post deployment of technologies to meet emergency COVID-19 use | Rowan and Laffey (2020); Derraik et al. (2020) |
| There are limited technologies suitable for PPE reuse that reflects matched efficacy for reprocessing | Rubio-Romero et al. (2020) |
| Differences in priority usage and decontamination technologies between higher risk medical environment (PPE) and lower risk community settings (face coverings) that have informed selection of technologies and approaches used | Derraik et al. (2020) |
| Evidence that PPE can be effectively reprocessed using technologies not readily available to public such as VH2O2, O3, low pressure UVC such as UVGI (2000 mJ/cm2) where variance in determining efficacy of UV dose between UV modalities influencing harmonious acceptance). Generally, is greater disinfection using UVA over UVB and UVA. | Derraik et al. (2020); Rowan, 2019; Rubio-Romero et al. (2020) |
| High throughput VH2O2 can effectively disinfect, for example,2500 N95 respirators per 12 h shift at 3000–750 ppm hydrogen peroxide | Rubio-Romero et al. (2020),Mackenzie (2020); Perkins et al. (2020) |
| Recommendation for wearing of face masks and coverings to prevent spread of COVID-19 | CDC (2020); Ministry of Health of Spain (2020); |
| Choice of technologies for reprocessing of PPE healthcare depends on the type and complexity of PPE (functionality, fit test, deformation, filtration efficacy) that are typically single use and thermally-sensitive with increasing challenges in the order face shields, gowns, FFRs (including disposable N95 respirators,) | Derraik et al. (2020); Rubio-Romero et al. (2020) |
| Evidence of extended use of N95 respirators such as 4 h (France, New Zealand and Sweden) to 40 h (Mexico) | Kobayashi et al. (2020) |
| Physical irradiation technologies (gamma) and ethylene oxide (EO) are not appropriate for PPE reuse due to non-compatability with material composition or concerns over lingering residual toxic end-points produced during EO | Rowan and Laffey (2020) |
| Barriers to reuse of PPE by healthcare workers include lack of knowledge to inform acceptance and discomfort over prolonged usage with potential for social marketing studies to inform trust and associated decision making | Rimmer (2020); Mitchell et al. (2012) |
| Evaluation of fitted filtration efficiency (FFE) showed that surgical masks with ties (71.5 ± 5.5%) and procedural mask with ear loops (38.1 ± 11.4%) exhibit lower FFE post VH2O2 treatment is lower than N95 respirators (98.5 ± 0.4%). This suggests potential benefits of using head ties instead of ear loops for homemade face coverings and would help prevent slippage below nose during wearing. | Sickbert-Bennett et al., 2020 |
| Disinfection performance studies for evaluating PPE reuse over single or several cycles use surrogate viruses or bacterial endospore indicators (bioburden typically at or below 106), where most SARS-COV-2 strain(s)are studied using in vitro tissue culture infection models. Most researcher won't have access to level 3 containment facilities | Rowan and Laffey (2020); Derraik et al. (2020) |
| Evaluation of facemask and variety of commonly available non-certified face coverings for filtering expelled droplets during speech, sneezing and coughing revealed that variability from below 0.1% (fitted N95 mask) to 110% (fleece mask). Sequence of decreasing efficacy N95 respiratory, combining cotton-polypropylene-cotton mask; combining layer cotton in pleated style mask; combining 2 layer cotton with pleated style mask; use of single layer cotton masks; knitted masks; double layer bandana; and fleece. | Fischer et al., 2020 |
| Improvised face masks and face coverings should be used as a last solution and for low risk situation as increased duration of wearing may increase risks of virus infiltration due to humidity, liquid diffusion and virus retention | European Centre for Disease Prevention and Control (2020) |
| Use of common washing machine (ca 60 °C for 30 min) combined with use of spin dryer appear effective for face cloth decontamination and reuse | Zhao et al. (2020); Rubio-Romero et al. (2020); HSE (2020) |
| SARS-COV2 is sensitive to commonly-used disinfectants on surface. However, lower environmental temperatures promote longer survival on surfaces, which may influence efficacy of mask wearing such as over winter flu season | Derraik et al. (2020) |
| Face shields are inferior to use of face masks where the latter is particularly relevant for combined use in healthcare settings to prevent infection through the eyes. | Rowan and Laffey (2020) |
| An increasing trend towards development of smart coatings on materials for inactivation of SARS-CoV-2 and against other future potential pandemic viruses, along with provision for incorporation in PPE, mobile phones and so forth | Behzadinasab et al., 2020. |
| Over 50 countries are now recommending facemasks by public that presents a new form single-use plastic waste | Silva et al., 2020 |
| Influence of soiling on critical PPE – up to 14 days survival and retention of SARS-CoV-2 on surgical gowns. | Kasloff et al., 2020. |
| There are opportunities for innovation in new bioplastic-based PPE and waste management as there is likely to be a high demand for PPE post COVID-19 | Ilyas et al., 2020 |
| There is an increase trend towards modelling recovery scenarios to investigate the potential impact of lockdown duration that is implemented to protect frontline HCWs against COVID-19 that may include provision for PPE costings against the cost associated with medical staff absenteeism or illness due to inadequate PPE. | Guan et al. (2020); Thomas et al. (2020); Ivanov (2020); Mukerji et al. (2017) |
| Use of artificial intelligence and deep learning could help identify high-risk patients and suggest appropriate types and use of PPE | Boškoski et al. (2020) |
Hydrogen peroxide in vapour (VH2O2), used alone or combined with ozone, has been used for high-throughput decontamination and reuse of complex PPE such as important filtering facepiece respirators (FFRs), such as the popular N95 respirator. VH2O2 has been certified for PPE decontamination under emergency use authorization (EUA) by the FDA in the United States in order to address shortages in supplies arising for ongoing COVID-19 pandemic. Many countries are permitting decontamination and reuse of PPE leaving decision to heath service managers. Ultraviolet (UV) light procedures, particularly using UVC wavelength, have also been reported as potentially appropriate decontamination technology for PPE, but this technology is unlikely to inactivate SARS-CoV-2 if this virus penetrates lower layers of material of FFRs where they would be hidden from the UV. Also, there is significant variability in the reporting of important UV dose (mJ/cm2) by different researchers using various UV technologies that prevents the harmonization of processes; but, this review provides guidance on standardized reporting. Moist heating at 60 °C to 70 °C combined with high humidity for 60 min is also a promising decontaminating procedure for PPE as it would also enable scalability and high-throughput processing. Use of microwave generated steam would not provide scale for PPE decontamination. Physical irradiation technologies, such as gamma and electron beam, along with ethylene oxide, are not suitable for PPE reprocessing.
Surgical face masks with head ties have been reported to offer superior filtration effectiveness over commonly used procedural face masks that have ear loops. This new information may inform the the effectiveness of commercial and homemade cloth face coverings that may be improved by using ties instead of ear loops that would also help prevent slippage of the face coverings below the nose and mouth. The incorporation of a nose bridge and increase number of layers in face cloth design, used by the community, would also improve performance against SARS-CoV-2.
Best published information would also suggest that SARS-CoV-2 will tolerated cold weather, such as 4 °C (refrigeration), better and survival beyond 14 days on materials, which has implications for environmental wearing of masks and face cloths as we enter the winder flu season. Use of a washing machine and spin dryer (60 °C for 60 min) with detergent is likely to decontaminate SARS-CoV-2 on contaminated face cloths where the risk of contamination by this virus is low. Wearing of face coverings by the general public to prevent the person-to-person spread of SARS-CoV-2 is important given increasing evidence that asymptomatic community transmission is contributing to many new cases in countries across the globe. There is a greater need to develop trust and confidence in reuse of PPE by HCWs that can be addressed through engagement in social marketing and knowledge sharing. There is a pressing need to consider new waste management strategies for the huge surge in single-use-plastic PPE that is contaminating our environment. This will create opportunities for using alternative bioplastics that are more environmentally friendly. There are also emerging opportunities to address real-time data analytics through digitization in order to obtain a better holistic understanding of critical shortage of critical supply chain for PPE during this COVID-19 and for future pandemics.
CRediT authorship contribution statement
NR and JGL conceptualised the manuscript. NR drafted the manuscript. Both authors read, edited, and approved the final manuscript.
Funding
The author received no financial support for the research, authorship, and/or publication of this article.
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Editor: Damia Barcelo
References
- 3M Science of Life. Disinfection of filtering facepiece respirators, announcement, 2020. https://multimedia.3m.com/mws/media/1816576O/disinfection-of-disposable-respirators-technical-bulletin.pdf (accessed 4th July, 2020).
- Aeschelmann F., Carus M. Bio-based building blocks and polymers in the world: capabilities, production, and applications – status quo and trends towards 2020. Ind. Biotechnol. 2020;11(3) doi: 10.1089/ind.2018.28999.fae. [DOI] [Google Scholar]
- AFNOR . Association française de Normalisation AFNOR; 2020. AFNOR SPEC S76-001 Barrier masks. Guide to minimum requirements, test methods, preparation and use for mass manufactured and homemade masks.https://iv.revistalocal.es/wp-content/uploads/AFNORSpec-S76-001- MascarillasDeProteccion.pdf Available on 9th of July 2020 at. [Google Scholar]
- African Centre for Disease Control and Prevention (2020). https://africacdc.org/covid-19/ (accessed 22nd July, 2020).
- Ahmed O., Brockmeier D., Lee K., Chapman W.C., Doyle M.B. Organ donation during the Covid-19 pandemic. Am. J. Transplant. 2020 doi: 10.1111/ajt.16199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerswald H., Yann S., Dui S., In S., Dussart P., Martin N.J., Karlsson E.A., Garcia-Rivera J.A. 2020. Assessment of Inactivation Procedures for SARS-CoV-2. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceló D. An environmental and health perspective for COVID-19outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. Journal of Environmental Chemical Engineering. 2020;8 doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batéjat C., Grassin Q., Manuguerra J.-C., Leclercq I. 2020. Heat Inactivation of the Severe Acute Respiratory Syndrome Coronavirus 2. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelle Final report for the bioquell hydrogen peroxide vapor (HPV) decontamination for reuse of N95 respirators. 2016. 2016. https://www.fda.gov/emergency-preparedness-and-response/mcm-regulatory-science/investigating-decontamination-and-reuse-respirators-public-health-emergencies Available from.
- Behzadinasab S., Chin A., Hosseini M., Poon L.L.M., Ducker W.A.A. A surface coating that rapidly inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces. 2020;12:34723–34727. doi: 10.1021/acsami.0c11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley K., Dove B.K., Parks S.R., Walker J.T., Bennett A.M. Hydrogen peroxide vapour decontamination of surfaces artificially contaminated with Norovirus surrogate feline Calcivurus. J. Hospital Infection. 2016;80(2\):116–121. doi: 10.1016/j.jhin.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Bergman, M., et al., (2010). Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. Journal of Engineered Fibers and Fabrics, 2010. 5(4): p. 33–41.
- Bergman M., et al. Impact of three cycles of decontamination treatments on filtering facepiece respirator fit. Journal of the International Society for Respiratory Protection. 2011;28(1):48–59. [Google Scholar]
- Bergman M.S., et al. Development of an advanced respirator fit-test headform. J. Occup. Environ. Hyg. 2014;11(2):117–125. doi: 10.1080/15459624.2013.816434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukov, J., Boydston, J.A., Dunning, R.A., Yeager, J.J., Wood, S., Reese, A.L., Ferris, A., Miller, D., Weaver, W et al. (2020). Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. MSpere, 5, e00441-20. DOI: 10.1128/msphere.00441-20. [DOI] [PMC free article] [PubMed]
- Bodell K., Buckakldian A.H., Perlmin S. Efficacy of an automated multiple whole room ultraviolet C disinfection system against coronavirus MHV and MERS-CoV. Infection Control Hospital Epidemiology. 2016;37:598–599. doi: 10.1017/ice.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boškoski, I., Gallo, C., Wallace, M. B., Costamagna, G. (2020). COVID-19 pandemic and personal protective equipment shortage: protective efficacy comparing masks and scientific methods for respirator reuse. Gastrointest. Endosc.. https?//doi.org/10.1016/j.gie.2020.04.0048. [DOI] [PMC free article] [PubMed]
- Carbon Commentary (2020). Bioplastics – an important component for global sustainability. https://www.carboncommentary.com/blog/2011/09/02/bioplastics-an-important-component-of-global-sustainability (accessed 8th July, 2020).
- Card K.J., Crozier D., Dhawan A., Dinh M., Dolson E., Farrokhian N., Gopalakrishnan V., Ho E., King E.S., Krishnan N., Kuzmin G., Maltas J., Pelesko J., Scarborough J.A., Scott J.G., Sedor G., Weaver D.T. UV sterilization of personal protective equipment with idle laboratory biosafety cabinets during the Covid-19 pandemic. MedRxiv. 2020 doi: 10.1101/2020.03.25.20043489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell, S. (2020). https://www.irishtimes.com/news/health/coronavirus-ireland-has-no-significant-herd-immunity-study-shows-1.4308216 (accessed 20th July, 2020).
- Centers for Disease Control and Prevention (2020). Coronavirus disease – decontamination and reuse https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html (accessed 10th July, 2020).
- Centres for Disease Control and Prevention (2020). Coronavirus disease (COVID-19): guidance for K-12 school administrators on the use of cloth face coverings in schools. https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/cloth-face-cover.html (Accessed 25th August, 2020).
- Chan K.H., Sridhar S., Zhang R.R., Chu H., Fung A.Y.F., Chan G., Chan J.F.W., To K.K.W., Hung I.F.N., Cheng V.C.C., Yeun K.Y. Factors affecting stability and infectivity of SARS-CoV-2. J. Hosp. Infect. 2020;106:226–231. doi: 10.1016/j.jhin.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Yang H. Current status of medical wastes disinfection and disposal technologies. Chen. J. Disinfect. 2016;33:171–174. [Google Scholar]
- Chen Y., Neff M., McEvoy B., Cao Z., Pezzoli R., Murphy A., Gately N., Hopkins M., Rowan N.J., Devine D.M. 3D printed polymers are less stable than injected moulded counterparts when exposed to terminal sterilization processing using novel vaporized hydrogen peroxide and electron beam processes. Polymer. 2019;183 doi: 10.1016/j.polymer.2019.121870. [DOI] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peris M., Poon L.L.M. Stability of SARS-Cov-2 in different environmental conditions. Lancet Microbe. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, N. (2020). Face masks, gloves and a new breed of plastic pollution. https://www.aljazeera.com/indepth/features/face-masks-gloves-breed-plastic-pollution-200701154052002.html (accessed 8th July, 2020).
- Cocking, S. (2020). AIT and NUIG explore PPE decontamination amid global shortage for COVID19. https://irishtechnews.ie/ait-and-nui-galway-ppe-decontamination-covid-19/ (accessed 8th July, 2020).
- Cook M. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic – a narrative review. Anaesthesia. 2020;75:920–927. doi: 10.1111/anae.15071. [DOI] [PubMed] [Google Scholar]
- Daeschler S.C., Manson N., Joachim K., Chin A.W.H., Chan K., Chen P.Z., Tajdaran K., Minroeini K., Zhang J.J., Maynes J.T., et al. Effect of moist heat reprocessing on N95 respirators on SARS-CoV-2 inactivation and respirator function. CMAJ. 2020 doi: 10.1503/cmaj.201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangaville T., Spann K., Celina M. Opinion to address the personal and protective equipment shortage in global community during COVID-10 outbreak. Polym. Degrad. Stab. 2020;176 doi: 10.1016/j.polymdegradstab.2020.109162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M.E., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. Journal of Virology Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis B., Cashion A., Emanuel S., Hubbard D. Ozone Gas: Scientific Justification and Practical Guidelines for Improvised Disinfection using Consumer-Grade Ozone Generators and Plastic Storage Boxes. Journal of Science and Medicine. 2020;1(2) doi: 10.37714/JOSAM.V211.35. [DOI] [Google Scholar]
- Derraik J.G.B., Anderson W.A., Connelly E.A., Anderson Y.C. Rapid Review of SARS-CoV-1 and SARS-CoV-2 Viability, Susceptibility to Treatment, and the Disinfection and Reuse of PPE, Particularly Filtering Facepiece Respirators. Int. J. Environ. Res. Public Health. 2020;17:6117. doi: 10.3390/ijerph17176117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhadi, M., Msherghi, A., Alkeelani, M., Alsuyihili, A., Khaled, A., Buzreg, A., Boughididah, T., Abukhashem, M., Alhashimi, A., Khel, S., Gaffaz, R., Ben Saleim, N., Bahroun, S., Elharb, A., Eisay, M., Alnafati, N., Almiqlash, B., Biala, M., Alghanai, E., Concerns for low-resource countries, with under-prepared intensive care units, facing the COVID-19 pandemic, Infection, Disease & Health, doi: 10.1016/j.idh.2020.05.008. [DOI] [PMC free article] [PubMed]
- Emadian S.M., Onay T.T., Demirel B. Biodegradation and bioplastics in natural environments. Waste Manag. 2016;59:526–536. doi: 10.1016/j.wasman.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Euronews, 2020. Surge in marine plastic waste as people discard PPE used to ward off COVID-19. https://www.euronews.com/2020/06/25/surge-in-marine-plastic-waste-as-people-discard-ppe-used-to-ward-off-covid-19 (accessed 8th July, 2020).
- European Centre for Disease Prevention and Control (2020). COVID-19 situation update worldwide, as of 22nd July 2020. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (Accessed 22nd July, 2020).
- European Commission. (2020). Waste management in the context of the coronavirus crisis. https://ec.europa.eu/info/sites/files/waste_management_guidance_dg-env.pdf (Accessed 20th July, 2020).
- Faridi S. A field indoor air measurement of SARS-CoV-2 in the patient rooms with confirmed Covid-19 in the largest hospital in Iran. Sci. Total Environ. 2020;725(2020) doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsaci, L. (2020). Coronavirus Ireland: cost of PPE for healthcare workers to hit €1 billion https://www.dublinlive.ie/news/health/coronavirus-ireland-cost-ppe-healthcare-18230287 (accessed 8th July, 2020).
- FDA, (2020) Emergency use authorization (EUA) information, and list of all current EUAs https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#covidppe (Accessed 8th July, 2020).
- FDA, (2020c). Enforcement policy for face masks and respirators during the coronavirus disease (COVID19) public health emergency (revised), April, 2020 https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-face-masks-and-respirators-during-coronavirus-disease-covid-19-public-health (Accessed 4th April, 2020).
- Fischer E.P., Fischer M.C., Grass D., Henrion I., Warren S.W., Westman E. Low cost measurement of facemask efficiency for following expelled droplets during speed. Sci. Adv. 2020 doi: 10.1126/sciadv.abd3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E.M., Shaffer R.E. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. J. Appl. Microbiol. 2011;110(910):287–295. doi: 10.1111/j.1365-2672.2010.04881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon J.E., Sagripanti J.L. Analysis of the survival of Venezualen equine encephalomyelitis virus and possible viral simulants in liquid suspensions. J. Appl. Microbiol. 2008;105:1477–1483. doi: 10.1111/j.1365-2672.2008.03919.x. [DOI] [PubMed] [Google Scholar]
- Flanagan S.T., Ballard D.H. 3-D printed face shields – a community response to the COVID-19 global pandemic. Acad. Radiol. 2020;27(6) doi: 10.1016/j.acra.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen F., Gerard C., Cozma-Petrut A., Viera-Pinto M., Jambrak A.R., Rowan N.J., Paulsen P., Rozycki M., Tsynes K., Rodriguez-Lazaro D., Robertson L. Inactivation of parasite transmission stages – efficacy of treatments on food of animal origin. Trends Food Sci. Technol. 2019;83:114–128. [Google Scholar]
- Garvey M., Hayes J., Clifford E., Rowan N. Ecotoxicological assessment of pulsed ultraviolet light-treated water containing microbial species and Cryptosporidium parvum using a microbiotest battery. Water and the Environment Journal. 2015;29(1):27–35. [Google Scholar]
- Gerard C., Franssen F., La Carbona S., Menterio S., Cozma-Petrut A., Utaaker K.S., Jabmrak A.R., Rowan N.J., Rodriguez-Lazaro D., Nasser A., Tysnes K., Robertson L.J. Inactivation of parasite transmission stages – efficacy of treatments on foods of non-animal origin. Trends Food Sci. Technol. 2019;91:12–23. [Google Scholar]
- Ghodrat M., Rashidi M., Samali B. Life cycle assessments of incineration treatment for sharp medical waste. Energy Technology. 2017:131–143. [Google Scholar]
- Global News Wire (2020). Medtronic increasing ventilator production to address COVID-19 pandemic.https://www.globenewswire.com/news-release/2020/03/19/2003071/0/en/Medtronic-Increasing-Ventilator-Production-to-Address-COVID-19-Pandemic.html (accessed 8th July, 2020).
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Guyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., et al. The species Severa acute respiratory shyndrom-related conronavirus: calssifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of Ireland When to wear cloth face coverings and how to make them. 2020. https://www.gov.ie/en/publication/aac74c-guidance-on-safe-use-of-face-coverings/ (Accessed 9th September, 2020)
- Grossmann J., Pierce A., Mody J., Gagne J. Institution of a novel process for N95 respiratory disinfection with vaporized hydrogen peroxide in the setting of the COVID-19 pandemic at a large academic medical center. J. Am. Coll. Surg. 2020 doi: 10.1016/j.jamcollsurg.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, D., Wang, D., Hallegatte, S., Davis, S.J., Huo, J., Li, S, Bai, Y., Lei, J., Xue, Q,
- Health Products Regulatory Authority (2020). Ventilators use in the treatment of COVID-19 – information collated by the HPRA. http://www.hpra.ie/homepage/medical-devices/covid-19-updates/ventilators (accessed 8th July, 2020).
- Hegde S. Which type of personal protective equipment (PPE) and which method of donning or doffing PPE carries the least risk of infection for healthcare workers? Evidence Based Dentistry. 2020;21:74–76. doi: 10.1038/s41432-020-0097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilingloh G.S., Aufderhorst U.W., Schipper L., Dittmer U., Witzke O., et al. 2020. Susceptibility of SARS-CoV-2 to UV Irradiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbuch B.K., et al. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am. J. Infect. Control. 2011;39(1):e1–e9. doi: 10.1016/j.ajic.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Heimbuch B., Harnish D. Applied Research Associates; Panama City, FL, USA: 2019. Research to Mitigate a Shortage of Respiratory Protection Devices During Public Health Emergencies; p. 2019. [Google Scholar]
- Holland M., Zaloga D.J., Friderici C.S. COVID-19 Personal Protective Equipment (PPE) for the emergency physician. Vis. J. Emerg. Med. 2020;19 doi: 10.1016/j.visj.2020.100740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, A.A., Barnes, D.K.A. (2020). Microplastic pollution in a rapidly changing world: implications for remote and vulnerable marine ecosystems. Sci. Total Environ.. 738. https:// 10.1016/j.scitotenv.2020.140349. [DOI] [PubMed]
- Howie R., Alfa M.J., Coombs K. Survival of enveloped and non-enveloped viruses on surfaces compared with other micro-organisms and impacts of suboptimal disinfection exposure. J. Hospital Infection. 2008;69(4):368–376. doi: 10.1159/000484899. [DOI] [PubMed] [Google Scholar]
- HSE (2020). Face coverings, medical masks and disposable gloves. https://www2.hse.ie/conditions/coronavirus/face-masks-disposable-gloves.html (Accessed 2 Sept, 2020).
- Hutti-Kaul R., Nillson L.J., Zhang B., Rehnberg N., Lundmark S. Designing biobased recycling polymers for plastics. Trends Biotechnol. 2020;38:56–67. doi: 10.1016/j.tibtech.2019.04.011. [DOI] [PubMed] [Google Scholar]
- Ilyas S., Srivastava R.R., Kim H. Disinfection technology and strategies for COVID-19 hospital and bio-medical waste management. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki H., Saito A., Sugiyama H., Okabayashi T., Fujimoto S. Rapid inactivation of SARS-CoV-2 with deep-UV LED irradiation. Emerging Microbes & Infections. 1744-1747;9:1. doi: 10.1080/22221751.2020.1796529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Medical Center of Beijing Lets talk about protective function of ´ masks. 2020. http://www.imcclinics.com/english/index. php/news/view?id=83 Accessed on 18th July 2020 at.
- Ivanov D. Predicting the impacts of epidemic outbreaks on global supply chains: a simulation-based analysis of the COVID-19/SARS-CoV2 case. Transp. Res. E. 2020 doi: 10.1016/j.tre.2020.101922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambeck J.R., Geyer R., Wilcox C., Siegler T.R., Perryman M., Andrady A., Narayan R., Low K.L. Plastic waste inputs from land into the sea. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- Jatta M., Kiefer C., Patolia H., Pan J., Harb C., Marr L.C., Baffoe-Bonnie A. N95 reprocessing by low temperature sterilization with 59% vaporized hydrogen peroxide during the 2020 COVID-19 pandemic. AJIC: American Journal of Infection Control. 2020 doi: 10.1016/j.ajic.2020.06.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop Z.M., Dobbs T.D., Ali S.R., Combellack E., Clancy R., Ibrahim N., Jovic T.H., Kaur A.J., Nijran A., O’Neill T.B., Whitaker I.S. Personal protective equipment for surgeons during COVID-19 pandemic: systematic review of availability, usage and rationing. BJS. 2020 doi: 10.1002/bjs.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinadatha C., Simmons S., Dale C., et al. Disinfecting personal protective equipment with pulsed xenon ultraviolet as a risk mitigation strategy for health care workers. Am J Infect Control. 2015;43(4):412–414. doi: 10.1016/j.ajic.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaemder S., Steinmann E. Persistence of coronavirus on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–257. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan H., Funk C., Grabert M., Oey M., Hankamer B. Green bioplastics as part of circular economy. Trends Plant Sci. 2019;24(3):237–249. doi: 10.1016/j.tplants.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Kasloff S.B., Strong J.E., Funk D., Cutts T.A. Stability of SARS-Cov-2 on critical personal protective equipment. MedRxiv. 2020 doi: 10.1101/2020.06.11.20128884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney P.A., Chan B.K., Kortright K., Cinteon M., Havill N., Russi M., Epright J., Lee L., Balcezak T., Martinello R. Hydrogen peroxide vapor sterilization of N95 respirators for reuse [e-pub ahead of print] MedRxiv. 2020 doi: 10.1001/jamaoto.2020.1423. Accessed 28th July, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi L.M., Marins B.M., dos Santos Costa P.C. Extended use or reuse of N95 respirators during COVID-19 pandemic: an overview of national regulatory authority recommendations. Infect. Control Hosp. Epidemiol. 2020 doi: 10.1017/ice.2020.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.Y., Cheng P.K., Lim W.W. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005;41:e67–e71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annual Review of Virology. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Bao Z., Liu S., Zuang D., Liu Y., Zhang W., Jiang L. Survival study of SARS virus in vitro. Chin. J. Disinfect. 2003;20:110–112. [Google Scholar]
- Li, Y-K., Peng, S., Li, L-Q., Wang, Q., Ping, W., Zhang, N., Fu, X-N. 2020. Clinical and transmission characteristics of COVID-19 – a retrospective study of 25 cases from a single thoracic surgery department. Current Medical Science, 40 (2), 295-30. [DOI] [PMC free article] [PubMed]
- Liao L., Xiao W., Zhao M., Yu X., Wang H., Wang Q., Chu S., Cui Y. Can N95 respirators be reused after disinfection? How many times? ACS Nano. 2020;14:6348–6356. doi: 10.1021/acsnano.0c03597. [DOI] [PubMed] [Google Scholar]
- Lindsley W.G., Martin S.B., Thewlis R.E., Sarkisian K., Nwoko J.O., Mead K.R., Noti J.D. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J. Occup. Environ. Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Ren N., Li C., Shan S., Miao F., Yang I., Feng I., Meng X., Wen X., Gong R.E., Xiong X., Fu C., Wu A. Status of management of medical waste in 125 medical institutions. Chin. J. Noscomial. 2017;27:4265–4269. [Google Scholar]
- Lockhart S.L., Duggan L.V., Wax R.S., et al. Personal protective equipment (PPE) for both anesthesiologists and other airway managers: principles and practice during the COVID-19 pandemic. Can J Anesth/J Can Anesth. 2020;67:1005–1015. doi: 10.1007/s12630-020-01673-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J.J., Paladino K.D., Farke J.D., Boulter K., Cawcutt K., Emodi M., Gibbs S., Hankins R., Hinkle L., Micheels T., Schwedhelm S., Vasa A., Wadman M., Watson S., Rupp M.E. N95 Filtering Facemask Respirator Ultraviolet Germicidal Irradiation (UVGI) Process for Decontamination and Reuse. Nebrasca Medicine. 2020. https://cleanhospital.com/wp-content/uploads/ 2020/03/N-95-decon-process.pdf Available on 10th August 2020 at.
- Ma Q-X, Shan H, Zhang C-M. (2020).Decontamination of face masks with steam for mask reuse in fighting the pandemic COVID-19: experimental supports. J. Med. Virol. 2020;1–4. doi: 10.1002/jmv.25921. [DOI] [PMC free article] [PubMed]
- MacIntyre C.R., Seale H., Dung T.C., Hien N.T., Nga P.T., Chughtai A.A., et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5(4) doi: 10.1136/bmjopen-2014-006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie D. Reuse of N95 masks. Engineering. 2020;6:593–596. doi: 10.1016/j.eng.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Man P., van Straten B., van den Dobbelsteen J., van der Eijk V. Sterilization of disposable face masks by means of standardized dry and steam sterilization processes; an alternative in the fight against mask shortages due to COVID-19. J. Hosp. Infect. 2020;105:356–357. doi: 10.1016/j.jhin.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Rodríguez F., et al. Blood biomarkers for assessing headaches in healthcare workers after wearing biological personal protective equipment in a COVID-19 field hospital. Research Square. 2020 doi: 10.21203/rs.3.rs-55229/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy B., Rowan N.J. Terminal sterilization of medical devices using vaporized hydrogen peroxide: a review of current methods and emerging opportunities. J. Appl. Microbiol. 2019;127(5):1403–1420. doi: 10.1111/jam.14412. [DOI] [PubMed] [Google Scholar]
- Meo S.A., Alhowikan A.M., Khlaiwi T.A.L., Meo I.M., Halepoto D.M., Iqbal M., Usmani A.M., Hajjar W., Ahmed N. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 2020 doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- Messerele V.E., Moose A.L., Ustimenko A.B. Processing of biomedical waste in plasma gasifier. Waste Manag. 2018;79:791–799. doi: 10.1016/j.wasman.2018.08.048. [DOI] [PubMed] [Google Scholar]
- Mills D., Delbert A., Harnish M.S. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am. J. Infect. Control. 2018;46(7):49–55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health of Spain Action procedure for occupational risk prevention services against exposure to SARS-COV-2. 2020. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/ documentos/PrevencionRRLL_COVID-19.pdf Available on 12th August 2020 at.
- Mitchell R., Ogunreai T., Astratianakis S., Bryce E., Gervais R., Gravel D., Johnson L., Leduc S., Roth V., Taylor G., Vearcombe M., Weir C. Impact of the 2009 influenza A (H1N1) pandemic on Canadian health care workers: a survey on vaccination, illness, absenteeism, and personal protective equipment. Am. J. Infect. Control. 2012;40(7):611–616. doi: 10.1016/j.ajic.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Mueller A.V., Eden M.J., Oakes J.M., Bellini C., Fernandez L.A. Quantitative Method for Comparative Assessment of Particle Removal Efficiency of Fabric Masks as Alternatives to Standard Surgical Masks for PPE. Matter. 2020;3(3):950–962. doi: 10.1016/j.matt.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji S., MacIntyre C.R., Seale H., et al. Cost-effectiveness analysis of N95 respirators and medical masks to protect healthcare workers in China from respiratory infections. BMC Infect. Dis. 2017;17:464. doi: 10.1186/s12879-017-2564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E.J., Masterson C., Rezoagli E., O’Toole D., Major I., Stack G.D., Lynch M., Laffey J.G., Rowan N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects —implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.139330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N95DECON Example Processes. 2020. https://www.n95decon.org/example-processes Available on 20th April 2020 at.
- N95DECON COVID N95 decon & reuse. UV-C. 2020. https://static1.squarespace.com/static/5e8126f89327941b9453eeef/t/ 5e85902c0d94fa0516aafa2a/1585811501243/200401_N95DECON_UV_factsheet_ v1.2_final.pdf Available on 10th April 2020 at.
- NGO Oceans Asia (2020). No shortage of surgical masks at the beach. https://oceansasia.org/beach-mask-coronavirus/ (accessed 20th July, 2020).
- NSAI Guidance on manufacturing and importing PPE and medical devices - ensuring compliance during the COVID-19 pandemic. 2020. https://www.nsai.ie/covid-19-ppe-md/ (Accessed 12th August, 2020)
- Nzediaegwu C., Chang S.X. Improper solid waste management increases potential for COVID-19 spread in developing countries. Resour. Conserv. Recycl. 2020 doi: 10.1016/j.resconrec.2020.104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hearn K., Gertsman S., Sampson M., Webster R., Tsampalieros A., Ng R., et al. 2020. Decontaminating N95 masks with Ultraviolet Germicidal Irradiation (UVGI) does not impair mask efficacy and safety: A Systematic Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hearn K., Sampson M., Webster R., Tsampalieros A., Ng R., Gibson J., Logos A.T., Acharya N., Agarwal A., Boggs S., Chamerlain G., Staykov E., Sirkora L., McNally J.D. Decontaminating N95 and SN95 masks with ultraviolet germicidal irradiation does not impair mask efficacy and safety. J. Hosp. Infect. 2020;106:163–173. doi: 10.1016/j.jhin.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill E., Stejskal V., Clifford E., Rowan N.J. Novel use of peatlands as future locations for the sustainable intensification of freshwater aquaculture production – a case study from the Republic of Ireland. Sci. Total Environ. 2020;706 doi: 10.1016/j.scitotenv.2019.136044. [DOI] [PubMed] [Google Scholar]
- Ou Q., Pei C., Kim S.C., Abell E., Pui D.Y.H. Evaluation of decontamination methods for commercial and alternative respirator and mask materials – view from filtration aspect. J. Aerosol Sci. 2020 doi: 10.1016/j.jaerosci.2020.105609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozog D., Parker-Miller S.B., Kohli I., Lyons A.B., Narla S., Torres A.E., Levesque M., Lim H.W., Hamzavi I.H. The importance of fit testing in decontamination of N95 respirators: a cautionary note. J. Am. Acad. Dermatol. 2020;83:672–674. doi: 10.1016/j.jaad.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagat A.-M., Seux-Goepfort R., Lutsch C., Lecouturier V., Saluzzo J.-F., Kuster I.C. Evaluation of SARS-coronavirus decontamination procedures. Appl. Biosafety. 2007;12:100–108. [Google Scholar]
- Park Personal protective equipment or healthcare workers during the COVID-19 pandemic. Infection and Chemotherapy. 2020;52(2):165–182. doi: 10.3947/ic.2020.52.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. Heat Inactivation of Different Types of SARS-CoV-2 Samples: What Protocols for Biosafety, Molecular Detection and Serological Diagnostics? Viruses. 2020;12 doi: 10.3390/v12070735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perencevich E.N., Diekema D.J., Edmond M.B. Moving personal protective equipment into the community: face shields and containment of COVID-19. JAMA. 2020;323(22):2252–2253. doi: 10.1001/jama.2020.7477. [DOI] [PubMed] [Google Scholar]
- Perkins D.J., Villescas S., Wu T.H., Muller T., Bradfute S., Hurwitz I., Cheng Q., Wilcox H., Weiss M., Bartlett C., Langsjoen J., Seidenberg P. COVID-19 global pandemic planning: decontamination and reuse processes for N95 respirators. Exp. Biol. Med. 2020;245:933–939. doi: 10.1177/1535370220925768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon A., Vialette M. Survival of viruses in water. Intervirology. 2018;61:214–222. doi: 10.1159/000484899. [DOI] [PubMed] [Google Scholar]
- Prata J.C., Silva A.L.P., Walker T.R., Duarte A.C., Rocha-Santos T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol. 2020;54(13):7760–7765. doi: 10.1021/acs.est.0c02178. [DOI] [PubMed] [Google Scholar]
- Price A., Chu L. Addressing COVID-19 Face Mask Shortages [v1.3]. COVID-19 Evidence Service. Stanford Medicine. 2020. https://elautoclave.files.wordpress.com/2020/03/stanford-2020.pdf Accessed on 18th July 2020 at.
- Price, A., Cui, Y., Liao, L., Xiao, W., Yu, X., Wang, H., Zhao, M., Wang, Q., Chu, S., Chu,
- Radonovich L.J., Simberkoff M.S., Bessesen M.T., et al. N95 Respirators vs Medical Masks for Preventing Influenza Among Health Care Personnel: A Randomized Clinical Trial. JAMA. 2019;322(9):824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranney M.L., Griffeth V., Jha A.K. Critical supply shortage – the need for ventilators and personal and protective equipment during the COVID-19 pandemic. New Englang Journal of Medicine. 2020 doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- Ratnesar-Shumate S., Williams G., Green B., Krause M., Holland B., Wood S., Bohannon J., Boydston J., Freeburger D., Hooper I. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. Journal of Infectious Disease. 2020;222:214–222. doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy S., Eimer B., Shaffer R.E. Simple Respiratory Protection—Evaluation of the Filtration Performance of Cloth Masks and Common Fabric Materials Against 20–1000 nm Size Particles. The Annals of Occupational Hygiene. 2010;54(7):789–798. doi: 10.1093/annhyg/meq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuters (2020). Discarded coronavirus masks clutter Hong Kong's beaches and trails. https://www.reuters.com/article/us-healthcoronavirus-hongkong-environme/discarded-coronavirus-masksclutter-hong-kongs-beaches-trails-idUSKBN20Z0PP.2020 (Accessed 20th July, 2020).
- Rhee S.W. Management of used personal and protective equipment related to COVID-19 in South Korea. Waste Management and Research. 2020:1–5. doi: 10.1177/0734242X20933343. [DOI] [PubMed] [Google Scholar]
- Rimmer A. Covid-19: Experts question guidance to reuse PPE. BMJ. 2020;369:m1577. doi: 10.1136/bmj.m1577. (Published 20 April 2020) [DOI] [PubMed] [Google Scholar]
- Rizman M., Mujtaba G., Memon S.S., Lee K., Rashid N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renewable and Sustainable Energy Reviews. 2018;92:394–404. September 2018. [Google Scholar]
- Rowan N.J. Pulsed light as an emerging technology to cause disruption for food and adjacent industries – Quo Vadis? Trends Food Sci. Technol. 2019;88:316–332. [Google Scholar]
- Rowan N.J., Galanakis C.M. Unlocking challenges and opportunities presented by COVID-19 pandemic for cross-cutting disruption in agri-food and green deal innovations: Quo Vadis? Sci. Total Environ. 2020;748 doi: 10.1016/j.scitotenv.2020.141362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan N.J., Laffey J.G. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic – Case study from the Republic of Ireland. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Romero J.C., del Carmen M., Ferreira P., Torrecilla J.A., Santiago G., Castro C. Disposable masks: disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Sci. Total Environ. 2020 doi: 10.1016/j.ssci.2020.104830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Salmón I., Margallo M., Laso J., Villanueva-Rey P., Mariño D., Quinteiro P., Dias A.C., Nunes M.L., Marques A., Feijoo G., Moreira M.T., Loubet P., Sonnemann G., Morse A., Cooney R., Clifford E., Rowan N.J., Méndez-Paz D., Parga X., Anglada C., Martin J.C., Rubén-Aldaco A. Addressing challenges and opportunities of the European seafood sector under a circular economy framework. Current Opinion in Environmental Science & Health. 2020;13:101–106. [Google Scholar]
- Saini Vikram, Sikri V., Batra S.D., Kalra P., Gautam K. Development of a highly effective low-cost vaporized hydrogen peroxide-based method for disinfection of personal protective equipment for their selective reuse during pandemics. Gut Pathogens. 2020 doi: 10.1186/s13099-020-00367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Stiegell M., Greeson N., Vogel A., Thomann W., Brown M., Sempowski G.D., Alderman A., Condreay J.P., Burch J., Wolfe C., Smith D., Lewis S. Decontamination and reuse of N95 respirators with hydrogen peroxide vapor to address worldwide personal protective equipment shortages during the SARSCoV-2 (COVID-19) pandemic. Applied Biosafety: Journal of ABSA International. 2020;25(2):67–70. doi: 10.1177/1535676020919932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya K., Noyes A., Kallin R., et al. Evaluating the efficacy of cloth facemasks in reducing particulate matter exposure. J Expo Sci Environ Epidemiol. 2017;27:352–357. doi: 10.1038/jes.2016.42. [DOI] [PubMed] [Google Scholar]
- Sickbert-Bennett E.E., Samet M., Clapp P.W. Filtration efficiency of hospital face mask alternatives available for use during the COVID-19 pandemic. JAMA Intern. Med. 2020 doi: 10.1001/jamaintermed.2020.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.L.P., Prata J.C., Walker T.R., Campos T.R.W., Duarte A.C., Soares A.M.V.M., Barceló D., Rocha-Santos T. 2020. Rethinking and Optimising Plastic Waste Management under COVID-19 Pandemic: Policy Solutions Based on Redesign and Reduction of Single-Use Plastics and Personal Protective Equipment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Tang Y., Ogunseitan O.A. Environmentally sustainable management of used personal and protective equipment. Environ. Sci. Technol. 2020;54:8500–8502. doi: 10.1021/acs.est.0c03022. [DOI] [PubMed] [Google Scholar]
- Skrzypczak N.G., Tanikella N.G., Pearce J.M. Open source high-temperature reprap for 3-D printing heat-sterilizable PPE and other applications. Basel. 2020 doi: 10.20944/preprints202005.0479.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Pan B., Kan H. Evaluation of heat inactivation of virus contamination on medical masks. J. Microbes Infect. 2020;15:31–33. [Google Scholar]
- Spanish Society of Preventive Medicine, Public Health and Hygiene Descontaminación de respiradores de partículas ante desabastecimiento debido a la pandemia COVID-19. 2020. https://www.sempsph.com/ images/REPROCESADO%20FFPS%201.pdf Available on 12th August 2020 at.
- Srinivasan, S., Peh, WCG. (2020). N95 filtering facepiece respirators during the COVID-19 pandemic: basics, types, and shortage solutions. Malaysian. Orthopaedic Journal 2020 Vol 14 No 2. doi: doi: 10.5704/MOJ.2007.002. [DOI] [PMC free article] [PubMed]
- Steinberg, B.E., Aoyama, K., McVey, M., Levin, D., Siddiqui, A., Munshey, F., Goldenber, N.M., Faraoni, D., Maynes, J. (2020). Efficacy and safety of decontamination for N95 respirator reuse – a systematic literature search and narrative synthesis. Can J. Anaesthesia. https://doi.org.1007/s12630-020-01770-w. [DOI] [PMC free article] [PubMed]
- Straub J. In search of technology readiness level (TRL) 10. Aerosp. Sci. Technol. 2015;46:312–320. [Google Scholar]
- Suanda J., Cawley D., Domegan C., Brenner N., Rowan N.J. A review of the perceived barriers within the health belief model on pap smear screening as a cervical cancer prevention measure. Journal of Asian Scientific Research. 2013;3:677–692. [Google Scholar]
- Suanda J., Cawley D., Brenner M., Domegan C., Rowan N. In: Social Marketing – Rebels with a Cause. Hastings Gerard, Domegan Christine., editors. Taylor and Francis Group Publishers; 2017. Identification of behavioural change strategies to prevent cervical cancer among Malay women in Malaysia; pp. 555–567. [DOI] [Google Scholar]
- Suppan L., Abbas M., Stuby L., Cottet P., Larribau R., Golay E., Iten A., Harbarth S., Gartner B., Suppan M. Effect of an E-learning module on personal protective equipment proficiency among prehospital personnel: web-based randomized controlled trial. J. Med. Internet Res. 2020;22(8) doi: 10.2196/21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakuv S., Chaudhary J., Sharma B., Verma A., Tamulevinus S., Thakur V.K. Sustainability of bioplastics – opportunities and challenges. Current Opinion in Green, Sustainable Chemistry. 2018;13:68–75. [Google Scholar]
- Thiruchelvi R., Kavitha K., Shankari K. New biotechnological routes for greener bioplastics from seaweed. Research Journal of Pharmacy and Technology. 2020;13(5) doi: 10.5958/0974-360X.2020.00444.8. [DOI] [Google Scholar]
- Thomas J.P., Srinivasan A., Wickranmarachchi C.S., Dhesi P.K., Hung Y.M., Kamath A. Evaluating the national personal and protective equipment guidance for NHS healthcare workers during the COVID-19 pandemic. Clinical Medicine. 2020;20(3) doi: 10.7861/clinmed.2020-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedeken, E.J., Tahar, A., McHugh, B., Rowan, N.J. (2017). Monitoring, sources, receptors, and control measures for three European Union watch list substances of emerging concern in receiving waters – a 20 year systematic review. Sci. Total Environ., 574-1140-1163. [DOI] [PubMed]
- Tobías A. Evaluation of the lockdown for the SAR-Cov2 epidemic in Italy and Spain after follow up. Sci. Total Environ. 2020;725:138539. doi: 10.1016/j.scitotenv.2020.138539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toon J. Ozone disinfection could safely allow reuse of personal protective equipment. Biomedical Safety & Standards. 2020;50(16):121–123. doi: 10.1097/01.BMSAS.0000696812.81177.fa. September 15, 2020. [DOI] [Google Scholar]
- Torres A.E., Lyons A.B., Narla S., Kohli I., Parks-Miller A., Ozog D., Hamzavi I.H., Lim H.W. 2020. Photochemistry and Photobiological Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Westhuizen, H.M., Kotze, K., Tonkin-Crine, S., Gobat, N., Greenhalgh, T. (2020). Face coverings for covid-19: from medical intervention to social practice. BMJ. http://dx.doi.org/10.1136/bmj.m3021. [DOI] [PubMed]
- Viscusi D.J., King W.P., Shaffer R.E. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J Int Soc Respir Prot. 2007;24:93–107. [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Lloyd-Smith J.O. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi D.J., Bergman M.S., Sinkule E., Shaffer R.E. Evaluation of the filtration performance of 21 N95 filtering face piece respirators after prolonged storage. Am. J. Infect. Control. 2009;37:381–386. doi: 10.1016/j.ajic.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi D.J., et al. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J. Occup. Environ. Hyg. 2011;8(7):426–436. doi: 10.1080/15459624.2011.585927. 2011. [DOI] [PubMed] [Google Scholar]
- Wang, J., Shen, J., Ye, D., Yan, X., Zhang, Y., Yang, W., Li, X., Wang, J., Zhang, L. Pan, L. 2020. Disinfection technology of hospital waste and wastewater suggestions for disinfection strategy during Coronavirus Disease-2019 (COVID-19) pandemic in China. Environ. Pollut., 262. https://doi.org./10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed]
- WHO (2020). Transmission of SARS-CoV2: implications for infection control prevention. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (Accessed 25th August, 2020).
- WHO, Shortage of personal protective equipment endangering.
- World Bank, World Development Indicators. (2019). Solid waste management. Retrieved on July 8th, 2020 from:https://www.worldbank.org/en/topic/urbandevelopment/ brief/solid-waste-management.
- World Health Organization. (2020). Director General's opening remarks at the media briefing on COVID-19, 11th March, 2020. https://www.who.int/dg/speeches/detail/who-direcot-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 (accessed 20th March, 2020).
- WWF (2020). Nello smalltimento di macherine e guanti serve responsabilità. https://www.wwf.int/scoule/?53500%2/FNello-smalltimento-di-macherine-e-guanti-serve-responsabilita World Wide Fund for nature (accessed 20th July, 2020).
- Yang Y., Wang H., Chen K., Zhao J., Deng S., Wang Y. Shelter hospital mode: how to prevent novel corona virus infection 2019(COVID-19) hospital-acquired infection? Infect. Control Hosp. Epidemiol. 2020 doi: 10.1017/ice.2020.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Jia-min, Zheng Chong-yi, Geng-fu Xiao, Yuan-quan Zhou, Gao Rong. Examination of the Efficacy of Ozone Solution Disinfectant in Inactivating SARS Virus. Chinese J. Disinfection. 2004 2004-01. [Google Scholar]
- Zhao Z., Zhang Z., Lanzarini L., Sinha S., Rho H., Herckes P., Westerhoff P. Germicidal ultraviolet light does not damage or impede performance of N95 masks upon multiple uses. Environmental Science and Technology Letters. 2020 doi: 10.1021/acs.estlett.0c00416. [DOI] [PubMed] [Google Scholar]
- Zheng J., Suh S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Chang. 2019;9:374–378. [Google Scholar]
- Zhu Z., Yan J.H., Jiang X.G., Lai Y.E., Cen K.F. Study on pyrolysis of typical medical waste materials by using TG-FTIR analysis. J. Hazard. Mater. 2008;153:670–676. doi: 10.1016/j.jhazmat.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Zorko David J., Gertsman Shira, O’Hearn Katie, Timmerman Nicholas, Ambu-Ali Nasser, Dinh Tri, Sampson Margaret, et al. Decontamination interventions for the reuse of surgical mask personal protective equipment: a systematic review. OSF Preprints. 2020 doi: 10.31219/osf.io/z7exu. April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]