Abstract
Background
Poor adherence to ART increases viremia, which leads to disease progression and transmission of drug-resistant HIV strains. This study aimed to assess the level of ART adherence and associated factors among adolescents and adult patients enrolled in ART care in Northern Ethiopia.
Methods
A retrospective analysis was conducted among 19,525 patients from April 2015 to March 2019. Data verification and filtration were done in Excel 2013 before exporting to STATA 14.0. Ordinal logistic regression was used to analyze the data.
Results
About 94.84%, 95% CI (94.52%, 95.14%) of the study subjects were in good adherence. However, about 1.46%, 95% CI (1.30%, 1.64%) and 3.70%, 95% CI (3.44%, 3.97%) of them had poor and fair adherence respectively. In the adjusted analysis, being male (AOR = 0.75; 95% CI: 0.0.65, 0.87), patients from general hospitals (AOR = 0.52; 95% CI: 0.39, 0.69), WHO staging IV (AOR = 0.57; 95% CI: 0.41, 0.81) and non-suppressed viral load (VL) status (AOR = 0.54; 95% CI: 0.47, 0.63) were negatively associated with good adherence. Whereas, age of 50+ years old (AOR = 1.68; 95% CI: 1.13, 2.50), recent CD4 count of 200–499 (AOR = 1.45; 95% CI: 1.21, 1.74) and recent CD4 count of 500 and above (AOR = 1.84; 95% CI: 1.47, 2.32) were positively associated with good ART drug adherence.
Conclusion
There was a higher level of adherence compared to the previous studies conducted in Ethiopia. Being male, patients from general hospitals, WHO staging II, II and IV and non-suppressed VL status were negatively associated with good adherence. Whereas, older ages, recent CD4 count of 200–499 and ≥500 CD4 count were positively associated with good ART drug adherence. The health system should recognize a higher need of younger age groups and males to design targeted counseling and support to encourage consistently high levels of adherence for a better ART treatment outcome.
Keywords: adherence and compliance, antiretroviral therapy, retreatment
Background
Standard antiretroviral therapy (ART) consists of at least three antiretroviral (ARV) drugs in combination to maximally suppress the HIV virus and stop the progression of HIV disease.1 Based on the 2017 World Health Organization’s (WHO) report, 36.7 million people were living with HIV/AIDS worldwide.2 Access to highly active antiretroviral therapy (HAART) in low- and middle-income countries has expanded dramatically.3 Subsequently, HAART coverage is increasing; and nearly 20.9 million people were taking HAART in June 2017.2 It is estimated that 1% of the Ethiopian population were living with HIV and 66% of the adults and adolescents aged 15 years and above were on ART treatment in 2018.4
The introduction of ART played a significant role in improving the lives of people living with HIV/AIDS (PLWHA).5 However, the efficacy and durability of the ART drug regimens are mainly determined on adherence to the drugs.6 ART requires near perfect adherence rates (as high as 95%).7,8 Poor adherence can cause serious consequences, such as increasing viremia which may lead to disease progression, development and transmission of drug-resistant HIV strains to others.9,10
Optimal adherence to HAART depends on socio-demographic (age, gender), clinical (WHO staging, VL and CD4 count) and health system-related factors.13–15 Although assessing the level of ART adherence and its determinant factors is crucial for further improvement of ART, there were no studies in Northern Ethiopia with a large representative sample size. Therefore, this study aimed to assess the level of self-reported adherence and associated factors among adolescent and adult patients enrolled in ART care in Northern Ethiopia. The finding of this study could supplement achieving the Sustainable Development Goal (SDG) to “end AIDS epidemic” by 2030.
Methods
Study Design, Setting and Data Source
A retrospective analysis was conducted from April 2015 to March 2019 in Tigray Health Research Institute (THRI), the only center of viral load (VL) determination in Tigray region and some parts of Afar region. Tigray region is the 4th most populous out of the 9 Regional States of Ethiopia (Figure 1). As part of the intensive program of the three 90s (by 2020; 90% of all people living with HIV will know their HIV status, 90% of all people with diagnosed HIV infection will receive sustained antiretroviral therapy, and 90% of all people receiving antiretroviral therapy will have viral suppression),14 ART treatment and response (the third 90) was being monitored by VL which was commenced in April 2015 in Tigray region. Data were obtained from the standard sample referral form of VL. The study was done among 19,525 patients, who had complete data on demographic, clinical, immunological and viral load in the database of THRI. Adherence was one of the independent variables in the previously published studies. However, the level of self-reported adherence and its determinants were not presented. To come up with the sample, all records in the database were reviewed and all the data that fulfill the eligibility criteria were included in the study.15–17
Figure 1.
Map of the study area.
Eligibility Criteria
Patients enrolled in ART care for at least 6 months were included in the study. Whereas, subjects younger than 15 years were excluded from the study.17
Operational Definitions
Adherence
The level of compliance to taking ART medications by the patients during ART care.
Good Adherence
Drug adherence of 95% or ≤2 missed drug doses of 30 doses or <3 missed drug doses of 60 doses.18
Fair Adherence
Drug adherence of 85–94% or 3–5 missed drug doses of 30 doses or 4–9 missed drug doses of 60 doses.18
Poor Adherence
Drug adherence of <85% or ≥6 doses of missed ART drug doses of 30 doses or >9 doses missed ART drug doses of 60 doses.18
Incomplete adherence
If the patients reported either poor or fair adherence.
Viral Non-Suppression
Elevated VL Ribonucleic Acid (RNA) copies of >1000 copies/mL in plasma in a patient who has been on ART for at least 6 months.18
Declined Immunological Response
A recent CD4 count of less than the baseline CD4 T-cells count where (Recent CD4 count minus the Baseline CD4 count = Negative).
Same Immunological Response
The recent and the baseline CD4 counts are the same where (Recent CD4 count minus Baseline CD4 count = 0).
Enhanced Immunological Response
A recent CD4 count of greater than the baseline CD4 T-cells count where (Recent CD4 count minus Baseline CD4 count = Positive).
Data Collection Tools and Procedure
All the data in the database of the VL were exported to Microsoft Excel 2013 and then data verification and filtration were done before exporting to STATA 14.0 for analysis. During the filtration, complete regimens, meaningful range of biological measures and clients from Tigray region health care facilities were filtered, and overall 19,525 subjects with compete data were filtered from the total 55,104 clients for the analysis. HIV-1 RNA was extracted from 0.2 mL of plasma using the Abbott m2000sp automated sample preparation system (Abbott Molecular, USA) at THRI. Extracted RNAs were measured using Abbott m2000rt quantitative Real Time HIV-1 assay (Abbott Molecular, USA) with an HIV-1 RNA detection level of 40 to 10 million copies/mL based on the manufacturer’s procedures. Adherence was measured by self-report and it was sent through the standard VL referral form. Immunological response after ART initiation was assessed by subtracting the baseline CD4 count from a recent CD4 count; categorized as deteriorated, the same and positively responded.
Data Quality Assurance
Data completeness and consistency were checked in Microsoft Excel. High and low positive controls were checked before performing the patient sample for VL determination. However, CD4 count quality controls were done based on low, medium and high controls to evaluate the run validity in each laboratory, where the CD4 count was done.
Data Management and Analysis
Analysis was done using STATA 14.0 to estimate the proportion of adherences and identify factors associated with adherences. Levels of adherences and the CD4 count were categorized based on the Ethiopian ART follow-up guideline and WHO respectively. The proportion of adherence levels was further evaluated by age group, sex, pregnancy status, breastfeeding status, WHO staging, CD4 baseline, CD4 recent, immunological response status, regimen, treatment line, reason for VL test, health facility ownership and health facility level. Cross tabulation was done for each of the independent variables with the outcome variable. Missed values were filtered and excluded for all variables; hence there was no issue of missing values in the final dataset.
The outcome variable has natural order, and hence the model analytic approach used to identify factors associated with adherence levels was the ordinal logistic regression. The outcome variable was adherence level (0 = Poor adherence, 1 = Fair adherence and 2 = Good adherence). Statistical significance was considered at 95% CI (two-sided) in the examination of variables and interpretation of crude and adjusted odds ratios. Unadjusted association was used to determine the strength of the association between the independent variables and the outcome variable in the bivariable ordinal logistic regression. Whereas, adjusted associations were used to determine the strength of associations in the multivariable ordinal logistic regression. All significant variables at P-value ≤0.05 in the bivariable ordinal logistic regression were entered into the multivariable ordinal logistic regression. As pregnancy and breastfeeding status apply to females, both variables were excluded from statistical model building in the multivariable ordinal logistic regression. Variables which have a collinearity effect (Variation Inflation Factor (VIF)) were removed/omitted in the statistical modeling.
Result
Level of Self-Reported ART Adherence
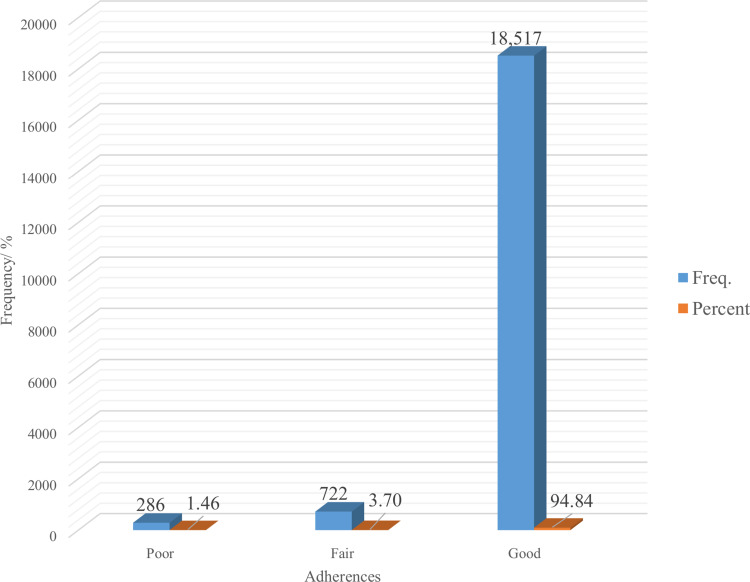
About 94.84%, 95% CI (94.52%, 95.14%) of the study subjects were at a good level of self-reported ART adherence. However, about 1.46%, 95% CI (1.30%, 1.64%) and 3.70%, 95% CI (3.44%, 3.97%) of them had poor and fair adherence respectively. The incomplete adherence level of this study was 5.16%, 95% CI (4.86%, 5.48%) (Figure 2).
Figure 2.
Levels of adherences of ART medication among HIV-positive adolescents and adults in Tigray region, North Ethiopia, 2015–2019 (n = 19,525).
Factors Associated with Self-Reported ART Drug Adherences
The bivariable ordinal logistic regression showed that age, gender, WHO stage, viral load status, 2h (TDF-3TC-ATV/R) regimen, baseline CD4 count and recent CD4 count were associated with self-reported ART drug adherence. After adjusting for possible effects of confounding variables in the multivariable ordinal logistic regression, being male (AOR = 0.75; 95% CI: 0.0.65, 0.87), patients from general hospitals (AOR = 0.52; 95% CI: 0.39, 0.69), WHO staging II (AOR = 0.47; 95% CI: 0.36, 0.60), WHO staging III (AOR = 0.25; 95% CI: 0.19, 0.34), WHO staging IV (AOR = 0.57; 95% CI: 0.41, 0.81) and non-suppressed VL status (AOR = 0.54; 95% CI: 0.47, 0.63) were negatively associated with good adherence. Whereas, age categories from 30 to 34 years (AOR = 1.54; 95% CI: 1.04, 2.26), 35–39 years (AOR = 1.47; 95% CI: 1.01, 2.15), 45–49 years (AOR = 1.87; 95% CI: 1.24, 2.83) and 50+ years old (AOR = 1.68; 95% CI: 1.13, 2.50), recent CD4 count of 200–499 (AOR = 1.45; 95% CI: 1.21, 1.74) and recent CD4 count of 500 and above (AOR = 1.84; 95% CI: 1.47, 2.32) were positively associated with good ART drug adherence (all associations assessed are presented in Table 1).
Table 1.
Bivariable and Multivariable Ordinal Logistic Regression Analysis of Adherence to Antiretroviral Therapy Among HIV-Positive Adolescents and Adults in Tigray Region, North Ethiopia, 2015–2019 (N = 19,525)
| Variables | Category | ART Adherence | Total | COR (95% CI) | AOR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Poor, n (%) | Fair, n (%) | Good, n (%) | |||||
| Age | 15–19 | 13 (4.55) | 23 (3.19) | 384 (2.07) | 420 (2.15) | 1 (Ref.) | 1 (Ref.) |
| 20–24 | 11 (3.85) | 15 (2.08) | 529 (2.86) | 555 (2.84) | 1.91 (1.13, 3.22)* | 1.55 (0.91, 2.62) | |
| 25–29 | 31 (10.84) | 65 (9.00) | 1736 (9.38) | 1832 (9.38) | 1.70 (1.14, 2.54)** | 1.33 (0.88, 2.01) | |
| 30–34 | 58 (20.28) | 119 (16.48) | 3539 (19.11) | 3716 (19.03) | 1.88 (1.29, 2.74)*** | 1.54 (1.04, 2.26)* | |
| 35–39 | 53 (18.53) | 163 (22.58) | 4014 (21.68) | 4230 (21.66) | 1.75 (1.21, 2.54)** | 1.47 (1.01, 2.15)* | |
| 40–44 | 56 (19.58) | 167 (23.13) | 3490 (18.85) | 3713 (19.02) | 1.48 (1.02, 2.14)* | 1.32 (0.90, 1.93) | |
| 45–49 | 28 (9.79) | 69 (9.56) | 2051 (11.08) | 2148 (11.00) | 1.99 (1.34, 2.97)*** | 1.87 (1.24, 2.83)** | |
| 50+ | 36(12.59) | 101 (13.99) | 2774 (14.98) | 2911 (14.91) | 1.91 (1.30, 2.81)*** | 1.68 (1.13, 2.50)* | |
| Gender | Female | 158 (55.24) | 412 (57.06) | 12,296 (66.40) | 12,866 (65.90) | 1 (Ref.) | 1 (Ref.) |
| Male | 128 (44.76) | 310 (42.94) | 6221 (33.60) | 6659 (34.10) | 0.66 (0.58, 0.75)*** | 0.75 (0.65, 0.87)*** | |
| Pregnant mother | No | 157 (99.37) | 410 (99.51) | 12,204 (99.25) | 12,771 (99.26) | 1 (Ref.) | |
| Yes | 1 (0.63) | 2 (0.49) | 92 (0.75) | 95 (0.74) | 1.42 (0.45, 4.51) | ||
| Lactating mother | No | 155 (98.10) | 402 (97.57) | 12,107 (98.46) | 12,664 (98.43) | 1 (Ref.) | |
| Yes | 3 (1.90) | 10 (2.43) | 189 (1.54) | 202 (1.57) | 0.67 (0.38, 1.18) | ||
| Facility ownership | Government | 277 (96.85) | 661 (91.55) | 17,733 (95.77) | 18,671 (95.63) | 1 (Ref.) | |
| NGO | 9 (3.15) | 61 (8.45) | 770 (4.16) | 840 (4.30) | 0.59 (0.46, 0.76) | ||
| Private | 0 | 0 | 14 (0.08) | 14 (0.07) | – | ||
| Facility type | Clinic | 0 | 0 | 43 (0.23) | 43 (0.22) | ||
| Health center | 69 (24.13) | 134 (18.56) | 5479 (29.59) | 5682 (29.10) | 1.05 (0.77, 1.41) | ||
| Primary hospital | 28 (9.79) | 81 (11.22) | 2022 (10.92) | 2131 (10.91) | 0.72 (0.52, 1.002) | ||
| General hospital | 163 (56.99) | 461 (63.85) | 9205 (49.71) | 9829 (50.34) | 0.57 (0.43, 0.76)*** | 0.52 (0.39, 0.69)*** | |
| Referral hospital | 16 (5.59) | 40 (5.54) | 1443 (7.79) | 1499 (7.68) | 1 (Ref.) | ||
| Other | 10 (3.50) | 6 (0.83) | 325 (1.76) | 341 (1.75) | 0.77 (0.44, 1.38) | ||
| Service provided at defense facility | No | 276 (96.50) | 692 (95.84) | 17,882 (96.57) | 18,850 (96.54) | 1 (Ref.) | |
| Yes | 10 (3.50) | 30 (4.16) | 635 (3.43) | 675 (3.46) | 0.86 (0.62, 1.19) | ||
| WHO staging | I | 220 (76.92) | 582 (80.61) | 17,027 (91.95) | 17,829 (91.31) | 1 (Ref.) | 1 (Ref.) |
| II | 23 (8.04) | 64 (8.86) | 688 (3.72) | 775 (3.97) | 0.37 (0.30, 0.47)*** | 0.47 (0.36, 0.60)*** | |
| III | 28 (9.79) | 50 (6.93) | 330 (1.78) | 408 (2.09) | 0.20 (0.15, 0.25)*** | 0.25 (0.19, 0.34)*** | |
| IV | 15 (5.24) | 26 (3.60) | 472 (2.55) | 513 (2.63) | 0.54 (0.39, 0.75)*** | 0.57 (0.41, 0.81)** | |
| Viral load test reason | First at 6 months | 177 (61.89) | 427 (59.14) | 11,355 (61.32) | 11,959 (61.25) | 1 (Ref.) | 1 (Ref.) |
| Routine annual VL test | 37 (12.94) | 175 (24.24) | 5820 (31.43) | 6032 (30.89) | 1.47 (1.25, 1.72)*** | 1.40 (1.19, 1.65)*** | |
| Suspected ART failure clinical | 0 | 4 (0.55) | 46 (0.25) | 50 (0.26) | 0.63 (0.23, 1.75) | ||
| Suspected ART failure– immunological | 8 (2.8) | 8 (1.11) | 65 (0.35) | 81 (0.41) | 0.21 (0.12, 0.36)*** | 0.45 (0.24, 0.82)** | |
| Suspected ART failure – initial viral load | 56 (19.58) | 92 (12.74) | 943 (5.09) | 1091 (5.59) | 0.33 (0.28, 0.41)*** | 0.55 (0.44, 0.69)*** | |
| Not indicated in the form | 8 (2.8) | 16 (2.22) | 288 (1.56) | 312 (1.60) | 0.64 (0.42, 0.97)* | 0.62 (0.41, 0.94) | |
| Viral load status | Suppressed | 123 (43.01) | 413 (57.20) | 13,836 (74.72) | 14,372 (73.61) | 1 (Ref.) | 1 (Ref.) |
| Non-suppressed | 163 (56.99) | 309 (42.80) | 4681 (25.28) | 5153 (26.39) | 0.38 (0.34, 0.43)*** | 0.54 (0.47, 0.63)*** | |
| Regimen | 1c (AZT–3TC–NVP) | 85 (29.72) | 222 (30.75) | 5525 (29.84) | 5832 (29.87) | 1 (Ref.) | 1 (Ref.) |
| 1d (AZT–3TC–EFV) | 28 (9.79) | 85 (11.77) | 1794 (9.69) | 1907 (9.77) | 0.88 (0.71, 1.10) | ||
| 1e (TDF–3TC–EFV) | 141 (49.30) | 309 (42.80) | 8940 (48.28) | 9390 (48.09) | 1.10 (0.95, 1.28) | ||
| 1f (TDF–3TC–NVP) | 29 (10.14) | 90 (12.47) | 2023 (10.93) | 2142 (10.97) | 0.95 (0.76, 1.18) | ||
| 1g (ABC–3TC–EFV) | 0 | 0 | 6 (0.03) | 6 (0.03) | – | ||
| 1h (ABC–3TC–NVP) | 0 | 1 (0.14) | 6 (0.03) | 7 (0.04) | 0.35 (0.04, 2.88) | ||
| 2a (ABC–ddl–LPV/R), 2c (TDF–ddl–LPV/R), 2d (TDF–ddl–NFV) and 2g (TDF–3TC–LPV/r) | 0 | 0 | 12 (0.06) | 12 (0.06) | – | ||
| 2f (AZT–3TC–ATV/r) | 0 | 3 (0.42) | 59 (0.32) | 62 (0.32) | 1.11 (0.35, 3.55) | ||
| 2h (TDF–3TC–ATV/R) | 3 (1.05) | 12 (1.66) | 152 (0.82) | 167 (0.86) | 0.57 (0.33, 0.98)* | 1.04 (0.59, 1.83) | |
| Baseline CD4 count | <200 | 164 (57.34) | 392 (54.29) | 9131 (49.31) | 9687 (49.61) | 1 (Ref.) | 1 (Ref.) |
| 200–499 | 103 (36.01) | 250 (34.63) | 7252 (39.16) | 7605 (38.95) | 1.25 (1.09, 1.43)** | 1.03 (0.88, 1.21) | |
| 500 and above | 19 (6.64) | 80 (11.08) | 2134 (11.52) | 2233 (11.44) | 1.32 (1.06, 1.64)* | 0.95 (0.71, 1.27) | |
| Recent CD4 count | <200 | 88 (30.77) | 206 (28.53) | 2749 (14.85) | 3043 (15.59) | 1 (Ref.) | 1 (Ref.) |
| 200–499 | 125 (43.71) | 325 (45.01) | 8459 (45.68) | 8909 (45.63) | 2.01 (1.73, 2.35)*** | 1.45 (1.21, 1.74)*** | |
| 500 and above | 73 (25.52) | 191 (26.45) | 7309 (39.47) | 7573 (38.79) | 2.96 (2.50, 3.52)*** | 1.84 (1.47, 2.32)*** | |
| Immunological response compared to baseline | Negative | 80 (27.97) | 180 (24.93) | 3279 (17.71) | 3539 (18.13) | 1 (Ref.) | 1 (Ref.) |
| No change | 6 (2.10) | 12 (1.66) | 232 (1.25) | 250 (1.28) | 0.72 (0.43, 1.21) | ||
| Positive response | 200 (69.93) | 530 (73.41) | 15,006 (81.04) | 15,736 (80.59) | 1.63 (1.41, 1.89)*** | 1.14 (0.93, 1.40) | |
Notes: *P-value ≤ 0.05; **P-value ≤ 0.01; ***P-value ≤ 0.001.
Abbreviations: 3TC, lamivudine; ABC, abacavir; AOR, adjusted odds ratio; ART, antiretroviral therapy; ATV/r, atazanavir/ritonavir; AZT, azidothymidine; CD4, cluster of differentiation 4; COR, crude odds ratio; ddl, didanosine; EFV, efavirenz; LPV/R, lopinavir/ritonavir; n, number; NFV, nelfinavir; NGO, non-governmental organization; NVP, nevirapine; P-value, precession value; Ref., reference; TDF, tenofovir disoproxil fumarate; WHO, World Health Organization; VL, viral load.
Discussion
The main aim of this study was to assess the level of adherence and associated factors among adolescent and adult patients on ART care in Northern Ethiopia. The good adherence rate in this study was 94.84%. This is near the current recommendation of at least 95% of ART adherence level required to suppress viral replication to achieve clinical improvement, and increased CD4 count.19 The finding of this study showed a better ART medication adherence compared to the studies conducted in Gobba, Southeast Ethiopia (90.8%),20 Eastern Ethiopia (85%),21 Hara Town and its Surroundings, Northeast Ethiopia (71.8%)22 and Gondar Referral Hospital, Northwest Ethiopia (88.2%).23 On the other hand, the incomplete adherence level of this study was 5.16%, 95% CI (4.86%, 5.48%), which is lower compared to the study conducted in Southern Ethiopia which was 13%, 95% CI (11%, 15%) of incomplete adherence level.24 The differences for these findings might be due to differences in the definition of incomplete adherence, the impact of the ART program over time in creating beneficiary awareness and care provider skill in the provision of counseling. The other possible explanation might be that many of the participants included in this study had longer ART duration, and those taking the drugs for a longer duration usually acquire skills on how to deal with some of the obstacles hindering their adherence. Further, other context variations may also explain the difference observed, like the method of adherence measuring.
With regard to the age of the patients, patients in the age groups 30–34, 35–39, 45–49 and 50+ years old, their odds of having good adherence compared with those who had fair and poor adherences combined were 1.54, 1.47, 1.87 and 1.68 times higher keeping all the other variables in the model constant. Studies from Gondar, Northwest Ethiopia,23 Northeast Ethiopia25 and Gobba Hospital, Southeast Ethiopia20 reported that age has no significant associations with adherences. However, a study from Kenya had found that adherence to ART increased with increased age and decreased as the age goes beyond 60 years.26 Another study from Rwanda has also shown that being in the age groups 18–24, 25–34 and 35–44 vs ≥44 years respectively were associated with non-perfect ART adherence.27 Moreover, there are different studies contradicting the finding of this study. A study from Eastern Ethiopia showed patients who were in the age group 35–44 years old were more adherent than older age groups (≥45 years).21 The differences of these findings might be due to differences in study design and measurements and the exact reasons for poor adherence were not explored in different age groups. However, there were previous studies that reported reasons for poor adherence among young people, including denial and fear of HIV infection, low self-esteem, and unstructured and chaotic lifestyles.28,29 Also, a few studies have documented high levels of risky sexual behavior among young age groups on ART.30 Poor adherence to ART among young individuals may increase treatment failure and the spread of drug-resistant HIV viruses. Incomplete treatment adherence may also affect, in the future, drug choice.
For male patients, the odds of good adherence compared with fair and poor adherences combined were 0.75 times less, keeping all the other variables in the model constant. Similar findings from sub-Saharan Africa and Rwanda evidenced that being male was the main determinant for ART non-adherence.27,31 Likewise, a prospective cohort observational study conducted in four health facilities in south Tigray revealed that being male was an independent risk factor for attrition from HIV care which translates into non-adherence.32 However, studies from Gobba Hospital, Southeast Ethiopia and Northeast Ethiopia have shown that gender was not significantly associated with ART adherence.20,25 The reason why males were prone to viral non-suppression might be due to low health-seeking behavior.32–34 Compared to females, males were more likely to forgo ART because of side effects.35 This indicates intensive counseling support to males is in need to minimize treatment failure and drug mutations.
The odds of good adherence versus the combined poor and fair adherence were 0.52 times less among patients served in general hospitals compared to those served in referral hospitals, given that all other variables in the model are held constant. Although there were no studies which contradict or support the finding of this study, the differences might be attributed due to variation in the level of setup, experience, training and skills of the service providers.
The odds of good adherence versus the combined poor and fair adherence were 1.45 times higher among patients with a recent CD4 count of 200–499 cells/mm3 compared to those patients with a recent CD4 count of <200 cells/mm3, given that all other variables in the model are held constant. Likewise, the odds of good adherence versus the combined poor and fair adherence were 1.84 times higher among patients with a recent CD4 count of ≥500 cells/mm3 compared to those patients with a recent CD4 count of <200 cells/mm3, given that all other variables in the model are held constant. A previous study evidenced that patients with higher recent CD4 count were significantly associated with adherence.23 A similar study also showed that low CD4 cell counts were associated with increased risk of non-adherence.35 This is because good adherence contributes to greater growth of CD4 cell counts.36 Or in those patients who miss building immunity, it is due to viral non-clearance from the blood in the absence of optimal adherence. Likewise, for patients with WHO staging II, III and IV compared with WHO staging I, the odds of good adherence versus the combined poor and fair adherence were 53%, 75% and 43% lower respectively, given that all of the other variables in the model held constant. However, a study from Northeast Ethiopia indicated that WHO clinical staging was not associated with medication adherence.25 This difference might be in study method difference, in which the later one was with a small sample size that cannot ensure external validity. On the other hand, for patients with viral non-suppression taking viral suppression as reference, the odds of good adherence versus the combined poor and fair adherence was 46% lower, given that all of the other variables in the model held constant. There were other similar studies which support non-adherence was associated with unsustained viral suppression patients.37 Likewise, higher viral loads are associated with increased risk of non-adherence.35 This is because as viremia increases, the probability of complication increases, which results in forgetting the medication time.
Strengths and Limitations of the Study
The strengths of the study are that the study was done in a relatively higher sample size with an appropriate analysis technique that provides important information regarding patients’ medication adherence to ART treatment in Northern Ethiopia. Despite these strengths, the study was not without limitation. Adherence was measured based on self-report and is susceptible to recall and social desirability bias. Due to the nature of secondary data, the analysis misses some important variables such as the existence of family support, disclosure, co-morbidity and grade of ART experience in HIV-infected patients.
Conclusion
There was a higher level of adherence compared to the previously conducted studies in Ethiopia. Being male, patients from general hospitals, WHO staging II, III and IV and non-suppressed VL status were negatively associated with good adherence. Whereas, older ages, recent CD4 count of 200–499 and ≥500 CD4 count were positively associated with good ART drug adherence. The health system should recognize a higher need of the younger age groups and males to design targeted counseling and support for this high-risk group. The present study supports the need to promote adherence and encourage consistently high levels of adherence to achieve a good treatment outcome and slow the development of drug resistance.
Acknowledgments
The authors would like to acknowledge the data clerks of Tigray Health Research Institute for their kind cooperation to access the required data.
Funding Statement
There was no specific fund for this study.
Data Sharing Statement
The data that support the findings of this study are available from the Tigray Regional Health Bureau and Tigray Health Research Institute but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data can be accessed from Tigray Health Research Institute, Institutional Review Board (IRB) via institutional.review.board.thri@gmail.com. Upon permission, de-identified data will be shared.
Ethics Approval and Informed Consent
Ethical clearance and approval were obtained from Tigray Health Research Institute (THRI) Institutional Review Board (IRB) with a reference no. of THRI/00132/19. The dataset owned by Tigray Regional Health Bureau (TRHB) and THRI and are the legally authorized representative of the patient’s data. Permission to use the data only for research purpose. Informed written consent was obtained from THRI and TRHB.
Patient Data Confidentiality and Compliance
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Personal identifiers were de-identified when extracting the data from the database to maintain privacy and confidentiality of the patients.
Author Contributions
All authors made a significant contribution to the conception, study design, execution, and acquisition of data, analysis and interpretation. All authors involved in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Authors’ declare that there are no competing interests.
References
- 1.WHO. Antiretroviral therapy. Geneva: WHO; Available from: https://www.who.int/hiv/topics/treatment/art/en/. Accessed May22, 2020. [Google Scholar]
- 2.World Health Organization. Antiretroviral Therapy (ART) Coverage Among All Age Groups. Switzerland, Genieva: WHO; 2017. [Google Scholar]
- 3.Weiler G. Global update on HIV treatment 2013: results, impact and opportunities. Kuala Lumpur, Malaysia: International AIDS Soiety (IAS); 2013. Available from: http://www.who.int/hiv/event s/2013/1_weile r_repor t_ias_v5. pdf. [Google Scholar]
- 4.UNAIDS. Over view in Ethiopia. Geneva: UNAIDS; 2018. Available from: https://www.unaids.org/en/regionscountries/countries/ethiopia. Accessed February27, 2020. [Google Scholar]
- 5.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on ART across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11(5):492–500. doi: 10.1097/COH.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton SS, Magagnoli J, Hardin JW. Odds of viral suppression by single-tablet regimens, multiple-tablet regimens, and adherence level in HIV/AIDS patients receiving antiretroviral therapy. Pharmacotherapy. 2017;37(2):204–213. doi: 10.1002/phar.1889 [DOI] [PubMed] [Google Scholar]
- 7.Amico KR, Toro-Alfonso J, Fisher JD. An empirical test of the information, motivation and behavioral skills model of antiretroviral therapy adherence. AIDS Care. 2005;17(6):661–673. doi: 10.1080/09540120500038058 [DOI] [PubMed] [Google Scholar]
- 8.Sarna A, Luchters S, Geibel S, Munyao P, Kaai S. Promoting Adherence to Antiretroviral Therapy Through a Directly Administered Antiretroviral Therapy (DAART) Strategy in Mombasa, Kenya. Nairobi, Kenya: Horizons Research Update, Population Council; 2005. [Google Scholar]
- 9.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the international antiviral society-USA panel. JAMA. 2014;312(4):410–425. doi: 10.1001/jama.2014.8722 [DOI] [PubMed] [Google Scholar]
- 10.Williams B, Wood R, Dukay V, et al. Treatment as prevention: preparing the way. J Int AIDS Soc. 2011;14(Suppl 1):S6. doi: 10.1186/1758-2652-14-S1-S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coetzee B, Kagee A, Bland R. Barriers and facilitators to paediatric adherence to antiretroviral therapy in rural South Africa: a multi-stakeholder perspective. AIDS Care. 2015;27(3):315–321. doi: 10.1080/09540121.2014.967658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reda AA, Biadgilign S. Determinants of adherence to antiretroviral therapy among HIV-infected patients in Africa. AIDS Res Treat. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta K, Ekstrand M, Heylen E, Sanjeeva G, Shet A. Adherence to antiretroviral therapy among children living with HIV in South India. AIDS Behav. 2016;20(5):1076–1083. doi: 10.1007/s10461-015-1207-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UNAIDS. 90-90-90: treatment for all. Geneva: UNAIDS; Available from: https://www.unaids.org/en/resources/909090. Accessed May22, 2020. [Google Scholar]
- 15.Desta AA, Wubayehu Woldearegay T, Berhe AA, Futwi N, Gebremedhn Gebru G, Godefay H. Immunological recovery, failure and factors associated with CD-4 T-cells progression over time, among adolescents and adults living with HIV on antiretroviral therapy in northern Ethiopia: a retrospective cross sectional study. PLoS One. 2019;14(12):e0226293. doi: 10.1371/journal.pone.0226293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desta AA, Mehari KK, Berhe AA, et al. Prediction of CD4 T-lymphocyte count using who clinical staging among ART-naïve HIV-infected adolescents and adults in northern ethiopia: a retrospective study. AIDS Res Treat. 2020;2020:2163486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desta AA, Woldearegay TW, Futwi N, et al. HIV virological non-suppression and factors associated with non-suppression among adolescents and adults on antiretroviral therapy in northern Ethiopia: a retrospective study. BMC Infect Dis. 2020;20(4). doi: 10.1186/s12879-019-4732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federal Ministry of Health (FMOH) Ethiopia. National Consolidated Guidelines for Comprehensive Hiv Prevention, Care and Treatment. Addis Ababa: FMOH Ethiopia; 2018. [Google Scholar]
- 19.WHO. Scaling up priority HIV/AIDS interventions in the health sector, progress report: towards universal access. Available from: www.who.int/hiv/mediacentre/universal_access_progress_report_en.pdf. Accessed April10, 2020.
- 20.Lencha B, Hasen K, Getachew K, Abdi M, Habtamu M. Adherence to antiretroviral therapy and associated factors among people living with HIV/AIDS at gobba hospital, southeast ethiopia: an institutional based study. Qual Prim Care. 2015;23(6):336–341. [Google Scholar]
- 21.Letta S, Demissie A, Oljira L, Dessie Y. Factors associated with adherence to antiretroviral therapy (ART) among adult people living with HIV and attending their clinical care, Eastern Ethiopia. BMC Int Health Hum Rights. 2015;15(1):33. doi: 10.1186/s12914-015-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legesse TA, Reta MA. Adherence to antiretroviral therapy and associated factors among people living with HIV/AIDS in Hara town and its surroundings, North-Eastern Ethiopia: a cross-sectional study. Ethiop J Health Sci. 2019;29(3):299–308. doi: 10.4314/ejhs.v29i3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molla AA, Gelagay AA, Mekonnen HS, Teshome D. Adherence to antiretroviral therapy and associated factors among HIV positive adults attending care and treatment in University of Gondar Referral Hospital, Northwest Ethiopia. BMC Infect Dis. 2018;18(1):266. doi: 10.1186/s12879-018-3176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teshome W, Belayneh M, Moges M, et al. Who Takes the Medicine? Adherence to Antiretroviral Therapy in Southern Ethiopia. Vol. 2015 Dove Medical Press Limited; 2015:9 Pages 1531—1537. doi: 10.2147/PPA.S9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mengistie A, Birhane A, Tesfahun E. Assessment of adherence to antiretroviral therapy among adult people living with HIV/AIDS in North East, Ethiopia. Appl Microb Res. 2019;2(2):21–27. [Google Scholar]
- 26.Anthony N. Factors that influence non-adherence to antiretroviral therapy among HIV and AIDs patients in central province, Kenya; 2011. Available from: http://ir-library.ku.ac.ke/handle/123456789/1725. Accessed August21, 2020.
- 27.Elul B, Basinga P, Nuwagaba-Biribonwoha H, et al. High levels of adherence and viral suppression in a nationally representative sample of HIV-infected adults on antiretroviral therapy for 6, 12 and 18 months in Rwanda. PLoS One. 2013;8(1):e53586. doi: 10.1371/journal.pone.0053586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonell K, Naar-King S, Huszti H, Belzer M. Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV. AIDS Behav. 2013;17(1):86–93. doi: 10.1007/s10461-012-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudy BJ, Murphy DA, Harris DR, Muenz L, Ellen J. Prevalence and interactions of patient-related risks for nonadherence to antiretroviral therapy among perinatally infected youth in the United States. AIDS Patient Care STDS. 2010;24(2):97–104. doi: 10.1089/apc.2009.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mhalu A, Leyna GH, Mmbaga EJ. Risky behaviours among young people living with HIV attending care and treatment clinics in Dar Es Salaam, Tanzania: implications for prevention with a positive approach. J Int AIDS Soc. 2013;16(1):17342. doi: 10.7448/IAS.16.1.17342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heestermans T, Browne JL, Aitken SC, Vervoort SC, Klipstein-Grobusch K. Determinants of adherence to antiretroviral therapy among HIV-positive adults in sub- Saharan Africa: a systematic review. BMJ Glob Health. 2016;1(4):e000125. doi: 10.1136/bmjgh-2016-000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bucciardini R, Fragola V, Abegaz T, et al. Retention in care of adult hiv patients initiating antiretroviral therapy in tigray, Ethiopia: a prospective observational cohort study. PLoS One. 2015;10(9):e0136117. doi: 10.1371/journal.pone.0136117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jobanputra K, Parker LA, Azih C, et al. Factors associated with Virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One. 2015;10(2):e0116144. doi: 10.1371/journal.pone.0116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penot P, He’ma A, Bado G, et al. The vulnerability of men to virologic failure during antiretroviral therapy in a public routine clinic in Burkina Faso. JIAS. 2014;17(1):18646. doi: 10.7448/IAS.17.1.18646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonsah JY, Njamnshi AK, Kouanfack C, et al. Adherence to antiretroviral therapy (ART) in yaounde´-cameroon: association with opportunistic infections, depression, ART regimen and side effects. PLoS One. 2017;12(1):e0170893. doi: 10.1371/journal.pone.0170893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Zhou J, Zhou J, et al. Consistent ART adherence is associated with improved quality of life, CD4 counts, and reduced hospital costs in Central China. AIDS Res Hum Retrovir. 2009;25(8):757–763. doi: 10.1089/aid.2008.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalichman SC, Grebler T, Amaral CM, et al. Viral suppression and antiretroviral medication adherence among alcohol using HIV positive adults. Int J Behav Med. 2014;21(5):811–820. doi: 10.1007/s12529-013-9353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]