Abstract
A causal relationship between elevated triglycerides and cardiovascular disease is controversial, as trials of triglyceride-lowering treatments have not shown significant impact on cardiovascular outcomes. However, hypertriglyceridemia is associated with atherogenesis and risk for acute cardiovascular events that persist despite optimal statin treatment. Although most trials of triglyceride-lowering treatments have been negative, in trials of niacin and fibrates, subgroup analyses in patients with higher baseline triglycerides and lower HDL-C levels suggest reduced incidence of cardiovascular endpoints. The REDUCE-IT trial demonstrated that addition of purified prescription eicosapentaenoic acid (icosapent ethyl) 4 g/day in high-risk patients with triglyceride levels 135–499 mg/dL and optimized statin treatment significantly reduced cardiovascular events versus placebo (hazard ratio 0.75; 95% confidence interval 0.68–0.83; P < 0.001). Benefit was seen regardless of baseline and on-treatment triglyceride levels, suggesting that other effects of eicosapentaenoic acid besides triglyceride reduction may have played a role.
Keywords: Triglycerides, REDUCE-IT, Icosapent ethyl, Eicosapentaenoic acid
Highlights
-
•
Hypertriglyceridemia is associated with atherogenesis & cardiovascular event risk.
-
•
Most triglyceride treatment cardiovascular outcomes trials have been negative.
-
•
However, icosapent ethyl significantly reduced cardiovascular events in REDUCE-IT.
-
•
Effects of icosapent ethyl beyond triglyceride reduction may play a role.
1. Introduction
The American Heart Association has long recognized that elevated triglyceride (TG) levels are a marker of cardiovascular disease (CVD) risk [1]. TG and TG-rich lipoproteins (TRLs) are among the atherogenic lipids and lipoproteins believed to be both causal and prognostic factors for atherosclerotic CV disease (ASCVD) [2,3]. Although there is pathophysiologic evidence for a role of TG in ASCVD, as well as observational and clinical trial data supporting the association between elevated TG levels and CV event risk [4,5], until recently primary endpoints of clinical trials of TG-lowering agents including fibric acid derivatives, niacin, and omega-3 fish oils have failed to show CV event risk reduction [[6], [7], [8], [9], [10], [11], [12], [13]]. The objective of this review is to examine the biochemical and clinical evidence for the role of elevated TG levels in ASCVD and to place the results of CV outcomes trials of TG-lowering agents, including the recent Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) [14], into proper context.
2. Biology of TG levels and ASCVD event risk
Although hypertriglyceridemia most commonly occurs secondary to conditions such as metabolic syndrome, diabetes mellitus, and central obesity, it can also be caused by various medications as well as primary genetic causes; these are summarized in Table 1 [15]. In some individuals, such as those with primary hypertriglyceridemia, excess alcohol consumption, or pregnancy, TG levels can rise to >1000 mg/dL, a significant risk factor for acute pancreatitis requiring immediate treatment [[15], [16], [17]].
Table 1.
Causes of hypertriglyceridemia.
| Primary Causes | |
| Genetic syndromes | Familial hypertriglyceridemia |
| Familial combined hyperlipidemia | |
| Lipoprotein lipase deficiency | |
| Apolipoprotein C-II deficiency | |
| Apolipoprotein AV deficiency | |
| Dysbetalipoproteinemia | |
| Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) deficiency |
|
| Secondary Causes | |
| Disease | Metabolic syndrome |
| Hypothyroidism | |
| Diabetes mellitus | |
| Central obesity | |
| Renal diseases | |
| Nephrotic syndrome | |
| Autoimmune disorders (e.g. lupus) | |
| HIV-associated dyslipidemia | |
| Pregnancy (third trimester) | |
| Medications | Non-selective beta blockers |
| Thiazides | |
| Corticosteroids | |
| Tamoxifen | |
| Raloxifene | |
| Oral estrogens | |
| Protease inhibitors | |
| Retinoic acid | |
| Isotretinoin | |
| Sirolimus | |
| l-asparaginase | |
| Bile acid resins | |
| Phenothiazines | |
| Second-generation antipsychotics | |
| Immunosuppressants | |
| Diet | Excess alcohol |
| Positive-energy balanced diet with saturated fat or high glycemic index/load content | |
Republished with permission of Bentham Science Publishers, from Hypertriglyceridemia - common causes, prevention and treatment strategies, Rygiel K, Curr Cardiol Rev 2018[15]; permission conveyed through Copyright Clearance Center, Inc.
Hypertriglyceridemia results from increased production of TRLs (ie, chylomicrons and very-low-density lipoproteins), decreased catabolism of TRLs, or a combination of the two, leading to an excess of TRLs and changes in the composition of key lipoprotein particles, including low- and high-density lipoproteins (LDL and HDL) [1]. In the setting of hypertriglyceridemia, cholesterol ester transfer protein transfers TGs out of TRLs in exchange for cholesterol esters from HDL and LDL particles. As the HDL and LDL particles become progressively more enriched with TGs, they become better substrates for lipolysis by hepatic lipase, resulting in HDL catabolism and increased formation of smaller, denser LDL particles. This produces “atherogenic dyslipidemia,” with high TRLs, increased numbers of small, dense LDL, and low serum levels of HDL. In people with hypertriglyceridemia, HDL may be dysfunctional and less likely to participate in reverse cholesterol transport, while small, dense LDL particles may be more susceptible to oxidative modification and reduced clearance. Hypertriglyceridemia is also associated with increased remnant lipoprotein particles (RLPs, which include very-low-density lipoproteins and intermediate-density lipoproteins) due to inadequate lipoprotein lipase (LPL) activity, such that RLPs are not lipolyzed and converted to LDL particles. RLPs are pro-atherogenic, as they contain apolipoprotein (apo) B, apo CIII, TGs, and cholesteryl esters. Similar to LDL particles, TRLs are trapped by glycosaminoglycans in the intima of blood vessels, where they are scavenged by macrophages to form foam cells and promulgate inflammation [18]. There is also evidence that free fatty acids released from hydrolyzed TGs and TRLs in the subendothelial space are pro-inflammatory and contribute to atherogenesis [19,20]. Remnants upregulate the expression of pro-inflammatory cytokines (eg, tumor necrosis factor-α, interleukin-6, vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and monocyte chemoattractant protein-1) and are directly cytotoxic to endothelium [[21], [22], [23]]. Ultimately, inflammation in the subendothelial space triggers formation of a pro-oxidative, pro-atherogenic milieu.
The possible causal role of elevated TG levels in ASCVD development and progression is supported by genetic, genome-wide analysis, and Mendelian randomization studies [3] that confirm elevated TG levels may play a causal role in atherogenesis. In a multivariable Mendelian randomization study of 20,000 individuals with myocardial infarction (MI), a one standard deviation increase in TG levels correlated with a 54% increase in risk for coronary heart disease (CHD) [24]. Common polymorphisms associated with higher TG levels and increased ASCVD event risk include loss-of-function mutations in APOA5 and APOC2, and gain-of-function mutations in APOC3, angiopoietin-like proteins-3 and -4, and LPL variants [25]. In contrast, genetic studies have demonstrated that genetically lower TG levels, irrespective of causal polymorphism, correlate with lower risk of ASCVD [[26], [27], [28], [29]].
3. Clinical evidence for the association of TGs and CV event risk
Several studies have shown that elevated TG levels correlate with elevated ASCVD event risk. In an analysis of 1836 consecutive patients who underwent coronary revascularization, risk of CV death over the subsequent 10 years was significantly associated with higher TG levels (P = 0.002), even after adjusting for total cholesterol and HDL cholesterol (HDL-C) [30].
The Strong Heart Study reported the effects of TGs in a community-based, prospective cohort of 3216 Native Americans and found that elevated (≥150 mg/dL) fasting TG levels and low (<40 mg/dL) HDL-C levels were associated with increased risk of incident CHD and ischemic stroke, particularly in individuals with diabetes or LDL cholesterol (LDL-C) levels ≥130 mg/dL, independent of other risk factors [31]. A recent analysis from the Atherosclerosis Risk in Communities Study and the Framingham Offspring Study found that ASCVD risk rose rapidly across the entire range of TG levels starting below 100 mg/dL [32].
Two claims-based analyses of the Optum Research Database examined the association between hypertriglyceridemia (TG levels 200–499 mg/dL, TG levels ≥150 mg/dL) and CV events in statin-treated individuals aged ≥45 years with documented diabetes and/or ASCVD [33,34]. The cohort with TG levels 200–499 mg/dL was more likely to have an initial major CV event than a propensity-matched cohort with TG levels <150 mg/dL (hazard ratio [HR] 1.349; 95% confidence interval [CI] 1.23–1.49; P < 0.001) [33]. Likewise, the cohort with TG levels ≥150 mg/dL was more likely to have an initial major CV event than the propensity-matched cohort (HR 1.26; 95% CI 1.19–1.34; P < 0.001) [34]. These results remained statistically significant even after adjusting for HDL-C levels.
In an analysis of baseline fasting TG levels from 2307 patients with type 2 diabetes and CVD in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial, higher TG levels were associated with major adverse CV events [35]. While elevated TG levels are an established marker for increased risk of CVD, the causal relationship is less certain. A large meta-analysis of epidemiologic data found that although higher TG levels are associated with increased CHD risk, the correlation was substantially attenuated after adjusting for established risk factors, especially HDL-C [36].
Data also suggest that lower TG levels are associated with reduced CV event risk. In the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22 trial (PROVE-IT TIMI 22), patients who received statin treatment following hospitalization for acute coronary syndrome had a lower risk of further CHD events if on-treatment TG levels were <150 mg/dL [4]. For each 10% lowering of on-treatment TG levels, there was a 2.7% reduction in incidence of subsequent CHD events (P = 0.003) [4]. In addition, Mendelian randomization analyses evaluating participants in the UK Biobank study or in one of 62 cohort, case-controlled, or cross-sectional studies conducted in North America or Europe found that TG-lowering LPL variants and LDL-C–lowering LDL-receptor variants were associated with a similar lower risk of CHD per unit difference in apo B [37].
Elevated RLPs, which are associated with hypertriglyceridemia, have also been correlated with CV events. An analysis of the Copenhagen City Heart Study found that a 39 mg/dL increase in non-fasting RLPs was associated with a 2.8-fold risk in ischemic heart disease [38]. Similar results were reported in an analysis of the Jackson Heart and Framingham Offspring Cohort studies [39]. Even after adjustment for other CV event risk factors, increased RLP remained a predictor of CHD (HR 1.18; 95% CI 1.00–1.39; P = 0.049).
Taken together, these data indicate that elevated TG and TRL levels and related markers are at least prognostic for ASCVD risk, and are potentially causative. A recent National Health and Nutrition Examination Survey (NHANES)-based analysis found that >25% of US adults have elevated TG levels, including stain-treated patients; it was estimated that over the next decade, there will be >3.4 million ASCVD events in patients with LDL-C levels <100 mg/dL who have TG levels ≥150 mg/dL, with ~1 million of these events occurring in statin users [40]. This high number of events predicted in statin-treated patients highlights the presence of CV event risk even in patients with controlled LDL-C [2].
4. CV outcomes trials of niacin and fibrates
Although there is evidence that lower TG levels are associated with reduced ASCVD event risk, most CV outcomes trials of TG-lowering treatments, such as niacin and fibrates, have failed to show clinical benefit (Table 2). None of these studies sought to randomize patients with hypertriglyceridemia, and neither of the 2 studies evaluating the efficacy of combining niacin with statin treatment demonstrated CV event risk reduction. The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) investigators studied the effect of niacin in patients with elevated TG levels and low HDL-C levels who received intensive statin therapy [41]. In the niacin group, median TG levels decreased from 164 mg/dL at baseline to 120 mg/dL after 3 years, while median HDL-C levels increased from 35 mg/dL to 42 mg/dL. Despite improvements in the plasma lipid profile with niacin, there was no difference between treatment groups in the composite primary CV endpoint (HR 1.02; 95% CI 0.87–1.21; P = 0.80). However, in a subgroup of patients with TG levels ≥200 mg/dL and HDL-C levels <32 mg/dL, CV event risk decreased with niacin versus placebo (HR 0.64; P = 0.032) [42]. The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), which investigated niacin in combination with laropiprant when added to simvastatin ± ezetimibe, also failed to show clinical benefit with niacin for major vascular events (rate ratio 0.96; 95% CI 0.90–1.03; P = 0.29) [7]. An exploratory subgroup analysis of HPS2-THRIVE in patients with low HDL-C levels and TG levels >151 mg/dL found that niacin was not associated with reduction in incidence of major vascular events.
Table 2.
Cardiovascular outcomes trials of niacin and fibrates.
| Trial | Intervention | Patient Population | Primary Endpoint | Primary Endpoint Met? | HR/RR for Intervention |
|---|---|---|---|---|---|
| Trials of Niacin | |||||
| AIM-HIGH [41] | Extended-release niacin 1500–2000 mg/day plus simvastatin vs placebo plus simvastatin | 3414 adults aged ≥45 years receiving high-intensity statin therapy with established CV disease, HDL-C <40 mg/dL, and TG 150–400 mg/dL | CHD death, non-fatal MI, ischemic stroke, hospitalization >23 h for ACS, or symptom-driven coronary or cerebral revascularization | No | HR 1.02; 95% CI 0.87–1.21; P = 0.80 |
| HPS2-THRIVE [7] | Niacin ER 1 g/day in combination with laropiprant 20 mg for 4 weeks followed by ER niacin 2 g/day in combination with laropiprant 40 mg/day for 3–6 weeks vs placebo for secondary prevention | 25,673 adults aged 50–80 years with a history of MI, cerebrovascular disease, peripheral artery disease, or diabetes mellitus with evidence of symptomatic coronary disease; all patients were on statins ± ezetimibe. No lipid level-based inclusion criteria. | Major vascular events (non-fatal MI, death from coronary causes, stroke of any type, or coronary or non-coronary revascularization) | No | RR 0.96; 95% CI 0.90–1.03; P = 0.29 |
| Trials of Fibrates | |||||
| FIELD [9] | Fenofibrate 200 mg micronized per day vs placebo for primary prevention | 9795 adults aged 50–75 years with type 2 diabetes and not using a statin or other lipid-modifying drugs; total cholesterol 116–251 mg/dL, plus either a total cholesterol/HDL-C ratio of ≥4.0:1 or TG 88.6–442.9 mg/dL | First occurrence of non-fatal MI or death from CHD | No | HR 0.89; 95% CI 0.75–1.05; P = 0.16 |
| ACCORD Lipid [8] | Fenofibrate 160 mg/day initially (later adjusted to eGFR) + simvastatin vs placebo + simvastatin | 5518 adults with type 2 diabetes aged 40–79 years if evidence of clinical CV disease, or aged 55–79 years if evidence of subclinical CV disease or at least 2 additional risk factors; LDL-C 60–180 mg/dL and HDL-C <55 mg/dL for women and African-American patients (<50 mg/dL for all other groups) | First occurrence of major CV event, including non-fatal MI, non-fatal stroke, or death from CV causes | No | HR 0.92; 95% CI 0.79–1.08; P = 0.32 |
| BIP [43] | Bezafibrate 400 mg/day vs placebo | 3122 adults aged 45–74 years with a history of MI ≥ 6 months to <5 years before enrollment, and/or stable angina pectoris with total serum cholesterol 180–250 mg/dL, LDL-C ≤180 mg/dL (≤160 mg/dL for patients <50 years of age), HDL-C ≤45 mg/dL, and TG ≤ 300 mg/dL; excluded patients using lipid-modifying drugs | Fatal MI, non-fatal MI, or sudden death | No | 9.4% risk reduction; P = 0.26 |
ACCORD, Action to Control Cardiovascular Risk in Diabetes; ACS, acute coronary syndrome; AIM-HIGH, Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes; BIP, Bezafibrate Infarction Prevention; CHD, coronary heart disease; CI, confidence interval; CV, cardiovascular; ER, extended release; FIELD, Fenofibrate Intervention and Event Lowering in Diabetes; HDL-C, high-density lipoprotein cholesterol; HPS2-THRIVE, Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; RR, rate ratio; TG, triglycerides.
Studies of fibrates have yielded equivocal results. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid study, in which fenofibrate was added to open-label simvastatin in patients with diabetes, found that although the fenofibrate group experienced a mean reduction in TG levels of 42 mg/dL, there was no corresponding benefit in the primary composite CV endpoint (HR 0.92; 95% CI 0.79–1.08; P = 0.32) [8]. There was, however, a trend toward better outcomes in fenofibrate-treated patients who had TG levels in the upper tertile (≥204 mg/dL) and HDL-C levels in the lowest tertile (≤34 mg/dL) (P = 0.057) [8]. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial randomized patients with type 2 diabetes to fenofibrate or placebo [9]; after 4 months, there was a 29% decrease in TG, 5% increase in HDL-C, and a 12% decrease in LDL-C levels. The primary CV endpoint, first occurrence of non-fatal MI or CHD death, was not significantly different between groups (HR 0.89; 95% CI 0.75–1.05; P = 0.16). The overall finding of the FIELD study was likely impacted by the fact that more patients in the placebo arm (17%) were treated with a non-study lipid-lowering drug (mainly statins) at any point during the study than the fenofibrate arm (8%). A significant reduction in total CVD events was observed (HR 0.89; 95% CI 0.80–0.99; P = 0.035) and driven by reduction of non-fatal MI and coronary revascularization. In the Bezafibrate Infarction Prevention (BIP) study [43], patients with a history of MI and/or stable angina were randomized to bezafibrate or placebo. Although there was a 21% reduction in TG and an increase in HDL-C levels sustained for 5 years, there was no significant reduction in risk of the composite CV endpoint after a mean 6.2 years of follow-up (risk reduction 9.4%; P = 0.26). A subgroup analysis of the BIP study by TG level found that elevated baseline TG levels were significantly associated with a greater reduction in CV endpoints with bezafibrate, with a 39.5% reduction in the primary endpoint versus a 7.9% reduction in those with TG levels <150 mg/dL (relative risk [RR] 0.57; 95% CI 0.35–0.93) [43]. The ongoing Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) trial will provide additional information on fibrates in managing patients with TG levels ≥200 and < 500 mg/dL and low HDL-C levels [44].
5. OMEGA-3 fatty acids and CV risk
There has been interest in omega-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) for prevention of CVD due to a low CVD incidence in populations that consume high amounts of fatty fish [45]. Short-term studies have shown that omega-3 fatty acids (2–4 g/day) can reduce plasma TG levels [[46], [47], [48], [49], [50]]. A number of CV outcomes studies have been conducted in patients receiving omega-3 fatty acids (Table 3 and discussed below).
Table 3.
Cardiovascular outcomes trials of omega-3 fatty acids.
| Trial | Intervention | Patient Population | Statin Use | Primary Endpoint | Primary Endpoint Met? | HR/OR/RR for Intervention |
|---|---|---|---|---|---|---|
| Trials of DHA+EPA combinations | ||||||
| GISSI Prevenzione [51] | 1 g omega-3 fatty acids (EPA:DHA in a 1:2 ratio) vs no supplement for secondary prevention | 11,324 patients within 3 months after MI; no lipid level–based inclusion criteria | Not established as preventive care standard at time of study | Co-primary endpoints: (1) death, non-fatal MI, and non-fatal stroke; (2) CV death, non-fatal MI, and non-fatal stroke | Yes | (1) RR 0.85; 95% CI 0.74–0.98 (2) RR 0.80; 95% CI 0.68–0.95 |
| OMEGA [10] | 1 g omega-3 fatty acids (DHA 380 mg + EPA 460 mg) vs 1 g olive oil for secondary prevention | 3851 patients who had experienced acute MI within 3–14 days of randomization; no lipid level–based inclusion criteria | >90% on statins at discharge post-MI | Sudden cardiac death | No | OR 0.95; 95% CI 0.56–1.60; P = 0.84 |
| VITAL [12] | 1 g omega-3 fatty acid (DHA 380 mg + EPA 460 mg) vs placebo for primary prevention | 25,871 men ≥50 years of age or women ≥55 years of age; no lipid level–based inclusion criteria | ~35% on statins | MI, stroke, and CV death | No | HR 0.92; 95% CI 0.80–1.06; P = 0.24 |
| ASCEND [11] | Omega-3 fatty acids (DHA 380 mg + EPA 460 mg) vs placebo | 15,480 diabetic patients aged ≥40 years, but with no evidence of CV disease; no lipid level–based inclusion criteria | ~75% on statins | Serious vascular events | No | RR 0.97; 95% CI 0.87–1.08; P = 0.55 |
| Trials of EPA alone | ||||||
| JELIS [60] | EPA 1.8 g/day + statin vs statin alone for primary and secondary prevention | 5859 men aged 40–75 years and 12,786 postmenopausal women up to 75 years with hypercholesterolemia (total cholesterol ≥251 mg/dL; corresponding to LDL-C ≥170 mg/dL) | 98% on statins | Major coronary events including sudden cardiac death, fatal and non-fatal MI, unstable angina pectoris, angioplasty, stenting, or coronary artery bypass grafting | Yes | HR 0.81; 95% CI 0.69–0.95; P = 0.011 |
| REDUCE-IT [14] | Icosapent ethyl 4 g/day + statin vs placebo + statin | 8179 adults aged ≥45 years with established CV disease or ≥50 years with type 2 diabetes and ≥1 additional CV event risk factor; TG 135–499 mg/dL and LDL-C 41–100 mg/dL | >99% on statins | Composite of CV death, non-fatal MI, non-fatal stroke, coronary revascularization, or unstable angina in a time-to-event analysis | Yes | HR 0.75; 95% CI 0.68–0.83; P < 0.001 |
ASCEND, A Study of Cardiovascular Events in Diabetes; CI, confidence interval; CV, cardiovascular; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; GISSI-Prevenzione, Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico; HR, hazard ratio; JELIS, Japan EPA Lipid Intervention Study; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; OMEGA, Randomized, Placebo-Controlled Trial to Test the Effect of Highly Purified Omega-3 Fatty Acids on Top of Modern Guideline-Adjusted Therapy After Myocardial Infarction; OR, odds ratio; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial; RR, rate ratio; TG, triglycerides; VITAL, Vitamin D and Omega-3 Trial.
5.1. CV outcomes studies of DHA+EPA combination omega-3 fatty acids
An early omega-3 fatty acid open-label clinical trial, Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI)-Prevenzione, had promising findings. Patients with MI in the previous 3 months (n = 11,324) were randomized in an open-label fashion to combination omega-3 fatty acids (EPA:DHA 1 g/day in a 1:2 ratio), vitamin E, vitamin E + omega-3 fatty acids, or placebo. In a 4-way analysis, omega-3 fatty acids alone were associated with a significant reduction in death, non-fatal MI, and non-fatal stroke (RR 0.85; 95% CI 0.74–0.98) and CV death, non-fatal MI, and non-fatal stroke (RR 0.80; 95% CI 0.68–0.95) [51]. Given that statins were not yet a standard background therapy when GISSI-Prevenzione was conducted, these findings are not generalizable to the contemporary era of CV event risk management.
Despite the positive results of GISSI-Prevenzione, a number of comprehensive meta-analyses and reviews failed to show consistent impact of omega-3 consumption on CV outcomes or CVD mortality [2,[52], [53], [54], [55]]. Several high-profile CV outcomes studies of combination omega-3 formulations (DHA+EPA) failed to meet primary endpoints. The Randomized, Placebo-Controlled Trial to Test the Effect of Highly Purified Omega-3 Fatty Acids on Top of Modern Guideline-Adjusted Therapy After Myocardial Infarction (OMEGA) was a secondary prevention study in patients who experienced acute MI 3–14 days from randomization [10]. Patients received combination omega-3 fatty acids (DHA 380 mg + EPA 460 mg) or placebo, in addition to standard-of-care treatment. There was no difference between treatment arms in the rate of the primary endpoint of sudden cardiac death (1.5%, both groups; odds ratio [OR] 0.95; 95% CI 0.56–1.60; P = 0.84). In A Study of Cardiovascular Events in Diabetes (ASCEND) [11], patients with diabetes received combination omega-3 fatty acids (DHA 380 mg + EPA 460 mg) or placebo. After a mean follow-up of 7.4 years, there was no difference in occurrence of serious vascular events (8.9%, omega-3 fatty acid group, 9.2%, placebo; RR 0.97; 95% CI 0.87–1.08; P = 0.55). Most recently, in the Vitamin D and Omega-3 Trial (VITAL), patients received 1 g/day of combination omega-3 fatty acids (DHA 380 mg + EPA 460 mg), vitamin D3 supplementation, or placebo [12,13]. After 5.3 years of follow-up, neither the omega-3 fatty acid product nor vitamin D3 was associated with a significantly lower incidence of major CV events (HR 0.97; 95% CI 0.85–1.12; P = 0.69). Most recently, the Outcomes Study to Assess Statin Residual Risk Reduction With Epanova in High CV Risk Patients With Hypertriglyceridemia (STRENGTH) trial [56], a phase 3 study of combination omega-3 carboxylic acids (4 g/day EPA+DHA), was discontinued prior to completion given that the drug was unlikely to demonstrate a clinical benefit among patients with dyslipidemia (TG levels ≥180 mg/dL and <500 mg/dL; HDL-C <42 mg/dL in men or <47 mg/dL in women) considered to be at high risk for future CV disease events [[56], [57], [58]].
Thus, the collective clinical data do not support the efficacy of DHA+EPA combination omega-3 fatty acids for ASCVD event risk reduction. As with most studies of fibrates and niacin, these DHA+EPA studies, with the exception of STRENGTH, did not specifically enroll patients with hypertriglyceridemia. In addition, doses of omega-3 fatty acids were generally low (≤1 g/day), and may have been inadequate for CV event risk reduction. However, the discontinued STRENGTH trial did enroll patients with elevated TG levels, and employed a higher dose of 4 g/day EPA+DHA, yet still failed to demonstrate benefit [[56], [57], [58]]. The ongoing Omega-3 Fatty Acids in Elderly Patients With Myocardial Infarction (OMEMI) study will provide additional insights into 1.8 g/day DHA+EPA combination omega-3 fatty acids in statin-treated patients for reduction of persistent CV event risk specific to the elderly population [59].
5.2. CV outcomes studies of EPA-only omega-3 fatty acids
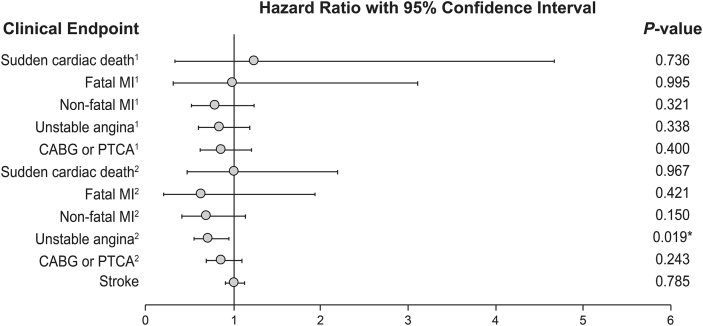
Studies of purified EPA in combination with statins have yielded more favorable results (Table 3). The open-label Japan EPA Lipid Intervention Study (JELIS) included 18,645 patients with hypercholesterolemia [60]. Patients received EPA 600 mg three times daily on a background of pravastatin 10 mg or simvastatin 5 mg. After 4.6 years of follow-up, EPA plus statin demonstrated a 19% reduction in the primary CV composite endpoint of major coronary events versus statin alone in the total study population (HR 0.81; 95% CI 0.69–0.95; P = 0.011); however, this remained statistically significant only for secondary prevention and not primary prevention. In addition, the only individual endpoint that reached statistical significance was hospitalization for unstable angina (HR 0.72; 95% CI 0.55–0.95, Fig. 1) [60]. JELIS was restricted to a Japanese population and thus may not be generalizable to other populations. Additional limitations include the open-label design, very low doses of background statin therapy, and use of a relatively low EPA dose (1.8 g/day).
Fig. 1.
Hazard Ratios of Clinical Endpoints for the Japan EPA Lipid Intervention Study (JELIS). Superscript 1 and 2 indicate primary and secondary prevention endpoints, respectively [60]. ∗P < 0.05. Reprinted from The Lancet, 369, Yokoyama M, Origasa H, Matsuzaki M et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis, 1090–1098. Copyright 2007, with permission from Elsevier.
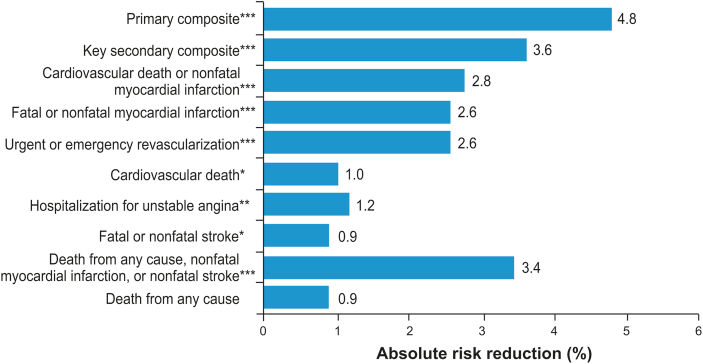
The recently published multicenter, international study REDUCE-IT investigated icosapent ethyl (high-purity EPA) 2 g twice daily versus placebo in patients with TG levels 135–499 mg/dL who were either ≥45 years of age with established CVD or ≥50 years of age with type 2 diabetes and at least one additional CV risk factor [14]. This was an aggressively statin-treated population with mean baseline LDL-C level of 75 mg/dL. After a median 4.9 years of follow-up, a primary composite CV endpoint (defined as CV death, non-fatal MI, non-fatal stroke, coronary revascularization, or unstable angina) occurred in 17.2% of patients in the icosapent ethyl group versus 22.0% in the placebo group (HR 0.75; 95% CI 0.68–0.83; P < 0.001; Fig. 2). Similar results were seen for the key secondary endpoint of CV death, non-fatal MI, or non-fatal stroke, which occurred in 11.2% of patients in the icosapent ethyl group versus 14.8% in the placebo group (HR 0.74; 95% CI 0.65–0.83; P < 0.001). Overall, icosapent ethyl reduced CV deaths by 20% compared with placebo. Among patients enrolled in REDUCE-IT in the United States, there was a significant 30% and 34% relative risk reduction in all-cause and CV-related mortality, respectively, in the icosapent ethyl group compared with the placebo group [61].
Fig. 2.
Absolute Risk Reduction (ARR) in REDUCE-IT Endpoints. Percentages were calculated by taking absolute differences in endpoint rates between icosapent ethyl and control groups [ARR = (n/N)placebo – (n/N)icosapent ethyl]. Primary composite endpoint events: cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina. Key secondary composite endpoint events: cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke [14]. ∗P < 0.05; ∗∗P ≤ 0.01; ∗∗∗P < 0.001 [14].
REDUCE-IT was the first international outcomes study of a lipid-lowering agent to demonstrate significant CV event risk reduction when targeting persistent risk beyond LDL-C lowering in statin-treated patients. As a result, several leading health authorities issued guideline updates incorporating REDUCE-IT findings, and the US Food and Drug Administration approved icosapent ethyl as an adjunct to statin therapy to reduce the risk of MI, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated TG levels (≥150 mg/dL) and established CVD or diabetes mellitus and two or more additional risk factors for CVD. Health Canada has also recently approved icosapent ethyl for all five major adverse CV events, including CV death, as part of the prespecified REDUCE-IT composite endpoint. The American Diabetes Association, the (US) National Lipid Association, and the European Society of Cardiology and European Atherosclerosis Society all recommend that addition of icosapent ethyl be considered in high-risk patients who have TG levels 135–499 mg/dL despite statin treatment [[62], [63], [64]]. The American Heart Association issued a Science Advisory stating that the results of REDUCE-IT support use of icosapent ethyl for reducing ASCVD risk in high-risk patients with hypertriglyceridemia on statin therapy [65]. The effectiveness of EPA treatment in REDUCE-IT, coupled with the discontinuation of the STRENGTH trial given that the EPA+DHA carboxylic acid compound used was deemed unlikely to demonstrate a benefit in patients, suggest that these effects may be specific to EPA, rather than a class effect of omega-3 fatty acids [57,61].
The clinical benefit seen with icosapent ethyl in REDUCE-IT was achieved regardless of baseline or on-treatment TG levels [14]. Similarly, the relatively modest decrease in TG levels in JELIS does not fully explain the observed clinical benefit of EPA treatment [60]. A recent meta-analysis of omega-3 fatty acid studies including REDUCE-IT supported a reduction in the risk of CVD endpoints [66]; however, a meta-analysis of TG-lowering treatments suggested that the CVD risk reduction associated with TG level lowering alone was relatively modest after exclusion of REDUCE-IT [67]. While elevated TG levels have been identified in some studies as an independent CVD risk factor [68,69], there is some evidence to the contrary. For example, another recent meta-analysis found that while an elevated TG level in patients with type 2 diabetes was associated with an increased risk of CVD, this association was lost after adjusting for other blood lipid parameters [70]. In REDUCE-IT, changes in EPA levels, not in lipid biomarker and inflammatory parameters, accounted for most of the study’s relative risk reduction for the primary and secondary endpoints [71]. Nonetheless, patients in REDUCE-IT with elevated TG levels and the associated high risk of ASCVD events were shown to benefit from EPA treatment. Taken together, this suggests that pleiotropic effects of EPA beyond TG lowering may contribute to CV event risk reduction.
The open-label, parallel-group Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy–Statin and Eicosapentaenoic Acid (RESPECT-EPA) [72] and the Effect of Vascepa on Improving Coronary Atherosclerosis in People with High Triglycerides Taking Statin Therapy (EVAPORATE) trial [73] will provide additional insights into the role of EPA in statin-treated patients for reduction of persistent CV event risk and progression of coronary atherosclerosis, respectively. A 9-month interim analysis of EVAPORATE showed that icosapent ethyl at 4 g/day did not reduce progression of low attenuation plaque volume (74% vs 94%; P = 0.47) or fibrofatty plaque volume (87% vs 25%; P = 0.65) versus placebo, respectively (n = 30, icosapent ethyl; n = 37, placebo) [74]. However, icosapent ethyl did reduce progression of total plaque versus placebo (15% vs 26%; P < 0.001). The study is designed to conclude after 18 months of therapy [74].
6. Pleiotropic effects of EPA
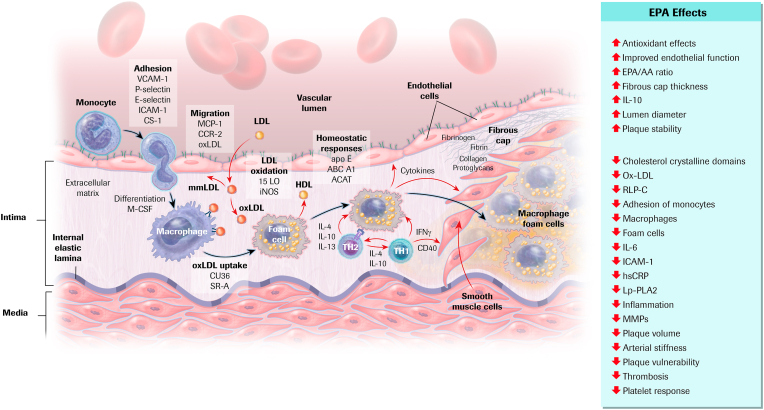
Putative pleiotropic effects of EPA beyond TG lowering include reduction of inflammatory markers (eg, high-sensitivity C-reactive protein) and inflammatory cytokines [46,47,75]. In addition, EPA has been shown to have beneficial effects on endothelial function, oxidative stress, foam cell formation, plaque formation/progression, cholesterol crystal formation and plaque rupture, platelet aggregation, and thrombus formation (Fig. 3) [75,76]. These collective effects may attenuate development and progression of atherosclerotic plaque.
Fig. 3.
Cellular and Molecular Mechanisms of Atherosclerosis and Role of EPA. Mechanisms are depicted in the illustration; effects of EPA are listed to the right indicating increases (↑) or decreases (↓). LDL is subject to oxidative modification, progressing from mm-LDL to ox-LDL. Monocytes attach to endothelial cells, migrate into the subendothelial space, and differentiate into macrophages. Ox-LDL cholesterol uptake leads to foam cell formation. Interactions between macrophage foam cells, Th1 cells, and Th2 cells establish a chronic inflammatory process. Cytokines secreted by lymphocytes and macrophages exert both pro- and anti-atherogenic effects on each of the cellular elements of the vessel wall. SMCs migrate from the medial portion of the arterial wall, proliferate, and secrete extracellular matrix proteins that form a fibrous plaque [75,76]. ACAT, acyl CoA:cholesterol acyltransferase; Apo E, apolipoprotein E; CCR, C–C chemokine receptor; CD, clusters of differentiation; CS, connecting segment; EPA, eicosapentaenoic acid; EPA/AA, eicosapentaenoic acid/arachidonic acid ratio; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; ICAM, intercellular adhesion molecule; IFN, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase; LDL, low-density lipoprotein; LO, lipoxygenase; Lp-PLA2, lipoprotein-associated phospholipase A2; MCP, monocyte chemotactic protein; mm-LDL, minimally modified LDL; MMP, matrix metalloproteinase; ox-LDL, oxidized LDL; RLP-C, remnant-like lipoparticle cholesterol; SMC, smooth muscle cell; Th, T helper; VCAM, vascular cell adhesion molecule. Adapted with permission from Atherosclerosis, 242(1), Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis, 357–366, copyright 2015, with permission from Elsevier [75].
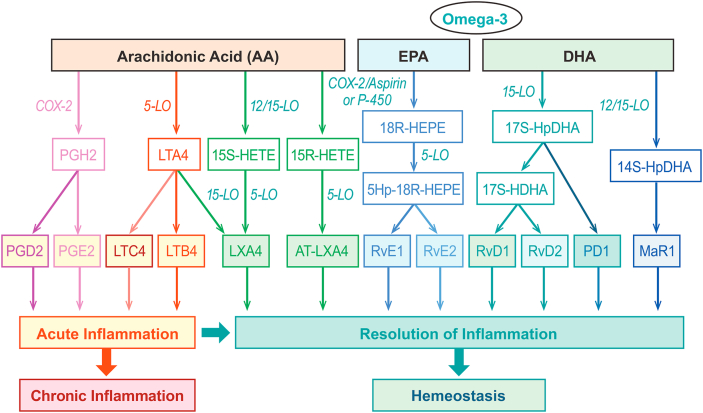
The possible mechanism underlying these effects may involve EPA acting as a competitive inhibitor of arachidonic acid (AA) for cyclooxygenase and lipoxygenase enzymes [77]. This competitive inhibition results in EPA-derived compounds with slightly different structures than AA-derived compounds and, importantly, less potent inflammatory effects (Fig. 4) [78]. Resolvins are a family of molecules that function as an “off switch” for inflammation, and AA and EPA both give rise to different specialized pro-resolvin mediators: AA is a precursor for lipoxins LXA4 and LXB4, whereas EPA is a precursor for E-series resolvins, RvE1 and RvE2 [79,80]. EPA can also compete with AA for incorporation into membrane phospholipids [81]. Thus, increasing plasma content of EPA may play a role in creating a less inflammatory environment, potentially impacting development and severity of inflammatory diseases, including atherosclerosis and other CVDs [75,77,81]. In addition, whereas AA has been shown to promote activity of genes in the pro-inflammatory NF-κB pathway [82], EPA downregulates expression of inflammatory cytokines and the NF-κB pathway [77,83]. EPA has a more potent effect on this gene expression than DHA, a precursor of D-series resolvins, protectins, and maresins [80], or combinations of DHA+EPA [83]. Consistent with data supporting the potential pleiotropic effects of EPA, a recent meta-analysis of 24 TG-lowering clinical trials (including 13 omega-3 fatty acid trials) noted that the magnitude of lipid profile reduction did not fully account for the CV benefit of EPA therapy [67].
Fig. 4.
Biosynthetic Cascades and Actions of Selected Lipid Mediators Derived From Arachidonic Acid (AA), Eicosapentaenoic Acid (EPA), and Docosahexaenoic Acid (DHA). COX, cyclooxygenase; HDHA, hydroxy-docosahexaenoic acid; HETE, hydroxyeicosatetraenoic acid; LO, lipoxygenase; LT, leukotriene; LX, lipoxin; MaR, maresin; PD, protectin; PG, prostaglandin; Reproduced with permission from Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011; 111(10):5922–5943, copyright 2011 American Chemical Society [78].
The anti-inflammatory effects of EPA and other omega-3 fatty acids may have potential for mitigating the symptoms of the novel 2019 coronavirus (COVID-19) infection. The production of resolvins and protectins by omega-3 fatty acids, as well as reducing reactive oxygen species and pro-inflammatory cytokines, such as tumor-necrosis factor-α and interleukin-1β, −6, and −8, have been shown to have a role in ameliorating outcomes in lung infection [84,85]. At least three clinical trials designed to investigate the effects of icosapent ethyl on inflammatory markers in patients with COVID-19 infection and the potential for prevention in high-risk individuals are ongoing (NCT04505098 [86], NCT04412018 [87], and NCT04460651 [88]). In addition, the efficacy of EPA free fatty acid gastro-resistant capsules is being evaluated in hospitalized patients with confirmed SARS-CoV-2 viral pneumonia (NCT04335032 [89]).
7. Discussion and conclusions
TG biochemistry and observational data support elevated TG levels as an ASCVD risk marker, and suggest a potentially causative role. Despite this, many studies of TG-lowering modalities including niacin, fibrates, and DHA+EPA combination omega-3 fatty acids failed to demonstrate CV event risk reduction. Among factors that may have impacted CV outcomes trial results are lack of enrollment of hypertriglyceridemic patients and lack of focus on persistent TG-related CV event risk. Nonetheless, subgroup analyses from several of these studies have been hypothesis-generating, suggesting potential benefit to TG level reduction in patients with hypertriglyceridemia and providing clinical support for targeting this population, as in REDUCE-IT. In fact, a meta-analysis of 24 TG-lowering clinical trials, which included REDUCE-IT, concluded that lowering TG levels was associated with a lower risk of major vascular events, even after adjusting for lowering LDL-C [67]. The effect of this meta-analysis was mostly powered by the REDUCE-IT trial. Another recent meta-analysis including REDUCE-IT concluded that data support routine dietary supplementation with omega-3 fatty acids to prevent vascular events and mortality and improve cardiometabolic risk factors [66]. The association between elevated TG and TRL levels and ASCVD risk is fueling research into treatments targeting different aspects of hypertriglyceridemia, including monoclonal antibodies that bind inhibitors of lipoprotein lipase, such as apo CIII and angiopoietin-like proteins-3 and -4 [90]. Similar to those performed for PSCK9, randomized clinical trials are needed to evaluate the risks and benefits of these therapies in humans and determine whether they will best serve as adjuvant agents to statins or as primary therapies.
REDUCE-IT demonstrated that icosapent ethyl 4 g, an EPA-only omega-3 fatty acid, reduced CV events in high-risk, statin-treated patients with elevated TG levels [14]. In contrast, most studies using combination DHA+EPA have not demonstrated CV event risk reduction, possibly because doses of omega-3 fatty acids in many of these prior investigations (generally ≤1 g) may have been inadequate for CV event risk reduction. However, the STRENGTH trial, which was evaluating EPA+DHA 4 g daily, was recently discontinued prior to completion given that the drug was unlikely to demonstrate clinical benefit among patients with dyslipidemia. Currently, EPA is the only omega-3 fatty acid that has demonstrated ASCVD risk reduction in statin-treated patients. A possible reason for this is that, beyond its TG-lowering effects, EPA has pleiotropic anti-inflammatory and anti-thrombotic effects that may contribute to CV event risk reduction. A number of ongoing studies will provide additional insights into the role of omega-3 fatty acids in statin-treated patients.
Funding
This work was funded by Amarin Pharma, Inc., Bridgewater, NJ, USA.
Disclosures
Peter P. Toth: Consultant: Amarin Pharma, Inc., Amgen, Kowa, Resverlogix, Theravance; Speakers bureau: Amarin Pharma, Inc., Amgen, Novo-Nordisk.
P.K. Shah: Dr. Shah reports no potential conflicts of interest, including specific financial interests, relationships, and affiliations, relevant to the subject matter or materials discussed in the manuscript.
Norman E. Lepor: Speakers bureau, research support, stock shareholder: Amarin Pharma, Inc.
None of the authors received any honoraria for this work.
Author contributions
Peter P. Toth: Conceptualization; Writing: review & editing.
P.K. Shah: Conceptualization; Writing: review & editing.
Norman E. Lepor: Conceptualization; Writing: review & editing.
Acknowledgments
Medical writing assistance was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, and funded by Amarin Pharma, Inc., Bridgewater, NJ, USA.
References
- 1.Miller M., Stone N.J., Ballantyne C., Bittner V., Criqui M.H., Ginsberg H.N., Goldberg A.C., Howard W.J., Jacobson M.S., Kris-Etherton P.M., Lennie T.A., Levi M., Mazzone T., Pennathur S. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 2.Ganda O.P., Bhatt D.L., Mason R.P., Miller M., Boden W.E. Unmet need for adjunctive dyslipidemia therapy in hypertriglyderidemia management. J. Am. Coll. Cardiol. 2018;72:330–343. doi: 10.1016/j.jacc.2018.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am. J. Cardiol. 2016;118:138–145. doi: 10.1016/j.amjcard.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Miller M., Cannon C.P., Murphy S.A., Qin J., Ray K.K., Braunwald E. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J. Am. Coll. Cardiol. 2008;51:724–730. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz G.G., Abt M., Bao W., DeMicco D., Kallend D., Miller M., Mundl H., Olsson A.G. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J. Am. Coll. Cardiol. 2015;65:2267–2275. doi: 10.1016/j.jacc.2015.03.544. [DOI] [PubMed] [Google Scholar]
- 6.The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH) Am. Heart J. 2011;161:471–477. doi: 10.1016/j.ahj.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The HPS2-THRIVE Collaborative Group Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 8.The ACCORD Study Group, Ginsberg H.N., Elam M.B., Lovato L.C., Crouse J.R., III, Leiter L.A. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The FIELD Study Investigators, Keech A., Simes R.J., Barter P., Best J., Scott R. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 10.Rauch B., Schiele R., Schneider S., Diller F., Victor N., Gohlke H., Gottwik M., Steinbeck G., Del C.U., Sack R., Worth H., Katus H., Spitzer W., Sabin G., Senges J. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 11.ASCEND Study Collaborative Group, Bowman L., Mafham M., Wallendszus K., Stevens W., Buck G. Effects of n-3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 2018;379:1540–1550. doi: 10.1056/NEJMoa1804989. [DOI] [PubMed] [Google Scholar]
- 12.Manson J.E., Cook N.R., Lee I.M., Christen W., Bassuk S.S., Mora S., Gibson H., Albert C.M., Gordon D., Copeland T., D’Agostino D., Friedenberg G., Ridge C., Bubes V., Giovannucci E.L., Willett W.C., Buring J.E. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manson J.E., Cook N.R., Lee I.M., Christen W., Bassuk S.S., Mora S., Gibson H., Gordon D., Copeland T., D’Agostino D., Friedenberg G., Ridge C., Bubes V., Giovannucci E.L., Willett W.C., Buring J.E. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatt D.L., Steg G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Doyle R.T., Jr., Juliano R.A., Jiao L., Granowitz C., Tardif J.C., Ballantyne C.M. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 15.Rygiel K. Hypertriglyceridemia - common causes, prevention and treatment strategies. Curr. Cardiol. Rev. 2018;14:67–76. doi: 10.2174/1573403X14666180123165542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Pretis N., Amodio A., Frulloni L. Hypertriglyceridemic pancreatitis: epidemiology, pathophysiology and clinical management. United Euro Gastroenterol. J. 2018;6:649–655. doi: 10.1177/2050640618755002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashid N., Sharma P.P., Scott R.D., Lin K.J., Toth P.P. Severe hypertriglyceridemia and factors associated with acute pancreatitis in an integrated health care system. J. Clin. Lipidol. 2016;10:880–890. doi: 10.1016/j.jacl.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Nordestgaard B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ. Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 19.März W., Scharnagl H., Winkler K., Tiran A., Nauck M., Boehm B.O., Winkelmann B.R. Low-density lipoprotein triglycerides associated with low-grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease: the Ludwigshafen risk and cardiovascular health study. Circulation. 2004;110:3068–3074. doi: 10.1161/01.CIR.0000146898.06923.80. [DOI] [PubMed] [Google Scholar]
- 20.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 21.Domoto K., Taniguchi T., Takaishi H., Takahashi T., Fujioka Y., Takahashi A., Ishikawa Y., Yokoyama M. Chylomicron remnants induce monocyte chemoattractant protein-1 expression via p38 MAPK activation in vascular smooth muscle cells. Atherosclerosis. 2003;171:193–200. doi: 10.1016/j.atherosclerosis.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Twickler T.B., Dallinga-Thie G.M., Visseren F.L., de Vries W.R., Erkelens D.W., Koppeschaar H.P. Induction of postprandial inflammatory response in adult onset growth hormone deficiency is related to plasma remnant-like particle-cholesterol concentration. J. Clin. Endocrinol. Metab. 2003;88:1228–1233. doi: 10.1210/jc.2002-020470. [DOI] [PubMed] [Google Scholar]
- 23.Doi H., Kugiyama K., Oka H., Sugiyama S., Ogata N., Koide S.I., Nakamura S.I., Yasue H. Remnant lipoproteins induce proatherothrombogenic molecules in endothelial cells through a redox-sensitive mechanism. Circulation. 2000;102:670–676. doi: 10.1161/01.cir.102.6.670. [DOI] [PubMed] [Google Scholar]
- 24.Musunuru K., Kathiresan S. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ. Res. 2016;118:579–585. doi: 10.1161/CIRCRESAHA.115.306398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dron J.S., Hegele R.A. Genetics of triglycerides and the risk of atherosclerosis. Curr. Atherosclerosis Rep. 2017;19:31. doi: 10.1007/s11883-017-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewey F.E., Gusarova V., Dunbar R.L., O’Dushlaine C., Schurmann C., Gottesman O., McCarthy S., Van Hout C.V., Bruse S., Dansky H.M., Leader J.B., Murray M.F., Ritchie M.D., Kirchner H.L., Habegger L., Lopez A., Penn J., Zhao A., Shao W., Stahl N., Murphy A.J., Hamon S., Bouzelmat A., Zhang R., Shumel B., Pordy R., Gipe D., Herman G.A., Sheu W.H.H., Lee I.T., Liang K.W., Guo X., Rotter J.I., Chen Y.I., Kraus W.E., Shah S.H., Damrauer S., Small A., Rader D.J., Wulff A.B., Nordestgaard B.G., Tybjaerg-Hansen A., van den Hoek A.M., Princen H.M.G., Ledbetter D.H., Carey D.J., Overton J.D., Reid J.G., Sasiela W.J., Banerjee P., Shuldiner A.R., Borecki I.B., Teslovich T.M., Yancopoulos G.D., Mellis S.J., Gromada J., Baras A. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N. Engl. J. Med. 2017;377:211–221. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.TG and HDL Working Group of the Exome Sequencing Project NH, Lung, and Blood Institute Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman M.J., Ginsberg H.N., Amarenco P., Andreotti F., Boren J., Catapano A.L., Descamps O.S., Fisher E., Kovanen P.T., Kuivenhoven J.A., Lesnik P., Masana L., Nordestgaard B.G., Ray K.K., Reiner Z., Taskinen M.R., Tokgozoglu L., Tybjaerg-Hansen A., Watts G.F. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur. Heart J. 2011;32:1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegele R.A., Ginsberg H.N., Chapman M.J., Nordestgaard B.G., Kuivenhoven J.A., Averna M., Boren J., Bruckert E., Catapano A.L., Descamps O.S., Hovingh G.K., Humphries S.E., Kovanen P.T., Masana L., Pajukanta P., Parhofer K.G., Raal F.J., Ray K.K., Santos R.D., Stalenhoef A.F., Stroes E., Taskinen M.R., Tybjaerg-Hansen A., Watts G.F., Wiklund O. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2014;2:655–666. doi: 10.1016/S2213-8587(13)70191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasai T., Miyauchi K., Yanagisawa N., Kajimoto K., Kubota N., Ogita M., Tsuboi S., Amano A., Daida H. Mortality risk of triglyceride levels in patients with coronary artery disease. Heart. 2013;99:22–29. doi: 10.1136/heartjnl-2012-302689. [DOI] [PubMed] [Google Scholar]
- 31.Lee J.S., Chang P.Y., Zhang Y., Kizer J.R., Best L.G., Howard B.V. Triglyceride and HDL-C dyslipidemia and risks of coronary heart disease and ischemic stroke by glycemic dysregulation status: the Strong Heart Study. Diabetes Care. 2017;40:529–537. doi: 10.2337/dc16-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aberra T., Peterson E.D., Pagidipati N.J., Mulder H., Wojdyla D.M., Philip S. The association between triglycerides and incident cardiovascular disease: what is “optimal”? J. Clin. Lipidol. 2020;14(4):438–447. doi: 10.1016/j.jacl.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toth P.P., Granowitz C., Hull M., Liassou D., Anderson A., Philip S. High triglycerides are associated with increased cardiovascular events, medical costs, and resource utilization: a real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toth P.P., Philip S., Hull M., Granowitz C. Association of elevated triglycerides with increased cardiovascular risk and direct costs in statin-treated patients. Mayo Clin. Proc. 2019;94:1670–1680. doi: 10.1016/j.mayocp.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 35.Nelson A.J., Navar A.M., Mulder H., Wojdyla D., Philip S., Granowitz C., Peterson E.D., Pagidipati N.J. Association between triglycerides and residual cardiovascular risk in patients with type 2 diabetes mellitus and established cardiovascular disease (from the Bypass Angioplasty Revascularization Investigation 2 Diabetes [BARI 2D] trial) Am. J. Cardiol. 2020 doi: 10.1016/j.amjcard.2020.07.005. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 36.Sarwar N., Danesh J., Eiriksdottir G., Sigurdsson G., Wareham N., Bingham S., Boekholdt S.M., Khaw K.T., Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 37.Ference B.A., Kastelein J.J.P., Ray K.K., Ginsberg H.N., Chapman M.J., Packard C.J., Laufs U., Oliver-Williams C., Wood A.M., Butterworth A.S., Di Angelantonio E., Danesh J., Nicholls S.J., Bhatt D.L., Sabatine M.S., Catapano A.L. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. J. Am. Med. Assoc. 2019;321:364–373. doi: 10.1001/jama.2018.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varbo A., Benn M., Tybjaerg-Hansen A., Jorgensen A.B., Frikke-Schmidt R., Nordestgaard B.G. Remnant cholesterol as a causal risk factor for ischemic heart disease. J. Am. Coll. Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 39.Joshi P.H., Khokhar A.A., Massaro J.M., Lirette S.T., Griswold M.E., Martin S.S. Remnant lipoprotein cholesterol and incident coronary heart disease: the Jackson Heart and Framingham Offspring Cohort studies. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan W., Philip S., Granowitz C., Toth P., Wong N. Hypertriglyceridemia in statin-treated US adults: the National Health and Nutrition Examination Survey. J. Clin. Lipidol. 2019;13:100–108. doi: 10.1016/j.jacl.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 41.The AIM-HIGH Investigators, Boden W.E., Probstfield J.L., Anderson T., Chaitman B.R., Desvignes-Nickens P. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 42.Guyton J.R., Slee A.E., Anderson T., Fleg J.L., Goldberg R.B., Kashyap M.L. Relationship of lipoproteins to cardiovascular events: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes) J. Am. Coll. Cardiol. 2013;62:1580–1584. doi: 10.1016/j.jacc.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.BIP Study Group Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 44.Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) [ NCT03071692] ClinicalTrials.gov; 2017. [DOI] [PubMed] [Google Scholar]
- 45.Newman W.P., Middaugh J.P., Propst M.T., Rogers D.R. Atherosclerosis in Alaska natives and non-natives. Lancet. 1993;341:1056–1057. doi: 10.1016/0140-6736(93)92413-n. [DOI] [PubMed] [Google Scholar]
- 46.Bays H.E., Ballantyne C.M., Kastelein J.J., Isaacsohn J.L., Braeckman R.A., Soni P.N. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial) Am. J. Cardiol. 2011;108:682–690. doi: 10.1016/j.amjcard.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 47.Ballantyne C.M., Bays H.E., Kastelein J.J., Stein E., Isaacsohn J.L., Braeckman R.A., Soni P.N. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study) Am. J. Cardiol. 2012;110:984–992. doi: 10.1016/j.amjcard.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 48.Park Y., Harris W.S. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J. Lipid Res. 2003;44:455–463. doi: 10.1194/jlr.M200282-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Mori T.A., Burke V., Puddey I.B., Watts G.F., O’Neal D.N., Best J.D., Beilin L.J. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am. J. Clin. Nutr. 2000;71:1085–1094. doi: 10.1093/ajcn/71.5.1085. [DOI] [PubMed] [Google Scholar]
- 50.Egert S., Kannenberg F., Somoza V., Erbersdobler H.F., Wahrburg U. Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J. Nutr. 2009;139:861–868. doi: 10.3945/jn.108.103861. [DOI] [PubMed] [Google Scholar]
- 51.GISSI Prevenzione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 52.Siscovick D.S., Barringer T.A., Fretts A.M., Wu J.H.Y., Lichtenstein A.H., Costello R.B., Kris-Etherton P.M., Jacobson T.A., Engler M.B., Alger H.M., Appel L.J., Mozaffarian D. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017;135:e867–e884. doi: 10.1161/CIR.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexander D.D., Miller P.E., Van Elswyk M.E., Kuratko C.N., Bylsma L.C. A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clin. Proc. 2017;92:15–29. doi: 10.1016/j.mayocp.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Abdelhamid A.S., Brown T.J., Brainard J.S., Biswas P., Thorpe G.C., Moore H.J., Deane K.H., AlAbdulghafoor F.K., Summerbell C.D., Worthington H.V., Song F., Hooper L. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018;11 doi: 10.1002/14651858.CD003177.pub4. Cd003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aung T., Halsey J., Kromhout D., Gerstein H.C., Marchioli R., Tavazzi L., Geleijnse J.M., Rauch B., Ness A., Galan P., Chew E.Y., Bosch J., Collins R., Lewington S., Armitage J., Clarke R. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. J. Am. Med. Assoc. Cardiol. 2018;3:225–234. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicholls S.J., Lincoff A.M., Bash D., Ballantyne C.M., Barter P.J., Davidson M.H., Kastelein J.J.P., Koenig W., McGuire D.K., Mozaffarian D., Pedersen T.R., Ridker P.M., Ray K., Karlson B.W., Lundstrom T., Wolski K., Nissen S.E. Assessment of omega-3 carboxylic acids in statin treated patients with high levels of triglycerides and low levels of high density lipoprotein cholesterol: rationale and design of the STRENGTH trial. Clin. Cardiol. 2018;41:1281–1288. doi: 10.1002/clc.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.2020. AstraZeneca ends cardiovascular outcomes study of Epanova as unlikely to show benefit [press release]: FirstWord Pharma.https://www.firstwordpharma.com/node/1693240 Available at: [Google Scholar]
- 58.Ferrieres J. The return of triglycerides and the omega-3 fatty acids revival. Arch. Cardiovasc. Dis. 2020;113:369–373. doi: 10.1016/j.acvd.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Laake K., Myhre P., Nordby L.M., Seljeflot I., Abdelnoor M., Smith P., Tveit A., Arnesen H., Solheim S. Effects of omega3 supplementation in elderly patients with acute myocardial infarction: design of a prospective randomized placebo controlled study. BMC Geriatr. 2014;14:74. doi: 10.1186/1471-2318-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., Kita T., Kitabatake A., Nakaya N., Sakata T., Shimada K., Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 61.Bhatt D.L., Miller M., Brinton E.A., Jacobson T.A., Steg G., Ketchum S.B., Doyle R.T., Juliano R.A., Jiao L., Granowitz C., Tardif J.C., Olshansky B., Chung M.K., Gibson C.M., Giugliano R.P., Budoff M.J., Ballantyne C.M. REDUCE-IT USA: results from the 3,146 patients randomized in the United States. Circulation. 2020;141:367–375. doi: 10.1161/CIRCULATIONAHA.119.044440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., Graham I.M., Halliday A., Landmesser U., Mihaylova B., Pedersen T.R., Riccardi G., Richter D.J., Sabatine M.S., Taskinen M.R., Tokgozoglu L., Wiklund O. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 63.American Diabetes Association . American Diabetes Association; Arlington County, VA: 2019. Living Standards of Medical Care in Diabetes.https://care.diabetesjournals.org/living-standards Available at: Accessed July 9, 2020. [Google Scholar]
- 64.NLA Position on the Use of Icosapent Ethyl in High and Very-High Risk Patients. National Lipid Association; Jacksonville, FL: 2019. https://www.lipid.org/nla/nla-position-use-icosapent-ethyl-high-and-very-high-risk-patients Available at: Accessed July 9, 2020. [Google Scholar]
- 65.Skulas-Ray A.C., Wilson P.W.F., Harris W.S., Brinton E.A., Kris-Etherton P.M., Richter C.K., Jacobson T.A., Engler M.B., Miller M., Robinson J.G., Blum C.B., Rodriguez-Leyva D., de Ferranti S.D., Welty F.K. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673–e691. doi: 10.1161/CIR.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 66.Mazidi M., Mikhailidis D.P., Banach M. Omega-3 fatty acids and risk of cardiovascular disease: systematic review and meta-analysis of randomized controlled trials with 127,447 individuals and a mendelian randomization study [abstract 20948] Circulation. 2019;140:e975–e976. [Google Scholar]
- 67.Marston N.A., Giugliano R.P., Im K., Silverman M.G., O’Donoghue M.L., Wiviott S.D., Ference B.A., Sabatine M.S. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019;140:1308–1317. doi: 10.1161/CIRCULATIONAHA.119.041998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klempfner R., Erez A., Sagit B.Z., Goldenberg I., Fisman E., Kopel E. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ. Cardiovasc. Qual. Outcomes. 2016;9:100–108. doi: 10.1161/CIRCOUTCOMES.115.002104. [DOI] [PubMed] [Google Scholar]
- 69.Austin M.A., Hokanson J.E., Edwards K.L. Hypertriglyceridemia as a cardiovascular risk factor. Am. J. Cardiol. 1998;81:7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 70.Ye X., Kong W., Zafar M.I., Chen L.L. Serum triglycerides as a risk factor for cardiovascular diseases in type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Cardiovasc. Diabetol. 2019;18:48. doi: 10.1186/s12933-019-0851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhatt D.L., Miller M., Steg G., Brinton E.A., Jacobson T.A., Ketchum S.B. Annual Scientific Session of the American College of Cardiology; Chicago, IL: 2020. EPA levels and cardiovascular outcomes in the reduction of cardiovascular events with icosapent ethyl-intervention trial [oral presentation] [Google Scholar]
- 72.Randomized trial for evaluation in secondary prevention efficacy of combination therapy - statin and eicosapentaenoic acid UMIN000012069. UMIN Clinical Trials Registry; 2018. [DOI] [PubMed] [Google Scholar]
- 73.Budoff M., Brent Muhlestein J., Le Pa V.T., May H.T., Roy S., Nelson J.R. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200-499 mg/dL) on statin therapy: rationale and design of the EVAPORATE study. Clin. Cardiol. 2018;41:13–19. doi: 10.1002/clc.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Budoff M.J., Muhlestein J.B., Bhatt D.L., Le Pa V.T., May H.T., Shaikh K. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: a prospective, placebo-controlled randomized trial (EVAPORATE): interim results. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa184. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 75.Borow K.M., Nelson J.R., Mason R.P. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242:357–366. doi: 10.1016/j.atherosclerosis.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 76.Glass C.K., Witztum J.L. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 77.Calder P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 78.Serhan C.N., Petasis N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dalli J., Serhan C.N. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dyall S.C. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siriwardhana N., Kalupahana N.S., Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012;65:211–222. doi: 10.1016/B978-0-12-416003-3.00013-5. [DOI] [PubMed] [Google Scholar]
- 82.Camandola S., Leonarduzzi G., Musso T., Varesio L., Carini R., Scavazza A., Chiarpotto E., Baeuerle P.A., Poli G. Nuclear factor kB is activated by arachidonic acid but not by eicosapentaenoic acid. Biochem. Biophys. Res. Commun. 1996;229:643–647. doi: 10.1006/bbrc.1996.1857. [DOI] [PubMed] [Google Scholar]
- 83.Allam-Ndoul B., Guenard F., Barbier O., Vohl M.C. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016;15:69. doi: 10.1186/s12944-016-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Messina G., Polito R., Monda V., Cipolloni L., Di Nunno N., Di Mizio G., Murabito P., Carotenuto M., Messina A., Pisanelli D., Valenzano A., Cibelli G., Scarinci A., Monda M., Sessa F. Functional role of dietary intervention to improve the outcome of COVID-19: a hypothesis of work. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Das U.N. Response to: bioactive lipids and coronavirus (COVID-19)-further discussion. Arch. Med. Res. 2020;51:445–449. doi: 10.1016/j.arcmed.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.A Pragmatic Randomized Trial of Icosapent Ethyl for High-Cardiovascular Risk Adults in the Era of Coronavirus Disease 2019 (MITIGATE) [ NCT04505098] ClinicalTrials.gov; 2020. [Google Scholar]
- 87.An Investigation on the Effects of Icosapent Ethyl (VascepaTM) on Inflammatory Biomarkers in Individuals with COVID-19 [ NCT04412018] ClinicalTrials.gov; 2020. [Google Scholar]
- 88.PREPARE-IT . ClinicalTrials.gov; 2020. Prevention of COVID19 with EPA in Healthcare Providers at Risk - Intervention Trial (PREPARE-IT) [ NCT04460651] [Google Scholar]
- 89.EPA-FFA to Treat Hospitalised Patients with COVID-19 (SARS-CoV-2) [ NCT04335032] ClinicalTrials.gov; 2020. [Google Scholar]
- 90.Sathiyakumar V., Kapoor K., Jones S.R., Banach M., Martin S.S., Toth P.P. Novel therapeutic targets for managing dyslipidemia. Trends Pharmacol. Sci. 2018;39:733–747. doi: 10.1016/j.tips.2018.06.001. [DOI] [PubMed] [Google Scholar]