Abstract
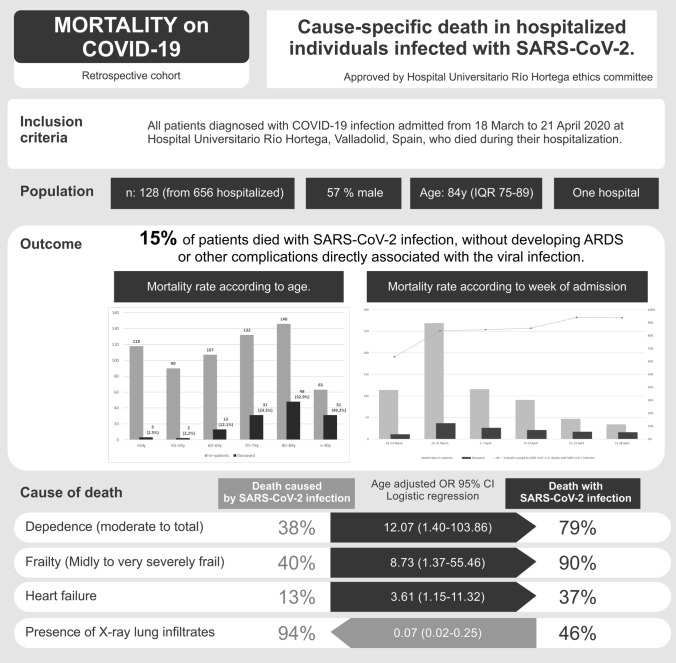
Infection with SARS-CoV-2 is becoming the leading cause of death in most countries during the 2020 pandemic. The objective of this study is to assess the association between COVID-19 and cause-specific death. The design is retrospective cohort study. We included data from inpatients diagnosed with COVID-19 between March 18 and April 21, 2020, who died during their hospital stay. Demographic, clinical and management data were collected. Causes of death were ascertained by review of medical records. The sample included 128 individuals. The median age was 84 (IQR 75–89), 57% were men. In 109 patients, the death was caused by SARS-CoV-2 infection, whereas in 19 (14.8%, 95 CI 10–22%), the infection acted only as a precipitating factor to decompensate other pathologies. This second group of patients was older (88y vs 82, p < 0.001). In age-adjusted analysis, they had a greater likelihood of heart failure (OR 3.61 95% CI 1.15–11.32), dependency in activities of daily living (OR 12.07 95% CI 1.40–103.86), frailty (OR 8.73 95% CI 1.37–55.46). The presence of X-ray infiltrates was uncommon (OR 0.07, 95% CI 0.02–0.25). A higher percentage of patient deaths from causes unrelated to COVID-19 complications occurred during the two first weeks of the pandemic. Fifteen percent of patients with COVID-19 infection died from decompensation of other pathologies and the cause of death was unrelated to COVID-19 severe complications. Most of these patients had more comorbidities and were frail and elderly. These findings can partially explain the excess mortality in older people.
Graphic abstract
Electronic supplementary material
The online version of this article (10.1007/s11739-020-02485-y) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, Cause of death, Age distribution, Risk factor, Frail elderly, Hospital mortality
Introduction
The first case of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in China in December 2019. Since then, increasing numbers of deaths have been related to SARS-CoV-2. The case fatality rate across countries and territories varies from less than 1% to over 10% [1]. These rates should be interpreted with caution, given the many variations and the number of factors influencing the rate [2, 3].
Many risk factors are associated with death in COVID-19 infection, with old age a common factor [4]. Case fatality rates ranged from 1.3% in patients < 70 years old to 8.0% in those 70–79 years old and 14.8% in patients aged > 80 years in a large Chinese series [5]. This is also true for critical cases, with a case fatality rate of 15% in patients ≤ 63 years old admitted to intensive care units vs 36% in patients > 63 years [6]. Underlying health conditions such as respiratory disease [odds ratio (OR) 2.46, 95% confidence interval (CI) 1.76–3.44], cardiovascular diseases (OR 3.42, 95% CI 1.88–6.22) and hypertension (OR 2.36, 95% CI 1.46 to 3,83) have also been associated with increased death risk [7], as have been obesity, smoking and male sex [8, 9].
The main complications associated with SARS-CoV-2 infection and death are acute respiratory distress syndrome (ARDS), disseminate intravascular coagulation and sepsis [10]. An excessive immune response causing a cytokine storm is involved in many cases where the disease progresses rapidly [11]. These acute complications due to the infection itself could explain the death rate in young people but leave open the question of the extremely high mortality in older people, especially in very old or frail people. This excess mortality in the oldest patients could partly explain the differences in death rates between countries and regions, with higher mortality in countries such as Spain and Italy, which have some of the highest life expectancies worldwide [12].
This retrospective cohort describes the cause of death in patients infected with SARS-CoV-2 in a single hospital. We aimed to differentiate the percentage of deaths directly associated with the infection and its complications (lung injury or thromboembolic events) and those where the illness acted as a trigger to decompensate previous comorbid conditions, with this decompensation being the cause of death.
Material and methods
Study population
This is a retrospective cohort study of all patients diagnosed with COVID-19 infection who were admitted at Hospital Universitario Río Hortega Valladolid, Spain, from 18 March to 21 April 2020 and died during their hospitalization. The hospital ethics committee approved this study. Due to the nature of the retrospective chart review, waived the need for informed consent from individual patients.
Patients were included if they were diagnosed with confirmed COVID-19 and died during the study period. Patients were diagnosed according to World Health Organization guidance [13]. Laboratory confirmation for SARS-CoV-2 was defined as a positive result of real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swabs or a positive serology test.
Data collection
A trained team of internal medicine physicians and specialist registrars reviewed and collected epidemiological, clinical and outcome data from electronic medical records. Patient confidentiality was protected by deidentification.
Every patient’s medical record was independently assessed by one of the researchers (MCS, MGM, PCM, IAJ, MRH, LHG, DVP, MCG, and MGF). The researchers were blinded to the outcome of interest during data collection. The assessment of the cause of death was done after they had gathered all the clinical information. When the cause of death was unclear, the medical record was assessed by a second researcher. Differences in opinion were resolved by discussion and consensus or by consulting one of the senior authors (LCG) if needed. Six patients’ cause of death should be assessed by a second researcher. All the patients whose cause of death was assessed as not caused directly by SARS-CoV-2 infection were double checked for the senior author. Three patients initially assigned to the death with SARS-CoV-2 infection group were finally switched to the death caused by SARS-CoV2 infection group after careful assessment of laboratory and imagen data.
Study outcomes and definitions
The primary outcome was the definition of the cause of death of the patients. As the secondary outcome, we assessed the development of ARDS, hyperinflammation response, sepsis or thromboembolic events.
Acute respiratory distress syndrome (ARDS) was identified according to the Berlin Definition [14]. All the following criteria were required:
Onset respiratory symptoms must begin within 1 week of a known clinical insult, or the patient must have new or worsening symptoms during the past week.
Chest imaging bilateral opacities must be present on a chest radiograph or computed tomographic (CT) scan. These opacities must not be fully explained by pleural effusions, lobar collapse, lung collapse or pulmonary nodules.
Origin of pulmonary infiltrates the patient’s respiratory failure must not be fully explained by cardiac failure or fluid overload. ARDS can be diagnosed once cardiogenic pulmonary oedema and alternative causes of acute hypoxemic respiratory failure have been excluded.
- Oxygenation impairment: a moderate to severe impairment of oxygenation must be present, as defined by the ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2), or the ratio of oxygen saturation to FiO2 (SaO2/FiO2) when PaO2 is not available. The severity of the hypoxemia defines the severity of ARDS:
- mild: PaO2/FiO2 200–300 mmHg or SaO2/FiO2 237–317 mmHg;
- moderate: PaO2/FiO2 100–200 mmHg or SaO2/FiO2 155–237 mmHg;
- severe: PaO2/FiO2 ≤ 100 mmHg or SaO2/FiO2 < 155 mmHg.
Hyperinflammation response we considered a hyperinflammatory response present in the context of moderate to severe COVID-19 infection with a lymphocyte count of < 1000 × 109/mL, and one of the following laboratory parameters:
serum ferritin > 1000 ng/mL;
C-reactive protein > 150 mg/L;
D-dimer > 800 μg/L;
Interleukin 6 > 20 pg/mL.
Cause-specific death in individuals infected with SARS-CoV-2 we defined clinical criteria for assessing COVID-related causes of death. It was not possible to support the diagnosis of death by necropsies, as they were not available. Patients were divided in two groups according their cause of death.
Deaths caused by SARS-CoV-2 infection we considered that death was directly caused by SARS-CoV-2 infection complications when the patient developed
ARDS or hyperinflammation response that led to their death;
acute respiratory failure leading to death without meeting ARDS and hyperinflammation response criteria, and severe lung injury likely due to COVID-19 on X-ray or CT;
acute respiratory failure or thrombotic events in the context of pulmonary embolism or other forms of venous thromboembolism that led to death;
septic shock due to SARS-CoV-2 that led to their death.
Death with SARS-CoV-2 infection we considered that COVID-19 infection was a precipitating factor to decompensate other chronic or acute pathologies and death was unrelated to severe complications of SARS-CoV-2 infection when
The patient developed acute respiratory failure caused by cardiogenic pulmonary oedema or other alternative causes of acute hypoxemic respiratory failure unrelated to COVID-19 that led to death. There were no lung injuries on X-ray or CT likely caused by COVID-19, or they were mild (only interstitial infiltrates of peripheral distribution, with neither alveolar infiltrates, nor consolidation, affecting less than 50% of lung in X-ray or CT scan: equivalent to Brixia score < 4 [15]).
The patient developed multiorgan failure associated with frailty that led to death. Septic shock was excluded. There were no lung injuries on X-ray or CT likely caused by COVID-19, or they were mild to moderate (interstitial or interstitial and alveolar infiltrates with interstitial predominance, affecting less than 50% of lung in X-ray or CT scan; equivalent to Brixia score < 7).
The patient's death was from any confirmed cause unrelated to COVID-19 complications, including septic shock due to another microorganism.
Patients’ grade of frailty was defined according Clinical Frailty Scale [16]. The grade of dependency was assessed using Barthel Index for activities of daily living [17].
Statistical analysis
The sample size was equal to the number of patients who died during the study period. Descriptive analysis of the continuous variables is expressed as the median and interquartile range (IQR). Categorical variables are presented as the number of patients (%) with 95% CI.
Differences in distributions of patient characteristics by outcome subgroup were reported using differences with 95% CIs. Categorical data were compared using the Chi square test or the Fisher exact test when needed. For crosstabs larger than 2 × 2 of 3 ordinal variables (age, performance in activities of daily living and clinical frailty scale), we used the linear-by-linear association test to assess for trends. Continuous data were compared using Student’s t test or the Mann–Whitney rank sum test with non-parametric variables. ORs adjusted by age are presented with 95% CIs for all the variables associated with the cause of death in univariate analysis. Adjustment by age was done using logistic regression.
All tests were two sided with statistical significance set at p < 0.05. All analyses were performed with SPSS, version 21.0 (IBM SPSS).
Results
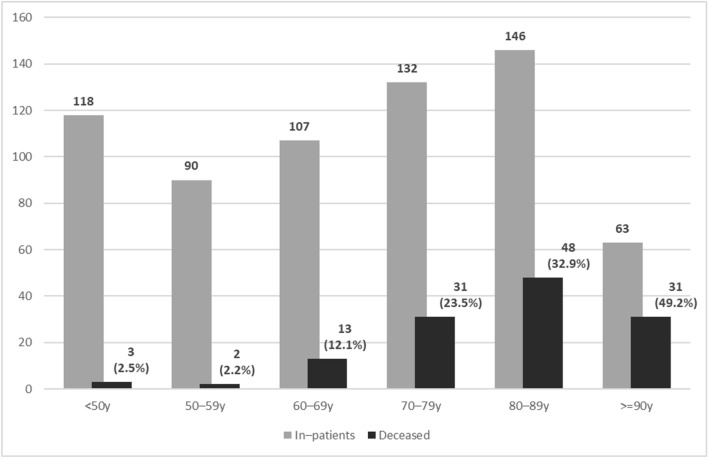
A total of 734 patients referred to our hospital were diagnosed as infected with SARS-CoV-2 during the study period. Of these, 656 (89%, 95% CI 87–91%) patients were admitted to hospital (median age 71 years, IQR 56–85; 52% were male). Seventy-eight patients were discharged on the same day directly from the emergency room because they had mild disease without pneumonia. One hundred and twenty-eight hospitalized patients died (19.5%, 95% CI 17–23%). The distributions of inpatients and the mortality rate according to age group are detailed in Fig. 1. At the time of writing, all the patients admitted during the study period had died or had been discharged from hospital.
Fig. 1.
Number of inpatients and mortality rate according to age
The median age of the patients who died was 84 years (IQR 75–89), and 57% were male. Basal characteristics, comorbidities and chronic treatment are detailed in Table 1. In 19 patients (14.8%, 95% CI 10–22%), the main cause of death was attributed to the decompensation of acute or chronic pathologies where COVID-19 infection acted as a precipitating factor. These patients were older, with more long-term conditions and higher Charlson comorbidity scores, and more frequently had heart failure, dementia or cerebrovascular disease. They had a higher grade of dependency and frailty.
Table 1.
Basal characteristics
| Characteristics | Total (n = 128) | Deaths caused by SARS-CoV-2 infection (n = 109) | Death with SARS-CoV-2 infection (n = 19) | p |
|---|---|---|---|---|
| Age, median (IQR), years | 84 (75–89) | 82 (74–89) | 88 (84–91) | < 0.001 |
| < 50 | 3 (2%) | 3 (3%) | 0 (0%) | 0.006 |
| 50–59 | 2 (2%) | 2 (2%) | 0 (0%) | |
| 60–69 | 13 (12%) | 13 (10%) | 0 (0%) | |
| 70–79 | 31 (24%) | 30 (28%) | 1 (5%) | |
| 80–89 | 48 (38%) | 37 (34%) | 11 (58%) | |
| ≥ 90 | 31 (24%) | 24 (22%) | 7 (37%) | |
| Males | 73 (57%) | 67 (62%) | 6 (32%) | 0.015 |
| Place of residence | ||||
| Home | 67 (52%) | 64 (58%) | 4 (21%) | 0.003 |
| Nursing home | 61 (48%) | 46 (42%) | 15 (79%) | |
| Comorbidities | ||||
| Hypertension | 91 (71%) | 74 (68%) | 17 (90%) | 0.055 |
| Dyslipidaemia | 48 (38%) | 42 (39%) | 6 (32%) | 0.563 |
| Myocardial infarction | 17 (13%) | 15 (14%) | 2 (11%) | 0.701 |
| Heart failure | 21 (16%) | 14 (13%) | 7 (37%) | 0.017 |
| Cerebrovascular disease | 15 (12%) | 10 (9%) | 5 (26%) | 0.048 |
| Dementia | 35 (27%) | 26 (24%) | 9 (47%) | 0.034 |
| COPD | 9 (7%) | 8 (7%) | 1 (5%) | 0.744 |
| Asthma | 6 (5%) | 5 (5%) | 1 (5%) | 0.898 |
| SAHS | 9 (7%) | 7 (6%) | 2 (11%) | 0.621 |
| Interstitial lung disease | 1 (1%) | 1 (1%) | 0 (0%) | 0.675 |
| Connective tissue disease | 1 (1%) | 0 (0%) | 1 (5%) | 0.148 |
| Peptic ulcer disease | 12 (12%) | 10 (12%) | 2 (13%) | 0.667 |
| Diabetes mellitus | ||||
| Uncomplicated | 36 (28%) | 31 (28%) | 5 (26%) | 0.849 |
| End organ damage | 4 (3%) | 3 (3%) | 1 (5%) | 0.479 |
| Chronic liver disease | ||||
| Mild | 5 (4%) | 5 (5%) | 0 (0%) | 0.341 |
| Moderate or severe | 2 (2%) | 2 (2%) | 0 (0%) | 0.552 |
| Chronic kidney disease | 20 (16%) | 17 (16%) | 3 (16%) | 0.983 |
| Solid tumour | ||||
| Localized | 17 (13%) | 16 (15%) | 1 (5%) | 0.465 |
| Metastatic | 2 (2%) | 2 (2%) | 0 (0%) | 0.552 |
| Leukaemia or lymphoma | 5 (4%) | 4 (4%) | 1 (5%) | 0.558 |
| Current or past smoker | 30 (23%) | 27 (25%) | 3 (16%) | 0.579 |
| Number of long-term conditions, median (IQR) | 3 (2–5) | 3 (2–4) | 3 (2–6) | 0.078 |
| Charlson comorbidity index, median (IQR) | 5 (3–6) | 4 (3–6) | 6 (4–7) | 0.004 |
| Performance in activities of daily living | ||||
| Independent | 59 (46%) | 58 (53%) | 1 (5%) | |
| Slight dependency | 21 (16%) | 18 (17%) | 3 (16%) | < 0.001 |
| Moderate dependency | 19 (15%) | 13 (12%) | 6 (32%) | |
| Severe dependency | 13 (10%) | 12 (11%) | 1 (5%) | |
| Total dependency | 16 (13%) | 8 (7%) | 8 (42%) | |
| Clinical Frailty Scale [16] | ||||
| Very fit | 2 (2%) | 2 (2%) | 0 (0%) | |
| Well | 21 (16%) | 21 (19%) | 0 (0%) | |
| Managing well | 16 (13%) | 15 (14%) | 1 (5%) | < 0.001 |
| Vulnerable | 29 (23%) | 28 (26%) | 1 (5%) | |
| Mildly frail | 21 (16%) | 17 (16%) | 4 (21%) | |
| Moderately frail | 12 (9%) | 9 (8%) | 3 (16%) | |
| Severely frail | 9 (7%) | 5 (5%) | 4 (21%) | |
| Very severely frail | 18 (14%) | 12 (11%) | 6 (32%) | |
| Chronic treatment | ||||
| ACEs | 34 (27%) | 24 (22%) | 10 (53%) | 0.005 |
| ARBs | 30 (23%) | 24 (22%) | 6 (32%) | 0.3850.372 |
| Statins | 38 (30%) | 34 (31%) | 4 (21%) | |
| Metformin | 23 (18%) | 18 (17%) | 5 (26%) | 0.334 |
| IDPP-4 | 18 (14%) | 17 (16%) | 1 (5%) | 0.308 |
| Insulin | 12 (9%) | 12 (11%) | 0 (0%) | 0.211 |
| Inhaled corticosteroids | 11 (9%) | 10 (9%) | 1 (5%) | 0.575 |
| Oral corticosteroids | 4 (4%) | 4 (4%) | 0 (0%) | 0.396 |
| Immunomodulators | 4 (4%) | 4 (4%) | 0 (0%) | 0.396 |
Bold indicate p values that achieve statistical significance (p < 0.05)
Deaths caused by SARS-CoV-2 infection: patients whose death was caused directly by SARS-CoV-2 infection. Death with SARS-CoV-2 infection: patients where SARS-CoV-2 acted as a precipitating factor to decompensate other chronic or acute pathologies that were the main cause of death
ACEs angiotensin-converting enzyme inhibitors, ARBs angiotensin II receptor blockers, COPD chronic obstructive pulmonary disease, RT-PCR reverse transcription polymerase chain reaction, SAHS sleep apnoea–hypopnea syndrome
The most reported symptoms are detailed in Table 2. Patients who died of COVID-19 complications were more frequently feverish or had fever or cough at admission. Dyspnoea was the most common symptom in both groups. Nearly half of the patients who died without COVID-19 complications had normal X-rays.
Table 2.
Initial clinical, radiological and laboratory parameters and specific COVID-19 treatment
| Characteristics | Total (n = 128) | Deaths caused by SARS-CoV-2 infection (n = 109) | Death with SARS-CoV-2 infection (n = 19) | p |
|---|---|---|---|---|
| Days from illness onset to hospital admission, median (IQR), days | 5 (2–7) | 5 (2–7) | 4 (2–7) | 0.944 |
| Signs and symptoms at diagnosis | ||||
| Shortness of breath | 103 (81%) | 86 (79%) | 17 (90%) | 0.363 |
| Fever | 89 (70%) | 80 (73%) | 9 (47%) | 0.023 |
| Cough | 54 (42%) | 50 (46%) | 4 (21%) | 0.043 |
| Diarrhoea or vomiting | 12 (9%) | 12 (11%) | 0 (0%) | 0.211 |
| Abdominal pain | 7 (6%) | 7 (6%) | 0 (0%) | 0.593 |
| Anosmia or ageusia | 22 (20%) | 20 (20%) | 2 (11%) | 0.404 |
| Asthenia | 17 (13%) | 14 (13%) | 3 (16%) | 0.718 |
| Arthromyalgia | 3 (2%) | 3 (3%) | 0 (0%) | 0.464 |
| Sore throat | 3 (2%) | 3 (3%) | 0 (0%) | 0.464 |
| Headache | 15 (12%) | 12 (11%) | 3 (16%) | 0.550 |
| X-ray/CT scan findings | ||||
| Normal | 16 (13%) | 6 (6%) | 10 (54%) | < 0.001 |
| Unilateral infiltrates | 21 (16%) | 16 (15%) | 5 (26%) | |
| Bilateral infiltrates | 91 (71%) | 87 (80%) | 4 (21%) | |
| Diagnosis | ||||
| RT-PCR positive | 123 (96%) | 106 (97%) | 17 (90%) | 0.123 |
| Ig test positive | 4 (3%) | 2 (2%) | 2 (10%) | |
| Presumptive diagnosis | 1 (1%) | 1 (1%) | 0 (0%) | |
| Laboratory parameters, median (IQR) | ||||
| SaFi | 390 (164–432) | 390 (289–433) | 390 (252–428) | 0.928 |
| PaFi | 233 (177–290) | 233 (169–295) | 233 (195–257) | 0.820 |
| White blood cells, × 109/mL | 6700 (4325–10,450) | 6500 (4300–10,100) | 7900 (5200–12,100) | 0.202 |
| Lymphocytes, × 109/mL | 700 (500–1000) | 700 (500–900) | 1000 (500–1300) | 0.074 |
| Platelets, × 109/mL | 173 (122–228) | 171 (115–227) | 196 (130–230) | 0.587 |
| Haemoglobin, g/dL | 130 (118–143) | 130 (116–142) | 131 (123–144) | 0.254 |
| D-dimer, mg/mL | 673 (440–1686) | 651 (422–1524) | 889 (556–3359) | 0.088 |
| CRP, mg/L | 120 (74–223) | 121 (75–211) | 104 (62–232) | 0.754 |
| Serum ferritin, ng/mL | 591 (350–1440) | 596 (359–1553) | 489 (330–931) | 0.321 |
| Creatinine, mg/dL | 1.22 (0.85–1.89) | 1.23 (0.85–1.68) | 1.19 (0.81–2.12) | 0.665 |
| Sodium | 136 (132–140) | 135 (131–140) | 137 (136–143) | 0.071 |
| ALT, U/l | 24 (17.37) | 24 (16–35) | 20 (18–49) | 0.728 |
| AST, U/l | 44 (32–65) | 44 (30–66) | 48 (33–58) | 0.494 |
| LDH, U/l | 429 (330–527) | 433 (327–530) | 406 (343–508) | 0.881 |
| Emergency room (ER) diagnosis | ||||
| SARS-CoV-2 was suspected in ER | 123 (95%) | 104 (95%) | 19 (100%) | 0.341 |
| Lung injury was diagnoses in ER | 88 (69%) | 82 (75%) | 6 (32%) | < 0.001 |
| Treatments during study period | ||||
| Antibiotic agent | 122 (95%) | 105 (96%) | 17 (90%) | 0.391 |
| Azithromycin | 107 (84%) | 91 (84%) | 16 (84%) | 0.356 |
| Lopinavir and ritonavir | 63 (49%) | 61 (56%) | 2 (11%) | < 0.001 |
| Hydroxychloroquine | 105 (82%) | 90 (83%) | 15 (80%) | 0.747 |
| Interferon | 14 (11%) | 14 (13%) | 0 (0%) | 0.128 |
| Tocilizumab | 4 (3%) | 4 (4%) | 0 (0%) | 1.000 |
| Corticosteroids | 34 (26%) | 33 (30%) | 1 (5%) | 0.238 |
| Anticoagulants | ||||
| LMWH prophylaxis | 84 (66%) | 71 (65%) | 13 (58%) | 0.752 |
| LMWH extended prophylaxis | 6 (5%) | 6 (6%) | 0 (0%) | |
| LMWH therapy | 16 (13%) | 13 (13%) | 2 (11%) | |
| Oral anticoagulants | 2 (2%) | 2 (2%) | 0 (0%) | |
| No anticoagulants | 20 (16%) | 16 (15%) | 4 (21%) | |
| Respiratory support | ||||
| High-flow oxygen | 2 (1%) | 2 (1%) | 0 (0%) | 0.552 |
| Non-invasive ventilation | 12 (9%) | 12 (11%) | 0 (0%) | 0.211 |
| Invasive mechanical ventilation | 26 (20%) | 26 (24%) | 0 (0%) | 0.013 |
| ECMO | 2 (2%) | 2 (2%) | 0 (0%) | 0.552 |
| DNR order | 20 (16%) | 12 (11%) | 8 (42%) | 0.002 |
Bold indicate p values that achieve statistical significance (p < 0.05)
Deaths caused by SARS-CoV-2 infection: patients whose death was caused directly by SARS-CoV-2 infection. Death with SARS-CoV-2 infection: Patients where SARS-CoV-2 acted as a precipitating factor to decompensate other chronic or acute pathologies that were the main cause of death.
ALT alanine aminotransferase, AST aspartate aminotransferase, CRP C-reactive protein, DNR Do not resucitate, ECMO extracorporeal membrane oxygenation, LDH lactate dehydrogenase, LMWH low molecular weight heparin, PaFi arterial partial pressure of oxygen/fraction of inspired oxygen, SaFi pulse oximetric saturation/fraction of inspired oxygen
The COVID-19 complications and the cause of death are detailed in Table 3. Nearly four in five patients who died with complications associated with SARS-CoV-2 infection developed ARDS or hyperinflammation.
Table 3.
COVID-19 complications
| Characteristics | Total (n = 128) | Deaths caused by SARS-CoV-2 infection (n = 109) | Death with SARS-CoV-2 infection (n = 19) | p |
|---|---|---|---|---|
| Days in hospital until death, median (IQR), days | 6 (3–10) | 7 (3–13) | 5 (1–9) | 0.035 |
| ARDS | 93 (77%) | 93 (85%) | 0 (0%) | < 0.001 |
| Hyperinflammation | 93 (77%) | 86 (79%) | 7 (37%) | < 0.001 |
| Septic shock | 12 (9%) | 11 (10%) | 1 (5%) | 0.505 |
| Pulmonary embolism | 3 (2%) | 3 (3%) | 0 (0%) | 0.464 |
| Place of death | ||||
| Hospital ward | 102 (80%) | 83 (76%) | 19 (100%) | |
| Intensive care unit | 26 (20%) | 26 (24%) | 0 (0%) | 0.013 |
| Main cause of death | ||||
| ARDS or severe lung injury (> 50% lung involvement in X-ray) caused by COVID | 104 (81%) | 104 (95%) | 0 (0%) | < 0.001 |
| Pulmonary thromboembolism | 2 (2%) | 2 (2%) | 0 (0%) | |
| Sepsis shock due to COVID | 3 (2%) | 3 (3%) | 0 (0%) | |
| Sepsis caused by bacterial infection without lung injury | 3 (3%) | 0 (0%) | 3 (16%) | |
| Wasting associated with age | 10 (8%) | 0 (0%) | 10 (53%) | |
| Heart failure without acute cardiac injury | 2 (2%) | 0 (0%) | 2 (11%) | |
| Kidney failure without lung injury or shock | 2 (2%) | 0 (0%) | 2 (11%) | |
| Bronchoaspiration | 1 (1%) | 0 (0%) | 1 (5%) | |
| Gastrointestinal bleeding | 1 (1%) | 0 (9%) | 1 (5%) | |
| Week of death | ||||
| 18–24 March | 11 (9%) | 7 (6%) | 4 (21%) | 0.042 |
| 25–31 March | 37 (29%) | 31 (28%) | 6 (32%) | |
| 1–7 April | 26 (20%) | 22 (20%) | 4 (21%) | |
| 8–14 April | 21 (16%) | 18 (17%) | 3 (16%) | |
| 15–21 April | 17 (13%) | 16 (15%) | 1 (5%) | |
| > 21 April | 16 (13%) | 15 (14%) | 1 (5%) | |
Bold indicate p values that achieve statistical significance (p < 0.05)
Deaths caused by SARS-CoV-2 infection: patients whose death was caused directly by SARS-CoV-2 infection. Death with SARS-CoV-2 infection: patients where SARS-CoV-2 acted as a precipitating factor to decompensate other chronic or acute pathologies that were the main cause of death
ARDS acute respiratory distress syndrome
The variables associated with the cause of death are shown in Table 4. OR are age adjusted. The confidence intervals are wide due to small sample size. They should be interpreted with caution.
Table 4.
Age adjusted odds ratios of the variables significantly associated with death with SARS-CoV-2 infection vs. death caused by SARS-CoV-2 infection
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI) | p |
|---|---|---|---|
| Heart failure | 3.96 (1.33–11.75) | 3.61 (1.15–11.32) | 0.028 |
| Cerebrovascular disease | 3.54 (1.05–11.86) | 2.46 (0.677–8.55) | 0.175 |
| Dementia | 2.87 (1.05–7.83) | 2.15 (0.76–6.10) | 0.150 |
| Dependence on activities of daily living | 20.47 (2.64–158.78) | 12.07 (1.40–103.86) | 0.023 |
| Frailty | 13.05 (2.87–59-34) | 8.73 (1.37–55.46) | 0.022 |
| Fever or feverish at admission | 0.33 (0.12–0.88) | 0.45 (0.16–1.28) | 0.134 |
| Cough | 0.32 (0.09–1.01) | 0.51 (0.15–1.74) | 0.282 |
| Presence of X-ray infiltrates | 0.05 (0.02–0.20) | 0.07 (0.02–0.25) | < 0.001 |
Bold indicate p values that achieve statistical significance (p < 0.05)
CI confidence interval, ns statistically not significant, OR odds ratio
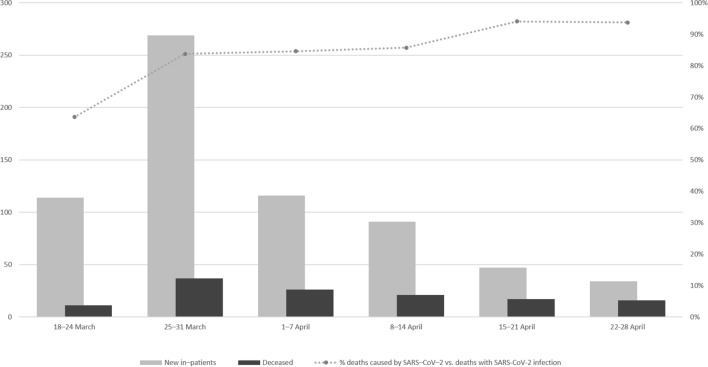
During the first weeks, with more pressure on the hospital from the admission of new patients with COVID-19, there was a higher ratio of deaths with SARS-CoV-2 infection vs. deaths caused by SARS-CoV-2 infection (Fig. 2). The number of new hospital admissions and the total number of deaths for week are also shown.
Fig. 2.
Evolution of the proportion of deaths caused directly by SARS-CoV-2 infection
Discussion
In this cohort study, we have reported the clinical characteristics of patients with COVID-19 admitted to a hospital who died during hospitalization. We analysed the cause of death to differentiate patients whose death was directly associated with development of COVID-19 complications (such is ARDS or pulmonary embolism) from infected patients who died of causes unrelated to any of these complications. Nearly 20% of patients died because of complications unrelated to COVID-19 infection. Most of these were old and frail patients, with only mild COVID-19 according to laboratory and X-ray findings. To the best of our knowledge, this is the first study analysing the cause of death of patients with COVID-19 to differentiate patients who died without developing COVID-19 complications.
The case fatality rate in patients with COVID-19 varies enormously across studies, from only 1.4% in the initial studies of the general population [3] to 21.9% in cohort studies of hospitalized patients with confirmed COVID-19 pneumonia [18] and more than 26% in patients admitted to intensive care units [6]. The mortality rates have been re-estimated [2]. The 15.2% rate of our study agrees with the expected mortality of 15.2% outside of China.
In all the literature, older people have a higher risk of death. However, the median age of 63 years in the first series in China [3] is far from our median age of 85 years. The marked aging of the population in our area and the greater life expectancy in Spain compared to China explain these differences. The older age of these COVID-19 victims brings the question of whether they have died prematurely [19]. For most young victims, there is no debate about the years of life lost but about their life quality. For older people, this is also true, as more than 80% of patients in our series died of causes directly related to COVID-19 and could have lived longer without the infection.
As might be expected, age, comorbidities, frailty and dependency were associated with other causes of death unrelated to COVID-19 complications. The ongoing acute severe conditions, infections [20] or adverse events associated with prescribed drugs [21] could have played a role in these patients’ deaths. Heart failure was the only comorbidity that emerged in the age adjusted regression analysis. Heart failure and dementia are two of the leading causes of death in older adults in Spain [22].
Differences existed in the drugs prescribed to the two groups. Lopinavir and ritonavir, tocilizumab, interferon and corticosteroids were practically not prescribed to the COVID-19 patients who died of unrelated causes. This is easy to explain, as these patients had only mild or no lung injury from SARS-CoV-2.
One of the most interesting associations (p = 0.042) was between death with SARS-CoV-2 infection and the time of hospital admission (Fig. 2). Unsurprisingly, most cases of unrelated cause of death occurred during the first 2 weeks of the epidemic in our area, when the hospital was in the process of organizing new healthcare procedures for COVID-19 patients and when the number of new admitted patients almost exceeded the hospital capacity (12 of 16 hospital wards were transformed into COVID-19 wards in only 2 weeks). The isolation in rooms of patients with dependency for activities of daily living, the low number of ward rounds compared with non-epidemic periods, the ban on relatives or friends accompanying the patients in their rooms, and the overload of hospitals and staff due to the high number of patients admitted every day may have played a negative role in the care of the patients admitted with COVID-19 infection. This negative effect was certainly magnified in frail patients, where the hospital care is sometimes much more important than the drugs intravenous fluids administered [23]. The way we should manage acute complications related to the disease should be different than the way we manage acute decompensation of comorbid conditions in patients with an acute viral infection with no severe disease. For many older patients with no severe COVID-19 disease, the health care, including stimulation, feeding or physical activity encouragement, as well as the management of their comorbidities, was the cornerstone of their treatment, more than the use of antivirals or immunomodulators. Several factors may be involved in the excess mortality of older people. The overload of the health system and consequent failure of hospital care standards and the need to increase isolation measures could have played a role in raising this mortality rate in patients with special care needs during admission.
Our study has several limitations. First, the causal contribution of SARS-CoV-2 infection to related deaths is a major question. It is difficult to differentiate between deaths with SARS-CoV-2 infection and deaths caused by SARS-CoV-2 infection due to the impossibility of performing necropsies. This forces interpreting our findings with caution, as exploratory and descriptive. Second, the study was conducted at a single-centre hospital with a limited sample size. Third, the study only included patients admitted to the hospital. Patients who died in their nursing homes were not included, which may lead to underestimating the real proportion of patients who died without developing COVID-19 complications. Fourth, some cases had incomplete clinical records and laboratory testing (supplementary material). To minimize the impact of missing data, we have incorporated all the electronic databases and scanned handwritten documents. Fifth, we found do not resuscitate orders (DNR) to allow natural death placed in patients with the highest grades of frailty and dependence. We do not know what the influence of DNR orders may had on the evolution of the disease and whether they could play a role in earlier deaths, just before severe symptoms of SARS-CoV-2 infection could develop. In sensitive analysis OR for dependence (OR 14.87, IC 95% 1.83–120.88) and frailty (OR 9.58, IC 95% 1.953–47.01) were statistically significant after being adjusted for the presence of DNR orders. We believe that one of the strengths of our study derives from the inclusion of all the patients who died during the study period.
Conclusion
Older and frail patients are at greater risk of death when infected by SARS-CoV-2. Although most deaths were associated with complications due to the own infection, a proportion of patients died from the decompensation of other acute or chronic pathologies. This partially explains the high fatality rate in the oldest groups of the population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
We thank Professor Félix Del Campo-Matías (Universidad de Valladolid) for useful discussion and comments on the manuscript.
We dedicate this manuscript to all our patients and especially to all their relatives and friends. We could not let most of you accompany your loved ones in their last moments. Be assured that they were never alone, and they always felt your support and affection close, thanks to the health workers who took care of them during hospitalization.
Author contribution
Substantial contributions to: (a) conception and design (LCG). (b) Acquisition of data (MCS, MGM, PCM, IAJ, MRH, LHG, DVP, MCG, and MGF). (c) Analysis and interpretation of data (LCG, MCS, MGM, PCM, IAJ, MRH, LHG, DVP, MCG, MGF, JPMG, TRA, JABC, and JFGCM). Drafting the article or revising it critically for important intellectual content (LCG, MCS, MGM, PCM, IAJ, MRH, LHG, DVP, MCG, MGF, JPMG, TRA, JABC, and JFGCM). Final revision of the version to be published (LCG, MCS, MGM, PCM, IAJ, MRH, LHG, DVP, MCG, MGF, JPMG, TRA, JABC, and JFGCM).
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
All procedures perfomed in human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Our study is a retrospective cohort. Due to the nature of the retrospective chart review, the hospital ethics committee waived the need for informed consent from individual patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marta Cobos-Siles, Email: mcoboss@saludcastillayleon.es.
Pablo Cubero-Morais, Email: pcuberom@saludcastillayleon.es.
Irene Arroyo-Jiménez, Email: irenearroyo21@gmail.com.
María Rey-Hernández, Email: mreyh@saludcastillayleon.es.
Laura Hernández-Gómez, Email: lauramhdezg@gmail.com.
Derly Judith Vargas-Parra, Email: dvargasp@saludcastillayleon.es.
María González-Fernández, Email: magonzalezfern@saludcastillayleon.es.
Marina Cazorla-González, Email: mcazorlagonzalezc@saludcastillayleon.es.
Miriam Gabella-Martín, Email: mgabellamarting@saludcastillayleon.es.
Tomás Ruíz-Albi, Email: truizal@saludcastillayleon.es.
José Angel Berezo-García, Email: jberezoga@saludcastillayleon.es.
Jesús Fernando García-Cruces-Méndez, Email: jgcruces@saludcastillayleon.es.
José Pablo Miramontes-González, Email: jpmiramontes@hotmail.com.
Luis Corral-Gudino, Email: corralgudino@yahoo.es.
References
- 1.Coronavirus Update (Live): 2,646,247 Cases and 184,352 Deaths from COVID-19 Virus Pandemic—Worldometer. https://www.worldometers.info/coronavirus/. Accessed 23 Apr 2020
- 2.Baud D, Qi X, Nielsen-Saines K, et al. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 10.Martins-Filho PR, Tavares CSS, Santos VS. Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. Euron J Int Med. 2020 doi: 10.1016/j.ejim.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontis V, Bennett JE, Mathers CD, et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389:1323–1335. doi: 10.1016/S0140-6736(16)32381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical management of severe acute respiratory infection when COVID-19 is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 2 Apr 2020
- 14.Definition Task Force ARDS, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 15.Borghesi A, Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med. 2020;125:509–513. doi: 10.1007/s11547-020-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 18.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Before their time—Would most covid-19 victims have died soon, without the virus? (Graphic detail) The Economist. https://www.economist.com/graphic-detail/2020/05/02/would-most-covid-19-victims-have-died-soon-without-the-virus?utm_campaign=coronavirus-special-edition&utm_medium=newsletter&utm_source=salesforce-marketing-cloud&utm_term=2020-05-02&utm_content=article-link-2, https://www.msn.com/es-es/?cobrand=acer17win10.msn.com&ocid=ACERDHP17&pc=ACTE. Accessed 2 May 2020
- 20.Wang CJ, Bair H, Yeh C-C. How to prevent and manage hospital-based infections during coronavirus outbreaks: five lessons from Taiwan. J Hosp Med. 2020 doi: 10.12788/jhm.3452. [DOI] [PubMed] [Google Scholar]
- 21.Bonow RO, Hernandez AF, Turakhia M. Hydroxychloroquine, coronavirus disease 2019, and QT prolongation. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1782. [DOI] [PubMed] [Google Scholar]
- 22.Soriano JB, Rojas-Rueda D, Alonso J, et al. The burden of disease in Spain: results from the global burden of disease 2016. Med Clin (Barc) 2018;151:171–190. doi: 10.1016/j.medcli.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Donnelly S. The elderly & COVID-19: cocooning or culling: -the choice is ours. QJM. 2020 doi: 10.1093/qjmed/hcaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.