Abstract
Benign metastasizing leiomyoma, originally reported in 1934 by Paul Steiner is a rare entity with less than 150 documented cases. While this entity has a favorable prognosis, without proper recognition it could be misdiagnosed as advanced stage metastasis. This case report discusses the relevant imaging findings of a case of benign metastasizing leiomyoma involving a 46-year-old woman which was detected in a preoperative work-up for hysterectomy. The patient presented with chronic cough for 2 years and a history of uterine fibroids. Because benign metastasizing leiomyoma was considered in this patient who presented with lung nodules and pelvic masses, a biopsy of the salient lesions was rapidly performed and enabled pathology to confirm a diagnosis of this entity. This case examines the differential diagnoses associated with multiple pulmonary nodules and provides an example of why the radiologist should consider benign metastasizing leiomyoma in that differential when these findings are identified in perimenopausal women.
Keywords: Fibroid, Benign metastasizing leiomyoma, Genitourinary, Gastrointestinal, Chest, Imaging
Abbreviations: CT, computed tomography; ACR, American College of Radiology; BML, benign metastasizing leiomyoma
Case Report
In addition to understanding the generic differential for multiple pulmonary nodules, radiologists are expected to tailor their differential to the patient's demographic. By familiarizing themselves with benign metastasizing leiomyomas, this can easily be excluded or included in a differential as it is associated with a very specific demographic. Further, because of its paucity of clinical symptoms, the radiologist is often the first to see the findings associated with this entity. In this case, we will discuss a clinical scenario in which these considerations assist in making a prompt and conclusive diagnosis avoiding additional confounding variables and workup.
A 46-year-old female presented to an outpatient clinic for preoperative workup in preparation for a hysterectomy. She complained of a dry cough for the previous 2 years and unintentional loss of 10 lbs. over the previous 1 year. There was no significant past medical history to explain the patient's symptoms. The patient was not a smoker and did not have a history of cancer. She did not have a contributory family history, history of recent travel, or significant occupational history. On examination, there was no fever, dyspnea, or shortness of breath on exertion. She underwent pulmonary function testing which demonstrated nonspecific mild restrictive and obstructive patterns. A preoperative chest X-ray was ordered which demonstrated innumerable nodular opacities throughout her lungs diffusely (Fig. 1). Of note, there was no evidence of lymphadenopathy or osseous lesions on the chest X-ray. This is relevant because the radiologist should initially consider that pulmonary nodules, whether solitary or multiple, are the most typical radiographic presentation of early lung cancer, and multiple pulmonary nodules of varying sizes may be the first sign of malignancy in a patient without a prior diagnosis [1]. While this patient was not a smoker and did not have significant risk factors for lung metastases or primary bronchogenic malignancy, in a high-risk patient, the differential considerations can be further confounded if the radiologist is not considering other benign entities [2].
Fig. 1.
PA and lateral chest X-ray (frontal and lateral views): there are innumerable nodular opacities throughout the lungs diffusely. No evidence of lymphadenopathy or bone lesions (arrows).
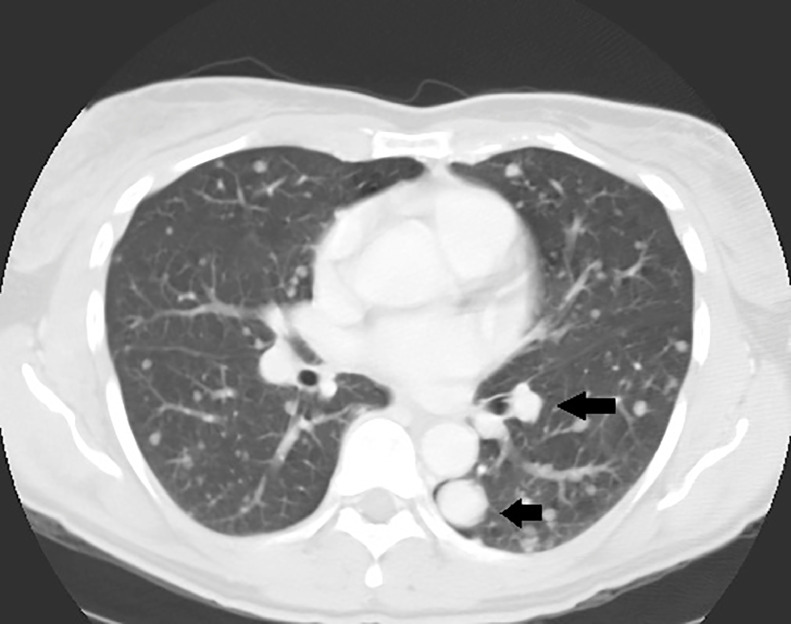
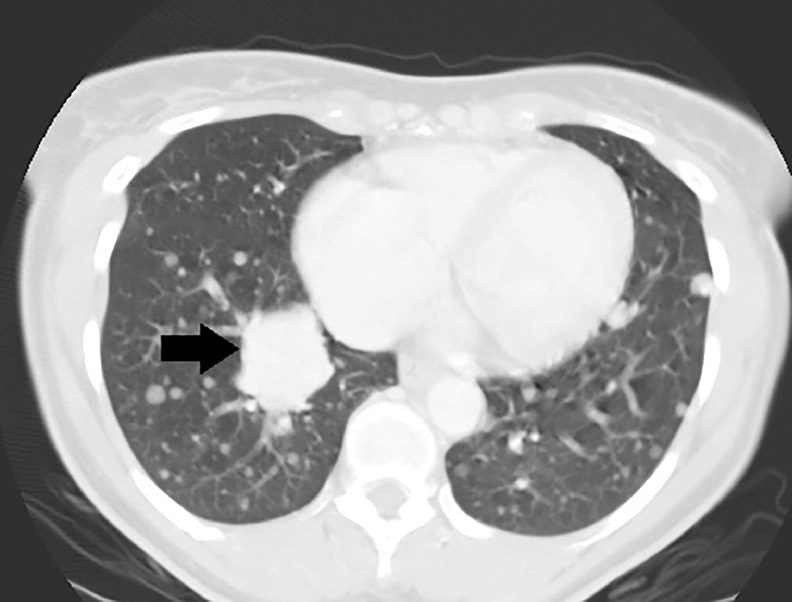
The next day, a CT chest, abdomen, and pelvis with intravenous and oral contrast was ordered using 5 mm slice thickness. The contrast-enhanced chest CT demonstrated innumerable soft-tissue density lesions throughout the lungs diffusely (Figs. 2 and 3), the largest in the right infrahilar region measured up to 4 cm (Fig. 3). There was no mediastinal/hilar lymphadenopathy and there were no pleural effusions or osseous lesions identified.
Fig. 2.
Contrast-enhanced chest CT (axial view/lung window): there are innumerable soft-tissue density lesions throughout the lungs of varying sizes (arrows).
Fig. 3.
Contrast-enhanced chest CT (axial view/lung window): the largest pulmonary lesion in the right infrahilar region measured up to 4 cm and was biopsied (arrow).
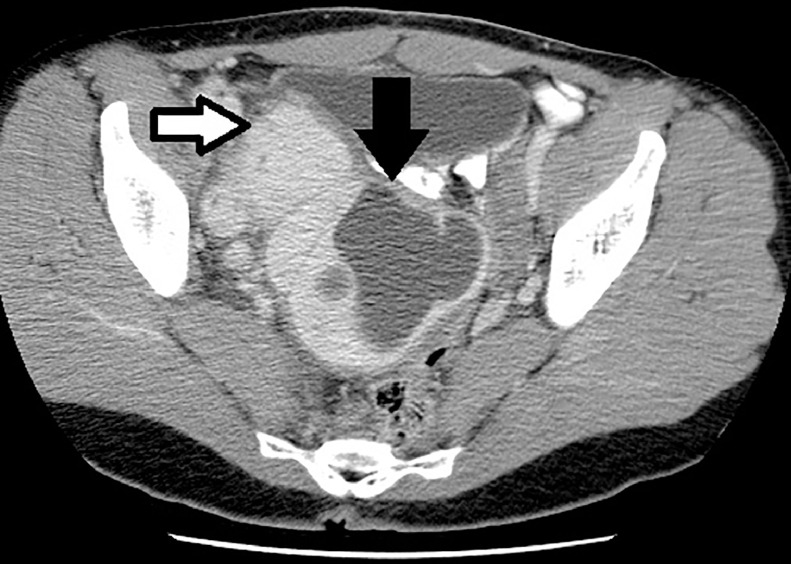
The contrast-enhanced abdomen and pelvis CT demonstrated a large soft tissue density mass in the central pelvis which measured up to 17 cm × 15 cm × 13 cm. There was an exophytic component arising from the right adnexa which measured up to 6.5 cm. There was a large nonenhancing area within the larger lesion including multiple thin, enhancing septations suggestive of necrosis (Fig. 4). There was no evidence of fat attenuation or calcification noted within the tumor. The adjacent fat planes were maintained and there was no lymphadenopathy present. There were no lytic or blastic lesions noted in the thorax, lumbosacral spine, or pelvic bones.
Fig. 4.
Contrast-enhanced abdomen and pelvis CT in 5 mm thickness slices (axial view/soft tissue window): a large soft tissue density mass in the central pelvis measured up to 17 cm × 15 cm × 13 cm and demonstrated a large necrotic component (black arrow). The smaller right adnexal lesion likely represents a pedunculated fibroid as per the surgical pathology report (white arrow). Surgical pathology report from pelvic lesions summarized: immunohistochemistry supportive of cellular leiomyoma which gave "metastasis" to lungs. (leiomyomatosis disseminata pulmonis) or benign metastasizing leiomyoma.
These findings along with the clinical symptoms and contrast-enhanced chest CT suggested a differential diagnosis of metastatic leiomyosarcoma versus benign metastasizing leiomyoma. A decision to obtain histopathological confirmation was made as metastatic leiomyosarcoma could not be completely excluded based on the imaging findings. After the risk and benefits were explained to the patient, a CT-guided needle biopsy was performed of the largest lung lesion. The patient opted to perform a hysterectomy during which both the central pelvic mass, right adnexal mass, and abdominal wall mass were removed and submitted to pathology. The lung lesions were thought to be self-limiting upon hysterectomy.
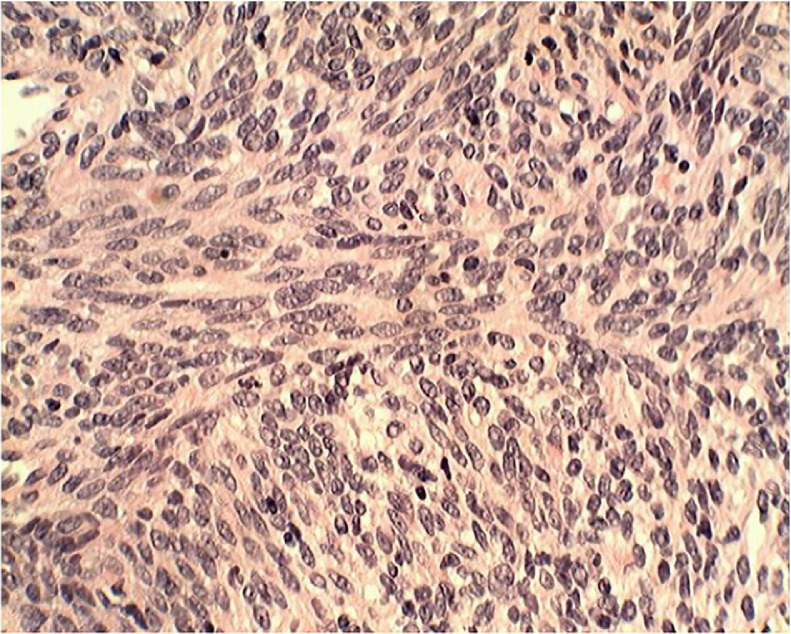
While the pathology slides for the central pelvic mass, right adnexal mass, and abdominal wall mass are unavailable for this case report, generous samples of the pelvic and abdominal wall masses were submitted to pathology. These samples demonstrated bland spindle cells without atypia. There was no apoptosis or necrosis; the sample was positive for desmin, estrogen, and progesterone; negative staining p53 and Ki-67. CT-guided needle biopsy of the pulmonary lesion demonstrated leiomyomatous tissue analogous to the pelvic and abdominal wall lesions. The surgical pathology report for these lesions is summarized as supportive of cellular leiomyoma which gave "metastasis" to lungs. A hematoxylin and eosin stain slide from CT-guided biopsy of the largest lung lesion is included and demonstrates both smooth muscle and fibrous tissue with their pseudocapsule demonstrating a “whorl-like,” trabeculated appearance compatible with leiomyoma (Fig. 5). The chest, abdominal wall, and pelvic findings on pathology and imaging confirmed a diagnosis of benign metastasizing leiomyoma (BML) without any evidence of sarcoma.
Fig. 5.
Pathology slide from lung lesion: hematoxylin and eosin stain demonstrating both smooth muscle and fibrous tissue with their pseudocapsule demonstrating a “whorl-like,” trabeculated appearance compatible with leiomyoma.
Discussion
There have been less than 150 documented cases of BML in the literature with the first documented case of this entity reported in a 36-year-old woman in 1934 by Dr. Paul Steiner. This patient presented with a history of dyspnea, nocturnal headaches and epistaxis, a chronic productive cough, and severe orthopnea. She also demonstrated swelling of the ankles and abdomen for 6 months. [3]. BML is typically seen in women who are in the perimenopausal age. Symptoms of chest pain, shortness of breath, and cough have been described. Although the clinical course is usually indolent, a more rapid progression to severe respiratory symptoms has also been reported, including the potential for pneumothorax [4]. One study attempted to discern a mean time between hysterectomy and presentation of pulmonary nodules because the clinical presentation usually originates from the presence of pulmonary nodules, manifesting as dyspnea or cough. This study didn't find a clinically useful association between time from hysterectomy and the presentation of pulmonary nodules or a meaningful association between a surgical history of hysterectomy and the presentation of pulmonary nodules. Leiomyomas are typically found in women of childbearing age and are often asymptomatic, but can be associated with abnormal uterine bleeding, mass effect on adjacent organs, pain, or infertility [5].
Leiomyomas are the most common type of benign uterine neoplasm and can be classified as subserosal, submucosal, and intramural in location. While they can be seen on many cross-sectional modalities, such as CT as in this case report, the standard of care for imaging these lesions is MRI. From a histopathological perspective, most leiomyomas are benign and consist of both smooth muscle and fibrous tissue with their pseudocapsule demonstrating a “whorl-like,” trabeculated appearance. These lesions begin at the cellular level and are extremely sensitive to high estrogen and progesterone environments such as the use of birth control and the premenopausal state. In rare instances where leiomyomas outgrow their blood supply, degeneration may occur giving them atypical imaging features such as fluid density or even calcification [5].
In addition to benign metastasizing leiomyomas, additional extrauterine growth patterns of leiomyomas include disseminated peritoneal leiomyomatosis, intravenous leiomyomatosis, parasitic leiomyoma, and retroperitoneal leiomyomatosis. Most of these are histologically and radiographically similar to leiomyomas, but are characterized by their unique locations. Disseminated peritoneal leiomyomatosis is characterized by hematogenous spread of leiomyomas along the peritoneum. This can be seen as a sequela of long-term oral contraceptive use and granuloma cell tumors of the ovary, but can also be seen in men and postmenopausal women where no excess hormone production is identified. Based on the radiographic appearance of this entity, the other differential consideration would be peritoneal carcinomatosis. Intravenous leiomyomatosis usually presents as a serpiginous mass within the IVC. Parasitic leiomyoma and retroperitoneal leiomyomatosis are primarily seen throughout the pelvis and retroperitoneum allowing for differentiation by location. Like BML, very little is understood about their pathogenesis [6].
The most obvious difference between BML and other extrauterine growth patterns of leiomyomas is that BML may also involve multiple systems which presents a unique diagnostic challenge if this entity is not included in the differential. As in this case, when there is pulmonary involvement, either a solitary nodular opacity or multiple nodular opacities can be identified. In rare cases, a miliary pattern of nodular opacities has been reported and, in the setting of necrotic pulmonary leiomyomas, cavitation may occur causing a pneumothorax. On contrast enhanced chest CT, these pulmonary nodules tend to be homogeneous and demonstrate some enhancement. In the pelvis, there will usually be uterine leiomyomas or the patient may have undergone a hysterectomy. The differential diagnosis for multiple nodules is broad and includes pulmonary metastases, pulmonary granulomas (calcification is rare in the setting of BML), nodular pulmonary sarcoid (mediastinal and hilar lymphadenopathy is rare in the case of BML), rheumatoid nodules, and amyloidosis (patients with BML tend to be younger than patients for whom amyloidosis is a consideration) [6].
The pathophysiology of extrauterine leiomyoma proliferation and, more commonly, diffuse uterine leiomyomatosis is similar. There are (2) predominate hypotheses which attempt to explain these entities: (1) the most widely accepted hypothesis includes the idea that the lesions hematogenously “metastasize” from a benign uterine leiomyoma via pelvic venous channels and (2) a less commonly accepted hypothesis suggests that the pathophysiology occurs at a cellular level which allows the smooth muscle cells to proliferate more freely because of increased sensitivity to hormonal factors [7]. Currently, there are limitations in histopathologic technology which prevent an understanding of this entity at the cellular level. However, there appears to be very little evidence that these cells undergo malignant transformation when the origin is benign. Additionally, there is no linear association between hysterectomy and proliferation or between time from hysterectomy and proliferation [8].
As in our case, hysterectomy is a common option for viable candidates. The lung lesions may regress or remain stable after the hysterectomy. In patients that cannot undergo surgery, there is a role for hormonal regulation to address further proliferation. However, because this disease entity is ultimately diagnosed pathologically, the safest lesions for biopsy need to be identified to make the diagnosis in a timely fashion. This is important for the psychological well-being of the patient and to ensure that an unnecessary workup for malignancy doesn't occur [9].
In summary, this case demonstrates some of the most common imaging findings in benign metastasizing leiomyoma. It also highlights the necessity of including this entity in the differential diagnosis for solitary or multiple pulmonary nodules. The management implications for identifying BML include avoiding unnecessary workup for malignancy, avoiding undue anxiety for the patient, and avoiding unnecessary interventional diagnostic procedures and their potential complications. This diagnosis, while made histopathologically, can be made in a timely manner if the radiologist considers this entity in the very specific demographic in which BML is associated—perimenopausal women with uterine leiomyomas. Future considerations include understanding the pathogenesis of extrauterine leiomyomatosis and diffuse leiomyomatosis at a cellular, biochemical, and biofeedback level because understanding the aberrant nature of this entity may give us an insight into its malignant counterparts and other hormonally driven malignancies in both male and female patients.
Informed consent
To whom it may concern,
At the time of the initial presentation of this case in tumor board in August 14, 2009, informed consent for publication of this case was obtained from the patient.
Conflict of interest
None.
Acknowledgments
Pathologist S. Svetoslav Bardorov, MD for his assistance in obtaining this diagnosis. Rachael R. Adair for her assistance in editing this paper.
References
- 1.Adekunle AM, Adair LB, Verly G, Jr Press HC. The solitary pulmonary nodule. J Natl Med Assoc. 1976;68(3):243–245. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2609667/ [PMC free article] [PubMed] [Google Scholar]
- 2.“2015 ACR appropriateness criteria® 1 radiologic management of thoracic nodules and masses.” American College of Radiology, https://acsearch.acr.org/docs/69343/Narrative/. [DOI] [PubMed]
- 3.Steiner PE. Metastasizing fibroleiomyoma of the uterus: report of a case and review of the literature. Am J Pathol. 1939;15(1):89–110. https://pubmed.ncbi.nlm.nih.gov/19970436/ 7. [PMC free article] [PubMed] [Google Scholar]
- 4.Maredia R, Snyder BJ, Harvey LA, Schwartz AM. Benign metastasizing leiomyoma in the lung. Radiographics. 1998;18(3):779–782. doi: 10.1148/radiographics.18.3.9599398. doi: 10.1148/radiographics.18.3.9599398. [DOI] [PubMed] [Google Scholar]
- 5.Murase E, Siegelman E, Outwater E, Perez-Jaffe L, Tureck R. Uterine leiomyomas: histopathologic features, MR imaging findings, differential diagnosis, and treatment. Radiographics. 1999;19(5):1179–1197. doi: 10.1148/radiographics.19.5.g99se131179. [DOI] [PubMed] [Google Scholar]
- 6.Fasih N, Prasad shanbhogue AK, Macdonald DB et-al. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics. 28 (7): 1931-48. https://doi:10.1148/rg.287085095. [DOI] [PubMed]
- 7.Abramson S, Gilkeson RC, Goldstein JD, Woodard PK, Eisenberg R, Abramson N. Benign metastasiz- ing leiomyoma: clinical, imaging, and pathologic correlation. AJR Am J Roentgenol. 2001;176(6):1409–1413. doi: 10.2214/ajr.176.6.1761409. [DOI] [PubMed] [Google Scholar]
- 8.Barnaś E, Książek M, Raś R, Skręt A, Skręt-Magierło J, Dmoch-Gajzlerska E. Benign metastasizing leiomyoma: a review of current literature in respect to the time and type of previous gynecological surgery. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0175875. Published 2017 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh DM, Burn PR, King DM. Benign metastasizing leiomyoma with intracaval leiomyomatosis. Br J Radiol. 2000;73(868):435–437. doi: 10.1259/bjr.73.868.10844871. [DOI] [PubMed] [Google Scholar]