Abstract
Childhood adversity is heterogeneous with potentially distinct dimensions of violence exposure and social deprivation. These dimensions may differentially shape emotion-based neural circuitry, such as amygdala–PFC white matter connectivity. Amygdala–orbitofrontal cortex (OFC) white matter connectivity has been linked to regulation of the amygdala’s response to emotional stimuli. Using a preregistered analysis plan, we prospectively examined the effects of childhood exposure to two dimensions of adversity, violence exposure and social deprivation, on the adolescent amygdala–PFC white matter connectivity. We also reproduced the negative correlation between amygdala–PFC white matter connectivity and amygdala activation to threat faces. 183 15−17-year-olds were recruited from the Fragile Families and Child Wellbeing Study — a longitudinal, birth cohort, sample of predominantly low-income youth. Probabilistic tractography revealed that childhood violence exposure and social deprivation interacted to predict the probability of adolescent right hemisphere amygdala–OFC white matter connectivity. High violence exposure with high social deprivation related to less amygdala–OFC white matter connectivity. Violence exposure was not associated with white matter connectivity when social deprivation was at mean or low levels (i.e., relatively socially supportive contexts). Therefore, social deprivation may exacerbate the effects of childhood violence exposure on the development of white matter connections involved in emotion processing and regulation. Conversely, social support may buffer against them.
Keywords: Diffusion MRI, Early adversity, Emotion processing, Amygdala, OFC, Longitudinal
1. Introduction
Childhood adversity is common and predicts a host of negative mental and physical health outcomes (Sacks and Murphy, 2018). Such experiences also shape the neural circuitry underlying emotion processing and regulation (Hein and Monk, 2016). Here, we examined a predominantly low-income sample of adolescents who have been followed since birth to better understand how specific dimensions of early adversity prospectively shape adolescent white matter connectivity between the amygdala and subregions of the prefrontal cortex (PFC), as well as the association between this white matter and amygdala reactivity during socioemotional processing.
Examining dimensions of adversity, that are modeled separately from socioeconomic status, may elucidate how complex experiences influence the brain and may contribute to negative consequences (Amso and Lynn, 2017; McLaughlin et al., 2014). Previous research has highlighted two core dimensions – threat and deprivation – that have roots in neurobiological research (McLaughlin et al., 2014). Further, behavioral research using this dimensional framework found that modeling the dimensions as cumulative exposure compared to a binary incidence variable (i.e., whether the person had experienced threat or deprivation) better predicted child outcomes (Wolf and Suntheimer, 2019). The present study examined two similar dimensions: (1) violence exposure and (2) social deprivation. Violence exposure is characterized by witnessing or being the victim of home and community violence. Social deprivation is defined as a lack of home and community emotional support (Hein, 2019). These dimensions exist on continua: violence exposure ranges from low (i.e., safety) to high and social deprivation from low (i.e., high levels of home/neighborhood support) to high (i.e., lack of support). Violence exposure is posited to alter regions of the brain involved in fear learning and emotion regulation, including the amygdala and PFC (McLaughlin et al., 2014). Compounding the stress of violence exposure, co-occurring social deprivation may exacerbate the effects of violence exposure and, conversely, low social deprivation (i.e., social support) may act as a buffer (Sheridan et al., 2018; Sonuga‐Barke et al., 2010).
Diffusion MRI (dMRI) work in this area is limited and has yielded mixed results (McLaughlin et al., 2019). Moreover, to date, the potential effects of different types of adversity (i.e., threat versus deprivation) on white matter connectivity have not been investigated simultaneously within the same analyses to understand how these complex exposures shape the brain. Retrospective reports of early social deprivation (i.e., neglect) have been associated with decreased strength of structural connections between the amygdala and PFC (Hanson et al., 2015). Additionally, one study found that retrospective reports of threat, specifically, trauma were associated with increased strength of the uncinate fasciculus (Gur et al., 2019), the major bundle of white matter connecting the PFC and subcortical regions (Olson et al., 2015). However, reported studies have also found null effects of threat, deprivation, or mixed exposures on the fronto-amygdala white matter (Bick et al., 2015; Dennison et al., 2016; Park et al., 2016). The vast majority of existing dMRI work examining adversity, though not all (Kim et al., 2019), has used diffusion tensor imaging (DTI) (Hanson et al., 2015) which measures bundles of white matter in aggregate. Much of the DTI literature on adversity has focused on the uncinate. However, DTI does not permit precise mapping of white matter tracts between specific structures, such as the amygdala and particular PFC subregions.
Probabilistic tractography uses dMRI to precisely map white matter tracts between structures (Behrens et al., 2007). This method, in a smaller subset of the current sample, showed that amygdala white matter connectivity with the orbitofrontal cortex (OFC – Brodmann’s Area (BA) 47, 11), dorsomedial PFC (BA10), and subgenual cingulate (BA25) was greater than amygdala white matter connectivity with other PFC regions, such as the dorsal anterior cingulate and dorsolateral PFC (Goetschius et al., 2019). Non-human primate studies also provide support for specific amygdala connectivity with the OFC, dmPFC, and subgenual cingulate (Ray and Zald, 2012; Zikopoulos et al., 2017). Additionally, our previous work revealed that adolescents with less white matter connectivity between the amygdala and the OFC (right BA47, left BA11) and dmPFC (bilateral BA10), but not the dorsolateral PFC, anterior cingulate, or subgenual cingulate, showed greater amygdala activation to threatening faces (Goetschius et al., 2019). Thus, the OFC, dmPFC, and subgenual cingulate seem to be well-connected via white matter to the amygdala. Additionally, amygdala–OFC and amygdala–dmPFC connectivity may play an important role in emotion processing and regulation; however, the effect of dimensional adversity on this white matter has not yet been examined.
Building on Goetschius et al. (2019), we used probabilistic tractography to assess whether violence exposure across childhood (ages 3, 5, 9 years) predicted adolescent (15–17 years) amygdala–PFC white matter connectivity with a focus on OFC, dmPFC, and subgenual cingulate subregions in a longitudinal, sample with a substantial representation of African American and low-income participants — populations that are underrepresented in neuroimaging research (Falk et al., 2013). We also examined whether the degree of social deprivation in childhood predicted adolescent amygdala–PFC white matter microstructure through interaction with violence exposure. We hypothesized the following: childhood violence exposure would be associated with adolescent amygdala–PFC white matter connectivity; and the interaction between childhood violence exposure and social deprivation would be associated with white matter connectivity such that the effects of high violence exposure would be buffered by decreasing social deprivation. In addition, because Goetschius et al. (2019) was conducted on a smaller subsample (N = 141) of the data used in the present study, and utilized a different diffusion data cleaning pipeline, we attempted to reproduce the associations observed between amygdala–PFC white matter connectivity and amygdala activation in the current, full sample (N = 152).
2. Materials & methods
These hypotheses, variables, and analyses were preregistered with the Open Science Framework (https://osf.io/spguw) and the data will be available on the NIMH Data Archive (https://nda.nih.gov/edit_collection.html?id=2106). Prior to preregistering these hypotheses, we had examined the diffusion MRI data on the 141 participants (Goetschius et al., 2019). In this analysis, we examined how the probability of amygdala–PFC white matter connectivity predicted amygdala reactivity to threatening faces; however, we had not evaluated any associations between the early environment and diffusion MRI data.
2.1. Participants
One hundred eighty-three adolescents (15–17 years) sampled from the Detroit, MI, Toledo, OH, and Chicago, IL sites of the Fragile Families and Childhood Wellbeing Study (FFCWS) were included in the present study (see Table 1 for sample demographics and exclusion criteria). The FFCWS is a population-based sample of children born in large US cities, with an oversample of non-marital births (∼3:1) (Reichman et al., 2001). When weighted, the FFCWS represents children born at the turn of the century in American cities of 200,000 or more. When not weighted (as here), given the oversample for non-marital births, the sample represents mostly low-income, urban families. Given the demographics and sample sizes in Detroit, Toledo and Chicago (Hein et al., 2018), a majority of the sample identified as African American. FFCWS families were interviewed at the birth of the focal child, and again when the child was 1, 3, 5, 9, and 15 years of age. The University of Michigan Medical School IRB approved this study (UM IRBMED: HUM00074392). Informed consent was obtained from the parent/legal guardian for both their participation and their teen’s participation and informed assent from the adolescent. These data overlap with prior work from our research group: fMRI and dMRI data, but no environmental data (Goetschius et al., 2019; Hein et al., 2018); violence exposure and social deprivation composites, but no MRI (Peckins et al., 2019).
Table 1.
Participant characteristics including: reasons for exclusion from analysis (upper) and the number of participants excluded for that reason; comparison between the demographic characteristics of the included and full samples for each hemisphere(lower).
| Exclusions | ||
|---|---|---|
| Reason | Number Excluded-Right | Number Excluded-Left |
| No dMRI Data | 41 | 41 |
| Preprocessing outliers > 5 % in diffusion data1 | 1 | 1 |
| No probabilistic tractography model convergence | 5 | 5 |
| Less than 70 % of voxels in PFC masks | 1 | 1 |
| Statistically influential outlier2 | 6 | 6 |
| Poor fMRI data3 | 31 | 31 |
| Included vs. Full Sample Comparison | |||
|---|---|---|---|
| Right Hemisphere Sample (N = 183) | Left Hemisphere Sample (N = 183) | Full Sample (N = 237) | |
| Age | M = 15.85 yrs | SD = 0.52 yrs | M = 15.85 yrs | SD = 0.53 yrs | M = 15.88 yrs | SD = 0.54 yrs |
| Puberty | M = 3.25 | SD = 0.58 | M = 3.26 | SD = 0.59 | M = 3.24 | SD = 0.59 |
| Gender | F = 98 | M = 85 | F = 99 | M = 84 | F = 125 | M = 112 |
| Race | African American: 132 | African American: 133 | African American: 170 |
| Caucasian: 26 | Caucasian: 26 | Caucasian: 34 | |
| Other: 25 | Other: 24 | Other: 33 | |
| Annual Income | $4999 or less: 23 | $4999 or less: 22 | $4999 or less: 28 |
| $5000 to $19,999: 31 | $5000 to $19,999: 31 | $5000 to $19,999: 41 | |
| $20,000 to $39,999: 54 | $20,000 to $39,999: 54 | $20,000 to $39,999: 66 | |
| $40,000 to $69,999: 33 | $40,000 to $69,999: 33 | $40,000 to $69,999: 46 | |
| $70,000 or more: 28 | $70,000 or more: 28 | $70,000 or more: 35 | |
| Not Report/Missing: 14 | Not Report/Missing: 15 | Not Report/Missing: 21 | |
These outlier slices were detected using the automated diffusion MRI cleaning method from MRtrix (v.3.0.R3). Slices with an average intensity four or more standard deviations lower than predicted by eddy's Gaussian process model were marked as outlier slices and replaced with model predictions.
The same number of participants were excluded in each hemisphere due to being a statistical outlier on their violence exposure and social deprivation composite scores; however, only one of the participants is an outlier in both hemispheres.
These participants are only excluded for the analyses looking at the association between amygdala–PFC white matter connectivity and amygdala activation. This is due to no functional MRI data (N = 6), artifacts in the data (N = 7), less than 70 % accuracy on the Faces task (N = 15), or less that 70 % of voxels included in the amygdala mask (N = 3).
2.2. Behavioral measures
2.2.1. Childhood violence exposure and social deprivation composite scores
Violence exposure and social deprivation were assessed using composite scores calculated using data from the Fragile Families and Child Wellbeing study at ages 3, 5, and 9 years. Both constructs included primary caregiver or mother report of experiences that directly (i.e., child physical and emotional abuse, child physical and emotional neglect) and indirectly (i.e., intimate partner emotional, physical, or sexual violence against mother, intimate partner support for mother, community violence, community support) affect the child. The primary caregiver was primarily a biological parent or family member. One participant’s primary caregiver was not a relative. No participants were in the foster care system. We considered violence exposure to exist on a continuum where higher scores represented more violence exposure and lower scores represented more safety. We considered social deprivation to exist on a continuum where higher scores (e.g., where the child experienced either more neglect or witnessed less social support for their mother or less neighborhood social cohesion) approximated deprivation and lower scores (e.g., where the child experienced less neglect or witnessed more social support for their mother or more neighborhood social cohesion) approximated social support. Our approach of including experiences with varying levels of proximity to the child across multiple time points allowed us to comprehensively assess the child’s cumulative, dimensional exposure to violence and social deprivation across childhood as has been done in previous research (Hein, 2019; McLaughlin and Sheridan, 2016). With this approach, we did not unpack the effect of proximal versus distal experiences, the effect of the developmental timing of exposures; however, those are important future research directions. These composite scores were first utilized in previous work from our group (Hein, 2019). All items at each time point were weighted equally. See Appendix A for specific items and the scales that they come from.
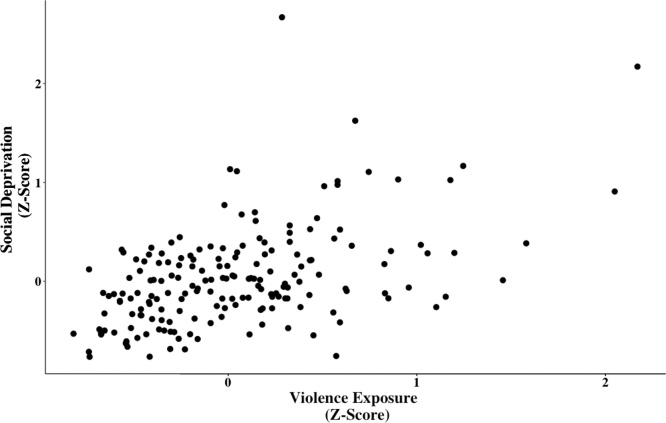
To calculate composite scores, the Z scores (zero-centered) for each of the childhood experiences (child abuse, exposure to intimate partner violence, community violence, child neglect, lack of romantic partner support, lack of neighborhood social cohesion) were summed for each of the childhood experiences within a cumulative dimension (violence exposure and social deprivation) (Song et al., 2013) and then divided by the number of childhood experiences within a dimension for each participant, thus maximizing the number of participants and the diversity of the sample by minimizing drop out due to missing data at any given wave. This means that a score of 0 is approximately average for the sample for that dimension. Scores greater than 0 represent higher than average violence or social deprivation and scores below 0 represent low violence or low social deprivation (i.e., social support). We then mean-centered the scores for violence exposure and social deprivation and created an interaction term (Hein, 2019). In our sample, violence exposure and social deprivation were correlated at r(181) = 0.50, t = 7.69, p < 0.001, but the variance inflation factor (VIF) was 1.326 (Fig. 1, Table 2). VIF reflects how much the estimated regression coefficients are increased due to collinear independent variables. Cutoffs are typically between 5–10, therefore, based on the VIF reported here, the multicollinearity of violence exposure and social deprivation was low (Craney and Surles, 2002; Sheather, 2009).
Fig. 1.
Scatterplot depicting the association between childhood exposure to violence and social deprivation. To calculate composite scores, the Z scores (zero-centered) for each of the childhood experiences were summed for each of the childhood experiences within a dimension (violence exposure and social deprivation) and then divided by the number of childhood experiences within a dimension for each participant. In our sample, violence exposure and social deprivation were correlated at r(181) = 0.50, t = 7.69, p < 0.001, but the VIF was 1.326.
Table 2.
Descriptive statistics for the main continuous predictor variables and covariates.
| Predictor | Mean (SD) | Minimum - Maximum |
|---|---|---|
| Violence Exposure1 | 0.04 (0.53) | −0.82 – 2.17 |
| Social Deprivation1 | 0.03 (0.50) | −0.76 – 2.67 |
| Internalizing Psychopathology2 | 0.02 (0.42) | −0.60 – 1.29 |
| Current Life Stress3 | 10.13 (5.35) | 0 – 25 |
| Maternal Education4 | 2.13 (1.03) | 1 – 4 |
To calculate composite scores, the Z scores (zero-centered) for each of the childhood experiences were summed for each of the childhood experiences within a dimension (violence exposure and social deprivation) and then divided by the number of childhood experiences within a dimension for each participant.
This variable is a multi-method, multi-informant latent factor that is constructed from the following measures: (1) K-SADS clinician report of past and current symptoms of dysthymia, social phobia, generalized anxiety disorder, major depression, and phobia and (2) parent and child report on the Mood and Feelings Questionnaire, Child Depression Inventory, and the Screen for Child Anxiety Related Disorders.
This variable is the sum of all of the items from the Adolescent Life Events Scale (ALES).
This is a self-report categorical variable with the following response options: 1 - less than high school, 2 - high school or equivalent, 3 - some college/technical school, 4 - college or graduate school.
2.2.2. Gender identification (faces) fMRI task
During fMRI data collection, participants completed an event-related emotional faces task in which they were instructed to identify to the gender of emotional faces displaying one of five emotions: fearful, happy, sad, angry, neutral. Details of the task are in the Appendix A (and see Goetschius et al., 2019; Hein et al., 2018). Participants who achieved less than 70 % accuracy on the Faces Task were excluded (N = 15). Average task accuracy was 94.74 %.
2.2.3. Covariates
To address potential confounds, the present analyses adjusted for race/ethnicity, maternal education at birth, and maternal marital status at birth. We controlled for maternal marital status at birth due to the oversampling of non-marital births in the FFCWS study (Reichman et al., 2001). Additionally, we adjusted for adolescent pubertal development, adolescent internalizing psychopathology, and adolescent life stress to ensure that observed effects were not driven by these adolescent factors. Adolescent internalizing psychopathology was assessed using a multi-method, multi-informant latent factor score constructed from the following measures: (1) K-SADS (Kaufman et al., 1997) clinician report of past and current symptoms of dysthymia, social phobia, generalized anxiety disorder, major depression, and phobia and (2) parent and child report on the Mood and Feelings Questionnaire (Angold et al., 1987), Child Depression Inventory (Helsel and Matson, 1984), and the Screen for Child Anxiety Related Disorders (Birmaher et al., 1997) (See Appendix A and Hein, 2019 for more detail including the CFA fit statistics). Current life stress was used as a covariate in the present analyses and was measured using the Adolescent Life Events Scale (adapted for Shaw et al., 2003 from Farrell et al., 1998 and Masten et al., 1994). This scale assesses the experience of common adolescent stressful life events in the past year. Descriptive statistics for all covariate variables are in Table 2. See Appendix A for more information on how covariates were measured. All analyses were done with and without covariates.
2.3. MR measures - adolescence
MR images were acquired using a GE Discovery MR750 3 T scanner with an 8-channel head coil located at the UM Functional MRI Laboratory. Head movement was minimized through: (a) instructions to the participant and (b) padding and pillows placed around the head, which are well-tolerated, yet limit motion. These procedures have been outlined in previous work (Goetschius et al., 2019; Hein et al., 2018).
T1-weighted gradient echo images were taken before the functional scans using the same field of view (FOV) and slices as the functional scans (TR = 12 ms, TE =5 ms, TI =500 ms, flip angle = 15°, FOV =26 cm; slice thickness = 1.4 mm; 256 × 192 matrix; 110 slices). DMRI data were collected using a spin-echo EPI diffusion sequence (scan parameters: TR 7250 ms, Minimum TE, 128 × 128 acquisition matrix, 22 cm FOV, 3 mm thick slices (no gap), 40 slices acquired using an alternating-increasing order, b value = 1000s/mm2, 64 non-linear directions, five b = 0 s/mm2 T2 images (b0) acquired). Functional MRI (fMRI) T2*-weighted BOLD images were acquired using a reverse spiral sequence (Glover and Law, 2001) of 40 contiguous axial 3 mm slices (TR =2000 ms, TE = 30 ms, flip angle = 90°, FOV =22 cm, voxel size = 3 mm × 3 mm × 3 mm, interleaved ascending acquisition).
Slices were prescribed parallel to the AC-PC line (same locations as structural scans). Images were reconstructed into a 64 × 64 matrix. Slices were acquired contiguously, which optimized the effectiveness of the movement post-processing algorithms. Images were reconstructed off-line using processing steps to remove distortions caused by magnetic field inhomogeneity and other sources of misalignment to the structural data, which yields excellent coverage of subcortical areas of interest.
2.3.1. dMRI processing
Diffusion images were converted from DICOM to NIFTI format using MRIcron (dcm2niix – 2MAY2016) for offline analysis using MRtrix (v.3.0.R3) (Veraart et al., 2016) and the FSL (v. 5.0.9) FMRIB's Diffusion Toolbox (FDT) (v. 3.0) (Jenkinson et al., 2012) (see Appendix A for more processing details).
DMRI data were then processed using probabilistic tractography in FSL. This involved building a distribution of diffusion parameters at each voxel using bedpost (Hernández et al., 2013) and estimating the probability of amygdala–PFC white matter connectivity for 4 PFC ROIs bilaterally (8 total) using probtrackx (Hernandez-Fernandez et al., 2016) (Appendix A). Those ROIs were BA10, BA11, BA25, and BA47 and they were selected due to a previous stronger likelihood of amygdala white matter connectivity in our previous work (Goetschius et al., 2019). ROIs, including both seed amygdalae (AAL Atlas) and target PFC regions (TD Brodmann’s Areas) were created from masks in WFU PickAtlas (Maldjian et al., 2003). The maximum-likelihood of amygdala–PFC connectivity was then extracted for each individual from a group-level peak (6 mm sphere around peak point) identified for each ROI (Greening and Mitchell, 2015) (details in Appendix A). The MNI coordinates (x,y,z) for the peak for each target are as follows: BA10 (left: −30, −4, −14; right: 32, −2, −12), BA11 (left: −30, −4, −14; right: 32, −2, −14), BA25 (left: −16, 0, −14, right: 18, 0, −14), BA47: (left: −30, −4, −14; right: 34, 0, −20).
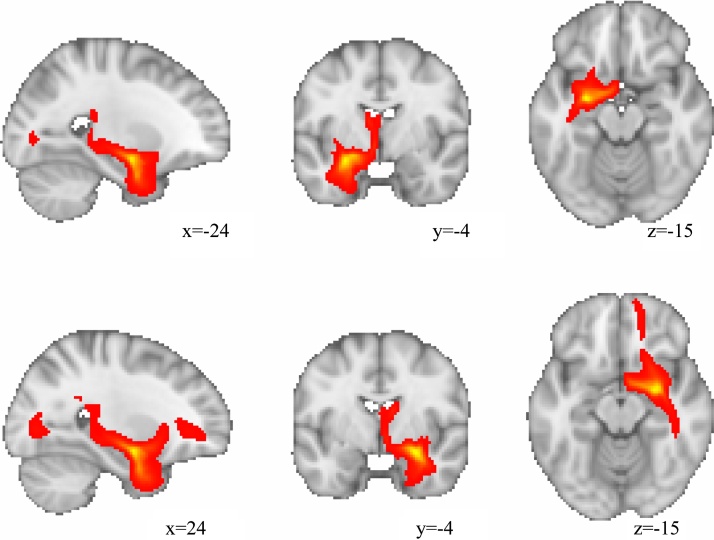
In the present study, we did not use waypoint or termination masks in the probabilistic tractography analysis. Thus, we cannot guarantee that streamlines did not cross the midline or enter the temporal pole. However, this does not appear to be the case for the measured connections here based on the average streamline images (Fig. 2).
Fig. 2.
Image representing the average streamlines reaching each voxel with the left (top) and right (bottom) amygdalae as the seed region. This can be thought of as quantifying the connectivity from the seed region. These images are thresholded at 1000 streamlines.
The dMRI processing approach used (i.e., from bedpostx through data extraction) was identical to a previously reported analysis; however, the present study’s sample size was larger (N = 152 with both usable fMRI and dMRI data compared to N = 141) because the dMRI data were processed for artifacts using a different, more reliable, and automated method that allowed us to retain more subjects (Andersson et al., 2017; Andersson and Sotiropoulos, 2016; Veraart et al., 2016). Due to the increased sample size and different dMRI cleaning method, we needed to reproduce the associations with amygdala activation seen in Goetschius et al. (2019).
2.3.2. fMRI processing
First-level statistical analyses for functional activation were performed using the general linear model implemented in SPM12. For each participant, conditions were modeled with the SPM12 canonical hemodynamic response function. Incorrect trials were modeled as a separate condition and were not included in subsequent analysis. A statistical image for each condition contrast in the Faces Task at each voxel was generated. Mean activation was extracted for both the left and right amygdala using MarsBaR (v. 0.44) (Brett et al., 2002) from the contrast image representing a combination of threat (fear + anger) trials vs. baseline (Goetschius et al., 2019; Hein et al., 2018). ROI masks used in the extraction were created using the left and right amygdala from the AAL Atlas in WFU Pickatlas (Maldjian et al., 2003).
2.4. Statistical analysis
2.4.1. Preregistered analyses
To determine how childhood exposure to violence and social deprivation at ages 3, 5, and 9 years were associated with amygdala–PFC white matter connectivity, we performed eight multiple regression analyses – one for each amygdala-PFC target pair (bilateral BA10, BA11, BA25, BA47). In each regression analysis, we first ran the analysis without any covariates. Then, we controlled for a list of preregistered covariates, including participant gender (male or female), race (African American, Caucasian, or Other), maternal education at birth, maternal marital status at birth). Additionally, in a separate analysis, we controlled for three variables that we did not pre-register, pubertal status, current life stress, and the internalizing disorders latent factor score (Hein, 2019), in addition to the preregistered covariates, though none of these variables changed the overall effect. We used a Bonferroni-corrected significance threshold based on those eight ROIs (p < 0.05/8 tests per hemisphere = 0.0063). To interpret significant interactions, simple slope and regions of significance analyses were conducted to determine the nature of the interaction and ensure that the interaction was within our observable data using methods outlined by Preacher et al. (2006).
Our main preregistered analysis plan proposed a structural equation model (SEM) where childhood dimensions of early adversity predicted internalizing psychopathology in a way that was mediated by amygdala–PFC white matter connectivity. We did not continue with this analysis plan when white matter connectivity was not significantly associated with internalizing psychopathology (Appendix A). Thus, we proceeded with our secondary analysis plan to examine the pieces of the SEM using multiple regression, including the violence exposure x social deprivation interaction. We did not have adequate statistical power to perform a moderated-mediation model to examine the interaction in a larger SEM framework given the likely small effect size (Preacher et al., 2007).
2.4.2. Non-preregistered analyses
Due to the use of an automated diffusion MRI data cleaning and artifact detection method which increased sample size, we reproduced the associations between amygdala–PFC white matter connectivity and amygdala activation that were previously reported where amygdala–OFC (right BA47, left BA11) and amygdala–dmPFC (bilateral BA10) white matter connectivity was associated with amygdala reactivity (Goetschius et al., 2019). To do this, we performed eight regressions predicting ipsilateral amygdala activation to threat faces from amygdala–PFC white matter connectivity – one for each amygdala-PFC target pair (bilateral BA10, BA11, BA47, BA25). In these regressions, we used a Bonferroni-corrected significance threshold (p = 0.05/8 tests = 0.0063).
3. Results
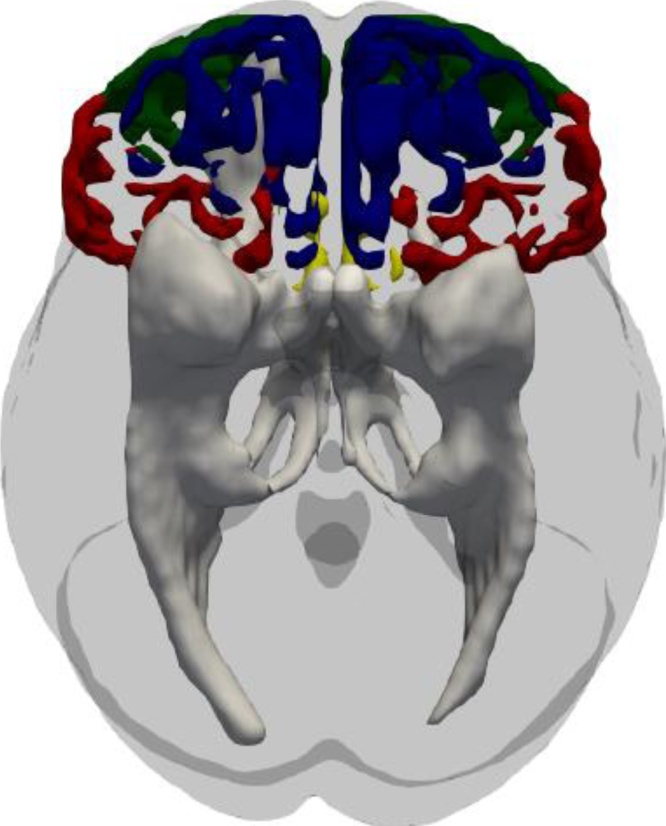
Probabilistic tractography was used to estimate the white matter connecting the amygdala with all eight PFC targets (bilateral BA10, BA11, BA25, BA47). For a visual representation, see Fig. 3.
Fig. 3.
Visual representation of the white matter tracts (gray-white) coming from the left and right amygdalae in our probabilistic tractography analysis. For illustrative purposes, the Brodmann’s Area (BA) masks used as targets are superimposed on the brain in different colors: BA10 (green), BA11 (blue), BA25 (yellow), BA47 (red). (For interpretation of the references to colour in this figure caption, the reader is referred to the web version of this article).
3.1. Violence Exposure x Social Deprivation predicted right hemisphere amygdala–OFC white matter connectivity
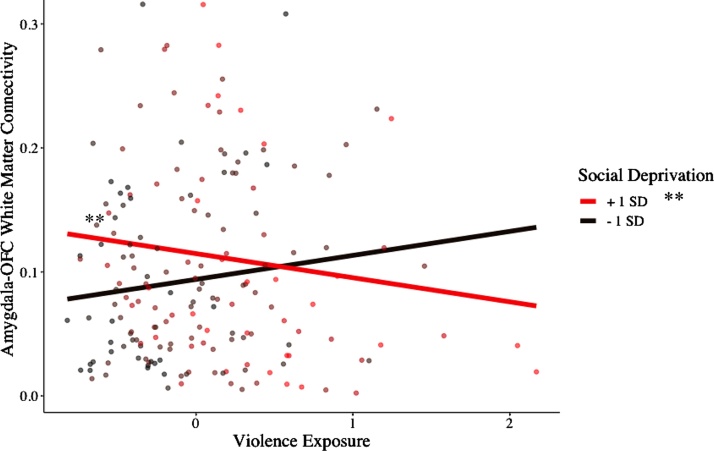
The interaction between violence exposure and social deprivation significantly predicted the probability of right hemisphere amygdala–BA47 (OFC) white matter connectivity (Table 3). This association held when adjusting for our pre-registered covariates (gender, race, maternal education at birth, and maternal marital status at birth) (β =−0.319, p = 0.004) and non-preregistered covariates (pubertal status, current life stress, and internalizing psychopathology in addition to the preregistered covariates) (β =−0.317, p = 0.005). Contrary to our preregistered hypotheses, there were no main effects of violence exposure or social deprivation. To better understand the interaction in the context of our data, simple slopes and regions of significance are plotted in Fig. 4. Simple slopes analysis revealed that when social deprivation was 0.78 or greater, violence exposure and probability of white matter were inversely related (β = −0.29, p = 0.048). When social deprivation was 1 standard deviation below the mean, there was no association between violence exposure and amygdala–OFC white matter connectivity (β = 0.02, p = 0.209). Thus, in our data, at relatively high values of social deprivation, violence exposure was related to a lower likelihood of amygdala–OFC connectivity, suggesting that violence exposure had the greatest association with amygdala–OFC white matter connectivity when social deprivation was also high.
Table 3.
Stepwise regression results using right amygdala–BA47 white matter connectivity as the criterion. These additive models show the base model with only covariates, the R2 change when adding the non-significant main effects of violence exposure and social deprivation, and then the R2 change when adding the significant interaction between violence exposure and social deprivation when predicting right amygdala–BA47 white matter connectivity.
| Predictor | b |
b 95 % CI [LL, UL] |
beta |
beta 95 % CI [LL, UL] |
Fit | Difference |
|---|---|---|---|---|---|---|
| (Intercept) | 0.14 | [0.02, 0.25] | ||||
| Race_12 | 0.00 | [−0.04, 0.05] | 0.02 | [−0.19, 0.23] | ||
| Race_22 | −0.01 | [−0.04, 0.03] | −0.03 | [−0.24, 0.18] | ||
| Gender | 0.01 | [−0.02, 0.04] | 0.04 | [−0.16, 0.24] | ||
| Pubertal Status | 0.02 | [−0.01, 0.05] | 0.10 | [−0.07, 0.26] | ||
| Maternal Education | 0.00 | [−0.02, 0.03] | 0.03 | [−0.16, 0.22] | ||
| Maternal Marital Status | −0.00 | [−0.00, 0.00] | −0.10 | [−0.26, 0.06] | ||
| Current Life Stress | −0.00 | [−0.02, 0.01] | −0.07 | [−0.23, 0.10] | ||
| Internalizing Psychopathology | −0.01 | [−0.05, 0.02] | −0.08 | [−0.25, 0.09] | ||
| R2 = .022 | ||||||
| 95 % CI[.00,.03] | ||||||
| (Intercept) | 0.14 | [0.02, 0.25] | ||||
| Violence Exposure | −0.00 | [−0.03, 0.02] | −0.01 | [−0.19, 0.18] | ||
| Social Deprivation | 0.01 | [−0.02, 0.03] | 0.04 | [−0.14, 0.22] | ||
| Race_12 | 0.00 | [−0.04, 0.05] | 0.01 | [−0.20, 0.23] | ||
| Race_22 | −0.01 | [−0.04, 0.03] | −0.04 | [−0.25, 0.18] | ||
| Gender | 0.01 | [−0.02, 0.04] | 0.04 | [−0.16, 0.24] | ||
| Pubertal Status | 0.02 | [−0.01, 0.05] | 0.09 | [−0.08, 0.26] | ||
| Maternal Education | 0.00 | [−0.02, 0.03] | 0.03 | [−0.16, 0.23] | ||
| Maternal Marital Status | −0.00 | [−0.00, 0.00] | −0.10 | [−0.26, 0.07] | ||
| Current Life Stress | −0.00 | [−0.02, 0.01] | −0.06 | [−0.23, 0.11] | ||
| Internalizing Psychopathology | −0.01 | [−0.05, 0.02] | −0.08 | [−0.25, 0.09] | ||
| R2 = .023 | ΔR2 = .001 | |||||
| 95 % CI[.00,.01] | 95 % CI[−.01, .01] | |||||
| (Intercept) | 0.14 | [0.03, 0.26] | ||||
| Violence Exposure | 0.01 | [−0.02, 0.04] | 0.07 | [−0.12, 0.25] | ||
| Social Deprivation | 0.02 | [−0.01, 0.05] | 0.13 | [−0.06, 0.32] | ||
| Interaction1 | −0.04* | [−0.08, −0.01] | −0.26 | [−0.44, −0.08] | ||
| Race_12 | 0.01 | [−0.03, 0.06] | 0.07 | [−0.15, 0.28] | ||
| Race_22 | −0.01 | [−0.04, 0.03] | −0.05 | [−0.26, 0.16] | ||
| Gender | 0.01 | [−0.02, 0.04] | 0.04 | [−0.16, 0.24] | ||
| Pubertal Status | 0.02 | [−0.01, 0.05] | 0.09 | [−0.08, 0.26] | ||
| Maternal Education | 0.00 | [−0.02, 0.03] | 0.03 | [−0.17, 0.22] | ||
| Maternal Marital Status | −0.00 | [−0.00, 0.00] | −0.09 | [−0.25, 0.07] | ||
| Current Life Stress | −0.00 | [−0.02, 0.01] | −0.05 | [−0.21, 0.12] | ||
| Internalizing Psychopathology | −0.02 | [−0.05, 0.01] | −0.09 | [−0.25, 0.08] | ||
| R2 = .069 | ΔR2 = .046** | |||||
| 95 % CI[.00,.09] | 95 % CI[−.01, .11] |
Note. A significant b-weight indicates the beta-weight and semi-partial correlation are also significant. b represents unstandardized regression weights. beta indicates the standardized regression weights. LL and UL indicate the lower and upper limits of a confidence interval, respectively.
Significant predictor using a Bonferroni corrected threshold (p < 0.05/8 tests = 0.0063).
indicates p < .01.
Interaction between Violence Exposure/Victimization and Social Deprivation.
Dummy coded variables represented 3 category race variable (African American, Caucasian, Other).
Fig. 4.
Plot illustrating the interaction between childhood violence exposure and social deprivation (ages 3, 5, 9) in predicting the probability of white matter connectivity between the amygdala and orbitofrontal cortex (OFC – Brodmann’s Area 47) in the right hemisphere (adolescence). The continuous moderator (social deprivation) has been plotted at a +/- 1 standard deviation (SD) interval. A Johnson-Neyman interval shows that violence exposure and white matter connectivity are significantly, inversely correlated when social deprivation = 0.78 and greater. The range of social deprivation values (zero-centered where 0 is the mean) in the data are [−0.76 2.67]. This figure illustrates that at relatively high values of social deprivation, violence exposure and likelihood of amygdala–OFC connectivity are negatively correlated.
3.2. Violence exposure x social deprivation predicted right hemisphere amygdala–dmPFC white matter connectivity
The interaction between violence exposure and social deprivation significantly predicted the probability of right hemisphere amygdala–BA10 (dorsomedial prefrontal cortex - dmPFC) white matter connectivity (β =−0.268, p = 0.011). This interaction, however, did not remain significant when controlling for the demographic covariates (β =−0.185, p = 0.091) (Table 1 in Appendix B). There were no main effects of violence exposure or social deprivation on right hemisphere amygdala–BA10 white matter connectivity.
3.3. Greater amygdala–OFC and amygdala–dmPFC white matter connectivity was related to attenuated amygdala reactivity
We reproduced results from previous analyses (Goetschius et al., 2019). The probability of amygdala white matter connectivity significantly predicted ipsilateral amygdala activation to threatening (fearful and angry) faces for the four PFC regions where it was previously related (bilateral BA10, left BA11, right BA47), even when adjusting for the specified covariates, using a hemisphere Bonferroni-corrected significance level (0.05/8 = 0.0063) such that increased probability of white matter was associated with decreased amygdala activation (Table 4). Additionally, amygdala–PFC white matter connectivity was not related to amygdala reactivity in regions where it had not been related in our previous report (right BA11, bilateral BA25, left BA47) (Table B8 in Appendix B).
Table 4.
Regression results from amygdala-prefrontal cortex white matter connectivity predicting ipsilateral amygdala activation to threat faces adjusting for covariates.
| B | SEB | β | t | p | |
|---|---|---|---|---|---|
| Model: R. Amygdala Activation (Threat) ∼ R. Amygdala-BA47 White Matter Connectivity | |||||
| RAmy_BA47* | −2.578 | 0.706 | −0.290 | −3.651 | <0.001 |
| Internalizing | 0.184 | 0.146 | 0.115 | 1.261 | 0.209 |
| Pubertal Status | 0.068 | 0.120 | 0.059 | 0.570 | 0.569 |
| Gender | 0.177 | 0.150 | 0.131 | 1.182 | 0.239 |
| Race_11 | −0.030 | 0.204 | −0.016 | −0.149 | 0.882 |
| Race_21 | −0.013 | 0.160 | −0.009 | −0.082 | 0.935 |
| Current Life Stress | 0.017 | 0.011 | 0.139 | 1.614 | 0.110 |
| Maternal Education | −0.083 | 0.057 | −0.124 | −1.467 | 0.145 |
| Maternal Marital Status | 0.096 | 0.146 | 0.055 | 0.655 | 0.514 |
| F(9, 139) = 2.746, p = 0.005, R2 = 0.151 | |||||
| Model: R. Amygdala Activation (Threat) ∼ R. Amygdala-BA10 White Matter Connectivity | |||||
| RAmy_BA10* | −4.105 | 1.363 | −0.249 | −3.013 | 0.003 |
| Internalizing | 0.141 | 0.147 | 0.088 | 0.957 | 0.340 |
| Pubertal Status | 0.048 | 0.122 | 0.042 | 0.396 | 0.692 |
| Gender | 0.154 | 0.142 | 0.114 | 1.015 | 0.332 |
| Race_11 | −0.067 | 0.207 | −0.036 | −0.325 | 0.746 |
| Race_21 | 0.024 | 0.164 | 0.016 | 0.148 | 0.883 |
| Current Life Stress | 0.020 | 0.011 | 0.163 | 1.881 | 0.062 |
| Maternal Education | −0.089 | 0.058 | −0.133 | −1.542 | 0.125 |
| Maternal Marital Status | 0.156 | 0.147 | 0.091 | 1.061 | 0.290 |
| F(9, 139) = 2.238, p = 0.023, R2 = 0.127 | |||||
| Model: L. Amygdala Activation (Threat) ∼ L. Amygdala-BA10 White Matter Connectivity | |||||
| LAmy_BA10* | −12.567 | 3.165 | −0.322 | −3.970 | <0.001 |
| Internalizing | 0.175 | 0.130 | 0.122 | 1.342 | 0.182 |
| Pubertal Status | 0.006 | 0.104 | 0.006 | 0.054 | 0.958 |
| Gender | 0.077 | 0.131 | 0.065 | 0.590 | 0.556 |
| Race_11 | 0.072 | 0.176 | 0.043 | 0.406 | 0.685 |
| Race_21 | 0.119 | 0.140 | 0.091 | 0.864 | 0.389 |
| Current Life Stress | 0.012 | 0.009 | 0.112 | 1.287 | 0.200 |
| Maternal Education | −0.071 | 0.049 | −0.120 | −1.436 | 0.153 |
| Maternal Marital Status | 0.032 | 0.128 | 0.021 | 0.253 | 0.801 |
| F(9, 140) = 2.789, p = 0.005, R2 = 0.152 | |||||
| Model: L. Amygdala Activation (Threat) ∼ L. Amygdala-BA11 White Matter Connectivity | |||||
| LAmy_BA11* | −6.743 | 1.871 | −0.290 | −3.604 | <0.001 |
| Internalizing | 0.129 | 0.130 | 0.090 | 0.991 | 0.323 |
| Pubertal Status | 0.062 | 0.106 | 0.062 | 0.587 | 0.558 |
| Gender | 0.158 | 0.132 | 0.132 | 1.192 | 0.235 |
| Race_11 | 0.066 | 0.178 | 0.039 | 0.368 | 0.713 |
| Race_21 | 0.103 | 0.139 | 0.079 | .743 | 0.458 |
| Current Life Stress | 0.017 | 0.009 | 0.156 | 1.813 | 0.072 |
| Maternal Education | −0.078 | 0.050 | −0.132 | −1.556 | 0.122 |
| Maternal Marital Status | −0.010 | 0.130 | −0.007 | −0.80 | 0.936 |
| F(9, 140) = 2.463, p = 0.012, R2 = 0.137 | |||||
significant at p < 0.0063 (Bonferroni corrected significance level for 8 tests).
Dummy coded variables represented 3 category race variable (African American, Caucasian, Other).
3.4. Null findings
Violence exposure, social deprivation, or their interaction did not significantly predict the likelihood of left hemisphere amygdala–BA10 white matter connectivity, left hemisphere amygdala–BA47 white matter connectivity, bilateral amygdala–BA11 white matter connectivity, or bilateral amygdala–BA25 white matter connectivity (Tables B2–B7 in Appendix B).
4. Discussion
Using an open science framework and preregistered hypotheses, the present study examined how two dimensions of adversity - violence exposure and social deprivation - were associated with structural connectivity between the amygdala and OFC in the right hemisphere, a critical circuit for emotion processing and regulation. Whereas, contrary to our hypotheses, there were no main effects of the two dimensions on white matter connectivity, the interaction of violence exposure and social deprivation at ages 3, 5, and 9 prospectively predicted the degree of right amygdala–OFC white matter connectivity in adolescence. Specifically, the combination of more violence exposure and more social deprivation in childhood prospectively predicted less amygdala–OFC white matter connectivity in adolescence; however, violence exposure was not associated with white matter connectivity when social deprivation was at mean or low levels (i.e., when children were in relatively socially supportive contexts). Thus, social deprivation may exacerbate the effects of childhood violence exposure on the development of white matter connections whereas social support may act as a buffer. This interaction remained even after adjusting for gender, race, pubertal development, current internalizing psychopathology, current life stress, maternal marital status at birth, and maternal education at birth. Importantly, the work was conducted in a well-sampled cohort of adolescents with high rates of poverty and a large proportion of African Americans, groups that are understudied in neuroimaging research (Falk et al., 2013).
As a secondary objective, we reproduced in an expanded, overlapping sample (Goetschius et al., 2019) the finding that increased amygdala–OFC and amygdala–dmPFC white matter connectivity was associated with attenuated amygdala-reactivity to threat faces. This association remained after adjusting for gender, race, pubertal development, current internalizing psychopathology, current life stress, maternal marital status at birth, and maternal education at birth. When considered in conjunction with the violence exposure by social deprivation interaction, these findings suggest that early adversity shapes white matter connections that modulate the amygdala, a structure involved in threat processing (Phelps and LeDoux, 2005).
The association between violence exposure and decreased amygdala–OFC white matter connectivity in the context of social deprivation builds on prior work (for review, see McLaughlin et al., 2019). Extant dMRI research indicates that child maltreatment or trauma are generally, but not exclusively (Gur et al., 2019), associated with both weaker structural connectivity within the uncinate fasciculus (Govindan et al., 2010; Hanson et al., 2015) and weaker global structural connectivity, including within the OFC (Puetz et al., 2017). Additionally, consistent with the present findings, fMRI work found that violence exposure is associated with altered amygdala activation (Hein, 2019; McCrory et al., 2011) and amygdala–PFC functional connectivity (Herringa et al., 2013; Kaiser et al., 2018). Further, neural tract tracer research in nonhuman primates revealed that stress affects amygdala–OFC structural connections via increased levels of dopamine (Zikopoulos et al., 2017) and that amygdala–OFC connections serve as a primary inhibitory pathway for amygdala function (Ray and Zald, 2012). Last, research examining the cortisol response to a social stressor in this sample found a similar interaction where the effect violence exposure was exacerbated by high social deprivation (Peckins et al., 2019). Taken together with the increased specificity provided by the current study, childhood violence exposure, when combined with social deprivation, may act as a potent stressor that is associated with decreased white matter in adolescence between the amygdala and the OFC. Expanding on the current DMAP model, our results suggest that the effect of violence exposure (a specific subtype of threat) on fronto-amygdala white matter may depend on the concurrent degree of social deprivation or support.
Extant literature is consistent with the right hemisphere-specific effects of the present study. Amygdala–OFC structural connections are posited to play a role in automatic emotion regulation (Phillips et al., 2008) with right hemisphere connections being more heavily involved in fear extinction learning (Gottfried and Dolan, 2004). Further, in healthy adults, greater right hemisphere amygdala–OFC functional connectivity has been observed in response to unpredictable threat (Gold et al., 2015), supporting the potential inhibitory role of the structural connections observed here.
In addition to the exacerbating effects of social deprivation and violence exposure, the present findings indicate that low social deprivation (i.e., social support) may exert a “protective-stabilizing” (Proctor, 2006) effect against the negative behavioral sequelae of violence exposure (Foster and Brooks-Gunn, 2009; Ozer, 2005). Consistent with the idea of a protective-stabilizing factor, the present study found that social support was associated with a lessening of the negative association between violence exposure and amygdala–OFC connectivity that was observed in the context of social deprivation (i.e., low social support). The present findings suggest that policies aimed at boosting social support for youth in high violence environments may lessen the effect of violence exposure on a primary neural circuit for emotion regulation.
Similar to the interaction in the amygdala–OFC connectivity, we found a violence exposure-social deprivation interaction when predicting right hemisphere amygdala-dmPFC (BA10) connectivity. However, the association was not significant when adjusting for the demographic covariates. BA47, the OFC ROI used, is rostrally bordered by the dmPFC (Petrides and Pandya, 2002), and neighboring cortical regions are often connected (Bullmore and Sporns, 2012). Thus, amygdala-dmPFC tracts may pass through the OFC, explaining the weaker association with the dmPFC.
Importantly, in contrast to our hypotheses, there were no main effects of childhood violence exposure or social deprivation on adolescent amygdala–OFC, amygdala–d mPFC, or amygdala–subgenual cingulate connectivity. Thus, it may not be fruitful to consider dimensions of adversity in isolation and out of context of other salient ecological variables (McLaughlin and Sheridan, 2016). Rather, in order to construct a more complete picture of how early adversity influences the brain, it is important to measure and model the effects of multiple dimensions that have been established to impact development.
The present study had limitations worth noting. First, due to the population-based sampling methodology used in the FFCWS, youth were not preselected based on their ability or willingness to participate in an MRI study, a common procedure in many neuroimaging studies. Thus, 41 participants of the available sample were ineligible or refused to complete the dMRI scan. Although it is a limitation that our full sample could not participate, the group of excluded participants does not differ from the included participants on demographic factors. A second limitation is that due to demographics of the current sample, our findings may not generalize to more affluent, rural, or other race/ethnic populations. Third, due to changes in the FFCWS questionnaire at year 15, we were unable to control for current life stress using the composite scores we created for ages 3, 5, and 9 years (Hein, 2019). To compensate, we used a life stress scale to control for current stress and found that it did not impact our main findings, suggesting that the effects were unique to childhood, rather than adolescent, adversity. Additionally, the FFCWS study did not collect data between ages 9 and 15, so it was not possible to prospectively account for exposures during this important developmental period. Fifth, human neuroimaging methods precluded us from determining how white matter may influence the direction of signaling between the amygdala and OFC. Consistent with models from non-human primate neural tract tracer research (Ray and Zald, 2012; Zikopoulos et al., 2017), we posit that the OFC inhibits the amygdala; however, the influence may be bidirectional. Previous research identified white matter tracts outside of those preregistered in the present study connecting the amygdala and PFC that may be shaped by early adversity (Choi et al., 2012; Huang et al., 2012; Jackowski et al., 2008). Additionally, although the present work used Brodmann’s Areas for ROI selection, previous work has used different anatomical parcellations. Future research examining potential effects of violence exposure and its interaction with social deprivation on additional pathways and using more precise anatomical parcellations would help to better understand how early adversity shapes the brain. Last, the probability of amygdala – PFC white matter connectivity in the present study was not limited to direct connections. Therefore, the estimates of white matter connectivity likely include indirect paths which pass through other regions of the brain prior to reaching the target region.
Results from the present study clarify possible directions for future research. Although longitudinal environmental data was a strength of the present study, we only had imaging data at one timepoint. Future research with longitudinal MRI data (Casey et al., 2018) may be able to better examine potential directional relations between dimensional early adversity and the brain by charting trajectories of development. Additionally, future research could characterize possible effects of other dimensions of adversity. We conceptualized violence exposure and social deprivation as composites made up of multiple timepoints in development and sources of exposure to create a cumulative assessment of dimension exposure to violence and social deprivation during childhood. However, it is likely that the proximity of exposure to the child and its developmental timing influence the magnitude of its effect (Dunn et al., 2013). Future research could work to break down the composites for each dimension to determine the importance of source and timing of exposure. Last, the items included in the dimension encompassing social deprivation – social support do not include all potential sources of social support. Future research should work to account for additional sources of social support (i.e., school connectedness) which may influence white matter.
5. Conclusions
Exposures related to early adversity are complex and can be broken down into dimensions which may affect brain development in different ways. The present study shows, for the first time, that two dimensions of childhood adversity, violence exposure and social deprivation, interact to predict adolescent white matter connecting right hemisphere amygdala–OFC which is involved in socio-emotional function. Probability of white matter connectivity included both direct and indirect paths between the amygdala and OFC. High childhood violence exposure together with high social deprivation led to a lower probability of amygdala–OFC white matter in adolescence and, based on the negative correlation between amygdala–OFC white matter connectivity and amygdala reactivity, potentially less OFC regulation of the amygdala to threat. This association was not present with low social deprivation (i.e., social support), potentially implicating social support as a neuroprotective factor.
Funding
The research reported in this paper was supported by grants from the National Institutes of Health, R01MH103761 (Monk), T32HD007109 (McLoyd & Monk), and S10OD012240 (Noll), as well as a Doris Duke Fellowship for the Promotion of Child Well-Being (Hein).
Declaration of Competing Interest
The authors declare no competing financial interests.
Acknowledgments
We would like to acknowledge the past work of the Fragile Families and Child Wellbeing Study, the families for sharing their experiences with us, and the project staff for making the study possible.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100849.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Amso D., Lynn A. Distinctive mechanisms of adversity and socioeconomic inequality in child development: a review and recommendations for evidence-based policy. Policy Insights Behav. Brain Sci. 2017;4(2):139–146. doi: 10.1177/2372732217721933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Graham M.S., Drobnjak I., Zhang H., Filippini N., Bastiani M. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: within volume movement. Neuroimage. 2017;152:450–466. doi: 10.1016/j.neuroimage.2017.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A., Costello E.J., Pickles A., Winder F. Medical Research Council Child Psychiatry Unit; London: 1987. The Development of a Questionnaire for Use in Epidemiological Studies of Depression in Children and Adolescents. [Google Scholar]
- Behrens T.E.J., Berg H.J., Jbabdi S., Rushworth M.F.S., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J., Zhu T., Stamoulis C., Fox N.A., Zeanah C., Nelson C.A. A randomized clinical trial of foster care as an intervention for early institutionalization: long term improvements in white matter microstructure. JAMA Pediatr. 2015;169(3):211–219. doi: 10.1001/jamapediatrics.2014.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B., Khetarpal S., Brent D., Cully M., Balach L., Kaufman J., Neer S.M. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.-L., Valabregue R., Poline J.-B. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16(2):S497. [Google Scholar]
- Bullmore E., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;13(5):336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Jeong B., Polcari A., Rohan M.L., Teicher M.H. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. NeuroImage. 2012;59(2):1071–1079. doi: 10.1016/j.neuroimage.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craney T.A., Surles J.G. Model-dependent variance inflation factor cutoff values. Qual. Eng. 2002;14(3):391–403. doi: 10.1081/QEN-120001878. [DOI] [Google Scholar]
- Dennison M.J., Sheridan M.A., Busso D.S., Jenness J.L., Peverill M., Rosen M.L., McLaughlin K.A. Neurobehavioral markers of resilience to depression amongst adolescents exposed to child abuse. J. Abnorm. Psychol. 2016;125(8):1201–1212. doi: 10.1037/abn0000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn E.C., McLaughlin K.A., Slopen N., Rosand J., Smoller J.W. Developmental timing of child maltreatment and symptoms of depression and suicidal ideation in young adulthood: results from the national longitudinal study on adolescent health. Depress. Anxiety. 2013;30(10) doi: 10.1002/da.22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E.B., Hyde L.W., Mitchell C., Faul J., Gonzalez R., Heitzeg M.M., Keating D.P., Langa K.M., Martz M.E., Maslowsky J., Morrison F.J., Noll D.C., Patrick M.E., Pfeffer F.T., Reuter-Lorenz P.A., Thomason M.E., Davis-Kean P., Monk C.S., Schulenberg J. What is a representative brain? Neuroscience meets population science. Proc. Natl. Acad. Sci. U. S. A. 2013;110(44):17615–17622. doi: 10.1073/pnas.1310134110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell A.D., Ampy L.A., Meyer A.L. Identification and assessment of problematic interpersonal situations for urban adolescents. J. Clin. Child Psychol. 1998;27(3):293–305. doi: 10.1207/s15374424jccp2703_6. [DOI] [PubMed] [Google Scholar]
- Foster H., Brooks-Gunn J. Toward a stress process model of children’s exposure to physical family and community violence. Clin. Child Fam. Psychol. Rev. 2009;12(2):71–94. doi: 10.1007/s10567-009-0049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover G.H., Law C.S. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn. Reson. Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Goetschius L.G., Hein T.C., Mattson W.I., Lopez-Duran N., Dotterer H.L., Welsh R.C. Amygdala-prefrontal cortex white matter tracts are widespread, variable and implicated in amygdala modulation in adolescents. NeuroImage. 2019;191:278–291. doi: 10.1016/j.neuroimage.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold A.L., Morey R.A., McCarthy G. Amygdala–prefrontal cortex functional connectivity during threat-induced anxiety and goal distraction. Biol. Psychiatry. 2015;77(4):394–403. doi: 10.1016/j.biopsych.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried J.A., Dolan R.J. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat. Neurosci. 2004;7(10):1144. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Govindan R.M., Behen M.E., Helder E., Makki M.I., Chugani H.T. Altered water diffusivity in cortical association tracts in children with early deprivation identified with tract-based spatial statistics (TBSS) Cereb. Cortex. 2010;20(3):561–569. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening S.G., Mitchell D.G.V. A network of amygdala connections predict individual differences in trait anxiety. Hum. Brain Mapp. 2015;36(12):4819–4830. doi: 10.1002/hbm.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.E., Moore T.M., Rosen A.F.G., Barzilay R., Roalf D.R., Calkins M.E., Ruparel K., Scott J.C., Almasy L., Satterthwaite T.D., Shinohara R.T., Gur R.C. Burden of environmental adversity associated with psychopathology, maturation, and brain behavior parameters in youths. JAMA Psychiatry. 2019;76(9):966–975. doi: 10.1001/jamapsychiatry.2019.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Knodt A.R., Brigidi B.D., Hariri A.R. Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev. Psychopathol. 2015;27(4 Pt 2):1611–1619. doi: 10.1017/S0954579415000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein T.C. University of Michigan; 2019. Dimensions of Early Adversity As Distinct Predictors of Adolescent Brain Development.https://deepblue.lib.umich.edu/bitstream/handle/2027.42/149945/heint_1.pdf?sequence=1&isAllowed=y Doctoral Dissertation. [Google Scholar]
- Hein T.C., Monk C.S. Research Review: neural response to threat in children, adolescents, and adults after child maltreatment - a quantitative meta-analysis. J. Child Psychol. Psychiatry. 2016 doi: 10.1111/jcpp.12651. [DOI] [PubMed] [Google Scholar]
- Hein T.C., Mattson W.I., Dotterer H.L., Mitchell C., Lopez-Duran N., Thomason M.E., Peltier S.J., Welsh R.C., Hyde L.W., Monk C.S. Amygdala habituation and uncinate fasciculus connectivity in adolescence: a multi-modal approach. NeuroImage. 2018;183:617–626. doi: 10.1016/j.neuroimage.2018.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsel W.J., Matson J.L. The assessment of depression in children: The internal structure of the child depression inventory (CDI) Behav. Res. Ther. 1984;22(3):289–298. doi: 10.1016/0005-7967(84)90009-3. [DOI] [PubMed] [Google Scholar]
- Hernández M., Guerrero G.D., Cecilia J.M., García J.M., Inuggi A., Jbabdi S., Behrens T.E.J., Sotiropoulos S.N. Accelerating fibre orientation estimation from diffusion weighted magnetic resonance imaging using GPUs. PLoS One. 2013;8(4):e61892. doi: 10.1371/journal.pone.0061892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Fernandez M., Reguly I., Giles M., Jbabdi S., Smith S.M., Sotiropoulos S.N. A fast and flexible toolbox for tracking brain connections in diffusion MRI datasets using GPUs. 22nd Annual Meeting of the Organization for Human Brain Mapping (OHBM); Geneva, Switzerland; 2016. (2016, June) [Google Scholar]
- Herringa R.J., Birn R.M., Ruttle P.L., Burghy C.A., Stodola D.E., Davidson R.J., Essex M.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. U. S. A. 2013;110(47):19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Gundapuneedi T., Rao U. White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology. 2012;37(12):2693–2701. doi: 10.1038/npp.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski A.P., Douglas-Palumberi H., Jackowski M., Win L., Schultz R.T., Staib L.W., Krystal J.H., Kaufman J. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res. 2008;162(3):256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Clegg R., Goer F., Pechtel P., Beltzer M., Vitaliano G., Olson D.P., Teicher M.H., Pizzagalli D.A. Childhood stress, grown-up brain networks: corticolimbic correlates of threat-related early life stress and adult stress response. Psychol. Med. 2018;48(7):1157–1166. doi: 10.1017/S0033291717002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kim D.-J., Davis E.P., Sandman C.A., Glynn L., Sporns O., O’Donnell B.F., Hetrick W.P. Childhood poverty and the organization of structural brain connectome. NeuroImage. 2019;184:409–416. doi: 10.1016/j.neuroimage.2018.09.041. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Masten A.S., Neemann J., Andenas S. Life events and adjustment in adolescents: the significance of event independence, desirability, and chronicity. J. Res. Adolesc. 1994;4(1):71–97. doi: 10.1207/s15327795jra0401_5. [DOI] [Google Scholar]
- McCrory E.J., Brito S.A.D., Sebastian C.L., Mechelli A., Bird G., Kelly P.A., Viding E. Heightened neural reactivity to threat in child victims of family violence. Curr. Biol. 2011;21(23):R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A. Beyond cumulative risk: a dimensional approach to childhood adversity. Curr. Dir. Psychol. Sci. 2016;25(4):239–245. doi: 10.1177/0963721416655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Lambert H.K. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Weissman D., Bitrán D. Childhood adversity and neural development: a systematic review. Annu. Rev. Develop. Psychol. 2019;1(1):277–312. doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson I.R., Heide R.J.V.D., Alm K.H., Vyas G. Development of the uncinate fasciculus: implications for theory and developmental disorders. Dev. Cogn. Neurosci. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer E.J. The impact of violence on urban adolescents: longitudinal effects of perceived school connection and family support. J. Adolesc. Res. 2005;20(2):167–192. doi: 10.1177/0743558404273072. [DOI] [Google Scholar]
- Park S., Lee J.-M., Kim J.-W., Kwon H., Cho S.-C., Han D.H., Cheong J.H., Kim B.-N. Increased white matter connectivity in traumatized children with attention deficit hyperactivity disorder. Psychiatry Res. Neuroimaging. 2016;247:57–63. doi: 10.1016/j.pscychresns.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Peckins M.K., Roberts A.G., Hein T.C., Hyde L.W., Mitchell C., Brooks-Gunn J., McLanahan S.S., Monk C.S., Lopez-Duran N.L. Violence exposure and social deprivation is associated with cortisol reactivity in urban adolescents. Psychoneuroendocrinology. 2019:104426. doi: 10.1016/j.psyneuen.2019.104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 2002;16(2):291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips M., Ladouceur C., Drevets W. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry. 2008;13(9):829–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K.J., Curran P.J., Bauer D.J. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J. Educ. Behav. Stat. 2006;31(4):437–448. doi: 10.3102/10769986031004437. [DOI] [Google Scholar]
- Preacher K.J., Rucker D.D., Hayes A.F. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behav. Res. 2007;42(1):185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Proctor L.J. Children growing up in a violent community: the role of the family. Aggress. Violent Behav. 2006;11(6):558–576. doi: 10.1016/j.avb.2005.12.004. [DOI] [Google Scholar]
- Puetz V.B., Parker D., Kohn N., Dahmen B., Verma R., Konrad K. Altered brain network integrity after childhood maltreatment: A structural connectomic DTI-study. Hum. Brain Mapp. 2017;38(2):855–868. doi: 10.1002/hbm.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R., Zald D.H. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 2012;36(1):479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman N.E., Teitler J.O., Garfinkel I., McLanahan S.S. Fragile families: sample and design. Child. Youth Serv. Rev. 2001;23(4):303–326. doi: 10.1016/S0190-7409(01)00141-4. [DOI] [Google Scholar]
- Sacks V., Murphy D. 2018. The Prevalence of Adverse Childhood Experiences, Nationally, by State, and by Race or Ethnicity.https://www.childtrends.org/publications/prevalence-adverse-childhood-experiences-nationally-state-race-ethnicity [Google Scholar]
- Shaw D.S., Gilliom M., Ingoldsby E.M., Nagin D.S. Trajectories leading to school-age conduct problems. Dev. Psychol. 2003;39(2):189–200. doi: 10.1037/0012-1649.39.2.189. [DOI] [PubMed] [Google Scholar]
- Sheather S. Springer Science & Business Media; 2009. A Modern Approach to Regression With R. [Google Scholar]
- Sheridan M.A., McLaughlin K.A., Winter W., Fox N., Zeanah C., Nelson C.A. Early deprivation disruption of associative learning is a developmental pathway to depression and social problems. Nat. Commun. 2018;9(1):1–8. doi: 10.1038/s41467-018-04381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.-K., Lin F.-C., Ward S.E., Fine J.P. Composite variables: when and how. Nurs. Res. 2013;62(1):45–49. doi: 10.1097/NNR.0b013e3182741948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga‐Barke E.J., Schlotz W., Kreppner J. V. Differentiating developmental trajectories for conduct, emotion, and peer problems following early deprivation. Monogr. Soc. Res. Child Dev. 2010;75(1):102–124. doi: 10.1111/j.1540-5834.2010.00552.x. [DOI] [PubMed] [Google Scholar]
- Veraart J., Novikov D.S., Christiaens D., Ades-aron B., Sijbers J., Fieremans E. Denoising of diffusion MRI using random matrix theory. NeuroImage. 2016;142:394–406. doi: 10.1016/j.neuroimage.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S., Suntheimer N.M. A dimensional risk approach to assessing early adversity in a national sample. J. Appl. Dev. Psychol. 2019;62:270–281. doi: 10.1016/j.appdev.2019.03.004. [DOI] [Google Scholar]
- Zikopoulos B., Höistad M., John Y., Barbas H. Posterior orbitofrontal and anterior cingulate pathways to the amygdala target inhibitory and excitatory systems with opposite functions. J. Neurosci. 2017;37(20):5051–5064. doi: 10.1523/JNEUROSCI.3940-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.