Abstract
A disease emerged in the city of Wuhan, Hubei Province, Central China in the last month of 2019. It was pneumonia caused by a newly emerged coronavirus called COVID-19, later. Coronaviruses are enveloped RNA viruses belong to the Betacoronavirus family and infected birds, humans, and other mammals. In March 2020, the World Health Organization declared the COVID-19 outbreak could be characterized as a global pandemic because the disease spread, and a large number of people were infected and died in many countries on different continents by virtue of this new virus. Now, intensive work is underway about the pathogenic mechanisms and epidemiological properties of COVID-19, and a great effort is made to develop effective specific therapeutic drugs, vaccines, and/or treatment strategies against these diseases. Herein, we have focused on all treatment options available against COVID-19 pneumonia in this text.
Keywords: COVID-19, Pandemic, Drug, Immunotherapy, Cellular therapy, Antiviral
Introduction
Coronaviruses (CoVs) belong to the subfamily Orthocoronavirinae in the family of Coronaviridae. In this family, there are four types of viruses: α-coronavirus, β-coronavirus, γ-coronavirus, δ-coronavirus (Banerjee et al. 2019; Yang and Leibowitz 2015). The CoV genome is an enveloped, positive-sense, and single-stranded RNA whose size is 26–32 kb and it has the largest genome of known RNA viruses. It is known that α- and β-CoV types cause infections in mammals as δ- and γ-CoVs infect birds (Banerjee et al. 2019; Yang and Leibowitz 2015; Schoeman and Fielding 2019). Severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) belonging to β-CoVs are the most well-known aggressive strains of coronaviruses and cause viral pneumonia outbreaks, recently. SARS first appeared in China in 2002, spread and resulted in 8437 cases and 813 deaths with an 11% mortality rate according to the World Health Organization (WHO) reports (Song et al. 2019; Graham et al. 2013; Zhong et al. 2003). MERS, which emerged in Saudi Arabia in 2012 firstly, caused 2206 patients in 27 countries, 1831 cases in Saudi Arabia with 787 deaths with the mortality rate of 37% between June 2012 and April 2018 (Zumla et al. 2015; Hui et al. 2018; Su et al. 2015; Nassar et al. 2018).
In December 2019, some people had sickness with unknown etiology in Wuhan City where 11 million residents live, Hubei Province in China. This sickness was a kind of pneumonia and the sequence analysis revealed that the cause of it was a novel CoV whose genome structure was more than 82% identical to those of SARS-CoV, following this phenomenon was named as coronavirus disease 2019 (COVID-19) (Xu et al. 2020; Wong et al. 2020; Li et al. 2020; Zhang et al. 2020; Chan et al. 2020). Unfortunately, case numbers are increasing continuously day by day, the COVID-19 disease has spread primarily to China, the Islamic Republic of Iran, and Europe. It was determined that the COVID-19 disease had human-to-human transmission ability by the National Health Commission of China on 20 January 2020. COVID-19 outbreak a public health emergency of international concern (PHEIC) on 30 January 2020.
Beginning in March 2020, COVID-19 has spread in 72 countries quickly, caused over 90,000 confirmed cases and 2946 deaths (Li et al. 2020). As this outbreak turned into a global threat, the WHO announced that the COVID-19 outbreak could be characterized as a global pandemic, on 12 March 2020 (The World Health Organization Regional Office for Europe. Coronavirus disease (COVID-19) outbreak news). 22,256,220 cases and 782,456 deaths caused by the COVID-19 pandemic according to the WHO records on 21 August 2020 (The World Health Organization Home. Coronavirus disease 2019 current case numbers).
Currently, the man has no registered specific vaccines or therapy methods for COVID-19 accepted by the whole world. WHO stated that there were nine vaccine candidates against COVID-19 in the advanced stages of clinical trials (timesnownews.com). Russia government announced the world’s first COVID-19 vaccine called as Sputnik V (Table 1) was developed by the Gamaleya Research Institute of Epidemiology and Microbiology on 12 August (sciencemag.org/news), but WHO declared that there is not enough information about Sputnik V and they are in contact with Russia (timesnownews.com). That’s why, there is an urgent need to find drugs or vaccines which can be used in the treatment of COVID-19 infections, effectively. Nevertheless, there are some reports and data from studies related to the use of known drugs like chloroquine and remdesivir that have proved efficacy on COVID-19 pneumonia both in vitro and in animal models. In this context, we summarize therapeutic options available for the treatment of COVID-19.
Table 1.
Ongoing clinical trials of vaccine candidates for COVID-19 (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=vaccine&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=; https://www.biocentury.com/clinical-vaccines-and-therapies)
| Vaccine candidate | Modality | Sponsor | Study identifier/status | Study location |
|---|---|---|---|---|
| BCG (Bacillus Calmette-Guérin) | Live attenuated vaccines | UMC Utrecht | NCT04328441/Phase 3 | Netherlands |
| Ain Shams University | NCT04350931/Phase 3 | Egypt | ||
| Universidad de Antioquia | NCT04362124/Phase 3 | Colombia | ||
| Tuberculosis Research Centre, India | NCT04475302/Phase 3 | India | ||
| Radboud University | NCT04417335/Phase 4 | Netherlands | ||
| TASK Applied Science | NCT04379336/Phase 3 | South Africa | ||
| Hospital Universitario Dr. Jose E. Gonzalez | NCT04461379/Phase 3 | Mexico | ||
| Hellenic Institute for the Study of Sepsis | NCT04414267/Phase 4 | Greece | ||
| Murdoch Childrens Research Institute | NCT04327206/Phase 3 | Australia | ||
| University of Campinas, Brazil | NCT04369794/Phase 4 | Brazil | ||
| Bandim Health Project | NCT04373291/Phase 3 | Denmark | ||
| Texas A&M University | NCT04348370/Phase 4 | United States | ||
| Assiut University | NCT04347876/Unspecified | Egypt | ||
| Direction des Soins de Santé de Base | NCT04384614/Unspecified | Tunisia | ||
| Assistance Publique - Hôpitaux de Paris | NCT04384549/Phase 3 | France | ||
| MMR vaccine | Live attenuated bacteria | Louisiana State University Health Sciences Center in New Orleans | NCT04475081/Phase 3 | United States |
| Oral polio vaccine | Live attenuated bacteria | Bandim Health Project | NCT04445428/Phase 4 | Guinea-Bissau |
| VPM1002 | Live attenuated bacteria | University Health Network, Toronto | NCT04439045/Phase 3 | Canada |
| Vakzine Projekt Management GmbH | NCT04387409/Phase 3 | Germany | ||
| Vakzine Projekt Management GmbH | NCT04435379/Phase 3 | Germany | ||
| RUTI | Inactivated bacteria | Fundació Institut Germans Trias i Pujol | NCT04453488/Phase 3 | Germany |
| CoronaVac (PiCoVacc) | Inactivated virus | Sinovac Research and Development Co., Ltd. | NCT04383574/Phase 1 Phase 2 | China |
| mRNA-1273 | RNA vaccine | National Institute of Allergy and Infectious Diseases (NIAID) | NCT04283461/Phase 1 | United States |
|
ModernaTX, Inc. ModernaTX, Inc. |
NCT04405076/Phase 2 NCT04470427/Phase 3 |
United States United States |
||
| AZD1222 (ChAdOx1 nCoV-19) | Viral vector | AstraZeneca | NCT04516746/Phase 3 | United States |
| BNT162 | RNA vaccine | BioNTech SE | NCT04368728/Phase 2 Phase 3 | United States |
| BioNTech RNA Pharmaceuticals GmbH | NCT04380701/Phase 1 Phase 2 | Germany | ||
| Ad5-nCOV | Viral vector | CanSino Biologics Inc. | NCT04398147/Phase 1 Phase 2 | Canada |
| AG0301-COVID-19 | DNA vaccine | AnGes, Inc. | NCT04463472/Phase 1 Phase 2 | Japan |
| Ad26.COV2.S | Viral vector | Janssen Vaccines & Prevention B.V. | NCT04505722/Phase 3 | Japan |
| Janssen Pharmaceutical K.K. | NCT04509947/Phase 1 | United States | ||
| AV-COVID-19 | Cellular vaccine | Aivita Biomedical, Inc. | NCT04386252/Phase 1 Phase 2 | United States |
| Covaxin (BBV152) | Inactivated virus | Bharat Biotech International Limited | NCT04471519/Phase 1 Phase 2 | India |
| Sputnik V (Gam-COVID-Vac) | Viral vector | Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation | NCT04436471/Phase 1 Phase 2 | Russian Federation |
| Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation | NCT04437875/Phase 1 Phase 2 | Russian Federation | ||
| GX-19 | DNA vaccine | Genexine, Inc. | NCT04445389/Phase 1 Phase 2 | South Korea |
| INO-4800 | DNA vaccine | International Vaccine Institute | NCT04447781/Phase 1 Phase 2 | South Korea |
| Inovio Pharmaceuticals | NCT04336410/Phase 1 | United States | ||
| KBP-COVID-19 | Protein-based | Kentucky BioProcessing, Inc. | NCT04473690/Phase 1 Phase 2 | |
| LUNAR-COV19 (ARCT-021) | RNA vaccine | Arcturus Therapeutics, Inc. | NCT04480957/Phase 1 Phase 2 | Singapore |
| V-SARS | Inactivated virus | Immunitor LLC | NCT04380532/Phase 1 Phase 2 | Canada Mongolia |
| Bac-TRL-Spike | DNA vaccine | Symvivo Corporation | NCT04334980/Phase 1 | United States Canada |
| COVAX-19 | Protein-based | GeneCure Biotechnologies | NCT04428073/Phase 1 | |
| CVnCOV | RNA vaccine |
CureVac AG CureVac AG |
NCT04515147/Phase 2 NCT04449276/Phase 1 |
Belgium Germany |
| MVC-COV1901 | Protein-based | Medigen Vaccine Biologics Corp. | NCT04487210/Phase 1 | Taiwan |
| MF59 adjuvanted SARS-CoV-2 Sclamp vaccine | Protein-based | The University of Queensland | NCT04495933/Phase 1 | Australia |
| SCB-2019 (COVID-19 S-Trimer) | Protein-based | Clover Biopharmaceuticals AUS Pty Ltd | NCT04405908/Phase 1 | Australia |
| TMV-083 | Engineered live attenuated virus | Institut Pasteur | NCT04497298/Phase 1 | Belgium France |
Therapy
The pharmaceutical interventions available for COVID-19 therapy can be divided into several groups: immunotherapy, cellular therapy, antiviral and other drugs, and Chinese medicine.
Immunotherapy
Immunoglobulins
Immunoglobulins are used to treat for various diseases, for example, Guillain–Barre Syndrome, Kawasaki disease as well as some pathologic conditions such as idiopathic thrombocytopenia purpura and chronic inflammatory demyelinating polyneuropathy (Cherin et al. 2016). Various glycoproteins on virus surfaces or the protein shell of a non-enveloped virus could be recognized by extensively neutralizing antibodies, but HIV-1 (human immunodeficiency virus), dengue virus, hepatitis C, Ebola virus, and influenza viruses can mutate superficial glycoproteins, thus they avoid the antibody response. This condition creates an obstacle to develop new therapies for such infections (Srinivasan et al. 2016). Although intravenous gammaglobulin has adverse reactions, it is probably the safest immunomodulating drug. Intravenous gammaglobulin has been used extensively for patients with SARS in Singapore, but venous thromboembolism including pulmonary embolism has emerged in one-third of critically ill patients (Lew et al. 2003). Human immunoglobulin is testing in pneumonia patients with COVID-19 and there are 478 clinical trials concerning immunoglobulins, of them 18 trials were finished (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=immunoglobulin&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Interferons
Interferons (IFNs) are substances having protein structure that bind to receptors on cellular surfaces and initiate JAK-STAT signaling cascades (Schneider et al. 2014). IFNs are considered as two types: Type I and Type II IFNs. Type I IFNs, which make up of two different proteins: IFN-α and IFN-β are inclusive of natural immunity against viral infections. Type I IFN release forms are the first line of defense against infections caused by viruses. In the 7th published National Health Council (NHC) guideline, IFN-α single or in combination with other drugs are indicated in the clinical usages (Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition)). IFN-α included in Type I IFNs is grown very rapidly as part of the congenital immune response to infections caused by viruses and inhibits the transcription of animal and human coronaviruses (Mathias et al. 2010; ClinicalTrials.gov [Internet] 2020a). In addition, they support innate and adaptive immune responses against viral infections. IFN-α effectively inhibited SARS-CoV replication in vitro experiments (Ströher et al. 2004; Zorzitto et al. 2006). It was shown that Type I interferons with the inclusion of IFN-β could inhibit the replication of SARS-CoV in vitro, too (Morgenstern et al. 2005). It was revealed that SARS-CoV blocked IFN transcription in infected cells and these cells might partly repair their congenital immune responsiveness to SARS-CoV following small amounts of IFNs had been prepared (Kuri et al. 2009). It has been shown that cynomolgus monkeys are preserved from SARS-CoV infection via IFN-α therapy, too (Haagmans et al. 2004). In a pilot clinical trial, synthetic recombinant IFN-α was proved to be active against SARS (Loutfy et al. 2003). Additionally, IFN-α-2a and ribavirin combination prolonged the survival of people with severe MERS-CoV infection (Mustafa et al. 2018). The different controlled test has been launched to test the efficiency of lopinavir/ritonavir and IFN-α-2b in hospitalized people with SARS-CoV-2 infections (ChiCTR2000029308) (Martinez 2020). Several combinations of IFN-α or IFN-β with other antivirals for instance lopinavir/ritonavir and/or ribavirin have been studied for the treatment of patients with SARS. Nitazoxanide, a Type I IFN inducer used for parasitic infection in humans, has been tried as an antiviral agent due to the ability to inhibit the replication of several RNA and DNA viruses (Pillaiyar et al. 2020). As of now, there are 87 clinical trials are underway about IFNs against COVID-19, of them 6 clinical trials were completed (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=interferon&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=). Recently, new clinical data for IFN-1-β has been obtained and it has been concluded that there is insufficient data to advise either for or against the use of IFN-1-β for the therapy of early (<7 days from symptom onset) mild and moderate COVID-19 pneumonia (NIH COVID-19 Treatment Guidelines, https://www.covid19treatmentguidelines.nih.gov/whats-new/).
Convalescent plasma therapy
Antiviral antibodies, namely, IgG, IgA, IgM, IgE, and IgD available in convalescent plasma can be obtained from recovered patients and they can be used to treat patients viral infections, effectively. So far, this method has been widely utilized in some infectious diseases, for instance, poliomyelitis, influenza A (H5N1), and Ebola (Zhou et al. 2007; van Griensven et al. 2016; Rinaldo 2005). In addition to this, passive immunization might be useful by making use of convalescent plasma from people with SARS-CoV infection. In Taiwan and Hong Kong convalescent plasma therapy has been tested on a small number of SARS-CoV-infected patients during the early course of the outbreak and this method has been determined to be useful (Cheng et al. 2005; Wong and Yuen 2008; Yeh et al. 2005). In view of these findings, convalescent plasma therapy can be a promising option for the therapy and prevention of COVID-19 pneumonia cases.
Convalescent plasma may be called as passive immunotherapy, too. If no specific vaccines or medications are available for arising infection-related diseases, this therapy method is generally applied (Marano et al. 2016). The feasibility, safety, and clinical efficacy of this therapy method has been tested in critically ill MERS patients and it has been revealed to have an immunotherapeutic potential (Arabi et al. 2015). Similarly, convalescent plasma gained from recovered SARS patients has been useful to treat other SARS patients (Cheng et al. 2005; Soo et al. 2004). One important thing to say is this, World Health Organization under Blood Regulators Network recommends the use of convalescent plasma or serum if there is no vaccination and antiviral medications for an arising virus. Convalescent plasma therapy should be normally used against COVID-19 on critically ill patients if it is available (Zhang and Liu 2020). Now, there are 140 clinical trials to appreciate the efficacy and safety of convalescent plasma for the therapy of COVID-19 and, of them nine trials were completed stage (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=convalescent+plasma&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry==&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Monoclonal antibody
It was determined that one recombinant human monoclonal antibody (mAb) (single-chain variable region fragments, scFvs 80R) inhibited S1 domain of S protein of SARS-CoV (Sui et al. 2004). Depending on the development of monoclonal antibodies, the SARS-CoV-2 outbreak can be prevented. Several studies revealed that some monoclonal antibodies which aim to the SARS-CoV spike protein could prevent the virus entry into the host cells (ter Meulen et al. 2004; ter Meulen et al. 2006; Traggiai et al. 2004). Neutralizing monoclonal antibodies target the 193-amino acid (residues 318–510) receptor-binding domain (RBD) of the spike protein to perform their functions (Wong et al. 2004). Considering these works, neutralizing monoclonal antibodies alone or in combination could be a promising therapeutic candidate for the therapy of COVID-19 pneumonia.
Patients with severe COVID-19 infection experienced a cytokine storm with increased plasma concentrations of interleukins IL-6, IL-2, IL-7, and IL-10 and tumor necrosis factor TNF-α. This syndrome is described by fever and multiorgan failure. IL-6, interferon-gamma (IFN-γ), TNF-α, and IL-10 are cytokines including in cytokine release pathogenesis (Wang and Han 2018). Especially, IL-6 acts as the central mediator of cytokine release syndrome (Lee et al. 2014).
For the therapy of COVID-19 pneumonia, immunomodulant therapy is not recommended routinely. Nevertheless, considering the clinical and laboratory findings consistent with cytokine release syndrome and considering the formation of pulmonary edema and hyaline membranes, a targeted therapy could be useful in selected patients with severe pneumonia in association with respiratory support during a short time (Lombardy Section Italian Society Infectious and Tropical Diseases 2020).
Currently, there are 47 clinical trials are being conducted for monoclonal antibodies in the therapy of COVID-19 and, no study was been completed (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=monoclonal+antibody&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Tocilizumab
Tocilizumab (Fig. 1) is a monoclonal antibody that aims to the IL-6 receptor. This agent can be used against COVID-19 in patients with severe and extensive lung disease with elevated IL-6 levels. Thus, the systemic inflammatory response syndrome by the viral-induced cytokine storm can be discontinued (Lombardy Section Italian Society Infectious and Tropical Diseases 2020). As on date, 65 clinical trials are being carried out to know the efficacy of tocilizumab in COVID-19 pneumonia, of them 4 trials were finished (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=tocilizumab&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
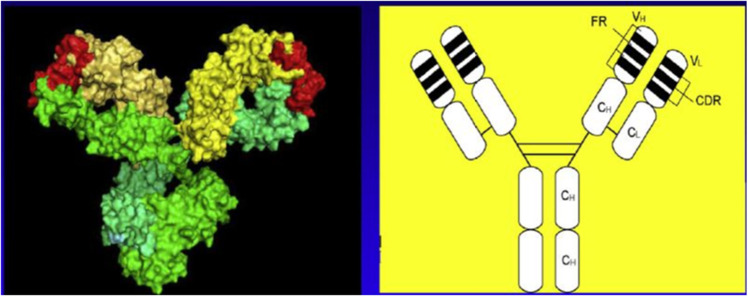
Fig. 1.
Molecular structure of tocilizumab. Right panel, schematic representation of the two-dimensional structure. Left panel, three-dimensional structural molecular model. (Produced by Drs. Ohta and Kobayashi, Chugai Pharmaceutical Co., Ltd.) (Ohsugi 2020)
Bevacizumab
Bevacizumab (Fig. 2), a humanized monoclonal antibody, aims vascular endothelial growth factor (VEGF) (ClinicalTrials.gov [Internet] 2020b; Wang et al. 2004). It has the ability to decrease the VEGF levels produced by hypoxia, severe inflammation, and upregulation of the infected respiratory tract epithelium; these symptoms might restrain the edema in patients suffered from COVID-19 pneumonia (Wang et al. 2004). There are only three clinical trials are underway about bevacizumab, at present (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=bevacizumab&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Fig. 2.
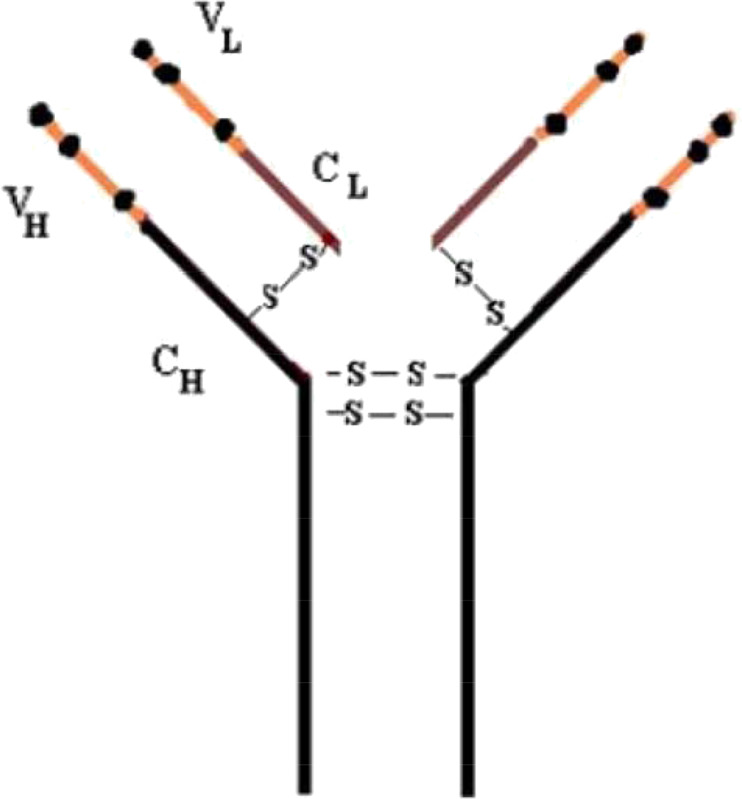
Structure of humanized bevacizumab antibody
Cyclosporine A
Cyclosporine A (Fig. 3) which has been used in transplantation widely as well as in the therapy of autoimmune disorders is an immunosuppressive drug. Because cyclosporine A has been used extensively, the survival rates of patients and grafts improved highly following solid-organ transplantation (Ziaei et al. 2016). It was claimed that nucleocapsid protein (NP) of SARS-CoV had a substantial role in the process of virus particle accumulation and release. In addition, it could connect to human cyclophilin A which is a key member of immunophilins. The function of these are to act as a cellular receptor for cyclosporine A (Luo et al. 2004; Dawar et al. 2017). Cyclophilin A facilitates or inhibits the replication of immunophilins in viral infections (Dawar et al. 2017). The inhibition of cyclophilins by using cyclosporine A might arrest the replication of coronaviruses such as SARS-CoV (Pfefferle et al. 2011), so the non-immunosuppressive cyclosporine A derivatives might have the potential as inhibitors against coronaviruses such as emerging newly COVID-19. Now, only 6 clinical trials are being carried out to reveal the efficacy of cyclosporine A against COVID-19 (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=cyclosporine+A&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e==&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Fig. 3.
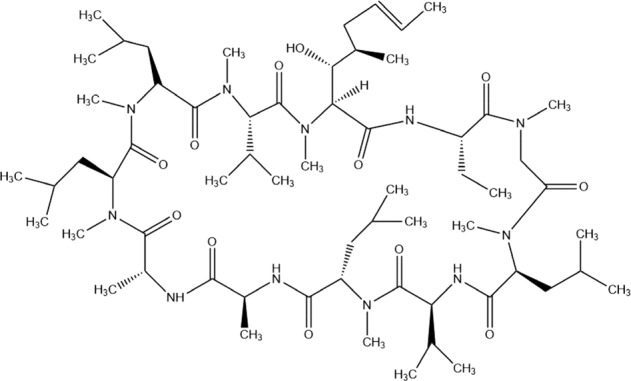
Chemical formula of cyclosporine A
Cellular therapy
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are strong anti-inflammatory and immunomodulatory. Besides, it has been determined that MSCs could improve acute/chronic lung injury and acute respiratory distress syndrome (ARDS), because they suppress the leakage of immune cells to pulmonary tissues and pro-inflammatory cytokine secretion (Ortiz et al. 2007; Gupta et al. 2007; Moodley et al. 2009; Matthay et al. 2010). Furthermore, MSCs contribute to decreasing lung fibrosis as well as increasing tissue repair (Kumamoto et al. 2009; El Agha et al. 2017). The patients with severe COVID-19 infections suffer from cytokine storm syndromes, ARDS, and acute lung injury so MSCs may be an encouraging therapeutic option to prevent these complications. There are 55 clinical trials ongoing about MSCs, of them only 2 trials were finished, now (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=mesenchymal+stem+cell&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=). However, the use of MSCs for the treatment of COVID-19 is not advised except in a clinical trial (NIH COVID-19 Treatment Guidelines, https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/blood-derived-products/mesenchymal-stem-cells/).
Natural killer cells
Natural killer (NK) cells are large granular lymphocytes and originated from bone marrow. Because NK cells have the ability to distinguish between normal cells and infected cells, it regulates a large number of immunological courses, for example, viral defense and immunological homeostasis. NK cells break down virus-infected cells by antibody-dependent cellular cytotoxicity (ADCC) process (Hammer et al. 2018). Therefore, NK cells show specificity to nearly all virus-infected cells. Some researches have indicated that NK cells can show antiviral efficiency against SARS-CoV, herpes simplex virus type 1 (HSV-1), cytomegalovirus, and HIV viruses through ADCC mediators (Dai and Caligiuri 2018; Arase et al. 2002; National Research Project for SARS, Beijing Group 2004).
Sorrento and Celularity declared that clinical cooperation has been started to be used CYNK-001, an allogeneic, off-the-shelf, obtained from navel cord blood NK cell therapy, as a new therapy method for the therapy and prevention of COVID-19 (Sorrento and Celularity to initiate emergency allogeneic NK cell therapy development for coronavirus infection Sorrento and Celularity to initiate emergency allogeneic NK cell therapy development for coronavirus infection). In order to rise immunity by using NK cell therapy until new treatment methods are found, NK cell therapy is of great importance against COVID-19 pneumonia so 28 clinical trials are underway about NK cell, at present (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=natural+killer+cell&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Antiviral drugs
Remdesivir
Remdesivir (Fig. 4), a nucleotide analog GS-5734, has capable of being incorporated into the new viral RNA leading to early termination. This mechanism constitutes the basis of the possible effectiveness of it against respiratory coronaviruses (Lombardy Section Italian Society Infectious and Tropical Diseases 2020). Remdesivir was determined to inhibit SARS-CoV and MERS-CoV in vivo (de Wit et al. 2020; Agostini et al. 2018). Lately, remdesivir has been shown to block SARS-CoV infection selectively at low-micromolar concentrations in vitro (Wang et al. 2020). Furthermore, when the patient with COVID-19 infection got worse, this patient was treated with remdesivir, intravenously in the USA (Holshue et al. 2020). Remdesivir has some benefits to treat COVID-19 infections, but randomized controlled trials are necessary to prove its activity and safety.
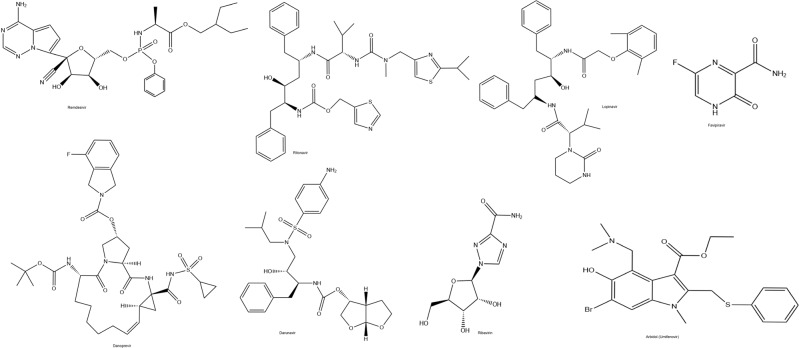
Fig. 4.
Chemical Structures of antiviral drugs
The suggested mechanism of remdesivir is the host cells’ post-entry stage (Wang et al. 2020). It was found that combined use of remdesivir and IFN-β created the higher an antiviral activity compared to the lopinavir/ritonavir-IFN-β combination against MERS-CoV virus in vitro and in vivo. Additionally, remdesivir could be better pulmonary function, cause to fall lung viral loads and severe lung pathology in mice, on the contrary, lopinavir/ritonavir-IFN-β could not (Sheahan et al. 2020).
Two clinical studies regarding the use of remdesivir have been carried out against severe or mild respiratory infections caused by COVID-19 (ClinicalTrials.gov [Internet] 2020c; NCT04252664 2020). Dyer et al. (2019) has shown that initial findings of a death rate were 33% in 499 patients under treatment by using remdesivir against the Ebola disease. Moreover, the mortality rate of non-treated infected patients was 75% (almost 1900 people) during the same epidemic period.
Recently, it was proclaimed that the United States Food and Drug Administration (FDA) approved remdesivir for emergency use to treat COVID-19 pneumonia (The FDA has approved emergency use of remdesivir to treat COVID-19).
Meanwhile, a molecular docking study including five antiviral drugs, ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir was conducted against SARS-CoV-2 RNA-dependent RNA polymerase (RdRp), these drugs showed promising results against COVID-19. Further optimization of these agents using a high-quality model of the SARS-CoV-2 RdRp might be useful for designing perfect compounds able to prevent this spreading infection (Elfiky 2020).
Now, 44 clinical trials are being carried out to reveal the efficacy of this drug against COVID-19, of them 5 trials were completed (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=remdesivir&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=). Considering the patient’s supplemental oxygen necessities and the manner of oxygen delivery, the use of remdesivir has been changed in the therapy of COVID-19 in July (NIH COVID-19 Treatment Guidelines, https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/remdesivir/).
Lopinavir/ritonavir
Lopinavir and ritonavir (Fig. 4) that inhibit the HIV-protease enzyme are antiretroviral drugs used for AIDS treatment. It is known that as a result of the combined use of ritonavir, which is used in low doses to increase the effectiveness of lopinavir, it decreases the mortality and morbidity values in HIV patients (Lombardy Section Italian Society Infectious and Tropical Diseases 2020).
Lin et al. (2020), reported that lopinavir and ritonavir could connect to the SARS-CoV-2 protease in the modeling study. This offers that lopinavir/ritonavir could be effective by inhibiting SARS-CoV-2 protein synthesis. Furthermore, several findings proved that treatment with lopinavir/ritonavir alone or in combination together with other antiviral agents developed severe patients with SARS or MERS (Chu et al. 2004; Sheahan et al. 2020; Zumla et al. 2016).
The combination of lopinavir/ritonavir and ribavirin has improved the course of the disease in SARS-CoV patients (Chu et al. 2004).
In other combination studies with lopinavir/ritonavir, ribavirin and IFN-α2a carried out in South Korea, a patient with MERS-CoV disease has been successfully treated (Kim et al. 2016). Now, lopinavir/ritonavir and IFN-β1b is in phase 2 for the MERS therapy in a randomized controlled trial (Arabi et al. 2018). Similarly, the therapeutic efficacy of the same combination is being investigated in a sequential randomized controlled trial (Arabi et al. 2020).
Despite the positive findings mentioned above, according to a recent study performed on patients with SARS-CoV-2 infection, it has been suggested that the lopinavir/ritonavir combination did not provide the clinical improvement compared to standard care processings (Cao et al. 2020). Actually, findings of lopinavir/ritonavir clinical efficacy remain limited and primarily anecdotal cases (Han et al. 2019). Although COVID-19 viral load can be decreased via lopinavir/ritonavir application very fast (Lim et al. 2020), the use of this combination for the therapy of COVID-19 is not advised by the COVID-19 Treatment Guidelines Panel except in a clinical trial (NIH COVID-19 Treatment Guidelines, https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/lopinavir-ritonavir-and-other-hiv-protease-inhibitors/).
Further research related the clinical efficacy about lopinavir/ritonavir combination in the therapy of COVID-19 is needed as current results contradict so 81 clinical trials are continuing on this combination, of them 8 trials were completed (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=lopinavir+%2F+ritonavir&cntry=&state=&city=&dist=).
Favipiravir
Favipiravir (Fig. 4) is a new antiviral drug that inhibits RNA-dependent RNA polymerase (RdRp). It was approved for use against novel influenza in February 2020 in China. Besides, its anti-influenza virus action, it stops the replication of RNA viruses such as flavi-, alpha-, filo-, bunya-, arena-, noroviruses (Delang et al. 2018). Favipiravir is a pro-drug which is converted into an active phosphoribosylated form (Favipiravir-RTP) in cells, then viral RNA polymerase identifies it as a substrate, resulting in inhibiting RNA polymerase function (Furuta et al. 2017). Eventually, favipiravir may have a potent antiviral activity for the therapy of COVID-19. The promising outcomes were achieved in a clinical trial with favipiravir against COVID-19 in China. The preliminary results from this study involving 80 patients (a total of the experimental and the control group) stated that favipiravir showed a more powerful antiviral activity than that of lopinavir/ritonavir. Adverse reactions did not observe in favipiravir therapy group considerably. Compared to the lopinavir/ritonavir group, it had considerably fewer adverse effects (https://www.szdsyy.com/News/0a6c1e58-e3d0-4cd1-867a-d5524bc59cd6.html).
Presently, 32 clinical trials are being carried out for using favipiravir in the treatment of COVID-19, of them 6 trials were completed and it is expected that promising results will be achieved in removing the virus (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=favipiravir&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=). Moreover, a pharmaceutical company has started Phase 3 clinical trials by using of tablet favipiravir in India and the complete study results are expected in August (News: Glenmark begins Phase 3 clinical trials on antiviral drug Favipiravir for COVID-19 patients in India).
Danoprevir
Danoprevir (Fig. 4), approved in China for the therapy of non-cirrhotic genotype 1b chronic hepatitis C together with some drugs, is a HCV N53 protease inhibitor. Only two clinical trials were completed using danoprevir combined with ritonavir in the treatment of SARS-CoV-2 infection in China (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=danoprevir&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Darunavir
Darunavir (Fig. 4) boosted with ritonavir or cobicistat are currently used for in HIV/AIDS treatment approved by the FDA. Thus, the pharmacokinetics and pharmacodynamics of darunavir or ritonavir are enhanced by cytochrome p450 (CYP3A) inhibition (Mathias et al. 2010; Santos et al. 2019). Cell experiments with darunavir showed that the drug inhibited viral replication of COVID-19 in vitro, significantly (News: Abidol and darunavir can effectively inhibit coronavirus). Although lopinavir/ritonavir used in the treatment of HIV/AIDS, has more efficacy and tolerability than darunavir, its use in COVID-19 is limited (Lombardy Section Italian Society Infectious and Tropical Diseases 2020). Currently, there are nine clinical trials at different phases of development on the efficacy of darunavir in the treatment of COVID-19 pneumonia (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=darunavir&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Ribavirin
Ribavirin (Fig. 4), has a broad-spectrum agent against virus diseases, is a nucleoside analog. It suppresses the action of inosine monophosphate dehydrogenase, thus, the synthesis of guanosine triphosphate (GTP) is affected, so the replication of RNA and DNA viruses could be prevented. Ribavirin was widely used during the outbreak of SARS in Hong Kong. For this, it was sometimes preferred with or without steroids (Wenzel and Edmond 2003; Jones et al. 2004; Peiris et al. 2003). The combination of ribavirin and interferon-β, which appears to inhibit SARS-CoV replication, has shown significant efficacy in the inhibition of SARS-CoV (Morgenstern et al. 2005). Ribavirin is being searched in 12 clinical trials, and 3 of them were completed, now (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=ribavirin&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=). In one of the completed clinical trials, Hung et al. (2020) reported that the ribavirin triple antiviral treatment was safe and superior compared to lopinavir-ritonavir combined therapy.
Arbidol (umifenovir)
Arbidol (Fig. 4) is an antiviral agent that has indol-derivative compound and is registered in Russia and China for prophylaxis and therapy of influenza and other respiratory viral infections. This drug prevents fusion between the virus and the cell membrane that is an important stage in the entry of the virus into the cell (Blaising et al. 2014). And also, it blocks hepatitis virus entry and replication in vitro (Pécheur et al. 2016). Arbidol and its derivative, arbidol mesylate, showed antiviral activity against SARS-CoV because they declined the reproduction of the virus in the cell cultures (Khamitov et al. 2008). Based on a few findings, arbidol tested alone or with some antiviral agents against COVID-19, and certain positive effects were observed (Wang et al. 2020; Zhang et al. 2020; Xu et al. 2020). Currently, arbidol alone or in combination with some antiviral drugs are tested through ten clinical trials, of them only one trial was completed (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=arbidol&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
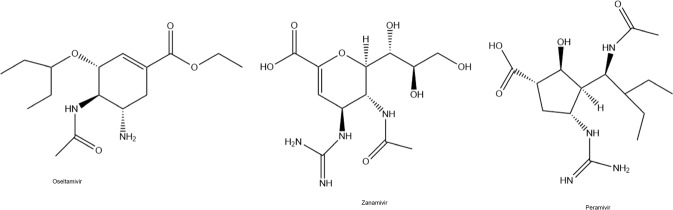
Neuraminidase inhibitors
Oseltamivir, zanamivir, and peramivir (Fig. 5) are antiviral drugs that inhibit the viral neuraminidase enzyme and block the release of viral particles out of host cells (Uyeki 2018). These agents called neuraminidase inhibitors are recommended for influenza (Chow et al. 2019). Neuraminidase inhibitors have been used as an empirical treatment in MERS-CoV infection (Bleibtreu et al. 2018). Furthermore, it was reported that oseltamivir has been used either with or without antibiotics and corticosteroids against COVID-19 (Wang et al. 2020). In a clinical trial, oseltamivir is tested with chloroquine and favipiravir (ClinicalTrials.gov [Internet] 2020d). Presently, 17 clinical trials are being conducted for using oseltamivir against COVID-19, of them only 1 trial was completed (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=oseltamivir&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist==&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Fig. 5.
Chemical formulas of neuraminidase inhibitors
Other drugs
Chloroquine and hydroxychloroquine
Chloroquine and hydroxychloroquine (Fig. 6) have been utilized against malaria for years. Besides, this chemical usage, they show antiviral effects against HIV. Their mechanism of action is to inhibit the virus entry into host cells. The second antiviral mechanism is thought to be relevant to the post-translation modification of freshly synthesized proteins over glycosylation inhibition (Rolain et al. 2007).
Fig. 6.
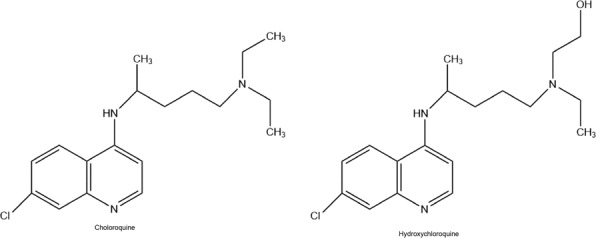
Chemical Structures of antimalarial drugs
Chloroquine and hydroxychloroquine blocked viral replication at multiple points in vitro experiments so these drugs have been found to be effective against SARS-CoV-2. Primarily, they come together in lysosomes, increase endosomal pH, and cut off the lysosome-endosome fusion. Therefore, the transport of virus is blocked (Yao et al. 2020). Furthermore, chloroquine and hydroxychloroquine have been determined to interfere with the angiotensin-converting enzyme 2 (ACE2) receptor, which has an important receptor for the attachment of COVID-19 into the cell by cell-surface protein glycosylation (Fantini et al. 2020). In addition, it was shown that chloroquine had higher efficacy than hydroxychloroquine (Liu et al. 2020).
Hydroxychloroquine combined with the macrolide antibiotic azithromycin cured 100% of patients with COVID-19 in a trial, lately. However, hydroxychloroquine alone cured only 57.1% of patients (Gautret et al. 2020). Nowadays, the efficacy of chloroquine and hydroxychloroquine will be tried in people with COVID-19 (National Library of Medicine (US), Bethesda (MD) 2020) in the healthcare setting (COPCOV) (ClinicalTrials.gov [Internet] 2020e), and chloroquine as preventative medicine against COVID-19. Now, 246 clinical trials are being carried out with hydroxychloroquine, of them 21 trials were completed (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=hydroxychloroquine&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
The antiviral activity of chloroquine phosphate has been shown against the SARS virus and avian flu in clinical studies in vitro and animal models (Savarino et al. 2006; Vincent et al. 2005; Yan et al. 2013). Because chloroquine has immunomodulatory action, this effect could enhance its antiviral activity in vivo. Chloroquine penetrates into the tissues well after oral administration. It was declared in February 2020 in China, the use of chloroquine improved the rate of clinical success, with a reduction of hospitalization and the improvement of the patient’s outcome (Multicenter collaboration group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia 2020). Presently, 84 clinical trials are being conducted with chloroquine, of them 9 trials were at the completed stage (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=chloroquine&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Since clinical data about the use of chloroquine and hydroxychloroquine in the treatment of COVID-19 pneumonia with severe and critical conditions is very limited, the COVID-19 Treatment Guidelines Panel does not advise the usage of these drugs in therapy, except in a clinical trial (NIH COVID-19 Treatment Guidelines, https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/chloroquine-or-hydroxychloroquine/).
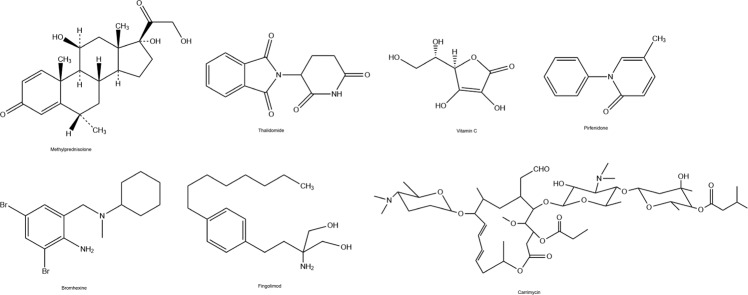
Methylprednisolone
Corticosteroid therapy (methylprednisolone (Fig. 7), dexamethasone, and hydrocortisone) has been shown to be useful for the therapy of SARS-CoV patients. This therapy method extended the survival period of clinical cases, significantly (Long et al. 2016). On the contrary, other researchers claimed that when corticosteroids used in the early steps of SARS infections it caused to increase values of viral load (Lee et al. 2004). Besides, researches where corticosteroids in the adjuvant therapy against MERS-CoV infections could not show the efficacy due to the death of all patients (Al-Tawfiq and Memish 2017). Previously, methylprednisolone has been used with antibiotics, oseltamivir, and oxygen therapy against COVID-19 (Huang et al. 2020).
Fig. 7.
Chemical Structures of some other drugs
Currently, a total of 32 clinical trials are underway with methylprednisolone in the treatment of COVID-19, of them 6 trials were completed (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=methylprednisolone&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Thalidomide
Thalidomide (Fig. 7) speeds up the deterioration of mRNA in blood cells, consequently shows anti-inflammatory activity. And also, it reduces TNF-α. Additionally, this agent has the ability to increase the secretion of interleukins (ILs), e.g., IL-12, and activate natural killer cells (Newfield 2018). Now, only 5 clinical trials are being conducted by researchers on thalidomide (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=thalidomide&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Vitamin C
Vitamin C (Fig. 7) is a water-soluble vitamin and known as ascorbic acid also. Because of its antioxidant efficiency, it may have a reducing effect on oxidative stress and inflammation (ClinicalTrials.gov [Internet] 2020f; Kashiouris et al. 2020). It has been revealed that vitamin C supported immune functions and protected against coronavirus infections (Hemila 2003). In human-controlled trials, vitamin C decreased the incidence of pneumonia, significantly. Thus, it was thought that vitamin C may be an anti-sensitiveness compound that prevents the sensitivity to lower respiratory tract infections under particular situations (Hemilä 1997). As of now, 32 clinical trials were being carried out on vitamin C, of them only 1 was completed (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=vitamin+c&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Since there are not enough data, it is not recommended to use or not use vitamin C in the treatment of COVID-19 patients (NIH COVID-19 Treatment Guidelines, https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/vitamin-c/).
Pirfenidone
Pirfenidone (Fig. 7) having anti-inflammatory and antioxidant activities have been used against idiopathic pulmonary fibrosis diseases, so far. Additionally, it inhibits IL-1β and IL-4, https://clinicaltrials.gov/ct2/show/NCT04282902?term=NCT04282902&draw=2&rank=1. Probably, its anti-inflammatory effects might be beneficial, but there are no clinical trials on pirfenidone, at present.
Bromhexine
Bromhexine (Fig. 7) inhibits a transmembrane protease serine that is responsible for the activation of S-glycoprotein found in SARS and MERS viruses. This glycoprotein provides the entry of the virus through the plasma membrane so 5 clinical trials are carried out on the efficacy of bromhexine against COVID-19 cases (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=bromhexine&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Fingolimod
Fingolimod (Fig. 7) is a sphingosine-1-phosphate receptor regulator. It has a potent modulator for the immune system thus, its use in multiple sclerosis is beneficial. Because some pathological evidences such as pulmonary edema and hyaline membrane formation are observed, it is thought that the utilization of drugs with immune modulator properties combined with the ventilator support is noteworthy to stop the progress of acute respiratory distress syndrome in severe patients. Only 1 clinical trial is being conducted by researchers on fingolimod, currently (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=fingolimod&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Imatinib
Blocking virus–host fusion is a promising target for the discovering of novel antiviral agents, so there are some studies on drugs that inhibit the Abl kinase pathway (Dong et al. 2020). In a study, imatinib, an Abl kinase inhibitor, was observed to block the replication of SARS and MERS viruses by blocking viral fusion in 2016 (Coleman et al. 2016). Hoffman et al. (2020) also showed that the COVID-19 used the SARS-coronavirus receptor ACE2 as well as the cellular protease TMPRSS2 for accessing into target cells, so TMPRSS2, transmembrane serine protease 2, inhibitory agents such as imatinib may be evaluated as therapy options for COVID-19 disease. And five clinical trials are underway on the safety and efficacy of imatinib for the treatment of COVID-19 pneumonia, at present (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=imatinib&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Carrimycin
Carrimycin (Fig. 7) is a macrolide class antibiotic and it is effective against some gram-positive bacteria, and Mycobacterium tuberculosis in vitro (Wang et al. 2019). The drug has been used for the therapy of patients with COVID-19 in a clinical study (NIH US National Library of Medicine clinical trial database, https://www.clinicaltrials.gov/ct2/show/NCT04286503?term=carrimycin&cond=Covid19&draw=2&rank=1).
Chinese medicine
The active compounds found in Chinese medicine, such as glycyrrhizin, hesperetin, baicalin, and quercetin (Fig. 8) are thought as considerable compounds in the prevention and treatment of COVID-19 disease (Li et al. 2020).
Fig. 8.
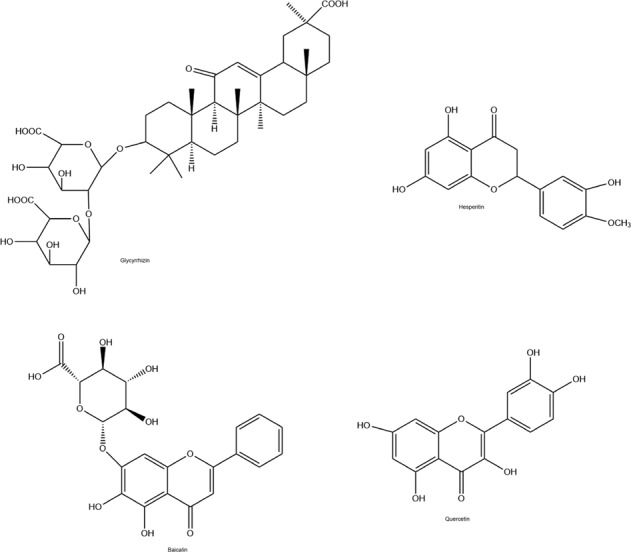
Chemical structures of Chinese medicine
Glycyrrhizin (Fig. 8), which is the active compound of liquorice roots used in Chinese medicine, can inhibit the transcription of SARS virus in vitro trials (Cinatl et al. 2003; Hoever et al. 2005). Lately, it was found that glycyrrhizin can bind to the ACE2 receptor (Chen and Du 2020). When given high doses of glycyrrhizin, it was clinically effective in clinical trials against the SARS virus (Lu et al. 2003; Wu et al. 2004). Moreover, it has been revealed that glycyrrhizin may inhibit the transcription of SARS-associated virus in vitro (Cinatl et al. 2003; Hoever et al. 2005). Only one clinical trial is carried out about glycyrrhizin, now (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=Glycyrrhizin&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
Hesperetin (Fig. 8) has a flavon-structure compound obtained from citrus fruits and it is a notable traditional Chinese medicine. It was revealed that hesperetin had the capability of alleviating the division action of the 3C-like protease (3CLpro) of SARS virus dose-dependently in cell-free as well as cell-based tests (Lin et al. 2005). 3CLpro is the viral parent proteinase that checks the actions of the coronavirus replication complex, thus it is an appealing target for treatment (3Clpro). Another study reported that hesperetin had the potential to inhibit ACE2 (Chen and Du 2020). For these reasons, it seems that hesperetin has the potential to block SARS-CoV infection.
Baicalin (Fig. 8) is one of the other active compounds used as Chinese medicine. Chen et al. (2004) revealed that baicalin can inhibit the SARS virus in vitro (Chen et al. 2004).
Quercetin (Fig. 8) which is widely used in Chinese medicine is a flavone obtained from plants. Quercetin inhibits the 3CLpro of the SARS virus, thus, blocks the accessing of the SARS virus into host cells (Yi et al. 2004). Currently, two clinical trials are being conducted to evaluate the efficacy of quercetin in COVID-19 patients (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=quercetin&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=).
In view of these works, Chinese medicine can play a primary role in the therapy and prevention of COVID-19 disease.
Vaccines
Vaccination offers a very convenient tool to prevent and to inhibit the infectious diseases spread. The vaccines stimulate humoral immune responses, in this way, neutralizing antibodies are started to be produced in the human body. Thus, it is possible to both limit infection and prevents reinfection. Vaccine development studies are continuing as well as any discovery of new drug or development treatment method to treat COVID-19 pneumonia. Revealing the genome sequence of SARS-CoV-2 has accelerated vaccine development studies. The methods used for developing the SARS-CoV-2 vaccine are the same as those used for the SARS-CoV vaccine. Both viruses bind the same host cell receptor (ACE2). In addition to this, they can share similar disease pathogeneses as well as restricted cross-neutralizing antibodies (Devaux et al. 2020; Shih et al. 2020). As of now (August 20, 2020), a total of 193 clinical trials are being conducted to develop a vaccine against COVID-19, of them 8 were at the completed stage (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=vaccine&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=). Information about the vaccine candidates against COVID-19 is presented at Table 1 (NIH US National Library of Medicine clinical trial database, https://clinicaltrials.gov/ct2/results?cond=Covid19&term=vaccine&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=; https://www.biocentury.com/clinical-vaccines-and-therapies).
Conclusion
The outbreak began with SARS-CoV in the year of 2002 firstly, then, extended to 32 countries and regions. Following one decade, the World encountered MERS-CoV, another coronavirus. Nowadays, the rising SARS-CoV-2 turned into a global threat so the WHO proclaimed that the COVID-19 outbreak was a global pandemic in March 2020.
COVID-19, previously known as SARS-CoV-2, targets lung cells by connecting to ACE2 protein. This protein is largely produced in some tissues such as bile duct, liver, gastrointestinal organs (e.g., small intestine, duodenum), esophagus, testis, and kidney as well as lung tissue (Zhang et al. 2020; Chai et al. 2020; Anti-2019-nCoV Volunteers et al. 2020; Fan et al. 2020). Thus, COVID-19 may damage these organs and tissues. Now, COVID-19 has extended to various countries speedily, caused severe sickness and these events made it a relevant and considerable public health-threatening. Taking into account the global threatening caused by COVID-19, efficient therapy methods against COVID-19 disease are promptly necessary. Nevertheless, the development of new agents for this disease is still a considerable problem for people in the world, and we have none formally approved agents against COVID-19 pneumonia now. Looking at the epidemiological properties of COVID-19, it is very important to cut off the extending of this virus owing to epidemic prevention as well as checking procedures. For this, the most recommended preventions are putting infected people in quarantine and checking the source of infection. The limited information available we have is needed to develop novel drugs and to find new therapy methods to prevent this outbreak and to treat COVID-19 pneumonia. Furthermore, the design and improvement of vaccines against COVID-19 are also necessary as well as discovering novel agents and clinical trials of known drugs.
In this review, we focused on some medicines that have already started with the repositioning for COVID-19 therapy. The repositioning clinical trials seem to be an attractive strategy, thus, the discovery of new classes of drug can be easier, the costs and time for reaching the market can decrease, the pharmaceutical supply chain is available for formulation and distribution, more therapy methods are possible by combining the drugs. We hope that the continuing studies may provide solutions for the prevention and therapy against the COVID-19 pandemic in 2020.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9:e00221–e002218. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq JA, Memish ZA. Update on therapeutic options for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Expert Rev Anti Infect Ther. 2017;15:269–275. doi: 10.1080/14787210.2017.1271712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anti-2019-nCoV Volunteers, Li Z, Wu M, Yao J, Guo J, Liao X, Song S, Li J, Duan G, Zhou Y, Wu X, Zhou Z, Wang T, Hu M, Chen X, Fu Y, Lei C, Dong H, Xu C, Hu Y, Han M, Zhou Y, Jia H, Chen X, Yan J (2020) Caution on kidney dysfunctions of COVID-19 patients. medRxiv. 10.1101/2020.02.08.20021212
- Arabi Y, Balkhy H, Hajeer AH, Bouchama A, Hayden FG, Al-Omari A, Al-Hameed FM, Taha Y, Shindo N, Whitehead J, Merson L, AlJohani S, Al-Khairy K, Carson G, Luke TC, Hensley L, Al-Dawood A, Al-Qahtani S, Modjarrad K, Sadat M, Rohde G, Leport C, Fowler R. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4:709. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi YM, Alothman A, Balkhy HH, Al-Dawood A, AlJohani S, Al Harbi S, Kojan S, Al Jeraisy M, Deeb AM, Assiri AM, Al-Hameed F, AlSaedi A, Mandourah Y, Almekhlafi GA, Sherbeeni NM, Elzein FE, Memon J, Taha Y, Almotairi A, Maghrabi KA, Qushmaq I, Al Bshabshe A, Kharaba A, Shalhoub S, Jose J, Fowler RA, Hayden FG, Hussein MA, The MIRACLE trial group Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi YM, Asiri AY, Assiri AM, Jokhdar HAA, Alothman A, Balkhy HH, AlJohani S, Al Harbi S, Kojan S, Al Jeraisy M, Deeb AM, Memish ZA, Ghazal S, Sarah Al F, Fahad A-H, Asim AS, Yasser M, Al Mekhlafi GA, Sherbeeni NM, Elzein FE, Almotairi A, Bshabshe AA, Kharaba A, Jose J, Al Harthy A, Al Sulaiman A, Mady A, Fowler RA, Hayden FG, Al-Dawood A, Abdelzaher M, Bajhmom W, Hussein MA, The Saudi Critical Care Trials group Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21:8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Kulcsar K, Misra V, Frieman M, Mossman K. Bats and coronaviruses. Viruses. 2019;11:pii: E41. doi: 10.3390/v11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Library of Medicine (US): Bethesda (MD) (2020) Mar 12–Identifier NCT04303507. Chloroquine prevention of Coronavirus disease (COVID-19) in the healthcare setting (COPCOV). https://clinicaltrials.gov/ct2/show/NCT04303507?term=NCT04303507&draw=2&rank=1. Accessed 15 Feb 2020
- Blaising J, Polyak SJ, Pécheur EI. Arbidol as a broad-spectrum antiviral: an update. Antivir Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleibtreu A, Jaureguiberry S, Houhou N, Boutolleau D, Guillot H, Vallois D, Lucet JC, Robert J, Mourvillier B, Delemazure J, Jaspard M, Lescure FX, Rioux C, Caumes E, Yazdanapanah Y. Clinical management of respiratory syndrome in patients hospitalized for suspected Middle East respiratory syndrome coronavirus infection in the Paris area from 2013 to 2016. BMC Infect Dis. 2018;18:331. doi: 10.1186/s12879-018-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BuzzFeedNews. The FDA has approved emergency use of remdesivir to treat COVID-19. https://www.buzzfeednews.com/article/danvergano/fda-remdesivir-coronavirus. Accessed 2 May 2020
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C (2020) A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Eng J Med. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed]
- Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Fan J, Lan F (2020) Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 10.1101/2020.02.03.931766
- Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Chan KH, Jiang Y, Kao RYT, Lu HT, Fan KW, Cheng VCC, Tsui WHW, Hung IFN, Lee TSW, Guan Y, Peiris JSM, Yuen KY. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Du Q. Potential natural compounds for preventing 2019-nCoV infection. Preprints. 2020;2020:2020010358. [Google Scholar]
- Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherin P, Marie I, Michallet M, Pelus E, Dantal J, Crave JC, Delain JC, Viallard JF. Management of adverse events in the treatment of patients with immunoglobulin therapy: a review of evidence. Autoimmun Rev. 2016;15:71–81. doi: 10.1016/j.autrev.2015.09.002. [DOI] [PubMed] [Google Scholar]
- ACC. Chinese Clinical Guidance for COVID-19 pneumonia diagnosis and treatment. 7th edn. http://kjfy.meetingchina.org/msite/news/show/cn/3337.html. Accessed 6 Mar 2020
- Chow EJ, Doyle JD, Uyeki TM. Influenza virus-related critical illness: prevention, diagnosis, treatment. Crit Care. 2019;23:214. doi: 10.1186/s13054-019-2491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY, HKU/UCH SARS Study Group Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov [Internet] (2020a) Identifier NCT04293887. Efficacy and safety of IFN-a2b in the treatment of novel coronavirus patients. National Library of Medicine (US), Bethesda (MD). https://clinicaltrials.gov/ct2/show/NCT04293887?term=NCT04293887&draw=2&rank=1Mar12. Accessed 8 Mar 2020
- ClinicalTrials.gov [Internet] (2020b) Identifier NCT04275414. Bevacizumab in severe or critical patients with Covid-19 pneumonia (BEST-CP). National Library of Medicine (US), Bethesda (MD). https://clinicaltrials.gov/ct2/show/NCT04275414?term=NCT04275414&draw=2&rank=1Mar12. Accessed 13 Feb 2020
- ClinicalTrials.gov [Internet] (2020c) Identifier NCT04257656. Severe2019-nCoV remdesivir RCT. National Library of Medicine (US), Bethesda (MD). https://clinicaltrials.gov/ct2/show/NCT04257656Mar12. Accessed 18 Feb 2020
- ClinicalTrials.gov [Internet] (2020d) Identifier NCT04303299, Various combination of protease inhibitors, Oseltamivir, Favipiravir, and Chloroquine for treatment of COVID-19: a randomized control trial (THDMS-COVID19). National Library of Medicine (US), Bethesda (MD), https://clinicaltrials.gov/ct2/show/NCT04303299Mar12. Accessed 15 Feb 2020
- ClinicalTrials.gov [Internet] (2020e) Identifier NCT04261517. Efficacy and safety of hydroxychloroquine for treatment of pneumonia caused by 2019-nCoV (HC-nCoV). National Library of Medicine (US), Bethesda (MD). https://clinicaltrials.gov/ct2/show/NCT04261517Mar12. Accessed 15 Feb 2020
- ClinicalTrials.gov [Internet] (2020f) Identifier NCT04264533. Vitamina C infusion for the treatment of severe 2019-nCoV infected pneumonia. National Library of Medicine (US), Bethesda (MD). https://clinicaltrials.gov/ct2/show/NCT04264533?term=NCT04264533&draw=2&rank=1Mar12. Accessed 15 Feb 2020
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2020 Mar 12 – Identifier NCT04282902, A study to evaluate the efficacy and safety of Pirfenidone with novel coronavirus infection. Available from: https://clinicaltrials.gov/ct2/show/NCT04282902?term=NCT04282902&draw=2&rank=1
- Coleman CM, Sisk JM, Mingo RM, Nelson EA, White JM, Frieman MB. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome Coronavirus and Middle East Respiratory Syndrome Coronavirus Fusion. J Virol. 2016;90:8924–8933. doi: 10.1128/JVI.01429-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai HS, Caligiuri MA. Molecular basis for the recognition of herpes simplex virus type 1 infection by human natural killer cells. Front Immunol. 2018;9:183. doi: 10.3389/fimmu.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawar FU, Tu J, Khattak MNK, Mei J, Lin L. Cyclophilin A: a key factor in virus replication and potential target for anti-viral therapy. Curr Issues Mol Biol. 2017;21:1–20. doi: 10.21775/cimb.021.001. [DOI] [PubMed] [Google Scholar]
- de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci USA. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delang L, Abdelnabi R, Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Dyer O. Two Ebola treatments halve deaths in trial in DRC outbreak. BMJ. 2019;366:I5140. doi: 10.1136/bmj.l5140. [DOI] [PubMed] [Google Scholar]
- El Agha E, Kramann R, Schneider RK, Li X, Seeger W, Humphreys BD, Bellusci S. Mesenchymal stem cells in fibrotic disease. Cell Stem Cell. 2017;21:166–177. doi: 10.1016/j.stem.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Elfiky AA. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Li K, Ding Y, Lu WL, Wang J (2020) ACE2 expression in kidney and testis may cause kidney and testis damage after 2019-nCoV infection. medRxiv. 10.1101/2020.02.12.20022418
- Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55:105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S, Colson P, Chabrière E, La Scola B, Rolain J-M, Brouqui P, Raoult D (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56:105949 [DOI] [PMC free article] [PubMed]
- Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11:836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Haagmans BL, Kuiken T, Martina BE, Fouchier RA, Rimmelzwaan GF, van Amerongen G, van Riel D, de Jong T, Itamura S, Chan KH, Tashiro M, Osterhaus AD. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer Q, Rückert T, Romagnani C. Natural killer cell specificity for viral infections. Nat Immunol. 2018;19:800–808. doi: 10.1038/s41590-018-0163-6. [DOI] [PubMed] [Google Scholar]
- Han W, Quan B, Guo Y, Zhang J, Lu Y, Feng G, Wu Q, Fang F, Cheng L, Jiao N, Li X, Chen Q. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol. 2019;92:461–463. doi: 10.1002/jmv.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä H. Vitamin C intake and susceptibility to pneumonia. Pediatr Infect Dis J. 1997;16:836–837. doi: 10.1097/00006454-199709000-00003. [DOI] [PubMed] [Google Scholar]
- Hemila H. Vitamin C and SARS coronavirus. J Antimicrob Chemother. 2003;52:1049–1050. doi: 10.1093/jac/dkh002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoever G, Baltina L, Michaelis M, Kondratenko R, Baltina L, Tolstikov GA, Doerr HW, Cinalt J., Jr Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J Med Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020;2020:929042. [Google Scholar]
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK, Washington State 2019-nCoV Case Investigation Team First case of 2019 novel coronavirus in the United States. N. Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.szdsyy.com/News/0a6c1e58-e3d0-4cd1-867a-d5524bc59cd6.html. Accessed 22 Feb 2020
- https://www.biocentury.com/clinical-vaccines-and-therapies. Accessed 20 Aug 2020
- https://www.sciencemag.org/news/2020/08/russia-s-approval-covid-19-vaccine-less-meets-press-release. Accessed 15 Aug 2020
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DS, Azhar EI, Kim YJ, Memish ZA, Oh MD, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18:e217–e227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, Ng Y-Y, Lo J, Chan J, Tam AR, Shum H-P, Chan V, Wu AK-L, Sin K-M, Leung W-S, Law W-L, Lung DC, Sin S, Yeung P, Yip CC-Y, Zhang RR, Fung AY-F, Yan EY-W, Leung K-H, Ip JD, Chu AW-H, Chan W-M, Ng AC-K, Lee R, Fung K, Yeung A, Wu T-C, Chan JW-M, Yan W-W, Chan W-M, Chan JF-W, Lie AK-W, Tsang OT-Y, Cheng VC-C, Que T-L, Lau C-S, Chan K-H, To KK-W, Yuen C-Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BM, Ma ESK, Peiris JSM, Wong PC, Ho JCM, Lam B, Lai KN, Tsang KWT. Prolonged disturbances of in vitro cytokine production in patients with severe acute respiratory syndrome (SARS) treated with ribavirin and steroids. Clin Exp Immunol. 2004;135:467–473. doi: 10.1111/j.1365-2249.2003.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiouris MG, L’Heureux M, Cable CA, Fisher BJ, Leichtle SW, Fowler AA. The emerging role of vitamin C as a treatment for sepsis. Nutrients. 2020;12:292. doi: 10.3390/nu12020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamitov RA, La Loginova S, Shchukina VN, Borisevich SV, Maksimov VA, Shuster AM. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol. 2008;53:9–13. [PubMed] [Google Scholar]
- Kim UJ, Won EJ, Kee SJ, Jung SI, Jang HC. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome. Antivir Ther. 2016;21:455–459. doi: 10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- Kumamoto M, Nishiwaki T, Matsuo N, Kimura H, Matsushima K. Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur Respir J. 2009;34:740–748. doi: 10.1183/09031936.00128508. [DOI] [PubMed] [Google Scholar]
- Kuri T, Zhang X, Habjan M, Martínez-Sobrido L, García-Sastre A, Yuan Z, Weber F. Interferon priming enables cells to partially overturn the SARS coronavirus-induced block in innate immune activation. J Gen Virol. 2009;90:2686–2694. doi: 10.1099/vir.0.013599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Allen Chan KC, Hui DS, Ng EKO, Wu A, Chiu RWK, Wong VWS, Chan PKS, Wong KT, Wong E, Cockram CS, Tam JS, Sung JJY, Lo YMD. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew TW, Kwek TK, Tai D, Earnest A, Loo S, Singh K, Kwan KM, Chan Y, Yim CF, Bek SL, Kor AC, Yap WS, Chelliah YR, Lai YC, Goh SK. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- Li H, Liu S-M, Yu X-H, Tang S-L, Tang C-K (2020) Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents 10.1016/j.ijantimicag.2020.105951 [DOI] [PMC free article] [PubMed]
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, Choe KW, Kang YM, Lee B, Park SJ. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-W, Tsai F-J, Tsai C-H, Lai C-C, Wan L, Ho T-Y, Hsieh C-C, Chao P-DL. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir Res. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Shen R, Guo X (2020) Molecular modeling evaluation of the binding abilities of ritonavir and lopinavir to Wuhan pneumonia coronavirus proteases. bioRxiv. 10.1101/2020.01.31.929695
- Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardy Section Italian Society Infectious and Tropical Diseases Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Le Infez Med. 2020;28:143–152. [PubMed] [Google Scholar]
- Long Y, Xu Y, Wang B, Zhang L, Jia D, Xue F, Duan G, He J, Xia J, Xu D. Clinical recommendations from an observational study on MERS: Glucocorticoids was benefit in treating SARS patients. Int J Clin Exp Med. 2016;9:8865–8873. [Google Scholar]
- Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B, Lho H, Pharm DH, Deif H, LaMere EA, Chang M, Kain KC, Farcas GA, Ferguson P, Latchford M, Levy G, Dennis JW, Lai EK, Fish EN. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- Lu H, Huo N, Wang G, Li H, Nie L, Xu X (2003) Clinical observation of therapeutic effect of compound glycyrrhizin on SARS. China Pharmacy 10:34–36
- Luo C, Luo H, Zheng S, Gui C, Yue L, Yu C, Sun T, He P, Chen J, Shen J, Luo X, Li Y, Liu H, Bai D, Shen J, Yang Y, Li F, Zuo J, Hilgenfeld R, Pei G, Chen K, Shen X, Jiang H. Nucleocapsid protein of SARS coronavirus tightly binds to human cyclophilin A. Biochem Biophys Res Commun. 2004;321:557–565. doi: 10.1016/j.bbrc.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, Grazzini G. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14:152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;64:e00399–20. doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias AA, German P, Murray BP, Wei L, Jain A, West S, Warren D, Hui J, Kearney BP. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin Pharm Ther. 2010;87:322–329. doi: 10.1038/clpt.2009.228. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Goolaerts A, Howard JP, Lee JW. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit Care Med. 2010;38:S569–S573. doi: 10.1097/CCM.0b013e3181f1ff1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, Boyd R, Trounson A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175:303–313. doi: 10.2353/ajpath.2009.080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J., Jr Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. 2005;326:905–908. doi: 10.1016/j.bbrc.2004.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multicenter collaboration group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for Chloroquine in the Treatment Of Novel Coronavirus Pneumonia Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E019. doi: 10.3760/cma.j.issn.1001-0939.2020.0019. [DOI] [PubMed] [Google Scholar]
- Mustafa S, Balkhy H, Gabere MN. Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): a review. J Infect Public Health. 2018;11:9–17. doi: 10.1016/j.jiph.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar MS, Bakhrebah MA, Meo SA, Alsuabeyl MS, Zaher WA. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: epidemiology, pathogenesis and clinical characteristics. Eur Rev Med Pharm Sci. 2018;22:4956–4961. doi: 10.26355/eurrev_201808_15635. [DOI] [PubMed] [Google Scholar]
- Natural Research Project for SARS, Beijing Group The involvement of natural killer cells in the pathogenesis of severe acute respiratory syndrome. Am J Clin Pathol. 2004;121:507–511. doi: 10.1309/WPK7Y2XKNF4CBF3R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04252664. Mild/moderate 2019-nCoV remdesivir RCT. https://clinicaltrials.gov/ct2/show/NCT04252664. Accessed 18 Feb 2020
- Newfield C (2018) New medical indications for thalidomide and its derivatives. Sci J Lander College Arts Sci 12:11–19
- News. Abidol and darunavir can effectively inhibit coronavirus. https://www.sd.chinanews.com/2/2020/0205/70145.html. Accessed 21 Feb 2020
- News. Glenmark begins Phase-3 clinical trials on antiviral drug Favipiravir for COVID-19 patients in India. https://www.thehindu.com/news/national/glenmark-begins-phase-3-clinical-trials-on-antiviral-drug-favipiravir-forcovid-19-patients-in-india/article31563198.ece. Accessed 25 May 2020
- NIH COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/vitamin-c/. Accessed 18 Aug 2020
- NIH COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/chloroquine-or-hydroxychloroquine/. Accessed 18 Aug 2020
- NIH COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/lopinavir-ritonavir-and-other-hiv-protease-inhibitors/. Accessed 17 Aug 2020
- NIH COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/remdesivir/. Accessed 17 Aug 2020
- NIH COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/blood-derived-products/mesenchymal-stem-cells/. Accessed 16 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=arbidol&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 21 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=bevacizumab&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 21 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=bromhexine&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 18 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=chloroquine&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 18 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=convalescent+plasma&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 21 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=cyclosporine+A&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 21 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=danoprevir&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 17 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=darunavir&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 17 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=favipiravir&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 17 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=fingolimod&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 21 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=Glycyrrhizin&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 18 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=hydroxychloroquine&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 18 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=imatinib&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 18 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=immunoglobulin&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 21 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=interferon&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 21 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=mesenchymal+stem+cell&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 21 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=methylprednisolone&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 17 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=monoclonal+antibody&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 21 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=natural+killer+cell&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 21 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=oseltamivir&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 17 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=quercetin&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 18 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=ribavirin&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 17 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=thalidomide&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 18 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/results?cond=Covid19&term=tocilizumab&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 21 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=vaccine&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 20 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=vitamin+c&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 18 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=lopinavir+%2F+ritonavir&cntry=&state=&city=&dist=. Accessed 17 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=remdesivir&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=. Accessed 17 Aug 2020
- NIH US National Library of Medicine clinical trial database. https://www.clinicaltrials.gov/ct2/show/NCT04286503?term=carrimycin&cond=Covid19&draw=2&rank=1. Accessed 21 Aug 2020
- Ohsugi Y. The immunobiology of humanized anti-IL6 receptor antibody: from basic research to breakthrough medicine. J Transl Autoimmun. 2020;3:100030. doi: 10.1016/j.jtauto.2019.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pécheur EI, Borisevich V, Halfmann P, Morrey JD, Smee DF, Prichard M, Mire CE, Kawaoka Y, Geisbert TW, Polyak SJ. The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. J Virol. 2016;90:3086–3092. doi: 10.1128/JVI.02077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, Nicholls J, Yee WKS, Yan WW, Cheung MT, Cheng VCC, Chan KH, Tsang DNC, Yung RWH, Ng TK, Yuen KY, SARS Study Group Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle S, Schöpf J, Kögl M, Friedel CC, Müller MA, Carbajo-Lozoya J, Stellberger T, von Dall’Armi E, Herzog P, Kallies S, Niemeyer D, Ditt V, Kuri T, Züst R, Pumpor K, Hilgenfeld R, Schwarz F, Zimmer R, Steffen I, Weber F, Thiel V, Herrler G, Thiel HJ, Schwegmann-Wessels C, Pöhlmann S, Haas J, Drosten C, von Brunn A. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011;7:e1002331. doi: 10.1371/journal.ppat.1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillaiyar T, Meenakshisundaram S, Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov Today. 2020;25:668–688. doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo CR., Jr Passive immunization against poliomyelitis: the Hammon gamma globulin field trials, 1951-1953. Am J Public Health. 2005;95:790–799. doi: 10.2105/AJPH.2004.040790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolain J-M, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JR, Curran A, Navarro-Mercade J, Ampuero MF, Pelaez P, Pérez-Alvarez N, Clotet B, Paredes R, Moltó J. Simplification of antiretroviral treatment from darunavir/ritonavir monotherapy to darunavir/cobicistat monotherapy: effectiveness and safety in routine clinical practice. AIDS Res Hum Retroviruses. 2019;35:513–518. doi: 10.1089/AID.2018.0178. [DOI] [PubMed] [Google Scholar]
- Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih H-I, Wu C-J, Tu Y-F, Chi C-Y. Fighting COVID-19: A quick review of diagnoses, therapies, and vaccines. Biomed J. 2020;S2319-4170:30085–300858. doi: 10.1016/j.bj.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, Zhu H, Zhao W, Han Y, Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:piiE59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo YO, Cheng Y, Wong R, Hui DS, Lee CK, Tsang KK, Ng MH, Chan P, Cheng G, Sung JJ. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10:676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrento and Celularity to initiate emergency allogeneic natural killer (NK) cell therapy development for coronavirus infection. https://seekingalpha.com/pr/17762358-sorrento-and-celularity-to-initiate-emergency-allogeneic-naturalkiller-nk-cell-therapy. Accessed 1 Apr 2020
- Srinivasan S, Ghosh M, Maity S, Varadarajan R. Broadly neutralizing antibodies for therapy of viral infections. Antib Technol J. 2016;6:1–15. [Google Scholar]
- Ströher U, DiCaro A, Li Y, Strong JE, Aoki F, Plummer F, Jones SM, Feldmann H. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-alpha. J Infect Dis. 2004;189:1164–1167. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Wong G, Liu Y, Gao GF, Li S, Bi Y. MERS in South Korea and China: a potential outbreak threat? Lancet. 2015;385:2349–2350. doi: 10.1016/S0140-6736(15)60859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Li W, Murakami A, Tamin A, Matthews LJ, Wong SK, Moore MJ, Tallarico AS, Olurinde M, Choe H, Anderson LJ, Bellini WJ, Farzan M, Marasco WA. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci USA. 2004;101:2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen J, Bakker AB, van den Brink EN, Weverling GJ, Martina BE, Haagmans BL, Kuiken T, de Kruif J, Preiser W, Spaan W, Gelderblom HR, Goudsmit J, Osterhaus AD. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363:2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen J, van den Brink EN, Poon LL, Marissen WE, Leung CS, Cox F, Cheung CY, Bakker AQ, Bogaards JA, van Deventer E, Preiser W, Doerr HW, Chow VT, de Kruif J, Peiris JS, Goudsmit J. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3:e237. doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World Health Organization Home. Coronavirus disease 2019 current case numbers. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Accessed 21 Aug 2020
- The World Health Organization Regional Office for Europe. Health topics. Health emergencies. Coronavirus disease (COVID-19) outbreak news. http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic Accessed 18 Apr 2020
- https://www.timesnownews.com/health/article/covid-19-vaccine-tracker-who-says-russian-sputnik-v-yet-to-complete-advanced-trials-updates-of-virus-jabs/636780. Accessed 15 Aug 2020
- Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo MR, Murphy BR, Rappuoli R, Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeki TM. Oseltamivir treatment of influenza in children. Clin Infect Dis. 2018;66:1501–1503. doi: 10.1093/cid/cix1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Griensven J, Edwards T, de Lamballerie X, Semple MG, Gallian P, Baize S, Horby PW, Raoul H, Magassouba N, Antierens A, Lomas C, Faye O, Sall AA, Fransen K, Buyze J, Ravinetto R, Tiberghien P, Claeys Y, De Crop M, Lynen L, Bah EI, Smith PG, Delamou A, De Weggheleire A, Haba N, Ebola-Tx Consortium Evaluation of convalescent plasma for Ebola virus disease in Guinea. N. Engl J Med. 2016;374:33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7:335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiang Y, Zhao C, He W (2019) Use of carrimycin in Mycobacterium tuberculosis infection resistance. China. US20190001160A1, 1 Mar 2019
- Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–8. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4. doi: 10.1186/s40364-018-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel RP, Edmond MB. Managing SARS amidst uncertainty. N. Engl J Med. 2003;348:1947–1948. doi: 10.1056/NEJMp030072. [DOI] [PubMed] [Google Scholar]
- Wong JEL, Leo YS, Tan CC (2020) COVID-19 in Singapore-current experience: critical global issues that require attention and action. JAMA. 10.1001/jama.2020.2467 [DOI] [PubMed]
- Wong SK, Li W, Moore MJ, Choe H, Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SS, Yuen KY. The management of coronavirus infections with particular reference to SARS. J Antimicrob Chemother. 2008;62:437–441. doi: 10.1093/jac/dkn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Xu X, Lu H, Lin X, Hou F, Yu Y, Wang G, Nie L (2004) Analysis of the chest X-ray manifestations in SARS patients treated with compound glycyrrhizin. China Pharmacy 1
- Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-W, Wu X-X, Jiang X-G, Xu K-J, Ying L-J, Ma C-L, Li S-B, Wang H-Y, Zhang S, Gao H-N, Sheng J-F, Cai H-L, Qui Y-Q, Li L-J. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Zou Z, Sun Y, Li X, Xu K-F, Wei Y, Jin N, Jiang C. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Leibowitz JL. The structure and functions of coronavirus genomic 3’ and 5’ ends. Virus Res. 2015;206:120–133. doi: 10.1016/j.virusres.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh KM, Chiueh TS, Siu LK, Lin JC, Chan PK, Peng MY, Wan HL, CHen JH, Hu BS, Perng CL, Lu JJ, Chang FY. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Li Z, Yuan K, Qu X, Chen J, Wang G, Zhang H, Luo H, Zhu L, Jiang P, Chen L, Shen Y, Luo M, Zuo G, Hu J, Duan D, Nie Y, Shi X, Wang W, Han Y, Li T, Liu Y, Ding M, Deng H, Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Cui X, Xiao J, Meng T, Zhou W, Liu J, Xu H (2020) The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. 10.1101/2020.01.30.927806
- Zhang J, Zhou L, Yang Y, Peng W, Wang W, Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8:e11–e12. doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wang L, Deng X, Liang R, Su M, He C, Hu L, Su Y, Ren J, Yu F, Du L, Jiang S. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92:408–417. doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong NS, Zheng BJ, Li YM, Poon LLM, Xie ZH, Chan KH, Li PH, Tan SY, Chang Q, Xie JP, Liu XQ, Xu J, Li DX, Yuen KY, Peiris JSM, Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N. Engl J Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- Ziaei M, Ziaei F, Manzouri B. Systemic cyclosporine and corneal transplantation. Int Ophthalmol. 2016;36:139–146. doi: 10.1007/s10792-015-0137-8. [DOI] [PubMed] [Google Scholar]
- Zorzitto J, Galligan CL, Ueng JJ, Fish EN. Characterization of the antiviral effects of interferon-alpha against a SARS-like coronavirus infection in vitro. Cell Res. 2006;16:220–229. doi: 10.1038/sj.cr.7310030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A, Chan JFW, Azhar EI, Hui DSC, Yuen K-Y. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]