Abstract
Background
Post-stroke depression (PSD) is a neuropsychiatric sequela that causes serious adverse effects on the prognosis of stroke patients. Our developed iPad application is a very innovative approach designed to improve participants' depressive symptoms by presenting positive words stimuli in a video. Although this application has fewer side effects than existing pharmacological and non-pharmacological interventions and is likely less burdensome for patients and caregivers, its efficacy for PSD has not been investigated. Here we present a pilot randomized controlled trial (RCT) protocol to investigate the therapeutic potential of this application intervention for PSD patients.
Methods
This study is designed as a 5-week, single-center, open-label, parallel-group, pilot RCT. Thirty-two patients with PSD will be randomly assigned to a combination of the iPad application and usual rehabilitation or usual rehabilitation alone (1:1 allocation ratio). The iPad application intervention lasts 3 min a day, and the usual rehabilitation lasts 3 h a day. The primary outcome is the change from baseline in The Center for Epidemiologic Studies Depression Scale score at the end of the 5-week intervention.
Discussion
This pilot RCT is the first study to investigate the potential of iPad application interventions to reduce depressive symptoms in PSD patients. This pilot RCT determines whether this is a viable and effective intervention and informs the design for a full-scale trial. If our hypothesis is correct, this trial can provide evidence to augment the standard practice of iPad application interventions to improve depressive symptoms in patients with PSD.
Abbreviations: API, Application Programming Interface; CBT, Cognitive Behavioral Therapy; CRW, Convalescent Rehabilitation Ward; OT, Occupational Therapists; PSD, Post-stroke Depression; PT, Physical Therapists; RCT, Randomized Controlled Trial; rTMS, Repetitive Transcranial Magnetic Stimulation; SPSRS, Subliminal Priming with Supraliminal Reward Stimulation; ST, Speech Therapists
Keywords: Mobile applications, Depression, Stroke, Rehabilitation
Highlights
-
•
PSD is a neuropsychiatric sequela that causes serious adverse effects on the prognosis of stroke patients.
-
•
We present a pilot RCT protocol to investigate the potential of iPad app intervention for PSD patients.
-
•
This trial may provide evidence to augment the standard practice of app interventions for PSD patients.
1. Introduction
Stroke is a serious medical condition that requires long-term rehabilitation (Tam and Bayley, 2018; Villa et al., 2018). Stroke patients have adverse health effects, including paralysis, dysphagia, language problems, and cognitive impairment (Kornfeld et al., 2017; Wang et al., 2018a; Salehi et al., 2019; Baumann et al., 2014; Volk et al., 2019; Chang et al., 2016). In addition, several neuropsychiatric problems may occur after a stroke, particularly post-stroke depression (PSD) (Fishman et al., 2019; Villa et al., 2018; Mitchell et al., 2017; Almeida and Xiao, 2007; Robinson and Jorge, 2016). PSD, a chronic disease with a significant negative impact on the prognosis of stroke patients, is a serious complication after stroke and affects approximately 30% of stroke patients (Tam and Bayley, 2018; Ayerbe et al., 2014; Hackett and Pickles, 2014; Hackett et al., 2005). PSD is also frequently unresponsive to convention treatment, and only 15%–50% of patients are reported to recover one year after PSD onset (Ayerbe et al., 2013; Berg et al., 2003; Kouwenhoven et al., 2011; Lenzi et al., 2008; Whyte and Mulsant, 2002). Clinical manifestations of PSD include depressed mood as the main symptom but also apathy, weight loss or gain, sleep disturbances, fatigue, feelings of worthlessness, and anhedonia (Feng et al., 2014). These symptoms can limit participation in rehabilitation and thus slow or reduce recovery of physical function (Villa et al., 2018; Gillen et al., 2001; Schulte-Altedorneburg and Bereczki, 2014). Additionally, PSD increases mortality, decreases quality of life (QOL), impairs activities of daily living (ADLs), increases care burden, and restricts social participation (Ellis et al., 2010; Williams et al., 2004; Dou et al., 2018; Han et al., 2017; Shi et al., 2016; Schulte-Altedorneburg and Bereczki, 2014; Jiao et al., 2016; Ayerbe et al., 2014; Zhang et al., 2017; Chau et al., 2009; Ezema et al., 2019; Matsuzaki et al., 2015; Gyagenda et al., 2015). Therefore, it is essential to develop safe, practical, and effective interventions to improve depressive symptoms.
The effectiveness of several treatment strategies has been investigated in PSD patients; however, therapeutic strategies have some limitations. Antidepressants are commonly used to treat PSD, but their use increases the risk of adverse events (AEs) (Paolucci, 2017; Hackett et al., 2008). Cognitive behavioral therapy (CBT) is widely used in PSD patients (Wang et al., 2018b). However, CBT requires specialized personnel, increasing cost and limiting the scale of treatment (Zhou et al., 2016). Further, a recent meta-analysis reporting the efficacy of CBT on PSD should be considered preliminary rather than definitive due to substantial heterogeneity across studies and potential bias (Wang et al., 2018b). Repetitive transcranial magnetic stimulation (rTMS) is a newer non-invasive intervention aiming to modulate neural excitability and induce plasticity by magnetic coil stimulation on the scalp (Gu and Chang, 2017; Duan et al., 2018; Slotema et al., 2010). A recent meta-analysis suggested that rTMS has beneficial effects on PSD (Shen et al., 2017). However, since rTMS is a special device, clinical application may be difficult depending on the treatment environment (Bucur and Papagno, 2019).
Given the limitations of these interventions, it is critical to develop new accessible and safe interventions to treat the depressive symptoms and other health problems of PSD patients. Interventions using mobile tablet computer applications can address many of these problems. Mobile tablet computers are widely used in medical practice, and unlike pharmaceuticals (Schooley et al., 2016) are not subject to strict regulations (Neugebauer et al., 2017). Moreover, mobile tablet computers are easily purchased, and there are many inexpensive or free mental health treatment applications (Pugliese et al., 2018). Therefore, these application interventions are likely much easier and less burdensome for patients than existing treatments. While no such applications exist for PSD (Pugliese et al., 2018), several have been reported to improve depressive symptoms in patients with clinical depression and subthreshold depression (Hung et al., 2016; Takahashi et al., 2019b; Ly et al., 2014; Ly et al., 2015; Fitzpatrick et al., 2017). To provide a therapeutic option that is easy to use, less burdensome for patients and caregivers, and with much lower risk of adverse effects, we have developed a motion picture-reproducing application called Subliminal Priming with Supraliminal Reward Stimulation (SPSRS) (Takahashi et al., 2019b). The SPSRS application automatically displays positive words in videos to improve depressive symptoms in patients with subthreshold depression. The SPSRS application allows the user to search for and watch videos using keywords. Additionally, the SPSRS application is free and uses the YouTube Application Programming Interface (API), so videos are plentiful, thus increasing patient enjoyment. Consequently, the SPSRS application is less burdensome for PSD patients and enables the intervention practitioner to perform a unified intervention. Previous studies have shown that the SPSRS application can reduce depressive symptoms in patients with subthreshold depression (Takahashi et al., 2019b). However, there are relatively few studies on the mechanisms underlying specific symptoms or clinical features of PSD (Robinson and Jorge, 2016). While PSD may be associated with unique neurobiological changes, necessitating specifically tailored interventions (Feng et al., 2014), the physical and non-physical symptoms of PSD are broadly similar to those of general depression (de Man-van Ginkel et al., 2015). Therefore, we speculated that the SPSRS application may also improve the depressive symptoms of PSD. However, intervention studies using the SPSRS application have not been performed on PSD patients. To evaluate the SPSRS application for PSD patients, we used the methodological framework of the Medical Research Council (MRC), which includes development, feasibility and piloting, evaluation, and implementation phases (Craig et al., 2008). Feasibility and piloting are presented in this study. Pilot studies are the best way to assess the feasibility of larger-scale and more expensive trials (Thabane et al., 2010). Conducting a pilot study before larger-scale trials also increases the likelihood of success by informing optimal design and scale (Thabane et al., 2010). Therefore, we will conduct a pilot randomized controlled trial (RCT) to investigate the potential of the SPSRS application intervention in PSD patients.
2. Methods
2.1. Trial design and study setting
The study is designed as a 5-week, single-center, open-label, pilot, randomized, parallel-group trial to compare the effectiveness of the iPad application plus usual rehabilitation to usual rehabilitation alone. The participants will be PSD patients admitted to the inpatient convalescent rehabilitation ward (CRW) of Kurashiki Heisei Hospital in Okayama Prefecture, Japan. In Japan, CRWs are settings where stroke patients work to improve ADL performance for home return under the guidance of physicians, nurses, physical therapists (PTs), occupational therapists (OTs), and speech therapists (STs) working in a cooperative and intensive fashion (Miyai et al., 2011). Patients are generally admitted to CRWs within two months of stroke onset, and the maximum stay can be up to six months (Miyai et al., 2011).
This study will follow the SPIRIT statement for conduct of clinical trials (Chan et al., 2013) and has been approved by The Ethics Review Committee of Kurashiki Heisei Hospital. Kurashiki Heisei Hospital is a private institution that specializes in the treatment and rehabilitation of cerebrovascular disorders, musculoskeletal diseases, and disuse syndrome. The CRW in Kurashiki Heisei Hospital contains 91 beds and conducts rehabilitation 365 days a year (i.e., every day) to promote independent ADLs and home discharge. For stroke patients in this CRW, PTs, OTs, and STs are assigned individually by the therapist in charge. If a therapist is absent or unable to intervene, an alternate PT, OT, or ST may provide rehabilitation. There are no specific departments in this hospital to treat mental illness.
2.2. Eligibility criteria
Participants will be selected based on the following eligibility criteria: (1) diagnosed with first stroke, (2) men and women, (3) 40 years or older, (4) Japanese as native language, (5) no cognitive impairment as evidenced by Mini-Mental State Examination-Japanese (MMSE-J) score ≥ 24 points (Sugishita et al., 2018), (6) presence of depression symptoms after stroke as defined by Center for Epidemiologic Studies Depression Scale (CES-D) score ≥ 16 points (Shima et al., 1985), and (7) providing informed consent.
Exclusion criteria are as follows: (1) major depressive disorder before stroke onset, (2) bilateral hemiplegia, (3) vision or hearing deficits that negatively impact everyday life, (4) severe aphasia, (5) severe unilateral spatial neglect, (6) diagnosis of neurodegenerative disorders such as Parkinson's disease and multiple system atrophy, and (7) current life-threatening severe organ failure, musculoskeletal disorders, or cancer.
2.3. Interventions
2.3.1. iPad application
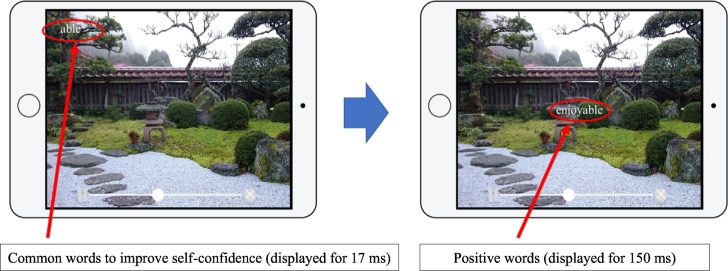
The experiment group in this study will receive a video viewing intervention using the iPad SPSRS application (Takahashi et al., 2019b). The SPSRS application allows users to search for and watch videos using keywords similar to a general video playback application. This SPSRS application uses a YouTube API so participants can freely watch videos on YouTube. The difference between this application and the standard YouTube application is that in all videos, the SPSRS is programmed to display common words to improve self-confidence such as “can”, “let us try”, “good luck”, “able”, and “do not worry” (Takahashi et al., 2019a). These words are randomly displayed subliminally (for 17 ms) in the four corners of the screen. Soon afterwards, positive words such as “nice”, “great”, “fantastic”, “satisfactory”, and “enjoyable” are displayed supraliminally (150 ms) in the middle of the screen (Takarada and Nozaki, 2014). All words are repeatedly displayed every 5 s (See Fig. 1). Therapists will use the SPSRS application according to the operation manual written by the authors. As a rule, the experimental group will receive SPSRS application intervention for approximately 3 min a day, seven days a week, for 5 weeks, for a total intervention time of 105 min or more. In this study, patients will be able to watch their favorite videos using an iPad managed by the therapists. The SPSRS application is filtered so patients cannot watch inappropriate videos published on YouTube. If there is no video that the patient wants to watch, the video will be selected by the therapists at their own discretion.
Fig. 1.
SPSRS application.
All SPSRS intervention viewing times will be recorded through a history function within the application to monitor adherence. All interventional therapists will be trained to use the SPSRS application and can refer to the ‘how-to guide’. Viewing adherence will be recorded once per week.
2.3.2. Usual rehabilitation
The participants in this study will receive a conventional rehabilitation program consisting of physical therapy, occupational therapy, and/or speech therapy as required under the direction of a physician for approximately 3 h every day. The specific rehabilitation protocols will be chosen in accordance with the 2015 Japanese Guidelines for the Management of Stroke (The Japan Stroke Society, 2015). In addition, clinical psychologists will provide support as necessary. Patient reports on conventional rehabilitation will be validated using electronic medical records. There will be no restrictions on conventional treatment for ethical reasons.
2.4. Criteria for discontinuing or modifying allocated interventions
The intervention will be stopped entirely or for individual participants for any of the following reasons:
-
(1)
A participant requests to withdraw from the trial or withdraws consent.
-
(2)
The entire clinical trial is discontinued.
-
(3)
A participant is deemed ineligible after registration.
-
(4)
Frequent AEs or other events that outweigh the potential benefits are observed.
-
(5)
The principal investigator, physicians, or therapists decide a participant should discontinue treatment for any reason.
The date and reason for the cancellation will be fully documented in the case report form to provide information about the feasibility and acceptability of the intervention and improve the standard practice of SPSRS application interventions for future full-scale trials. Whenever possible, the participants will be asked to participate in assessments related to the primary and secondary outcomes for determination of safety and efficacy of the intervention. If a patient refuses to undergo periodic evaluation or withdraws consent, they are considered to have dropped out. The data accrued to that point will be analyzed according to intention-to-treat principles.
All AEs from medical records and observations by the treating therapist will be included in the analysis. When an AE occurs, the investigator will assess and document the event's content, duration, severity, outcome, and relevance to study participation. Details regarding AEs will be immediately reported to the Ethics Review Committee of Kurashiki Heisei Hospital.
2.5. Outcomes
The primary outcome is the change in the CES-D score from baseline (Shima et al., 1985) at the end of the 5-week intervention. The secondary outcomes are the change in scores on the 36-item Short-Form Health Survey version 2 (SF-36v2) (Fukuhara et al., 1998a; Fukuhara et al., 1998b), Functional Reach Test (FRT) (Duncan et al., 1990), and Functional Independence Measure (FIM) (Linacre et al., 1994; Keith et al., 1987) from baseline to the end of the intervention. Intervention safety will be assessed by examining the incidence of AEs.
2.6. Recruitment timeline
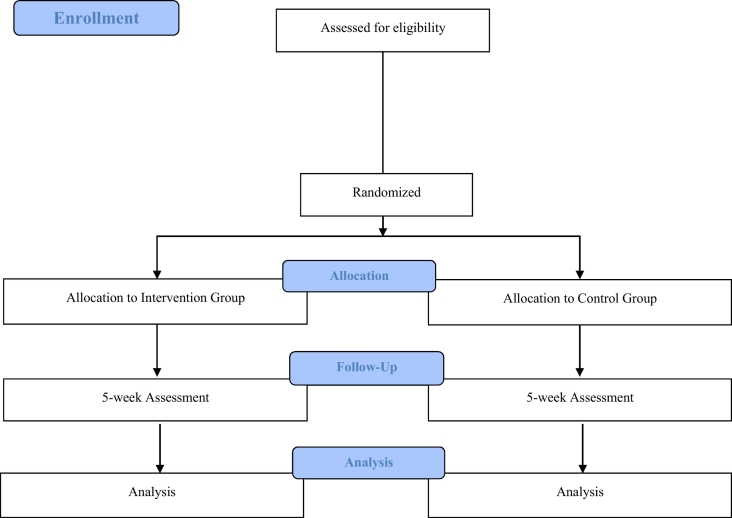
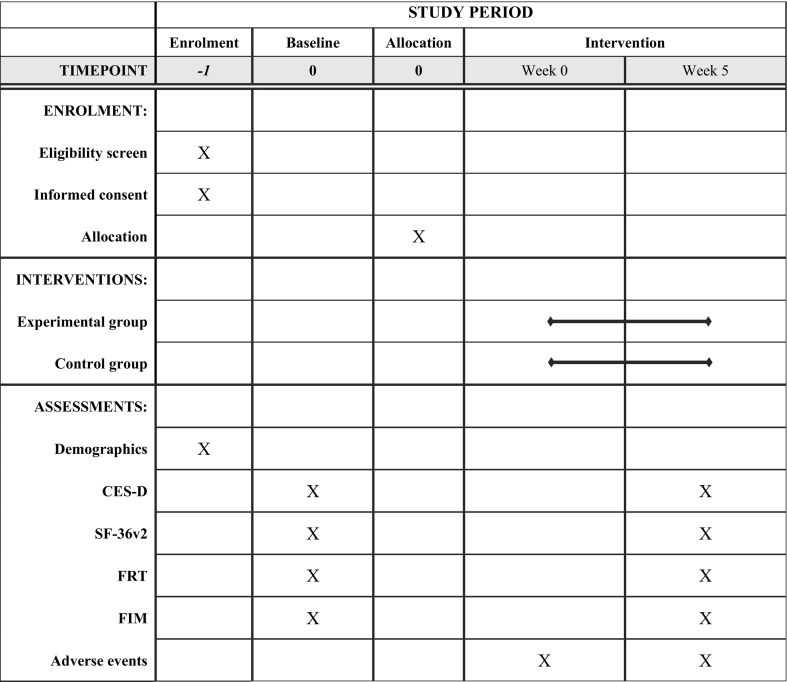
More than 200 stroke patients enter Kurashiki Heisei Hospital annually, so many participants are expected to be invited. The therapist will distribute a brochure that provides a brief description of the trial and then invite patients to participate. Eligible participants will be enrolled after baseline assessment and randomly assigned to the experimental intervention or control group. Recruitment and registration will be completed within a week of entering the CRW. The trial period is shown in the CONSORT diagram (Fig. 2) (Moher et al., 2010) and SPIRIT template (Table 1) (Chan et al., 2013). We estimate two registrations per month between May 2019 and March 2022.
Fig. 2.
CONSORT flowchart of the study design.
Table 1.
The assessment schedule.
CES-D, The Center for Epidemiologic Studies Depression Scale; SF-36v2, The short form 36 health survey version 2; FRT, Functional Reach Test; FIM, Functional Independence Measure.
2.7. Sample size
The calculation of sample size requires four factors: significance level, power, an estimate of the group difference, and an estimated standard deviation (Charles et al., 2009). However, there are no published RCTs on the potential efficacy of the SPSRS application intervention on depressive symptoms in PSD patients, so we have no way of estimating group differences and variances. In fact, one of the main purposes of this pilot study is to provide information on sample size calculations for subsequent full-scale trial implementation (Arain et al., 2010; Thabane et al., 2010). While no formal sample size calculation is required in a pilot trial, a sufficient enrollment number is necessary to determine whether a full-scale study is warranted (Thabane et al., 2010). To estimate effects from pilot studies, 15–20 participants per group are usually recommended (Hertzog, 2008). Therefore, our goal is to recruit 16 participants per group as the minimum sample size. The results of the primary and secondary outcomes in this study will then be used to calculate sample sizes for full-scale trials.
2.8. Allocation
All eligible patients will be randomly assigned in a 1:1 ratio to the experimental or control group at the end of the baseline evaluation by the Central Registry in the Project Management Office at Kibi International University. The allocation sequence will be generated by a third party independent of this study using Excel software and provided to the central registration center. Allocation sequence will be determined according to permuted block randomization of reasonable size to ensure treatment balance. The block size will not be disclosed until the trial is complete to ensure concealment. Throughout this process, patients, raters, and therapists will have no access to information that may reveal their assignments.
2.9. Blinding
It will be difficult to blind participants and therapists due to the explicit nature of the intervention. In addition, this study was designed as an open-label trial because no research staff are necessary to maintain blinding of the outcome assessors. However, baseline assessments will be performed prior to randomization. Furthermore, we will use standardized methods to measure the primary and secondary outcomes and perform procedures to audit and maintain data quality. These processes will reduce the potential for bias resulting from the inability to maintain blinding during the study.
2.10. Data collection
Standardized operating procedure materials and evaluation manuals related to data collection and storage will be provided to the Kurashiki Heisei Hospital, and training sessions will be held. These training sessions will last two hours. In addition, regular meetings will be held to discuss issues related to trial implementation. Outcome assessment will be conducted according to the schedule shown in Table 1.
This study will also collect demographic and other clinical data such as age, sex, body mass index, stroke type, hemiplegia levels (Brunnstrom recovery stages) (Brunnstrom, 1970), period from stroke onset to start of the trial, baseline cognitive function, pre-stroke living arrangement, education level, smoking habit, drinking habit, and presence of heart disease, diabetes mellitus, and hypertension as part of the baseline assessment. In addition, the presence or absence of interventions by clinical psychologists during the trial period will be recorded.
The MMSE-J consists of 10 items assessing orientation, registration, attention or calculation, recall, naming, repetition, comprehension, reading, writing, and construction (Sugishita et al., 2018). The total score ranges from 0 to 30 points with lower score indicative of more severe cognitive dysfunction. A score of 23 or less indicates the possibility of cognitive impairment (Sugishita et al., 2018). The reliability and validity of the MMSE-J has been previously reported (Shigemori et al., 2010; Sugishita et al., 2018).
The CES-D is a 20-item self-report questionnaire used to measure depressive symptoms (Shima et al., 1985). The CES-D consists of four subscales related to positive affect, somatic and retarded activity, depressed affect, and interpersonal problems. Each CES-D item is scored on a four-point Likert scale from 0 to 3. The total score ranges from 0 to 60 points, with higher score indicating stronger depressive symptoms. The cut-off value for the CES-D is 15/16 points (Shima et al., 1985). A score of 16 or higher indicates the possibility of depression. The CES-D is one of the most widely applied indicators of PSD(Meader et al., 2014), and its reliability and validity have been documented (Ohno et al., 2017; Shima et al., 1994; Shima et al., 1985; Meader et al., 2014; Towfighi et al., 2017; Shinar et al., 1986).
The SF-36v2 is a scale used to assess health-related QOL (Fukuhara et al., 1998a; Fukuhara et al., 1998b). This study will use the standard version of the SF-36v2, which consists of eight subscales: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. Three summary scores are calculated from these eight subscales: physical component summary, mental component summary, and role/social component summary. Each subscale and the three summary scores are calculated based on national standard values (50 national standard values and 10 standard deviations). A higher SF-36v2 score corresponds to better health-related QOL. The reliability and validity of the SF-36v2 have been demonstrated for stroke patients (Suzukamo et al., 2011; Fukuhara et al., 1998a; Fukuhara et al., 1998b; Carod-Artal, 2012; Dorman et al., 1998; Hagen et al., 2003; Forsberg and Nilsagård, 2013; Katona et al., 2015).
The FRT evaluates standing balance ability (Duncan et al., 1990) using the GB-200 rolling resistance device (OG Giken, Okayama, Japan). The participants flex their shoulder joints to 90 degrees, extend their fingers, and open both legs to shoulder width. The participants then fix both feet and reach out as far as possible. The change in distance of the third finger in the final posture is measured (cm). Higher FRT values reflect better standing balance. The FRT is measured twice, and the mean is used for analysis. The reliability and validity of the FRT for stroke patients have been demonstrated (Weiner et al., 1992; Merchan-Baeza et al., 2014; Duncan et al., 1992; Duncan et al., 1990; Merchán-Baeza et al., 2014; Martins et al., 2012; Smith et al., 2004).
The FIM measures activity limitations as the amount of assistance required to perform basic physical and cognitive activities by observing participant behaviors (Linacre et al., 1994; Keith et al., 1987). It consists of 13 motor item and 5 cognitive item subscales, each rated on a 7-point scale. Total score ranges from 18 to 126 points, with higher FIM score indicating less assistance needed and thus lower activity limitations. The FIM has documented reliability and validity for assessment of stroke patients (Estrada-Barranco et al., 2019; Dodds et al., 1993; Kucukdeveci et al., 2001; Hsueh et al., 2002; Keith et al., 1987; Gosman-Hedström and Svensson, 2000).
Safety will be defined as the percentage of patients experiencing any AEs during the study period, whether or not considered causally related to the study intervention, including death, life-threatening illnesses, and injuries requiring prolonged hospitalization or resulting in persistent disability. All AEs will be recorded by treating therapists. In addition, any AEs identified from the electronic medical records will be included in the analysis.
2.11. Plan to promote participant retention and complete follow-up
The results of the allocation will be communicated to the participants to increase study transparency. To prevent study dropout, the therapist will explain that the SPSRS interventions and outcome measures are available free of charge to the participants. In addition, the participants will be told that those assigned to the control group can receive the same SPSRS intervention as the experimental group, if necessary, after final outcome measurements. Participants who meet the criteria for discontinuation or who decline the intervention will also be invited to participate in the fifth week assessment. These requests will be emphasized and explained to the participants at the time of informed consent. No financial or physical incentives will be provided to the patients at any point during the study.
2.12. Data management, confidentiality, and access to data
We will use a two-step management system to ensure data accuracy. As the first step, the case report form will be copied to paper. The data will then be entered into an Excel spreadsheet by two independent researchers using a computer that is not connected to the Internet. The entered data will be saved on a USB memory device with password protection. As the management of the second step, the data will be regularly confirmed by researchers other than the first step researchers. All obtained data will be stored safely at Kurashiki Heisei Hospital. Collected paper documents will be stored separately for each participant. Paper materials related to each participant's evaluation will be stored in an evaluation file, and intervention materials will be stored in a separate intervention file. Files that contain the evaluation and intervention forms will be stored on a locked shelf at the Kurashiki Heisei Hospital. Any information identifying the participants will be excluded from the paper file, and other identifying information will be recorded as a participant number to further protect patient privacy. All other documents, including signed informed consent forms, will be stored on separate locked shelves. The paper files will be stored for 5 years after the trial. The USB memory devices(s) will also be stored on a locked shelf. Only data managers and principal investigators will have access to the complete data set prior to the publication of key results. Data managers and principal investigators will examine problems in the data and finalize a data set for statistical analysis. After publication, only the principal investigator and individuals approved by the principal investigator will be allowed access to the dataset. The statistical analysis will use a computer at Kibi International University that is not connected to the Internet.
2.13. Statistical methods
The primary outcome will be estimated from the mean value using linear mixed models (LMMs) with the restricted maximum likelihood estimate method for repeated measurement analysis, and both groups will be compared. LMM is a statistical method suitable for longitudinally designed clinical trials where data may be lost (Gueorguieva and Krystal, 2004). The primary outcome, the CES-D score, is considered a dependent variable. The fixed effect factors are group allocation, assessment time point, and interaction between group allocation and assessment time point. The random effect factors are the participants. We will set the statistical significance to p < 0.05 (two-sided) for all tests. The secondary outcomes will be analyzed in the same way. The safety analysis will use a cross table to compare the occurrence of AEs between groups. A subgroup analysis is not planned at this time. The latest version of SPSS will be used to analyze all data. We will also report the effect size (Hedge's g) between groups (Hedges, 1981; Hedges and Olkin, 1985). According to the intention-to-treat principle, all data from assigned participant will entered into the analysis.
2.14. Data monitoring and auditing
The size of this pilot RCT and the amount of personal information collected will be limited to facilitate analysis and minimize participant risk. Furthermore, no harmful effects or significant results are expected by the end of the intervention. Therefore, no formal data monitoring committee has been assigned and no data auditing is planned at this time. In addition, no interim analysis of intervention impact is planned.
2.15. Ethics and dissemination (approval, protocol amendments, and consent)
This trial has been approved by the Ethics Review Committee of Kurashiki Heisei Hospital (approval number: H30–038) and will be conducted in accordance with The Code of Ethics of the World Medical Association's Declaration of Helsinki. The details of this trial will be explained to the patients by the principal investigator or collaborator before participation using trial information materials. Changing the research plan requires approval from the Ethics Review Committee of Kurashiki Heisei Hospital. The researchers will briefly explain the potential risks and benefits of study participation before the patient enters the study. In addition, it will be documented that the patient understands the risks and benefits of the intervention. Written informed consent will be obtained from patients who have agreed to participate in the study. The signature and date on the informed consent form will be verified before the intervention.
2.16. Ancillary and post-trial care
The participants will be able to contact the researchers during the study period and from the end of participation until discharge. The researchers will observe the progress of the participants after the intervention is completed, and changes in physical condition will be noted. We cannot guarantee that unanticipated complications will not occur during or after completion of the study. In the unlikely event of serious complications or adverse health effects, appropriate measures will be taken according to standard treatment guidelines for adverse health effects during ordinary medical care. Medical expenses will be borne by patients in the same way as ordinary medical care.
2.17. Dissemination policy
The results of this study, whether showing efficacy or no efficacy, will be submitted to a peer-reviewed journal. Further dissemination will be achieved by reporting the findings and scientific significance at various academic society functions.
3. Discussion
PSD limits a patient's involvement in rehabilitation and slows clinical recovery, thereby reducing QOL, functional independence, and social participation (Chau et al., 2009; Zhang et al., 2017; Gyagenda et al., 2015; Matsuzaki et al., 2015; Ezema et al., 2019; Ayerbe et al., 2014; Jiao et al., 2016; Shi et al., 2016; Schulte-Altedorneburg and Bereczki, 2014; Gillen et al., 2001; Villa et al., 2018). Several interventions, such as drug therapy, CBT, and rTMS, have demonstrated efficacy against PSD (Shen et al., 2017; Hackett et al., 2008; Paolucci, 2017; Wang et al., 2018b), but all have side effects, require specialized staff for administration or monitoring, and incur substantial costs both to the patient and the healthcare system (Zhou et al., 2016; Bucur and Papagno, 2019; Paolucci, 2017; Hackett et al., 2008). Our SPSRS application for presenting positive word stimuli on YouTube videos is designed to improve depressive symptoms non-invasively and with little risk of AEs (Takahashi et al., 2019b). This SPSRS application can also provide a uniform intervention and reduce the burden on PSD patients and therapists. In addition, mobile tablet computers are inexpensive (Pugliese et al., 2018) and the SPSRS application is free (Takahashi et al., 2019b), which may allow for broad application for outpatients with limited mobility, rural patients, and patients in less developed countries where the expertise and equipment required for other interventions such as CBT and rTMS are unavailable. Previous studies have shown that the SPSRS application can reduce depressive symptoms in patients with subthreshold depression. It has been suggested that the physical and non-physical symptoms of PSD are broadly similar to those of general depression (de Man-van Ginkel et al., 2015). For this reason, we speculated that the SPSRS application developed for subthreshold depression may improve the depressive symptoms of PSD patients. However, no SPSRS application intervention has yet been implemented for PSD patients. Therefore, the purpose of this pilot RCT was to investigate the potential of a SPSRS intervention consisting of an iPad application aimed at improving depressive symptoms in PSD patients.
The results of this study will provide a foundation for future tablet-based application interventions for PSD patients. After the study is complete, we will be ready to design a full-scale RCT following the template of the pilot study, design a full-scale RCT with modifications, or decide that a full-scale RCT is not possible or warranted. Until positive results are available, it is still possible to prepare for a full-scale RCT, including at other centers managing the care of patients with PSD.
A recent systematic review reported insufficient data to evaluate the efficacy of mobile health applications (McKay et al., 2018). This may lead to potentially incomplete and inaccurate guidance. There are special barriers to conducting RCTs on medical devices, including trial design issues such as randomization, participant acceptance and compliance, blinding, and determining appropriate outcome measures (Neugebauer et al., 2017). Therefore, trials must be carefully designed to minimize the risk of bias. We designed this pilot trial to enhance the scientific validity of any future large-scale study. However, considering medical trial management regulations in Japan, stricter conditions for conducting the trial could not be applied. First, the study will not be blinded, which can bias intervention therapists, participants, and evaluators. Given the nature of the study, it is difficult to blind intervention therapists and participants. In full-scale trials, the evaluator must be blinded. Second, this pilot study will be conducted in only one hospital. Therefore, the findings may not be applicable to all PSD patients (Dechartres et al., 2011). Third, this study is the first to examine the efficacy of the SPSRS application for PSD patients, so no formal sample size calculation was performed (we will use a recommended minimum sample size for conducting pilot studies). This may limit the ability to evaluate group, time, and group × time interaction effects. For these reasons, it is important not to over-interpret the results of this pilot study.
4. Conclusion
This pilot RCT is the first study to investigate the potential of our SPSRS application displaying positive word stimuli in YouTube videos for reducing depressive symptoms in PSD patients. This pilot RCT is based on standardized, robust procedures and is designed according to rigorous methods of scientific investigation. Therefore, we believe the study can determine whether the SPSRS application is a potentially viable and effective intervention device warranting larger-scale studies. Further, this study will provide guidance for the design of such studies. If our hypothesis is correct, this trial may provide evidence that augmenting standard rehabilitation practice with SPSRS application interventions can improve depressive symptoms in patients with PSD.
Trial registration
ClinicalTrials.gov; Identifier: NCT03864484
Funding
This work was supported by JSPS KAKENHI Grant Number 19K19724.
Roles and responsibilities
All authors contributed to the concept and implementation of this clinical trial. HU and KH organized and designed this study. HU created a research plan and organized a research team, created a procedure for the intervention and evaluator, conducted training for the intervention and evaluator, and performed all aspects of study management. KH refined the study protocol and research implementation. HU, NF, YN, and YO are in charge of recruitment monitoring and patient recruitment. HU, KK, and YN are in charge of data management. HU, JK, YH, KK, MI, and SN are in charge of patient recruitment, intervention, and data collection. HU and KH are in charge of the statistical analysis plan. All authors have critically reviewed and approved the final version of the manuscript.
Declaration of competing interest
There are no conflicts of interest in this study.
References
- Almeida O.P., Xiao J. Mortality associated with incident mental health disorders after stroke. Aust N Z J Psychiatry. 2007;41:274–281. doi: 10.1080/00048670601172772. [DOI] [PubMed] [Google Scholar]
- Arain M., Campbell M.J., Cooper C.L., Lancaster G.A. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med. Res. Methodol. 2010;10:67. doi: 10.1186/1471-2288-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayerbe l., Ayis s., Wolfe c.D., Rudd A.G. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br. J. Psychiatry. 2013;202:14–21. doi: 10.1192/bjp.bp.111.107664. [DOI] [PubMed] [Google Scholar]
- Ayerbe L., Ayis S., Crichton S., Wolfe C.D., Rudd A.G. The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J. Neurol. Neurosurg. Psychiatry. 2014;85:514–521. doi: 10.1136/jnnp-2013-306448. [DOI] [PubMed] [Google Scholar]
- Baumann M., LE Bihan E., Chau K., Chau N. Associations between quality of life and socioeconomic factors, functional impairments and dissatisfaction with received information and home-care services among survivors living at home two years after stroke onset. BMC Neurol. 2014;14:92. doi: 10.1186/1471-2377-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A., Palomäki H., Lehtihalmes M., Lönnqvist J., Kaste M. Poststroke depression: an 18-month follow-up. Stroke. 2003;34:138–143. doi: 10.1161/01.str.0000048149.84268.07. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Harper & Row; New York: 1970. Movement Therapy in Hemiplegia: A Neurophysiological Approach. [Google Scholar]
- Bucur M., Papagno C. Are transcranial brain stimulation effects long-lasting in post-stroke aphasia? A comparative systematic review and meta-analysis on naming performance. Neurosci. Biobehav. Rev. 2019;102:264–289. doi: 10.1016/j.neubiorev.2019.04.019. [DOI] [PubMed] [Google Scholar]
- Carod-Artal F.J. Determining quality of life in stroke survivors. Expert Rev Pharmacoecon Outcomes Res. 2012;12:199–211. doi: 10.1586/erp.11.104. [DOI] [PubMed] [Google Scholar]
- Chan A.W., Tetzlaff J.M., Gotzsche P.C., Altman D.G., Mann H., Berlin J.A., Dickersin K., Hrobjartsson A., Schulz K.F., Parulekar W.R., Krleza-Jeric K., Laupacis A., Moher D. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. Bmj. 2013;346 doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, W. H., Sohn, M. K., Lee, J., Kim, D. Y., Lee, S. G., Shin, Y. I., OH, G. J., LEE, Y. S., JOO, M. C., HAN, E. Y., KANG, C. & KIM, Y. H. 2016. Predictors of functional level and quality of life at 6 months after a first-ever stroke: the KOSCO study. J. Neurol., 263, 1166–77. [DOI] [PubMed]
- Charles P., Giraudeau B., Dechartres A., Baron G., Ravaud P. Reporting of sample size calculation in randomised controlled trials: review. Bmj. 2009;338:b1732. doi: 10.1136/bmj.b1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau J.P., Thompson D.R., Twinn S., Chang A.M., Woo J. Determinants of participation restriction among community dwelling stroke survivors: a path analysis. BMC Neurol. 2009;9:49. doi: 10.1186/1471-2377-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P., Dieppe P., Macintyre S., Michie S., Nazareth I., Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Bmj. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechartres A., Boutron I., Trinquart L., Charles P., Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann. Intern. Med. 2011;155:39–51. doi: 10.7326/0003-4819-155-1-201107050-00006. [DOI] [PubMed] [Google Scholar]
- Dodds T.A., Martin D.P., Stolov W.C., Deyo R.A. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch. Phys. Med. Rehabil. 1993;74:531–536. doi: 10.1016/0003-9993(93)90119-u. [DOI] [PubMed] [Google Scholar]
- Dorman P., Slattery J., Farrell B., Dennis M., Sandercock P. Qualitative comparison of the reliability of health status assessments with the EuroQol and SF-36 questionnaires after stroke. United Kingdom Collaborators in the International Stroke Trial. Stroke. 1998;29:63–68. doi: 10.1161/01.str.29.1.63. [DOI] [PubMed] [Google Scholar]
- Dou D.M., Huang L.L., Dou J., Wang X.X., Wang P.X. Post-stroke depression as a predictor of caregivers burden of acute ischemic stroke patients in China. Psychol Health Med. 2018;23:541–547. doi: 10.1080/13548506.2017.1371778. [DOI] [PubMed] [Google Scholar]
- Duan X., Yao G., Liu Z., Cui R., Yang W. Mechanisms of transcranial magnetic stimulation treating on post-stroke depression. Front. Hum. Neurosci. 2018;12:215. doi: 10.3389/fnhum.2018.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan P.W., Weiner D.K., Chandler J., Studenski S. Functional reach: a new clinical measure of balance. J. Gerontol. 1990;45:M192–M197. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]
- Duncan P.W., Studenski S., Chandler J., Prescott B. Functional reach: predictive validity in a sample of elderly male veterans. J. Gerontol. 1992;47:M93–M98. doi: 10.1093/geronj/47.3.m93. [DOI] [PubMed] [Google Scholar]
- Ellis C., Zhao Y., Egede L.E. Depression and increased risk of death in adults with stroke. J. Psychosom. Res. 2010;68:545–551. doi: 10.1016/j.jpsychores.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Barranco C., Cano-De-La-Cuerda R., Molina-Rueda F. Construct validity of the Wisconsin Gait Scale in acute, subacute and chronic stroke. Gait Posture. 2019;68:363–368. doi: 10.1016/j.gaitpost.2018.12.020. [DOI] [PubMed] [Google Scholar]
- Ezema C.I., Akusoba P.C., Nweke M.C., Uchewoke C.U., Agono J., Usoro G. Influence of post-stroke depression on functional Independence in activities of daily living. Ethiop. J. Health Sci. 2019;29:841–846. doi: 10.4314/ejhs.v29i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., Fang M., Liu X.Y. The neurobiological pathogenesis of poststroke depression. ScientificWorldJournal. 2014;2014:521349. doi: 10.1155/2014/521349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman K.N., Ashbaugh A.R., Lanctot K.L., Cayley M.L., Herrmann N., Murray B.J., Sicard M., Lien K., Sahlas D.J., Swartz R.H. The role of apathy and depression on verbal learning and memory performance after stroke. Arch. Clin. Neuropsychol. 2019;34:327–336. doi: 10.1093/arclin/acy044. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick K.K., Darcy A., Vierhile M. Delivering cognitive behavior therapy to young adults with symptoms of depression and anxiety using a fully automated conversational agent (Woebot): A randomized controlled trial. JMIR Ment Health. 2017;4 doi: 10.2196/mental.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg A., Nilsagård Y. Validity and reliability of the Swedish version of the activities-specific balance confidence scale in people with chronic stroke. Physiother. Can. 2013;65:141–147. doi: 10.3138/ptc.2011-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Bito S., Green J., Hsiao A., Kurokawa K. Translation, adaptation, and validation of the SF-36 health survey for use in Japan. J. Clin. Epidemiol. 1998;51:1037–1044. doi: 10.1016/s0895-4356(98)00095-x. [DOI] [PubMed] [Google Scholar]
- Fukuhara, S., Ware, J. E., JR., Kosinski, M., Wada, S. & Gandek, B. 1998b. Psychometric and clinical tests of validity of the Japanese SF-36 health survey. J. Clin. Epidemiol., 51, 1045–53. [DOI] [PubMed]
- Gillen R., Tennen H., Mckee T.E., Gernert-Dott P., Affleck G. Depressive symptoms and history of depression predict rehabilitation efficiency in stroke patients. Arch. Phys. Med. Rehabil. 2001;82:1645–1649. doi: 10.1053/apmr.2001.26249. [DOI] [PubMed] [Google Scholar]
- Gosman-Hedström G., Svensson E. Parallel reliability of the functional independence measure and the Barthel ADL index. Disabil. Rehabil. 2000;22:702–715. doi: 10.1080/09638280050191972. [DOI] [PubMed] [Google Scholar]
- Gu S.Y., Chang M.C. The effects of 10-Hz repetitive transcranial magnetic stimulation on depression in chronic stroke patients. Brain Stimul. 2017;10:270–274. doi: 10.1016/j.brs.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R., Krystal J.H. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch. Gen. Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Gyagenda J.O., Ddumba E., Odokonyero R., Kaddumukasa M., Sajatovic M., Smyth K., Katabira E. Post-stroke depression among stroke survivors attending two hospitals in Kampala Uganda. Afr. Health Sci. 2015;15:1220–1231. doi: 10.4314/ahs.v15i4.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett M.L., Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int. J. Stroke. 2014;9:1017–1025. doi: 10.1111/ijs.12357. [DOI] [PubMed] [Google Scholar]
- Hackett M.L., Yapa C., Parag V., Anderson C.S. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- Hackett, M. L., Anderson, C. S., House, A. & Xia, J. 2008. Interventions for treating depression after stroke. Cochrane Database Syst Rev, Cd003437. [DOI] [PubMed]
- Hagen S., Bugge C., Alexander H. Psychometric properties of the SF-36 in the early post-stroke phase. J. Adv. Nurs. 2003;44:461–468. doi: 10.1046/j.0309-2402.2003.02829.x. [DOI] [PubMed] [Google Scholar]
- Han Y., Liu Y., Zhang X., Tam W., Mao J., Lopez V. Chinese family caregivers of stroke survivors: determinants of caregiving burden within the first six months. J. Clin. Nurs. 2017;26:4558–4566. doi: 10.1111/jocn.13793. [DOI] [PubMed] [Google Scholar]
- Hedges L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981;6:107–128. [Google Scholar]
- Hedges L.V., Olkin I. Academic Press; New York: 1985. Statistical Methods for Meta-Analysis. [Google Scholar]
- Hertzog M.A. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31:180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- Hsueh I.P., Lin J.H., Jeng J.S., Hsieh C.L. Comparison of the psychometric characteristics of the functional independence measure, 5 item Barthel index, and 10 item Barthel index in patients with stroke. J. Neurol. Neurosurg. Psychiatry. 2002;73:188–190. doi: 10.1136/jnnp.73.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung S., Li M.S., Chen Y.L., Chiang J.H., Chen Y.Y., Hung G.C. Smartphone-based ecological momentary assessment for Chinese patients with depression: an exploratory study in Taiwan. Asian J. Psychiatr. 2016;23:131–136. doi: 10.1016/j.ajp.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Jiao, J. T., Cheng, C., Ma, Y. J., Huang, J., DAI, M. C., JIANG, C., WANG, C. & SHAO, J. F. 2016. Association between inflammatory cytokines and the risk of post-stroke depression, and the effect of depression on outcomes of patients with ischemic stroke in a 2-year prospective study. Exp Ther Med, 12, 1591–1598. [DOI] [PMC free article] [PubMed]
- Katona M., Schmidt R., Schupp W., Graessel E. Predictors of health-related quality of life in stroke patients after neurological inpatient rehabilitation: a prospective study. Health Qual. Life Outcomes. 2015;13:58. doi: 10.1186/s12955-015-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith R.A., Granger C.V., Hamilton B.B., Sherwin F.S. The functional independence measure: a new tool for rehabilitation. Adv. Clin. Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- Kornfeld S., Studer M., Winkelbeiner S., Regenyi M., Boltshauser E., Steinlin M. Quality of life after paediatric ischaemic stroke. Dev. Med. Child Neurol. 2017;59:45–51. doi: 10.1111/dmcn.13295. [DOI] [PubMed] [Google Scholar]
- Kouwenhoven S.E., Kirkevold M., Engedal K., Kim H.S. Depression in acute stroke: prevalence, dominant symptoms and associated factors. A systematic literature review. Disabil. Rehabil. 2011;33:539–556. doi: 10.3109/09638288.2010.505997. [DOI] [PubMed] [Google Scholar]
- Kucukdeveci A.A., Yavuzer G., Elhan A.H., Sonel B., Tennant A. Adaptation of the functional Independence measure for use in Turkey. Clin. Rehabil. 2001;15:311–319. doi: 10.1191/026921501676877265. [DOI] [PubMed] [Google Scholar]
- Lenzi G.L., Altieri M., Maestrini I. Post-stroke depression. Rev. Neurol. (Paris) 2008;164:837–840. doi: 10.1016/j.neurol.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Linacre J.M., Heinemann A.W., Wright B.D., Granger C.V., Hamilton B.B. The structure and stability of the functional Independence measure. Arch. Phys. Med. Rehabil. 1994;75:127–132. [PubMed] [Google Scholar]
- Ly, K. H., Truschel, A., Jarl, L., Magnusson, S., Windahl, T., Johansson, R., Carlbring, P. & Andersson, G. 2014. Behavioural activation versus mindfulness-based guided self-help treatment administered through a smartphone application: a randomised controlled trial. BMJ Open, 4, e003440. [DOI] [PMC free article] [PubMed]
- Ly, K. H., Topooco, N., Cederlund, H., Wallin, A., BERGSTROM, J., MOLANDER, O., CARLBRING, P. & ANDERSSON, G. 2015. Smartphone-supported versus full Behavioural activation for depression: A randomised controlled trial. PLoS One, 10, e0126559. [DOI] [PMC free article] [PubMed]
- de Man-van Ginkel J.M., Hafsteinsdóttir T.B., Lindeman E., Geerlings M.I., Grobbee D.E., Schuurmans M.J. Clinical manifestation of depression after stroke: is it different from depression in other patient populations? PLoS One. 2015;10 doi: 10.1371/journal.pone.0144450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins E.F., DE Menezes L.T., DE Sousa P.H., De Araujo Barbosa P.H., Costa A.S. Reliability of the functional reach test and the influence of anthropometric characteristics on test results in subjects with hemiparesis. NeuroRehabilitation. 2012;31:161–169. doi: 10.3233/NRE-2012-0786. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S., Hashimoto M., Yuki S., Koyama A., Hirata Y., Ikeda M. The relationship between post-stroke depression and physical recovery. J. Affect. Disord. 2015;176:56–60. doi: 10.1016/j.jad.2015.01.020. [DOI] [PubMed] [Google Scholar]
- Mckay F.H., Cheng C., Wright A., Shill J., Stephens H., Uccellini M. Evaluating mobile phone applications for health behaviour change: A systematic review. J. Telemed. Telecare. 2018;24:22–30. doi: 10.1177/1357633X16673538. [DOI] [PubMed] [Google Scholar]
- Meader N., Moe-Byrne T., Llewellyn A., Mitchell A.J. Screening for poststroke major depression: a meta-analysis of diagnostic validity studies. J. Neurol. Neurosurg. Psychiatry. 2014;85:198–206. doi: 10.1136/jnnp-2012-304194. [DOI] [PubMed] [Google Scholar]
- Merchán-Baeza J.A., González-Sánchez M., Cuesta-Vargas A.I. Reliability in the parameterization of the functional reach test in elderly stroke patients: a pilot study. Biomed. Res. Int. 2014;2014:637671. doi: 10.1155/2014/637671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan-Baeza J.A., Gonzalez-Sanchez M., Cuesta-Vargas A.I. Reliability in the parameterization of the functional reach test in elderly stroke patients: a pilot study. Biomed. Res. Int. 2014;2014:637671. doi: 10.1155/2014/637671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A.J., Sheth B., Gill J., Yadegarfar M., Stubbs B., Yadegarfar M., Meader N. Prevalence and predictors of post-stroke mood disorders: A meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen. Hosp. Psychiatry. 2017;47:48–60. doi: 10.1016/j.genhosppsych.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Miyai I., Sonoda S., Nagai S., Takayama Y., Inoue Y., Kakehi A., Kurihara M., Ishikawa M. Results of new policies for inpatient rehabilitation coverage in Japan. Neurorehabil. Neural Repair. 2011;25:540–547. doi: 10.1177/1545968311402696. [DOI] [PubMed] [Google Scholar]
- Moher D., Hopewell S., Schulz K.F., Montori V., Gotzsche P.C., Devereaux P.J., Elbourne D., Egger M., Altman D.G. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Bmj. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer E.A.M., Rath A., Antoine S.L., Eikermann M., Seidel D., Koenen C., Jacobs E., Pieper D., Laville M., Pitel S., Martinho C., Djurisic S., Demotes-Mainard J., Kubiak C., Bertele V., Jakobsen J.C., Garattini S., Gluud C. Specific barriers to the conduct of randomised clinical trials on medical devices. Trials. 2017;18:427. doi: 10.1186/s13063-017-2168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Takahashi K., Inoue A., Takada K., Ishihara Y., Tanigawa M., Hirao K. Smallest detectable change and test-retest reliability of a self-reported outcome measure: results of the Center for Epidemiologic Studies Depression Scale, general self-efficacy scale, and 12-item general health questionnaire. J. Eval. Clin. Pract. 2017;23:1348–1354. doi: 10.1111/jep.12795. [DOI] [PubMed] [Google Scholar]
- Paolucci S. Advances in antidepressants for treating post-stroke depression. Expert. Opin. Pharmacother. 2017;18:1011–1017. doi: 10.1080/14656566.2017.1334765. [DOI] [PubMed] [Google Scholar]
- Pugliese M., Ramsay T., Johnson D., Dowlatshahi D. Mobile tablet-based therapies following stroke: A systematic scoping review of administrative methods and patient experiences. PLoS One. 2018;13 doi: 10.1371/journal.pone.0191566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R.G., Jorge R.E. Post-stroke depression: a review. Am. J. Psychiatry. 2016;173:221–231. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- Salehi S., Tahan N., Bagheban A.A., Monfared M.E. 2019. Quality of Life Within Three Months After Stroke: A Study in the City of Arak. (Iran. J Natl Med Assoc) [DOI] [PubMed] [Google Scholar]
- Schooley B., Walczak S., Hikmet N., Patel N. Impacts of mobile tablet computing on provider productivity, communications, and the process of care. Int. J. Med. Inform. 2016;88:62–70. doi: 10.1016/j.ijmedinf.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Schulte-Altedorneburg M., Bereczki D. Post-stroke depression. Orv. Hetil. 2014;155:1335–1343. doi: 10.1556/OH.2014.29968. [DOI] [PubMed] [Google Scholar]
- Shen X., Liu M., Cheng Y., Jia C., Pan X., Gou Q., Liu X., Cao H., Zhang L. Repetitive transcranial magnetic stimulation for the treatment of post-stroke depression: A systematic review and meta-analysis of randomized controlled clinical trials. J. Affect. Disord. 2017;211:65–74. doi: 10.1016/j.jad.2016.12.058. [DOI] [PubMed] [Google Scholar]
- Shi Y.Z., Xiang Y.T., Yang Y., Zhang N., Wang S., Ungvari G.S., Chiu H.F., Tang W.K., Wang Y.L., Zhao X.Q., Wang Y.J., Wang C.X. Depression after minor stroke: the association with disability and quality of life--a 1-year follow-up study. Int J Geriatr Psychiatry. 2016;31:421–427. doi: 10.1002/gps.4353. [DOI] [PubMed] [Google Scholar]
- Shigemori K., Ohgi S., Okuyama E., Shimura T., Schneider E. The factorial structure of the Mini-Mental State Examination (MMSE) in Japanese dementia patients. BMC Geriatr. 2010;10:36. doi: 10.1186/1471-2318-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima S., Shikano T., Kitamura T., Asai M. New self-rating scale for depression. Seishin Igaku (Clinical Psychiatry) 1985;27:717–723. [Google Scholar]
- Shima S., Kitagawa Y., Kitamura T., Fujinawa A., Watanabe Y. Poststroke depression. Gen. Hosp. Psychiatry. 1994;16:286–289. doi: 10.1016/0163-8343(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Shinar D., Gross C.R., Price T.R., Banko M., Bolduc P.L., Robinson R.G. Screening for depression in stroke patients: the reliability and validity of the Center for Epidemiologic Studies Depression Scale. Stroke. 1986;17:241–245. doi: 10.1161/01.str.17.2.241. [DOI] [PubMed] [Google Scholar]
- Slotema C.W., Blom J.D., Hoek H.W., Sommer I.E. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873–884. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- Smith P.S., Hembree J.A., Thompson M.E. Berg balance scale and functional reach: determining the best clinical tool for individuals post acute stroke. Clin. Rehabil. 2004;18:811–818. doi: 10.1191/0269215504cr817oa. [DOI] [PubMed] [Google Scholar]
- Sugishita, M., Koshizuka, Y., Sudou, S., Sugishita, K., Hemmi, I., Karasawa, H., Ihara, M., Asada, T. & Mihara, B. 2018. The Validity and Reliability of the Japanese Version of the Mini-Mental State Examination (MMSE-J) with the original procedure of the Attention and Calculation Task(2001). Japanese Journal of Cognitive Neuroscience, 20, 91–110.
- Suzukamo Y., Fukuhara S., Green J., Kosinski M., Gandek B., Ware J.E. Validation testing of a three-component model of short Form-36 scores. J. Clin. Epidemiol. 2011;64:301–308. doi: 10.1016/j.jclinepi.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Takada K., Inoue A., Ohno S., Tanigawa M., Ishihara Y., Uchida H., Hirao K. Identification of common words to improve self-confidence in Japanese students with subthreshold depression. Int J Adolesc Med Health. 2019;31(3):1–7. doi: 10.1515/ijamh-2017-0018. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Takada K., Hirao K. Feasibility and preliminary efficacy of a smartphone application intervention for subthreshold depression. Early Interv Psychiatry. 2019;13:133–136. doi: 10.1111/eip.12540. [DOI] [PubMed] [Google Scholar]
- Takarada Y., Nozaki D. Maximal voluntary force strengthened by the enhancement of motor system state through barely visible priming words with reward. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A.K.H., Bayley M.T. A narrative review of the impact of medical comorbidities on stroke rehabilitation outcomes. Disabil. Rehabil. 2018;40:1842–1848. doi: 10.1080/09638288.2017.1309465. [DOI] [PubMed] [Google Scholar]
- Thabane, L., Ma, J., Chu, R., Cheng, J., Ismaila, A., Rios, L. P., Robson, R., Thabane, M., Giangregorio, L. & Goldsmith, C. H. 2010. A tutorial on pilot studies: the what, why and how. BMC Med. Res. Methodol., 10, 1. [DOI] [PMC free article] [PubMed]
- The Japan Stroke Society . Tokyo; Kyowa kikaku: 2015. Japanese Guidelines for the Management of Stroke 2015. [Google Scholar]
- Towfighi A., Ovbiagele B., EL Husseini N., Hackett M.L., Jorge R.E., Kissela B.M., Mitchell P.H., Skolarus L.E., Whooley M.A., Williams L.S. Poststroke depression: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e30–e43. doi: 10.1161/STR.0000000000000113. [DOI] [PubMed] [Google Scholar]
- Villa R.F., Ferrari F., Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol. Ther. 2018;184:131–144. doi: 10.1016/j.pharmthera.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Volk G.F., Steinerstauch A., Lorenz A., Modersohn L., Mothes O., Denzler J., Klingner C.M., Hamzei F., Guntinas-Lichius O. Facial motor and non-motor disabilities in patients with central facial paresis: a prospective cohort study. J. Neurol. 2019;266:46–56. doi: 10.1007/s00415-018-9099-x. [DOI] [PubMed] [Google Scholar]
- Wang J., Li W., Li Y., Jin X., Niu Y., Tian X., Huo H. A novel endoscopic surgery for dysphagia after stroke. Surg. Endosc. 2018;32:127–133. doi: 10.1007/s00464-017-5647-1. [DOI] [PubMed] [Google Scholar]
- Wang S.B., Wang Y.Y., Zhang Q.E., Wu S.L., Ng C.H., Ungvari G.S., Chen L., Wang C.X., Jia F.J., Xiang Y.T. Cognitive behavioral therapy for post-stroke depression: a meta-analysis. J. Affect. Disord. 2018;235:589–596. doi: 10.1016/j.jad.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Weiner D.K., Duncan P.W., Chandler J., Studenski S.A. Functional reach: a marker of physical frailty. J. Am. Geriatr. Soc. 1992;40:203–207. doi: 10.1111/j.1532-5415.1992.tb02068.x. [DOI] [PubMed] [Google Scholar]
- Whyte E.M., Mulsant B.H. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol. Psychiatry. 2002;52:253–264. doi: 10.1016/s0006-3223(02)01424-5. [DOI] [PubMed] [Google Scholar]
- Williams L.S., Ghose S.S., Swindle R.W. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am. J. Psychiatry. 2004;161:1090–1095. doi: 10.1176/appi.ajp.161.6.1090. [DOI] [PubMed] [Google Scholar]
- Zhang, L., Sui, M., Yan, T., You, L., Li, K. & Gao, Y. 2017. A study in persons later after stroke of the relationships between social participation, environmental factors and depression. Clin. Rehabil., 31, 394–402. [DOI] [PubMed]
- Zhou, T., Li, X., Pei, Y., Gao, J. & Kong, J. 2016. Internet-based cognitive behavioural therapy for subthreshold depression: a systematic review and meta-analysis. BMC Psychiatry, 16, 356. [DOI] [PMC free article] [PubMed]