Abstract
Background
Aromatase inhibitors (AIs) are the preferred endocrine treatment for postmenopausal hormonal receptor-positive breast cancer. However, there is controversy on the long-term cardiovascular and cerebrovascular safety of AIs over that of tamoxifen.
Methods
We analyzed the National Health Information Database (NHID) of 281,255 women over a 20-year-old diagnosed with breast cancer between 2009 and 2016. Cardiovascular events (CVEs) were defined as the development of the following, acute coronary syndrome (ACS), ischemic and hemorrhagic stroke, defined by using insurance claim records. The model was constructed by Cox proportional hazard regression and this model was used to analyze the effects of AI and tamoxifen on CVE.
Results
We included 47,569 women for the final analysis. Patients were classified into ‘No hormonal treatment (n = 18,807), ‘Switch (n = 2097)’, ‘Tamoxifen (n = 7081)’ and ‘AI (n = 19,584)’. There were 2147 CVEs in 2032 patients (4.1%). Univariate analysis showed that women with tamoxifen had significantly lower risk for CVEs compared to no-treatment (hazard ratio (HR) 0.84, 95% confidence interval (CI) 0.74–0.97) while AI showed no such effect (HR 0.93, 95% CI 0.84–1.02). After adjusting for other risk factors (hypertension, dyslipidemia, family history), the use of tamoxifen was associated with significant protective effect against ACS (HR 0.63, 95% CI 0.47–0.84).
Conclusions
Our results, based on the NHID, supports the protective effect of tamoxifen against CVE in Korean breast cancer patients aged 55 and older that is not seen with AIs. Our results can guide the selection of adjuvant hormonal treatment agents for Korean breast cancer patients based on their risk of developing CVE.
Keywords: Breast cancer, Aromatase inhibitors, Tamoxifen, Cardiovascular disease, Cerebrovascular disease
Highlights
-
•
Controversy on the long-term cardiovascular and cerebrovascular safety of AIs over that of tamoxifen.
-
•
The protective effect of tamoxifen against cardiovascular events in elderly breast cancer patients that is not seen with AIs.
-
•
Choice of hormonal therapy depends on patient’s cardiovascular or cerebrovascular risks.
Introduction
More than 60% of all breast cancer patients have hormone-responsive tumors, defined by either estrogen or progesterone receptor expression, for whom adjuvant endocrine therapy can significantly reduce tumor recurrences [1,2]. For postmenopausal women with hormone-responsive breast cancer, the use of aromatase inhibitor is preferred over tamoxifen due to the lower rates of breast cancer recurrence and mortality [3,4]. However, there has been increasing concerns on the issue of cardiovascular or cerebrovascular effect of aromatase inhibitors as cardiovascular events (CVEs) are the major cause of death in breast cancer patients who remain free of the disease [5]. One major basis of this concern is that aromatase inhibitors can be associated with higher incidence of lipid metabolism disorders when compared to tamoxifen [6,7].
Several meta-analyses of randomized trials have shown the potential association between the use of aromatase inhibitors and increased risk of CVEs [[10], [11], [8], [9]]. The increased risk of CVEs in women taking aromatase inhibitor can be the results of either the cardio-protective effect of tamoxifen or the intrinsic cardiotoxic effect of aromatase inhibitors [10,11]. However, the randomized trials of aromatase inhibitors have heterogeneous definitions and report forms for CVEs, and the documented numbers of serious cardiac events are quite low since they are not the primary endpoints [6]. To overcome these issues by examining insurance claim records or patients’ medical records, several population-based or multi-institutional retrospective analyses were performed but the findings are not consistent in terms of the cardio-protective effect of tamoxifen or the relative risk of CVEs with aromatase inhibitors [[12], [13], [14], [15], [16]].
In the present study, we aimed to determine the potential effect of tamoxifen or aromatase inhibitors on CVEs in elderly women with breast cancer by using the Korean nationwide insurance database.
Material and methods
The National Health Information Database (NHID) is a public database formed by the National Health Insurance Service (NHIS) of Korea in 2012. National Health Insurance program is a legally designated health care system that provide health care coverage for all Korean citizens. The NHIS, as the single insurer for this National Health Insurance program, pays costs based on the billing records of health care providers, and therefore, the NHID contains records on all medical service and diagnostic information [17]. NHIS also provides biennial health examination screening service for Korean citizens over the age of 40 of which the information is stored in NHIS Health Examination Database. In 2014, more than 70% of the candidates for health examination screening used the service [17]. The information on family history, past medical history, and the use of medical prescription is collected during the health examination screening process as a non-mandatory questionnaire. NHIS provides NHID raw data for biomedical research purposes in anonymized forms.
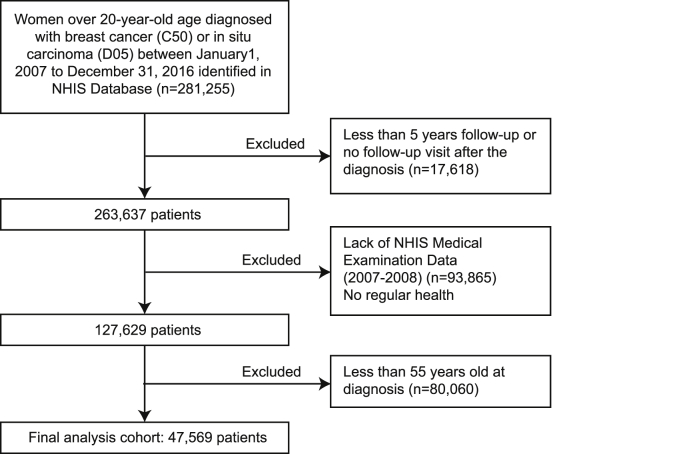
For the present study, the following inclusion criteria were used to identify the study population; 1) patients with the International Classification of Disease 10th edition (ICD-10) codes for breast malignancy, 2) patients diagnosed after the Jan 2009 since the NHIS Health Examination questionnaire has undergone a major modification in 2009, 3) patients with available NHIS Health Examination Data, 4) patients without pre-existing history of acute coronary syndrome (ACS) or stroke before the diagnosis of the breast malignancies as the newly developed events can be independent of the endocrine treatment in these patients and the aforementioned history can also affect the clinician’s choice of endocrine treatment options, and 5) patients who completed at least 5 years’ follow-up after the initial treatment (Fig. 1). The information on the pathologic characteristics of tumors such as molecular subtypes or stages and the data on disease recurrence were not available in the NHIS database. The information on the treatment, including the date for initial treatment, the use of various adjuvant therapeutic agents, for breast malignancies were obtained from the NHID dataset. We merged the identified NHID dataset with NHIS Health Examination database to obtain information on the family history, past medical history of diabetes hypercholesterolemia, or hypertension, and the use of medication for diabetes, hypercholesterolemia, or hypertension. Patients were considered to be hypertensive if they had diastolic blood pressures ≧90 mmHg or systolic ≧140 mmHg, or the use of antihypertensive medications. Diabetes was defined when a fasting blood glucose level ≧126 mg/dL or the use of antidiabetic medications. In the case of hypercholesterolemia, it was defined as the case where the concentration of low-density lipoprotein (LDL) cholesterol ≧130 mg/dL or the use of lipid-lowering agents. In our study, body mass index (BMI, kg/m2) was assigned as a continuous variable. The family history of heart disease and stroke were defined when the subject answered as “yes” to the corresponding question in the health screening questionnaire.
Fig. 1.
Patients selection from National Health Insurance Service of Korea.
For the outcomes, we collected information on the occurrence of ACS (ICD code I20–I25), ischemic stroke (IS, ICD code I63), and hemorrhagic stroke (HS, ICD code I60–I62). We defined the event based on the ICD code category and subcategory as well as on the insurance claim data of medical procedures such as angioplasty or bypass surgery. Continuous data were expressed as median with ranges and discrete data as numbers and percentages. The event-free survival was defined as the time interval between the date of the diagnosis of breast malignancies and the date of event occurrence. We defined the events as the first occurring CVEs and the recurrent events were not accounted. The follow-up duration for patients who did not develop events was between the date of diagnosis and the end of Dec 2016. We did not consider a separate time-varying Cox because the method of treatment for breast cancer is determined immediately after surgery depending on the subtype and surgical method. We calculated the cumulative probabilities of incidence of CVEs using the Kaplan-Meier method. Differences between Kaplan-Meier curves were compared using a log-rank test. The Cox proportional hazards model was used to quantify the effects of different treatments adjusted by the aforementioned covariates. The assumptions of proportionality were verified by comparing log-log survival curves. Statistical analysis was performed with the use of SAS 9.4 version (SAS Institute Inc. 2010) and R 3.5.0 version (R Core Team 2013) statistical software.
The institutional review board of the Seoul National University Hospital has waived the need to obtain informed consents for this analysis (IRB waiver No: E−1707-008-865).
2. Results
From the NHIS Database, we identified a total of 204,856 women with the diagnosis of malignant breast diseases between Jan 2009 and Dec 2016. After excluding cases of limited follow-up, lack of NHIS Medical Examination Data, or age information, we included 47,569 women for the final analysis (Fig. 1). According to the type of the endocrine treatments, patients were classified into ‘No hormonal treatment group (n = 18,807)’, ‘Switch group (n = 2097)’, ‘Tamoxifen group (n = 7081)’ and ‘Aromatase inhibitor group (n = 19,584)’. The demographic information of the studied patients is listed in Table 1.
Table 1.
Characteristics of the patients.
| Overall | No treatment | Switch | Tamoxifen | AIa | |
|---|---|---|---|---|---|
| Age (years) | 63.3 ± 6.9 | 63.2 ± 7.0 | 63.3 ± 7.0 | 63.8 ± 7.2 | 63.3 ± 6.6 |
| Types of hormonal therapy (n, %) | 47569 | 18807 (39.5) | 2097 (4.4) | 7081 (14.9) | 19584 (41.2) |
| Median time between diagnosis and initial treatment (months) | 3.5 | 1.8 | 5.8 | ||
| Exposure duration of therapy (years) | 3.6 ± 1.7 | 2.8 ± 1.7 | 3.0 ± 1.7 | ||
| BMIb (kg/m2) | 24.4 ± 3.0 | 24.1 ± 3.0 | 24.5 ± 2.9 | 24.3 ± 3.0 | 24.6 ± 3.0 |
| SBPc (mm Hg) | 125.4 ± 15.4 | 124.9 ± 15.5 | 125.7 ± 14.6 | 125.3 ± 15.0 | 125.9 ± 15.5 |
| DBPd (mm Hg) | 76.5 ± 9.8 | 76.2 ± 9.9 | 76.7 ± 9.5 | 76.4 ± 9.8 | 76.9 ± 9.9 |
| FBSe (mg/dL) | 99.5 ± 17.6 | 99.2 ± 18.2 | 100.1 ± 16.9 | 98.5 ± 16.7 | 100.2 ± 17.5 |
| LDLf (mg/dL) | 122.5 ± 46.7 | 123.4 ± 44.7 | 119.5 ± 39.5 | 120.5 ± 51.6 | 122.9 ± 47.5 |
| Hypertension (n, %) | 19934 (41.9) | 7489 (39.9) | 920 (43.9) | 2983 (42.1) | 8542 (43.6) |
| Diabetes (n, %) | 6688 (14.1) | 2505 (13.3) | 338 (16.1) | 958 (13.5) | 2887 (14.7) |
| Hyperlipidemia (n, %) | 21724 (45.7) | 8731 (46.4) | 915 (43.6) | 3016 (42.6) | 9062 (46.3) |
| Family history of stroke (n, %) | 3951 (8.3) | 1535 (8.2) | 165 (7.9) | 573 (8.1) | 1678 (8.6) |
| Family history of hypertension (n, %) | 1919 (4.0) | 788 (4.2) | 88 (4.2) | 273 (3.9) | 770 (3.9) |
AI, aromatase inhibitor.
BMI, body mass index.
SBP, systolic blood pressure.
DBP, diastolic blood pressure.
FBS, fasting blood sugar.
LDL, low density lipoprotein cholesterol.
The median interval from diagnosis of breast cancer to the start of the treatment was 3.5 months in the switch group, 1.8 months in the tamoxifen group and 5.8 months in the Aromatase inhibitor group. It is also listed in Table 1.
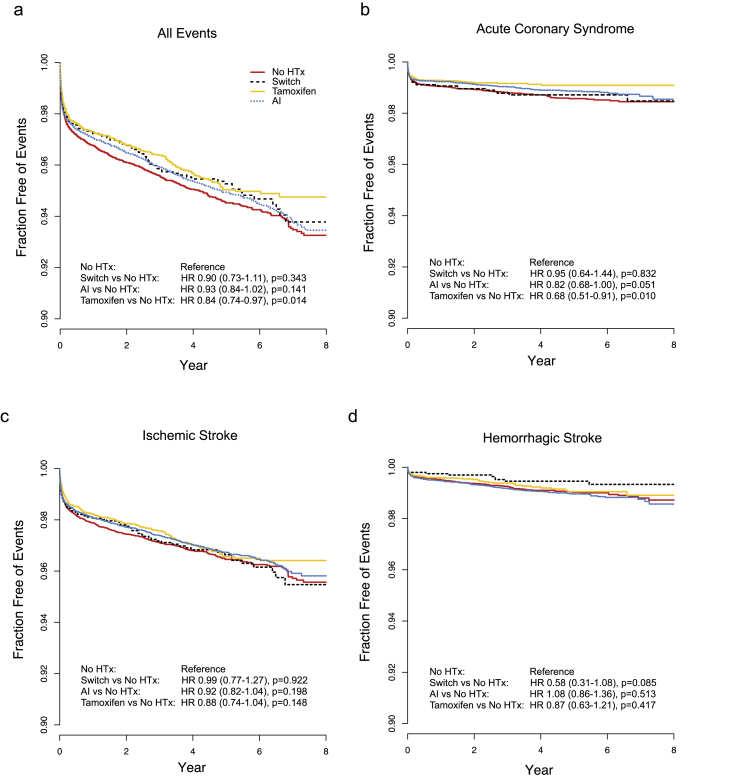
During the study period, a total of 2147 CVEs occurred in 2032 patients during their follow-up with 5-year cumulative rate of 4.1% (Supplementary Table 1). Seven patients had both ACS and HS, 55 had both ACS and IS, 55 patients had both HS and IS, and two patients had all three CVEs. IS was the most frequent event, followed by ACS and HS. Survival analysis on the event-free survival showed that patients treated with tamoxifen had significantly lower rates of CVEs when compared to patient who did not receive any hormonal treatment (Fig. 2a). There was no significant difference in event-free survival for patients with aromatase inhibitor or switch regimen compared to patients with no hormonal treatment. The significant protective effect of tamoxifen was only observed against ACS with 32% risk reduction while it was not significant for either HS or IS (Fig. 2b–d).
Fig. 2.
Survival curves for cardiovascular or cerebrovascular events. Kaplan-Meier curves for all cardiovascular and cerebrovascular events (a), acute coronary syndrome (b), ischemic stroke (c), and hemorrhagic stroke (d) is shown. P values and hazard ratios are calculated from log-rank test and univariate Cox regression analysis with no treatment group as reference group, respectively.
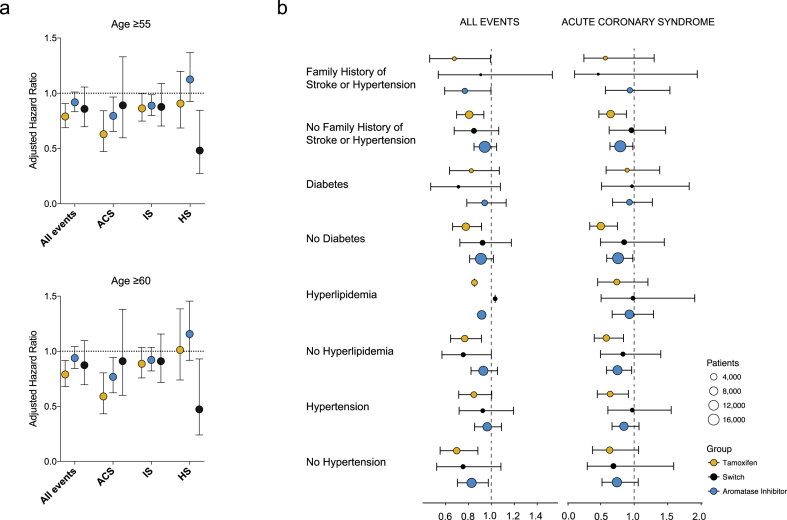
From the NHIS Database and NHIS Medical Examination Data, we were able to extract information on the potential risk factors for developing CVEs. Multivariate analysis using Cox proportional regression model has identified age, diabetes, hypertension, and family history of stroke of hypertensive diseases were independent risk factors for CVEs in breast cancer patients (Supplementary Table 2). After adjusting these factors, the use of tamoxifen was still significantly associated with decreased risk of developing CVEs (Fig. 3a, Supplementary Table 3). The protective effect of tamoxifen was strongest ACS with 36% risk reduction (p = 0.002, HR 0.630, 95% C.I. 0.472–0.841). Additionally, tamoxifen showed marginal protective effect against IS (p = 0.023, HR 0.863, 95% C.I. 0.748–0.996). Aromatase inhibitor was associated with marginal protective effect against ACS when compared to no hormonal treatment group (p = 0.041, HR 0.888, 95% C.I. 0.799–0.987, Fig. 3a), but showed non-significant effect in overall events. Additionally, we assumed death as a competing risk and performed a sensitivity test and Gray test. The sensitivity test, which performed the same analysis with the admission of cardiovascular disease except death, showed that the results were not different from those described above and are presented in Supplementary Table 7. The chi-square value was 25.1678 (Df = 3) and the p < 0.0001. Therefore, even though death was corrected between groups, there was a significant difference.
Fig. 3.
Adjusted hazard ratio of various endocrine therapies on cardiovascular and cerebrovascular events. Hazard ratios for cardiovascular and cerebrovascular events in women older than 55 (n = 47,569, upper panel) or 60 (n = 30,024, lower panel) adjusted for hypertension, diabetes, age, body mass index, and family history (a). The adjusted risk of various endocrine therapies for subgroups of known risk factors are shown (b). ACS: acute coronary syndrome, IS: ischemic stroke, HS: hemorrhagic stroke.
Although the median age of natural menopause in Korean women is around 50 [18], it was still possible that our cohort of over 55 might contain a small proportion of premenopausal women which may result in a bias toward protective effect for tamoxifen. To address this potential bias, we examined the effect of tamoxifen in a cohort of 30,024 patients who are over 60 years old, and observed a similar degree of significant risk reduction for patients taking tamoxifen (HR 0.790, 95% C.I. 0.680–0.917 for all events, HR 0.596, 95% C.I. 0.436–0.816 for ACS, Fig. 3). The marginal protective effect of aromatase inhibitor against ACS was also observed for women older than the age of 60.
To examine the association between various endocrine therapies and other cardiovascular prognostic factors, we analyzed the hazard ratio of each endocrine therapy in subsets of patients stratified by risk factors such as family history, diabetes, or hypertension. The degrees of protective effect of tamoxifen were similar across various subsets of patients (Fig. 3b). In most subgroups, tamoxifen showed the largest protective effect against ACS. Additionally, the protective effect of tamoxifen on CVEs remained to be significant after adjusting for the use of various adjuvant systemic therapies obtained from the NHIS database (Supplementary Table 5). Finally, to minimize the bias caused by the heterogeneity in time interval between the date of diagnosis and the date of NHIS Health Examination, we selected patients who had their NHIS Health Examination questionnaire answered within two years of their diagnosis since the median time interval was 2.15 years (±2.76). As shown in the Supplementary Table 6, we observed a consistent protective effect of tamoxifen in preventing CVE in this cohort of 14,402 patients.
3. Discussion
Although the meta-analysis of randomized trials have shown higher cardiovascular risk associated with aromatase inhibitor compared to tamoxifen [[10], [8], [9]], results from randomized trials have limited accuracy regarding the records of adverse events due to the heterogeneous nature of the report forms [6]. Several previous large-scaled retrospective studies failed to demonstrate the increased risk of CVEs associated with aromatase inhibitors compared to tamoxifen including the studies of 16,289 patients Danish Breast Cancer Cooperative Group and 11,045 or 13,273 patients from Kaiser Permanente Southern California [12,13,15]. In another study, there were no significant differences in the risk of CVEs between AI and tamoxifen users after adjustment for known risk factors [19,20]. In the present study, we demonstrate the protective effect of tamoxifen on ACS based on the nationwide health insurance of 47,569 elderly breast cancer patients. The use of tamoxifen was associated with 36% risk reduction against ACS while aromatase inhibitors showed marginal protective effect compared to no hormonal treatment group. The protective effect of tamoxifen seemed to persist across various subgroups of different risk factors. Our analysis is based on the nationwide insurance data collected by the National Health Insurance Service which provides mandatory health insurance coverage for all Korean citizens. Our results are inconsistent with the previous report from the population-based cohort study in Ontario comprising 9350 patients that showed significantly less incidence of myocardial infarction in women receiving tamoxifen compared to aromatase inhibitors [16].
Another important finding of our study is that the use of aromatase inhibitors was not associated with significantly increased risk of CVEs. In our dataset, the use of aromatase inhibitor was associated with a trend for protective effect compared to no hormonal treatment group. This finding is in contrast to the recent meta-analysis by Goldvaser et al. [11] that showed increased risk of CVE with aromatase inhibitor compared to placebo with borderline significance. Additionally, unlike the previous meta-analysis [21], we observed no significant increase for IS or HS in women taking tamoxifen. The lack of association between tamoxifen and the risk of stroke in our study can reflect the ethnic difference in pathogenesis of cerebrovascular diseases [22].
Our result showed that women with tamoxifen had a significantly lower risk for CVE compared to the no-treatment group while AI showed no such effect. After adjusting for other risk factors (hypertension, dyslipidemia, family history), the use of tamoxifen was associated with a significant protective effect against ACS. But our study has several limitations. First, our analysis is based on the anonymized insurance data gathered by National Health Insurance Service of Korea which make it impossible to evaluate the seriousness of individual events. However, for CVEs, we only included patients who underwent either angioplasty or bypass surgery for their diagnosis of ACS suggesting that the events used in the present study are likely to be substantially serious at least for those with ACSs. Second, the women without the NHIS health examination data were excluded from analysis to properly adjust for known risk factors such as hypertension or diabetes. The disadvantage of our approach is the potential selection bias toward healthier patients who were willing to undergo biennial health examination. Additionally, we were not able to assess how the patients with chronic disease such as hypertension or diabetes were managing their diseases. The degree of blood sugar control or blood pressure control may also affect the risk of future cardiovascular or cerebrovascular effects. Thirdly, various chemotherapeutic agents can cause a wide range of cardiac side effects including ACS [23]. Although we adjusted the use of systemic chemotherapy and trastuzumab, the detailed information on the dose and duration of the systemic therapy is not fully adjusted. Finally, the information on tobacco use and tumor characteristics such as subtypes or stages are not available in the NHIS database which could be significant confounding factors.
4. Conclusions
Our analysis of nationwide data shows a significant protective effect of tamoxifen against ACS in elderly breast cancer patients. Our data, along with observations from previous studies, might suggest that tamoxifen is protective in this population of Korean women who participated in this particular healthcare program and had no prior history of CVEs. Our results can guide the selection of adjuvant hormonal treatment agents for Korean breast cancer patients based on their risk of developing CVE.
Role of the funding source
No external funding was obtained.
Declaration of competing interest
The authors indicated no potential conflicts of interest.
Acknowledgements
This research was supported by National Research Foundation of Korea (NRF, 2019R1A2C2005277); National R&D Program for Cancer Control (HA15C0011); MSIT (Ministry of Science and ICT), Korea, under the ITRC (Information Technology Research Center) support program (IITP-2020-2018-0-01833) supervised by the IITP (Institute for Information & Communications Technology Planning & Evaluation).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.08.003.
Contributor Information
Sung Hyouk Choi, Email: sakebite@snu.ac.kr.
Kyoung-Eun Kim, Email: tonyk07213@daum.net.
Yujin Park, Email: shrck1015@gmail.com.
Young Wook Ju, Email: yawooya@gmail.com.
Ji-Gwang Jung, Email: mc2plato@naver.com.
Eun Shin Lee, Email: silvershoe99@gmail.com.
Han-Byoel Lee, Email: hblee80@gmail.com.
Wonshik Han, Email: hanw@snu.ac.kr.
Dong-Young Noh, Email: dynoh@snu.ac.kr.
Hyung-Jin Yoon, Email: hjyoon@snu.ac.kr.
Hyeong-Gon Moon, Email: moonhg74@snu.ac.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Moon H.G., Han W., Kim J.Y., Kim S.J., Yoon J.H., Oh S.J. Effect of multiple invasive foci on breast cancer outcomes according to the molecular subtypes: a report from the Korean Breast Cancer Society. Ann Oncol. 2013;24(9):2298–2304. doi: 10.1093/annonc/mdt187. [DOI] [PubMed] [Google Scholar]
- 3.Dowsett M., Cuzick J., Ingle J., Coates A., Forbes J., Bliss J. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28(3):509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 4.Burstein H.J., Temin S., Anderson H., Buchholz T.A., Davidson N.E., Gelmon K.E. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32(21):2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patnaik J.L., Byers T., DiGuiseppi C., Dabelea D., Denberg T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez E.A. Safety profiles of tamoxifen and the aromatase inhibitors in adjuvant therapy of hormone-responsive early breast cancer. Ann Oncol. 2007;18(Suppl 8):26–35. doi: 10.1093/annonc/mdm263. [DOI] [PubMed] [Google Scholar]
- 7.Nabholtz J.M., Gligorov J. Cardiovascular safety profiles of aromatase inhibitors : a comparative review. Drug Saf. 2006;29(9):785–801. doi: 10.2165/00002018-200629090-00003. [DOI] [PubMed] [Google Scholar]
- 8.Cuppone F., Bria E., Verma S., Pritchard K.I., Gandhi S., Carlini P. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta-analysis of randomized trials. Cancer. 2008;112(2):260–267. doi: 10.1002/cncr.23171. [DOI] [PubMed] [Google Scholar]
- 9.Amir E., Seruga B., Niraula S., Carlsson L., Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 10.Khosrow-Khavar F., Filion K.B., Al-Qurashi S., Torabi N., Bouganim N., Suissa S. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2017;28(3):487–496. doi: 10.1093/annonc/mdw673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldvaser H., Barnes T.A., Seruga B., Cescon D.W., Ocana A., Ribnikar D. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(1) doi: 10.1093/jnci/djx141. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez R.K., Sorensen H.T., Jacobsen J., Pedersen L., Lash T.L. Tamoxifen treatment in Danish breast cancer patients and 5-year risk of arterial atherosclerotic events: a null association. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2509–2511. doi: 10.1158/1055-9965.EPI-08-0570. [DOI] [PubMed] [Google Scholar]
- 13.Geiger A.M., Chen W., Bernstein L. Myocardial infarction risk and tamoxifen therapy for breast cancer. Br J Canc. 2005;92(9):1614–1620. doi: 10.1038/sj.bjc.6602562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiger A.M., Fischberg G.M., Chen W., Bernstein L. Stroke risk and tamoxifen therapy for breast cancer. J Natl Cancer Inst. 2004;96(20):1528–1536. doi: 10.1093/jnci/djh285. [DOI] [PubMed] [Google Scholar]
- 15.Haque R., Shi J., Schottinger J.E., Chung J., Avila C., Amundsen B. Cardiovascular disease after aromatase inhibitor use. JAMA Oncol. 2016;2(12):1590–1597. doi: 10.1001/jamaoncol.2016.0429. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Qadir H., Amir E., Fischer H.D., Fu L., Austin P.C., Harvey P.J. The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post-menopausal women with early stage breast cancer. Eur J Canc. 2016;68:11–21. doi: 10.1016/j.ejca.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Cheol Seong S., Kim Y.Y., Khang Y.H., Heon Park J., Kang H.J., Lee H. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol. 2017;46(3):799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park C.Y., Lim J.Y., Park H.Y. Age at natural menopause in Koreans: secular trends and influences thereon. Menopause. 2018;25(4):423–429. doi: 10.1097/GME.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 19.Kamaraju S., Shi Y., Smith E., Nattinger A.B., Laud P., Neuner J. Are aromatase inhibitors associated with higher myocardial infarction risk in breast cancer patients? A Medicare population-based study. Clin Cardiol. 2019;42(1):93–100. doi: 10.1002/clc.23114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ligibel J.A., O’Malley A.J., Fisher M., Daniel G.W., Winer E.P., Keating N.L. Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Canc Res Treat. 2012;131(2):589–597. doi: 10.1007/s10549-011-1754-1. [DOI] [PubMed] [Google Scholar]
- 21.Braithwaite R.S., Chlebowski R.T., Lau J., George S., Hess R., Col N.F. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18(11):937–947. doi: 10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi-Kwon S., Kim J.S. Lifestyle factors and risk of stroke in Seoul, South Korea. J Stroke Cerebrovasc Dis. 1998;7(6):414–420. doi: 10.1016/s1052-3057(98)80125-0. [DOI] [PubMed] [Google Scholar]
- 23.Bird B.R., Swain S.M. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Canc Res. 2008;14(1):14–24. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.