Abstract
Lung cancer is the most common cancer and leading cause of cancer mortality globally. Lung cancer is associated with significant morbidity, with symptoms often being poorly managed, causing significant symptom burden for both patients and their family caregivers. In people with life-limiting illnesses including advanced cancer, palliative care has been effective in improving symptom control, physical and mental wellbeing, quality of life, and survivorship; with benefits extending to caregivers while in the role and subsequently. Earlier integration of palliative care within oncology may be associated with improved patient outcomes, and has been supported by two Lancet commissions and national guidelines. The evidence for its effectiveness, however, has been mixed across the cancer spectrum. The aim of this review was to evaluate the current evidence for the effectiveness of early integrated palliative care in improving outcomes for people with lung cancer and their caregivers. Meta-analyses were performed where studies used the same measure. Otherwise, synthesis used a narrative approach. Similar to other types of advanced cancer, this review reveals mixed evidence for the effectiveness of early referral to palliative care and for the effectiveness of individual palliative interventions for people with lung cancer and their caregivers. Evidence that on-demand palliative care is equally, if not more effective than palliative care that is routinely provided, raises the question whether initiation and provision of palliative care as part of multidisciplinary lung cancer care ought to be guided by an early referral or need-based referral. Better understanding of what constitutes palliative care when delivered to people with lung cancer and their caregivers will help delineate the correlation with reported outcomes for these populations.
Keywords: Lung cancer, palliative care, supportive care, multidisciplinary care
Introduction
Globally, lung cancer is the most common cancer (2.09 million cases) and the leading cause of cancer mortality (1.76 million deaths) (1). In Australia lung cancer remains the leading cause of cancer mortality, accounting for nearly 1 in 5 (18%) cancer deaths, for both sexes (1 in 20 for males and 1 in 30 for females) before the age of 85 (2). Lung cancer is associated with the highest proportion of cancer burden and a poor 5-year survival rate of 17% (range, 68% stage I–3.2% for stage IV) (3), which is comparatively lower than other cancers (2). In 2016 to 2017, lung cancer was the second most common reason for a radiotherapy course (in both males and females) and the second most common type of cancer for palliative care hospitalization (13% of all cases) in Australia (2). Lung cancer is categorized as non-small cell lung cancer in more than 80% of cases and small cell lung cancer in about 15% of cases (3).
Lung cancer is associated with significant morbidity, with the most distressing symptoms commonly reported by people including breathlessness, pain, fatigue, and anorexia (4,5). Symptoms experienced by people with lung cancer are often poorly managed, causing significant symptom burden. Effectively managing these physical and psycho-social symptoms (6), which also affect caregivers (7), requires the input of a multidisciplinary team, including specialist palliative care. Specialist palliative care (at home or the hospital) has been shown to be associated with improved pain and symptom control, anxiety and reduced hospital admissions for people with advanced cancer (8), with benefits extending to caregivers while in the role and subsequently (9). There is limited evidence of its effectiveness on people’s quality of life, experience of care and economic cost (10).
The past few decades have seen a paradigm shift towards the provision of palliative care as integral to comprehensive care for people with advanced cancer. Earlier integration of palliative care within the oncology setting may be associated with improved patient outcomes (4,11,12). In a landmark study in 2010, Temel and colleagues (13) showed that early integration of palliative care reduced depression and symptom burden, and improved quality of life for people newly diagnosed with metastatic non-small cell lung cancer. Subsequently, two Lancet commissions have made recommendations for palliative care to be offered from the earliest stages in the disease trajectory, and concurrently with any curative and/or life-prolonging therapies (14,15). Earlier integration of palliative care into oncology care is also supported by national guidelines, including the American Society of Clinical Oncology (ASCO) Provisional Clinical Opinion (16) and the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (17).
A recent systematic review reported mixed results for the effectiveness of early palliative care both in the outpatient and community setting, across various conditions (18). While some randomized controlled trials (RCTs) reported improvements (including improved depression, patient and caregiver quality of life, caregiver burden; increased use of advance care directives, patient and family satisfaction; reduced aggressive end-of-life care, hospitalizations, hospital length of stay, and medical care costs), other RCTs reported no evidence of improvement (in symptoms, quality of life and resource utilization and costs), when compared to “usual” care (18). Given the heterogeneity in patient populations, it is important to identify the sub-populations who would most benefit from early palliative care as part of their lung cancer care.
The aim of this review is to appraise the current evidence for the effectiveness of early integrated palliative care in improving outcomes for people with advanced (metastatic) lung cancer and their caregivers.
Methods
A systematic review conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (19). We reviewed studies reporting on the effectiveness of palliative care interventions provided to adult patients (≥18 years) with advanced (metastatic) small cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC). Studies with mixed cancer cohorts were eligible for inclusion if they included participants with lung cancer clearly delineated in their sample size. Included studies were reports on phase II and phase III randomized controlled health service trials (RCTs) examining either (I) the efficacy of integrating early specialized palliative care alongside standard oncology care versus standard oncology care alone; or (II) providing individual palliative care interventions simultaneously with oncology care, for any outcome of interest. Secondary analyses and qualitative findings of RCTs were excluded, as were published protocols. Systematic reviews were also excluded, with primary studies screened for eligibility.
MEDLINE and PubMed were searched for primary studies published between 1 January 2010 and 31 July 2019, using Medical Subject Headings (MeSH) terms and text words for ‘lung cancer’ AND ‘palliative care’ OR ‘supportive care’ AND ‘randomized controlled trials’, limited to English. Database searches used the CareSearch palliative care filter (20,21). Database searching was supplemented with lateral searching of Google Scholar. Search results were imported into EndNote X9, and eligibility criteria applied to title/abstract screening and full text review (performed by S.K., in discussion with D.C.C.).
Data were extracted using an Excel proforma (Microsoft Office 2016) on: author, year, country, study design, sample (cancer stage; sample size), intervention/control, outcome measures, results, and author-identified conclusions. Intervention data were extracted using the Template for Intervention Description and Replication (TIDieR) checklist (22).
Synthesis used meta-analysis where results for the same outcome measure were reported in comparable ways in two or more trials. Random effects models were used to allow for the possibility that between-group differences varied according to differences in sample characteristics (23). Summary measures were mean differences in scores on the outcome measures between groups. These were estimated as change from baseline wherever possible, or else at follow-up only where the former was not reported. Ninety-five percent confidence intervals (CIs) were estimated. Heterogeneity was estimated using the Cochrane I2 statistic, and interpreted according to the Cochrane Handbook of Systematic Reviews as follows: 0% to 40% unimportant, 30% to 60% moderate, 50% to 90%: substantial, and 75% to 100% considerable heterogeneity (24).
For outcomes where meta-analysis was not possible, synthesis used narrative methods (25) grouped around the type of intervention, and patient and caregiver outcomes, defined in the broadest sense.
Results
Of 485 records identified, 13 papers were included in the final analysis, reporting on 11 randomized controlled trials (RCTs) (Figure 1). Six RCTs, reported in the eight papers presented in Table S1, evaluated the effectiveness of delivering specialized palliative care alongside standard oncology care vs. standard oncology care alone (13,26-32) (Table S1). Two of these trials (27,30) reported their patient (27,30) and caregiver (28,31) outcomes separately, and are presented as such in Table S1. Five RCTs (33-37) evaluated the effectiveness of providing individual palliative care interventions simultaneously with oncology care (Table S2). All 11 trials were included in a narrative synthesis, but the heterogeneity of measures and the ways in which these were reported limited meta-analysis to two trials (26,27).
Figure 1.
PRISMA flow diagram of included and excluded studies.
Table S1. Randomized controlled trials evaluating specialized palliative care interventions + standard oncology care vs. stand oncology care alone.
| Author, year, country | Aim | Sample | Intervention | Control | Outcome measures | Results | Conclusions |
|---|---|---|---|---|---|---|---|
| El-Jawahri et al., 2017 (28), USA, non-blinded randomized trial, single site | To evaluate the effects of early integrated palliative care on caregiver-reported outcomes in patients with newly diagnosed incurable cancers | Caregivers of people with incurable lung (NSCLC, SCLC, mesothelioma) or non-colorectal gastrointestinal (GI) cancers: total (n=275); intervention (n=137); control (n=138) | Early integrated palliative care & oncology care; meeting with a PC clinician at least once per month until death | Usual oncology care | Primary • Patient mood and anxiety—Hospital Anxiety and Depression Scale (HADS) • Patient QOL—Medical Health Outcomes Survey-Short Form (SF-36) Secondary • Caregiver mood and anxiety—HADS • Caregiver QOL—SF-36 |
? Improvement in caregivers’ total distress, depression subscale, but not anxiety subscale or QOL at week 12 ? No differences in caregivers’ outcomes at week 24 ? Significant effects on caregivers’ total distress (both anxiety and depression) at 3 and 6 months before patient death, but no difference in caregiver reported QOL |
Early involvement of palliative care for patients with newly diagnosed lung and gastrointestinal cancers leads to improvement in caregivers’ psychological symptoms; the benefits of early, integrated palliative care models in oncology care extend beyond patient outcomes and positively impact the experience of caregivers |
| Groenvold et al., 2017 (29), Denmark, randomized controlled trial, multi-site | To investigate the potential impact of early specialist palliative care in patients with advanced cancer and palliative care needs | 297 patients with stage IV cancer of any type, or stage III/IV cancers in the central nervous system (145 to intervention, 152 to control): cancer in the lung (39% in intervention, 30% in control), digestive system (14% in intervention, 25% in control), or the breast (21% in intervention, 23% in control), other cancers in stage IV or cancers in the CNS of grades III/IV (26% in intervention, 22% in control) | Early specialist palliative care was defined as ‘usual specialist palliative care’ initiated earlier than what otherwise would have been the case. Patients in intervention group were referred to a specialist palliative care team. Treatments and other interventions were determined by the patient’s needs | Standard care only. Standard care included palliative care provided by the departments of oncology, general practitioners (GPs) or home care services | Primary • Change in patient’s primary need—7 of 15 scales from EORTC-QLQ: physical function, role function, emotional function, nausea/vomiting, pain, dyspnea and lack of appetite Secondary • Changes in the seven QLQ-C30 scales |
? Early specialist palliative care had no significant effect on the primary outcome over 8 weeks (P=0.14) ? Separate analyses of each of the seven scales showed no differences between intervention and control groups; the exception was nausea/vomiting, showing the largest change (P=0.013, 0.01 threshold) favoring the intervention ? Survivorship did not differ between the two groups (P=0.16, P=0.39 in fully adjusted analysis) |
This RCT did not show beneficial or harmful effects of early specialist palliative care in advanced cancer patients with palliative care needs. |
| Temel et al., 2017 (27), USA, non-blinded randomized trial, single site | To evaluate the effects of early integrated palliative care on patient-reported outcomes in patients with newly diagnosed incurable cancers | ? People within 8 weeks of a diagnosis of incurable lung (NSCLC, SCLC, mesothelioma) or non-colorectal GI (pancreatic, esophageal, gastric or hepatobiliary) cancer: total (n=350); Intervention (n=175); Control (n=175). ? Lung cancer: intervention (n=96, 54.9%) control (n=95, 54.3%). ? Non-colorectal GI cancer: intervention (n=79, 45.1%); control (n=80, 45.7%) |
Early integrated palliative care & oncology care Meeting with a palliative care clinician at least once per month until death |
Usual oncology care | Primary • QOL—Functional Assessment of Cancer Therapy-General (FACT-G) scale Secondary • Mood and anxiety—Patient Health Questionnaire-9 (PHQ-9); HADS • Patients’ understanding of prognosis and report of communication with oncologists—Prognosis and Treatment Perceptions Questionnaire |
? Greater improvement in the intervention group in QOL baseline to week 24 (1.59 vs. −3.40; P=0.010) but not week 12 (0.39 vs. −1.13; P=0.339) • Lower depression in the intervention group at week 24, controlling for baseline scores (adjusted mean difference, −1.17; 95% CI, −2.33 to −0.01; P=0.048) • Improved QOL and depression at week 12 and 24 (patients with lung cancer; intervention) vs. deterioration in both (patients with lung cancer; usual care) • Improved QOL and mood by week 12 (patients with GI cancers; both groups) • Intervention vs. usual care patients: more likely to discuss their wishes with their oncologist if they were dying (30.2% vs. 14.5%; P=0.004) |
For patients with newly diagnosed incurable cancers, early integrated palliative care improved QOL and other salient outcomes, with differential effects by cancer type; early integrated palliative care may be most effective if targeted to the specific needs of each patient population |
| Bakitas et al., 2015 (30), USA, fast-track randomized controlled trial, multi-site | To compare the effect of early vs. delayed intervention timing on patient-reported outcomes, 1-year survival, and resource use | ? Total (n=207): early intervention (n=104), delayed intervention (n=103) ? Lung cancer: early group (n=46, 44.2%), delayed group (n=42, 40.8%) ? GI tract: early group (n=26, 25%), delayed group (n=24, 23.3.8%) ? Breast: early group (n=10, 9.6%), delayed group (n=13, 12.6%) ? Genitourinary tract: early group (n=10, 9.6%), delayed group (n=13, 12.6%) ? Hematologic malignancy: Early group (n=5, 4.8%), delayed group (n=5, 4.8%) |
Outpatient palliative care consultation and six structured weekly telephone coaching with an advanced practice nurse | Patients in the delayed group received initiation of PC months later than in early palliative care group. | ? Patient-reported: · Quality of Life—46-item Functional Assessment of Chronic Illness-Therapy—Palliative Care (FACIT-Pal) · Symptom impact—Quality of Life at End of Life (QUAL-E) · Mood—Centre for Epidemiologic Studies—Depression Scale (CES-D) • One-year and overall survival • Resource use and location of death |
? There were no significant differences between the early palliative care and delayed groups in QOL, symptom impact and moon 3 months after enrolment • There were no significant differences in analyses of decedents’ outcomes looking backward from death at 12, 6, or 3 months • There was a 15% difference in survival at 1 year (early group, 63% vs. delayed group, 48%; P=0.038) • Median survival was 18.3 months for the early group (n=50) and 11.8 months for the delayed group (n=59), but the log-rank test was not significant (P=0.18) • There were trends towards rates of hospital, ICU days and ED visits in the early group compared to the delayed group, but this was not significant • Use of chemotherapy in the last 2 weeks of life was not statistically different (1.57; 95% CI, 0.37 to 6.7; P=0.54) • There were no significant differences in the number of decedents who died at home in the early (n=27, 54%) or delayed intervention (n=28, 47%) |
This study supports the association between early palliative care and improved survival, but the mechanisms by which this occurs requires further research |
| Dionne-Odom et al. 2015 (31), USA, fast-track randomized controlled trial, multi-site | To determine the effect of early vs. delayed initiation of a palliative care intervention for family caregivers of people with advanced cancer | • 122 caregivers (early group, n=61; delayed group, n=61). • Lung (early group, n=28, 45.9%; delayed group, n=25, 41%); • Gastrointestinal (early group, n=14, 23.0%; delayed group, n=17, 27.9%); genitourinary (Early group, n=5, 8.2%; delayed group, n=5, 8.2%) • Breast (early group, n=5, 8.2%; delayed group, n=5, 8.2%) • Hematologic (early group, n=3, 4.9%; delayed group, n=4, 6.6%) • Other solid tumor (early group, n=6, 9.8%; delayed group, n=5, 8.2%) |
Outpatient palliative care consultation and telephone coaching specific to caregivers | Delayed group received palliative care 3 months after diagnosis | • Caregiver QOL (CQOL-C) • Caregiver Depressed mood – Center for Epidemiologic Study–Depression Scale (CESD) • Caregiver Objective, stress, and demand burdens—Montgomery-Borgatta CG Burden (MBCB) subscales |
? The intervention led to lower depression in the early group compared to the delayed group (mean difference, −3.4; SE, 1.5; d=−0.32; P=0.02) • There were no significant differences in QOL (mean difference =−2; SE =2.3; d=−0.13; P=−0.39) or burden (objective burden: mean difference =0.3; SE=0.7; d=0.09; P=0.64; stress burden: mean difference =−0.5; SE= 0.5; d=−0.2; P=0.29; demand burden: mean difference=0; SE=0.7; d= -0.01; P=0.97) compared with initiation 3 months later |
The telephone based and prompt provision of this intervention may have been key elements to the convenience of this intervention, and its ability to teach and foster skills in caregivers that could be successfully applied and integrated over time. Future work should further devise ways to alleviate the caregiver burden and optimize their physical health |
| McCorkle et al. 2015 (26), USA, cluster-randomized controlled trial, four disease-specific clinics at a single site | To evaluate the effects of a multidisciplinary intervention coordinated by advance practice nurses (APNs) on patient-reported outcomes in patients newly diagnosed with late-stage cancers | • Late-stage cancer diagnosis; post-biopsy or surgery with additional treatment recommended; at least one self-reported chronic condition • Total (n=146): intervention (n=66), control (n=80) • Randomized to the intervention: gynecologic (n=20) and lung (n=16) clinics • Randomized to the control: head and neck (n=17) and gastrointestinal (n=39) clinics |
Multidisciplinary intervention coordinated by an advanced practice nurse (APN) | Enhanced usual care group (usual multidisciplinary care plus a copy of the symptom management toolkit with instructions on its use) |
Primary and secondary • Symptom distress—Symptom Distress Scale (SDS) • Health distress—four-item scale developed by the Stanford Patient Education Research Center • Functional status—Enforced Social Dependency Scale (ESDS) • Self-rated health (physical and mental)—1st item of SF-12 • QOL—FACT-G) (version 4) • Anxiety—HADS • Depression—PHQ-9 • Uncertainty—Mishel Uncertainty in Illness Scale-Community Form (MUIS-C) • Self-efficacy—Self-Efficacy for Managing Chronic Disease Scale (SEMCD 6) |
? No differences between the two groups on the primary patient-reported outcomes at 1 and 3 months post-baseline: symptom distress, emotional distress, enforced social dependency (persona and social) • Both groups: significant improvement on personal competence • Only enhanced usual care: significant improvement on social competence • Both groups: worse perceptions of their own health over time • Physical and emotional symptoms remained stable or significantly improved from baseline for both groups • Overall, secondary outcomes remained stable within the groups; but patients who received the enhanced usual care reported significantly better self-efficacy at 1 month (P<0.0097) and less uncertainty at 1 month (P<0.0007) and 3 months (P<0.0.106) compared to the intervention group |
If patients newly diagnosed with late-stage cancer were managed by disease-specific multidisciplinary teams who palliated their symptoms, providing whole patient care, patient outcomes remained stable or improved |
| Zimmermann et al. 2014 (32), Canada, cluster-randomized controlled trial, single site | To assess the effect of early palliative care in patients with advanced cancer on several aspects of quality of life | • Advanced cancer prognosis 6–24 months: lung cancer (n=101; 43.81%), gastrointestinal (n=139; 60.4%), genitourinary (n=78; 33.7%), breast (n=72; 31.3%), gynecological (n=71; 30.8%) • Total (n=461) [Intervention (n=228), Control (n=233)] • Lung cancer: total (n=101); intervention (n=55; 24.1%), control (n=46; 19.7%) |
Consultation and follow-up in the oncology palliative care clinic by a palliative care physician and nurse | Standard care (oncologist and oncology nurses) | Primary and secondary • QOL—Functional Assessment of Chronic Illness Therapy Spiritual Well-Being (FACIT-Sp); QUAL-E • Symptom severity—Edmonton Symptom Assessment System (ESAS) • Satisfaction with care—FAMCARE-P16 • Problems with medical interactions—Cancer Rehabilitation Evaluation System Medical Interaction Scale (CARES-MIS) |
At 3 months: • No difference for FACIT-Sp; • Significant difference in QUAL-E and FAMCARE-P16 favoring the intervention group; • No difference in ESAS and CARES-MIS. At 4 months: • Improvement for all measures except CARE-MIS, favoring the intervention group |
Although the difference in quality of life was non-significant at the primary endpoint, this trial shows promising findings that support early palliative care for patients with advanced cancer |
| Temel et al. 2010 (13), USA, non-blinded randomized controlled trial, single center | To examine the effect of early palliative care integrated with standard oncologic care on patient-reported outcomes, the use of health services, and the quality of end-of-life care among patients with metastatic non-small cell lung cancer | • People with metastatic, non-small cell lung cancer • Total (n=151): Intervention (n=76), Control (n=75) |
Consultation and follow-up with palliative care physician/advance practice nurse; Guidelines for palliative care in the ambulatory setting (National Consensus Project for Quality Palliative Care) | Standard oncologic care alone | Primary • QOL—Trial Outcome Index (TOI); Functional Assessment of Cancer Therapy-Lung (FACT-L); Lung cancer subscale (LCS); Secondary • Anxiety and depression—HADS; PHQ-9 • Aggressive care at the end of life—chemotherapy within 14 days before death; no hospice care; or admission to hospice 3 days or less before death • Patients’ resuscitation preferences—documented in medical records |
The intervention group showed • Improved QOL TOI 6.0 (FACT-L mean score 98.0 vs. 91.5; P=0.03 and LCS 1.7) • Fewer depressive symptoms (16% vs. 38%; P=0.01) • Fewer people received aggressive end-of-life care (33% vs. 54%; P=0.05) • Longer median survival (11.6 vs. 9.8 months; P=0.02) |
Among patients with metastatic non-small cell lung cancer, early palliative care led to significant improvements in both quality of life and mood; compared with patients receiving standard care, patients receiving early palliative care had less aggressive care at the end of life but longer survival |
Table S2. Randomized controlled trials evaluating individual palliative care interventions vs. standard/usual oncology care.
| Author, year, country | Aim | Sample | Intervention | Control | Outcome measures | Results | Conclusions |
|---|---|---|---|---|---|---|---|
| Uster et al. 2018 (33); Switzerland; Two-arm, parallel group, randomized controlled trial; single site | To test the effects of a combined nutrition and physical exercise program on cancer patients with metastatic or locally advanced tumors of the gastrointestinal and lung tracts | • Metastatic or locally advanced tumors of the gastrointestinal (n=38) and lung (n=20) tracts • Total (n=58); Intervention (n=29); Control (n=29) • NSCLC: total (n=16); intervention (n=9; 31.0%); control (n=7; 24.1%) • SCLC: total (n=4); intervention (n=2; 6.9%); control (n=2; 6.9%) |
3 month nutrition and physical exercise program | Usual care (standard oncology care; maintain usual daily physical activity level; nutritional support provided only when medically indicated) | Primary • QOL—European Organization for Research and Treatment of Cancer Quality of Life Questionnaire V3.0 (EORTC QLQ-C30) Secondary • Dietary intake—3 day food diaries) • Nutritional status—body weight (bioelectrical impedance analysis) • Physical performance—handgrip strength, 6-min walk and timed sit-to-stand test • Clinical data—unplanned admission, total length of all hospital stays, performance status (ECOG) |
? No difference in global health status/quality of life (overall QoL) post intervention (improvement in both groups) • Reduced nausea and vomiting (P=0.023) and increased protein intake (P=0.01) in the intervention group • No statistical differences for energy intake, nutritional status and physical performance |
Good adherence to a combined nutrition and exercise program; the multimodal intervention did not improve overall QOL, but contributed to an adequate protein intake and to the general well-being of the patient by reducing nausea and vomiting |
| Yang et al. 2018 (34); Singapore; pilot, randomized phase II trial; single site | To determine feasibility and acceptability of the Enhancing Quality of Life in Patients (EQUIP) intervention; data completion rate of patient reported outcome measures in the trial; the estimated effect of the EQUIP intervention on quality of life and mood | • New diagnosis of stage 3 (n=21) or 4 lung cancer (n=48) • Total (n=69): Intervention (n=35); Control (n=34) • Adenocarcinoma: intervention (n=19; 54.3%); control (n=22; 64.7%) • SCLC: intervention (n=7; 20%); control (n=6; 17.7%) • Squamous cell carcinoma: intervention (n=4; 11.4%); control (n=4; 11.8%) • Others: intervention (n=5; 14.3%); control (n=2; 5.9%) |
Usual care plus patients individually received the EQUIP intervention (4 face-to-face educational sessions with a nurse) | Usual care (standard oncology care as well as referral for palliative care services if deemed appropriate by the primary oncologist) | Primary • QOL—Chinese validated Functional Assessment of Cancer Therapy-Lung (FACT-L); Lung Cancer Subscale (LCS); Trial Outcome Index (TOI) Secondary • Mood—Chinese validated Hospital Anxiety and Depression Scale (HADS) |
· No significant difference between intervention and control groups in quality of life and mood at 12 weeks after baseline • All patients were satisfied with the topics shared and felt they were useful |
Nurse-directed face-to-face educational sessions were feasible and acceptable to patients with advanced lung cancer; however, there was no indication of benefit of the EQUIP intervention on quality of life and mood (which could be due in part to a low prevalence of targeted symptoms) |
| Schellekens et al. 2017 (35); The Netherlands; parallel group randomized controlled trial; multi-site | To examine the effectiveness of mindfulness-based stress reduction (MBSR) added to care as usual (CAU) vs. CAU alone in reducing psychological distress in lung cancer patients and/or their partners | • Patients and/or partners of patients presenting with cytologically or histologically proven NSCLC or SCLC. Both curative and palliative stage were included, with stage being based on the intent of the anticancer treatment • Patients (n=63): CAU+MBSR (n=31); CAU (n=32) • Caregivers (n=44): CAU+MBRS (n=21); CAU (n=23) • NSCLC: intervention (n=28; 90%); control (n=26; 81%) • SCLC: intervention (n=2; 7%); control (n=5; 16%) • Mesothelioma: intervention (n=1; 3%); control (n=1; 3%) |
Care as usual plus mindfulness-based stress reduction (group-based training in which participants practice mindfulness and receive teaching on stress) | Care as usual | Primary • Psychological distress—Hospital Anxiety and Depression Scale (HADS) Secondary • QOL—EORTC QLQ-C30 • Caregiver burden—Self-Perceived Pressure from Informal Care • Patient-caregiver relationship satisfaction (Investment Model Scale-Satisfaction subscale) • Mindfulness skills—Five Facet Mindfulness Questionnaire • Self-compassion—Self-Compassion Scale • Post-traumatic stress symptoms—Impact of Event Scale |
• Significantly less psychological distress (P=0.008, d=0.69) in the intervention than the control • Baseline distress moderated outcome: those with more distress benefitted most from MBSR • Patients showed more improvements in quality of life, mindfulness skills, self-compassion, and rumination in the intervention than the control. In partners, no differences were found between groups |
Findings suggest that psychological distress in lung cancer patients can be effectively treated with MBSR; no effect was found in partners (possibly because they were more focused on patients’ well-being rather than their own) |
| Mosher et al. 2016 (36); USA; pilot randomized trial; Single site | To examine the preliminary efficacy of telephone-based symptom management (TSM) for symptomatic lung cancer patients and their family caregivers | • Diagnosis of SCLC or NSCLC; people receiving hospice care at the time of enrolment were excluded • Total: patients (n=106); caregivers (n=106) TSM: patients (n=51); caregivers (n=51) • Education/support: patients (n=55); caregivers (n=55) • NSCLC: TSM (n=44; 86.27%); Education/support (n=49; 89.09%) • SCLC: TSM (n=7; 13.73%); Education/support (n=6; 10.91%) |
4 sessions of telephone symptom management (TSM) consisting of cognitive-behavioral and emotion-focused therapy | 4 sessions of education/support | Primary • Patient and caregiver depressive and anxiety—Patient Health Questionnaire; Generalized Anxiety Disorder scale (GAD-7) • Patient physical symptoms—Brief Pain Inventory Short Form; Fatigue Symptom Inventory; Memorial Symptom Assessment Scale (frequency and severity of breathlessness and distress related to breathlessness) Secondary • Patients’ perceived ability to manage pain, other symptoms, and function & Caregiver confidence in their ability to manage symptoms—16-item standard self-efficacy scale modified from the arthritis literature • Caregivers’ self-efficacy to manage own emotions—8 items • Patient and caregiver perceived constraints on cancer-related disclosure from the other dyad member—5 item social constrains scale • Caregiver burden—Caregiver Reaction Assessment |
• No significant group differences for all patient outcomes and caregiver self-efficacy for helping the patient manage symptoms and caregiving burden at weeks 2 and 6 post-intervention • Small effects in favor of TSM regarding caregiver self-efficacy for managing their own emotions and perceived social constraints from the patient • No significant change over time for study outcomes in either group |
? Findings suggest that the brief telephone-based psychosocial intervention was not efficacious for symptomatic improvement in lung cancer patients and their family caregivers • Next steps include examining specific intervention components in relation to study outcomes, mechanisms of change, and differing intervention doses and modalities |
| Schofield et al. 2013 (37); Australia; two-group randomized controlled trial; single site | To test the effectiveness of a multidisciplinary supportive care program based on systematic needs assessment in people with inoperable lung cancer | • Diagnosis of inoperable lung or pleural (including mesothelioma) cancer; scheduled to receive palliative external beam radiotherapy, palliative chemotherapy or radical radiotherapy and chemotherapy • Total (n=108): Intervention (n=55); Control (n=53) • SCLC: intervention (n=4; 7.3%); control (n=5; 9.4%) • NSCLC: intervention (n=48; 87.3%); control (n=45; 84.9%) • Mesothelioma: intervention (n=3; 5.5%); control (n=3; 5.7%) |
2 consultations at treatment commencement and completion and the provision of a systematic needs assessment data to the patient’s multidisciplinary team (MDT) | Usual care (standard care as per hospital protocol –multidisciplinary meetings with referrals to allied health and palliative care as required; no systematic assessment/management patient need or systematic communication of patient needs) | Primary • Unmet needs—Needs Assessment for Advanced Lung Cancer Patients • Psychological morbidity—HADS • Global distress—Distress Thermometer (DT) • Health related QOL—EORTC QLQ-C30 V2.0 |
? Trial closed prematurely • No significant difference for any of the primary measures (all P>0.10) • Change score analysis indicated a relative benefit from the intervention for unmet symptom needs at week 8 and 12 post-assessment (effect size =0.55 and 0.40, respectively) |
Novel approach, but the hypothesis that the intervention would benefit perceived unmet needs, psychological morbidity, distress and health-related quality of life was not supported overall |
Study characteristics
All eleven trials were conducted in high income countries, with the majority conducted in the United States (n=5) (13,26-28,30,31,36), and the reminder conducted in Australia (37), Canada (32), Denmark (29), Singapore (34), Switzerland (33), and The Netherlands (35). Seven trials included people with various cancers (26-33,37), while four trials included lung cancer only (13,34-36). Four trials included people with cancer (‘patients’) and their family caregivers (‘caregivers’) (27,28,30,31,35,36) and seven trials included patients only (13,26,29,32-34,37).
Primary and secondary outcomes varied widely across studies, with the most common being quality of life and psychological symptoms (such as anxiety and depression). Others included symptom distress, functional status, and survivorship. For lung cancer specifically, the outcomes measured included: patient/caregiver quality of life and mood (including anxiety and depression); patient understanding of prognosis; patient-oncologist communication; aggressiveness of care at the end-of-life, patient resuscitation preferences, and survivorship (13,27,28).
The components of early palliative care interventions in the eight RCTs that evaluated the effectiveness of delivering specialized palliative care alongside standard oncology care compared to usual oncology care alone (13,26-32) were quite similar (Table S3). Overall, early referral interventions involved consultations with a specialist palliative care team (with various members) or palliative care physician or an advance practice nurse. Delivery was in person (in clinics or at home) or over the phone. Interventions were delivered: within 4 weeks of enrolment and at least monthly until death (27,28); within 3 weeks after recruitment and at least monthly until death (13); tailored to patient need (29); within 30–60 days after diagnosis (early intervention) or 3 months after diagnosis (delayed intervention) (30,31); within 24 hours of diagnosis followed by phone and in-person contacts (26); within 1 month of recruitment followed by a routine phone call one week after the initial consultations and thereafter as needed, as well as a monthly outpatient palliative care follow up (32).
Table S3. TIDieR table describing early palliative care interventions.
| Author, year | Intervention | Who provided | How | Where | When/how much | Tailoring | Modification | Fidelity |
|---|---|---|---|---|---|---|---|---|
| El-Jawahri et al. 2017 (28) | Patients assigned to early palliative care met with board certified palliative care physician or advance practice nurse | Certified palliative care physician or advance practice nurse | Face to face, or over the phone | In clinic | Within 4 weeks of patient enrolment, and at least monthly palliative care visits until death | Not available | Not available | (I) 229 and 183 caregivers completed the week 12 and week 24 assessments with a missing data rate of 16.7% and 33.5%, respectively. (II) Intervention: 110/137 caregivers completed 12 week follow up assessment; 89/110 completed 24 week follow-up assessment. (III) Control: 119/138 caregivers completed 12-week follow up assessment, 94/119 completed 24 week follow-up assessment |
| Groenvold et al. 2017 (29) | ‘Early specialist palliative care’ was defined as ‘usual specialist palliative care’ initiated at earlier time than otherwise. Patients in the intervention group were referred to a specialist palliative care team | Specialist palliative care teams depended on the different specialized palliative care centers in the study. Members included doctors, nurses, physiotherapist, psychologists, social workers, chaplains, secretary, and volunteers, or pharmacists | Face-to-face or over the phone | In clinic | Frequency was tailored to patient need | Primary outcome was tailored to the patient by being the patient’s most pronounced symptom/problem (‘primary need’); number and frequency of contacts with the specialist palliative care team and the treatments and other interventions were determined by the patient’s needs | Not available | (I) Intervention: 138/145 received allocated intervention; 32 were lost to follow up (15 died, 9 did not answer questionnaire, 8 due to administrative failure). (II) Control: 139/152 received the allocated intervention; 39 were lost to follow up (15 died, 20 did not answer questionnaire, 4 due to administrative failure) |
| Temel et al. 2017 (27) | Patients assigned to early palliative care met with board certified Palliative care physician or advance practice nurse | Certified palliative care physician or Advance practice nurse | Face to face, or over the phone | In clinic | Within 4 weeks of patient enrolment, and at least monthly palliative care visits until death | Not available | Not available | (I) Intervention: 148/175 patients completed 12 week assessment; 118/148 completed 24 week assessment. (II) Control: 153/175 patients completed 12 assessment; 125/153 completed 24 week assessment |
| Bakitas et al. 2015 (30) | ENABLE (Educate, Nurture, Advise, Before Life Ends) includes initial in-person, standardized outpatient palliative care consultation by a board-certified palliative care clinician and structured telephone coaching sessions by an APN using a manualized curriculum | Palliative care consultation was conducted by a board certified palliative care clinician; telephone coaching was conducted by advance practice nurses | Face-to-face palliative care visits; telephone coaching sessions | In clinic | Early group: within 30 to 60 days of being informed of an advanced cancer diagnosis, cancer recurrence, or profession, in the opinion of the oncologist, prognosis between 6 and 24 months. Delated group: patients were referred to first palliative care visit 3 months later | Not available | Not available | (I) Allocated to early intervention: 92/104 patients received the allocation intervention (9 did not start the intervention; 3 died before start); 33 patients discontinued the intervention (reasons: not interested (n=14), passive withdrawal (n=6), overwhelmed (n=6), moved care (n=3), too ill (n=2), too well (n=1), no reason (n=1)). (II) Allocated to delayed intervention: 81/103 patients received the allocation intervention (8 did not start the intervention; 14 died before start); 27 patients discontinued the intervention [reasons: not interested (n=11), passive withdrawal (n=4), overwhelmed (n=3), moved care (n=3), too ill (n=1), too well (n=5), no reason (n=0)] |
| Dionne-Odom et al. 2015 (31) | ENABLE includes initial in-person, standardized outpatient PC consultation and structured telephone coaching sessions designed for caregivers. Caregivers were encouraged to be present the in-person palliative care consultation | Consultations by a board-certified palliative care physician; coaching sessions delivered by advanced practice nurses | Face-to-face palliative care visits; telephone coaching sessions | In clinic or over the phone | (I) Early group: within 30 to 60 days of being informed of an advanced cancer diagnosis, cancer recurrence, or profession, in the opinion of the oncologist, prognosis between 6 and 24 months. (II) Delayed group: 3 months later. (III) Three caregiver educational sessions, once a week, delivered by nurses | Not available | Not available | (I) Early group: 61/63 enrolled caregivers provided data for analysis. 27 patients died with an enrolled caregiver. (II) Delayed group: all enrolled caregivers provided data, 39 patients died with an enrolled caregiver |
| McCorkle et al. 2015 (26) | 10-week standardized intervention which included monitoring patients’ status, providing symptom management, executing complex care procedures, teaching patients and family caregivers, clarifying the illness experience, coordinating care, responding to the family, enhancing QOL, and collaborating with other providers | Clinic advance practice nurses contacted patients, and trained physician assistants and medical social workers to be part of multidisciplinary team |
Face-to-face, or over the phone | In clinic | Clinic advance practice nurses initially contacted patients within 24 hours, and weekly phone and in-person contacts were scheduled (five clinic visits and five telephone calls in total) | Not available | Not available | (I) Fidelity was assessed and monitored by the study advance practice nurse coordinator through quantification of whether the scheduled patient contacts occurred according to the protocol’s timeframe and review of 10% of the documentation by the team members to ensure compliance with study protocols. (II) Intervention: 54/66 completed one-month follow-up assessment; 36/54 completed three-month follow-up assessment. (III) Control: 68/80 patients completed one-month follow-up assessment; 54/66 patients completed three-month follow-up assessment |
| Zimmerman et al. 2014 (32) | Consultation and follow-up in the oncology palliative care clinic. Intervention consisted of: (I) comprehensive, multidisciplinary assessment of symptoms; (II) routine telephone contact from a palliative care nurse; (III) monthly outpatient palliative care follow-up; and (IV) a 24-h on-call service for telephone management of urgent issues | Early palliative care intervention: palliative care physician and nurse, home palliative care physician (either for back up support to family physicians doing house calls or direct care if the family physician does not provide house calls). Standard care: oncologist and oncology nurses | Face-to-face visits in clinic (palliative care or oncology), telephone follow up, home visits (early palliative care only) | In clinics, over the phone or at patient homes (early palliative care only) | Assessment was within 1 month of recruitment (60–90 min in duration); routine phone call from palliative care nurse 1 week after first consultation and thereafter as needed; monthly outpatient palliative care follow-up | Ancillary interventions provided depended on the status of the patient | Not available | (I) Intervention: 201/228 patients completed at least one follow up assessment; 29 patients died, 41 patients withdrew; 131 patients completed the study. (II) Control: 192/233 patients completed at least one follow-up assessment; 9 patients died and 28 patients withdrew from follow up; 155 patients completed study |
| Temel et al. 2010 (13) | Patients assigned to early palliative care met with a member of the palliative care team within 3 weeks of enrolment and at least monthly thereafter until death | Board certified palliative care physicians and advance practice nurses | Face-to-face visits | In clinics | Within 3 weeks after enrolment and at least monthly thereafter in the outpatient setting until death | Additional visits with the palliative care service were scheduled at the discretion of the patient, oncologist, or palliative care provider | Not available | All the patients assigned to early palliative care, except for one patient who died within 2 weeks after enrolment, had at least one visit with the palliative care service by week 12 |
Synthesis of results
Effectiveness of providing specialized palliative care alongside standard oncology care vs. standard oncology care alone
Patients
(I) Survival
Survival data for early palliative care were drawn from two studies, one specifically evaluating outcomes in lung cancer (13), the other evaluating outcomes in a number of cancer populations including lung cancer (30). Compared to usual oncology care, early palliative care was associated with less premature mortality in people newly diagnosed with lung cancer (13). Longer one year survival was reported for people with advanced cancer (including lung cancer) for whom palliative care was initiated within 30 to 60 days of diagnosis, compared to those who received palliative care 3 months after their diagnosis (63% vs. 48%; difference, 15%; P=0.038) (30).
(II) Quality of life
Quality of life was an outcome of interest in three studies, including two which evaluated this in patients with metastatic/incurable lung cancer (13,27) and one which evaluated this in patients with advanced cancer prognosis including lung cancer (32). Early palliative care was associated with improved quality of life at 24 weeks, but not at 12 weeks (27). For people with lung cancer specifically, quality of life improved at week 12 and 24 for the intervention group but deteriorated for the control group (27). Compared to usual oncology care, early palliative care was found to improve quality of life for people newly diagnosed with lung cancer (13). Similar findings have been replicated in another trial which found that early palliative care improved quality of life and satisfaction with care for people with advanced lung cancer (32).
(III) Treatment and health care utilization
Four studies evaluated the effect of early palliative care on treatment preferences and health care utilization, in lung cancer (13) and mixed-cancer populations (27,30,32). Early referral to palliative care appeared to impact on the patient-treating clinician relationship, with people in the intervention group (compared to usual care) more likely to discuss their wishes with their oncologists if they were dying (27) and having less aggressive treatment at the end of life (13). However, another trial found that early palliative care did not change the issues discussed during medical interactions (32). For people with advanced cancer receiving palliative care early (within 30–60 days after diagnosis) or later (3 months after diagnosis), similar relative rates between the two groups were reported for hospital and intensive care unit days, emergency department visits, chemotherapy in the last 14 days, and home deaths (30).
(IV) Depression
Two studies with focus on lung cancer evaluated the impact of early palliative care on depression (13,27). Early palliative care was found to lower depression at 12 weeks (27). For people with lung cancer in particular, depression improved at week 12 and 24 when receiving early palliative care but deteriorated for those receiving usual oncology care only (27). Another trial reported fewer depressive symptoms for people in the intervention group receiving early palliative care (13).
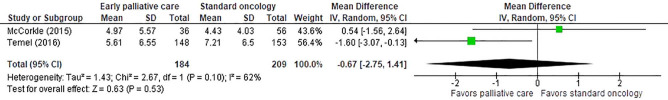
A meta-analysis could be conducted for the Patient Health Questionnaire 9 (PHQ-9) (38) used in two studies (26,27). PHQ-9 measures depression on a nine-item scale, with higher scores indicating greater depression. Scores could be converted into five ordinal descriptors of depression. Results from these two studies found a non-significant difference between early palliative care versus standard oncology (P=0.53); however, heterogeneity was substantial (Figure 2).
Figure 2.
Results from meta-analysis of Patient Health Questionnaire 9 (PHQ-9) used in two studies comparing early palliative care alongside oncology care versus standard oncology alone.
(V) Other symptoms
Symptom outcomes were measured in four studies evaluating the effectiveness of early palliative care compared to standard oncology care (29,32) or enhanced usual oncology care (26) or delayed palliative care (30). Patient-reported outcomes (symptom impact, mood) were not statistically significant for people with advanced cancer (including lung cancer) receiving early palliative care (within 30 to 60 days of diagnosis) vs. delayed palliative care (3 months after diagnosis) (30). For people with advanced lung cancers, early intervention did not improve symptom and emotional distress, and personal and social dependency (26) or reduce symptom severity (32). Early specialist palliative care did not improve patients’ primary needs (where the primary need was the patient-identified symptom or problem with highest intensity, including physical function, role function, emotional function, pain, nausea/vomiting, breathlessness, and lack of appetite) (29). In another trial, physical and emotional symptoms either remained stable or improved slightly for both the early intervention and usual care group (26).
(VI) Self-efficacy
Self-efficacy was a secondary outcome in one study evaluating the effects of multidisciplinary palliative care compared to enhanced usual care (26). For people with incurable (lung) cancer, personal competence (comprised of six daily living activities: eating, dressing, walking, traveling, bathing, and toileting) improved in both early palliative care intervention and usual care groups, while social competence (comprised of activities in the home, work activities, social and recreational roles, and communication) improved in the usual care group only (26). Perceptions of own health over time worsened for both groups (26). Enhanced usual care significantly improved self-efficacy and reduced uncertainty at 1 and 3 months (compared to the intervention) (26).
(VII) Communication
For people with advanced cancers (various), early involvement of specialist palliative care was not effective in improving medical interactions (32). However, people receiving early palliative care (compared to usual oncology care) were more likely to discuss their wishes with their oncologists if they were dying (27).
Caregivers
Early palliative care for people with lung cancer was effective in improving caregivers’ total distress and depression, but not anxiety or overall quality of life at 12 weeks (28). No differences in caregivers’ psychological and quality of life outcomes were observed at 24 weeks (28). Caregivers reported significantly lower depression and anxiety at 3 and 6 months before the death of the person with cancer; again, no such difference was reported for overall quality of life (28). For caregivers of people with advanced cancer (including lung cancer), early vs. delayed palliative care improved depression and stress burden in the terminal analysis; there were no differences in quality of life or burden for either group (31).
Effectiveness of providing individual palliative care interventions simultaneously with oncology care
Patients
(I) Quality of life
In people with metastatic or locally advanced lung or gastrointestinal cancers, an interventional program of nutrition and physical exercise did not improve overall health-related quality of life compared to usual care (33). In mixed patient populations (including people with lung cancer), a nurse-directed face-to-face educational intervention resulted in no significant difference between intervention and control groups in quality of life at 12 weeks after baseline (34). In people with inoperable lung cancer (predominantly non-small cell lung cancer), consultations and systematic needs assessment as part of patient’s multidisciplinary team meetings (vs. usual care where no such systematic assessment was provided at multidisciplinary team meetings) did not improve quality of life (37).
(II) Other symptoms
Nutrition and physical exercise (compared to usual care) reduced nausea and vomiting (P=0.023) and increased protein intake (P=0.01) (33). Compared to usual care, no statistical differences were found for energy intake, nutritional status and physical performance (33). A nurse-directed face-to-face educational intervention resulted in no significant difference between intervention and control groups in mood at 12 weeks after baseline (34). Providing systematic needs assessment during consultations and multidisciplinary team meetings (vs. usual care where no such systematic assessment was provided) found no improvement in unmet needs assessment, psychological morbidity or distress (37). Change score analysis indicated a relative benefit from the intervention for unmet symptom needs at week 8 and 12 post-assessment (effect size =0.55 and 0.40, respectively) (37).
(III) Communication
All patients undertaking a nurse-directed face-to-face educational intervention reported satisfaction with the topics of discussion (including symptom management, problem solving, communication, and advance care planning), finding them useful (34).
Caregivers
Providing mindfulness-based stress reduction training to people with lung cancer, found no effect on their caregivers’ psychosocial distress (including quality of life, mindfulness skills, self-compassion and rumination) (35). Offering telephone symptom management (TSM) (with cognitive-behavioral and emotion-focused therapy content) vs. education/support found no significant group differences in improving the caregiver self-efficacy for helping the person with lung cancer manage cancer symptoms as well as caregiver burden at 2 and 6 weeks post-intervention (36). A small improvement favoring TSM was observed in caregivers’ self-efficacy to manage their own emotions and perceived social constraints from the patient, but no significant changes were reported over time for either group (36).
Discussion
There is mixed evidence for the effectiveness of early referral to palliative care and individual palliative care interventions for people with lung cancer and their caregivers. The two major positive trials testing the effectiveness of early referral to palliative care focused on lung cancer (13,27). It is noteworthy that these trials were conducted at a single US site and in a country where concurrent palliative care and active oncology treatment has only recently been funded (39). All other trials were conducted with heterogeneous advanced cancer populations, at single or multiple centers, and some were conducted in countries where universal health care may also be available. The trials evaluating individual interventions were predominantly conducted at single sites, in countries with and without universal health care coverage. Four of the five trials focused exclusively on lung cancer. Their results were equally inconclusive in relation to the effectiveness of the proposed palliative care interventions.
This lack of conclusive evidence for lung cancer parallels findings for the effectiveness of early referral to palliative care in other cancer populations. Trials reporting advantages of providing early referral to palliative care include people newly diagnosed with gastrointestinal (GI; non-colorectal) cancer, where early referral decreased depression, improved patients’ quality of life and ability to cope with the prognosis, and enhanced patient-clinician communication about end-of-life preferences (27). Importantly, the trial reported that quality of life and mood at week 12 improved for both the intervention and control groups (27). Breathlessness support services were found to improve mastery of breathlessness in people with advanced diseases, including lung cancer (40). Early integration of palliative care also improved survival (at 6 months and overall), but only for people with chronic obstructive and pulmonary disease (COPD) and interstitial lung disease, and not those with lung cancer (40).
Mixed results or lack of improvement within trials have also been noted. In people with gastric cancer, systematic early palliative care (compared to on-demand palliative care) showed slight, but not significant benefit in improving quality of life; and no improvement in anxiety/depression and family satisfaction with care (41). Similarly, people with advanced pancreatic cancer receiving systematic (vs.on-demand) palliative care reported improved quality of life and symptom burden, and reduced hospitalization; but not overall survival (42). Early specialist palliative care compared to standard oncology care in people with malignant pleural mesothelioma (a cancer with high symptom burden and poor survival) did not improve quality of life nor psychological symptoms (depression and anxiety), suggesting that routine referral to specialist palliative care soon after diagnosis is not needed if access to such services can be provided when needed (43).
The same mixed benefits of integrating early palliative care in standard oncology care extends to caregivers. In gastrointestinal (non-colorectal) cancer, early palliative care was associated with improved caregiver total distress and depression, but not anxiety at 12 weeks (28). Although the same benefits were not observed at 24 weeks, significant benefits were observed for both depression and anxiety at 3 and 6 months before the patient’s death (28).
Despite these limitations, earlier integration of palliative care into oncology care is supported by national guidelines, including for people newly diagnosis with people with non-small cell lung cancer (17). The recommendations further state that screening for palliative care needs should be continuous, at appropriate intervals as clinically indicated (17).
Defining the intervention elements and measuring fidelity
Despite great excitement after the Temel et al. (13) trial, the demonstrated survival advantages have not been reproduced other than in another US trial conducted by Bakitas et al. (30). In determining the effects of palliative care on survivorship, it is worth asking if these trials observed increased survival in the intervention group or increased mortality in the group that did not get palliative care. It is possible that what has been observed in these trials is not improved survival but premature mortality in the control group who did not get access to palliative care (44). Given the (perceived or actual) benefits of palliative care for both patients and caregivers (during and after the caregiving period) (9,31), lack of access to palliative care is likely to increase the burden of the disease on people’s lives, including their survivorship. Delineating these effects in future trials is important, yet challenging, and will have implications for the design of future studies, as well as our understanding of the true net effect of palliative care for the cancer population in general, and lung cancer in particular.
Another important consideration when determining the correlation between the effects observed in these trials and the active intervention is understanding what element(s) within palliative care make the most difference for participant’s outcomes. The five trials included in this review testing individual palliative care interventions in the oncology setting (Table S3) integrated different therapeutic elements into their palliative care interventions. The use of mindfulness-based techniques was reported as a potentially helpful therapy to reduce patients’ distress, which is supported by previous research in other populations with cancer (45). However, more robust data is required to draw definite conclusions. Evidence is still conflicting for other interventions such as nutrition and exercise-based programs (46). This is important given the adverse impact of cachexia and muscle wasting on quality of life, survivorship and caregiver distress. Most trials evaluate different interventions as part of a comprehensive palliative care model of care. Thus, it might be difficult to determine the relevance and impact of each component on the patient/caregiver outcomes. Other factors [for example, who delivers palliative care, the oncologist or the palliative care specialist (4)] might also play a role.
Definitional issues are also relevant when trying to unpack the components of “early” palliative care. Lack of consensus around “early” palliative care (18) means that this is defined in relation to multiple entry points, and can include any of the following: initial consultation at time of diagnosis; being seen by a palliative care specialist less than 3 months after diagnosis of advanced cancer or greater than 3 months before death; at particular treatment time points specific to cancer type (for example, at the time of cisplatinum resistance for advanced ovarian cancer) (18). Early palliative care can also be tied to the presence of prognostic signs or symptoms; or defined in relation to the setting (e.g., outpatient vs. inpatient) or duration of continuity before death (>90, 31–90, 11–30, 1–10 days) (18). This variation will particularly be relevant in multi-site trials where local practices and models of care are likely to influence the components and delivery of the intervention and controlled arms (44).
Equally importantly, lack of consensus around what is best supportive care or usual/standard care means that often these are not clearly defined in trials (47). An international Delphi-consensus process identified four key domains of best supportive care in clinical trials: multi-disciplinary care; supportive care documentation; symptom assessment; and symptom management (48). Incorporating this in the design of the control (but also active) arm will have implications in how these trails are conducted and outcomes compared (44).
Implications for the provision of multidisciplinary care in lung cancer
If multidisciplinary care is the gold standard of optimal care for people with lung cancer, how does integrating early palliative care affect this model of care, if at all? Evidence suggest that multidisciplinary teams in lung cancer are effective in changing patient management, more so than in improving survival (for which there is limited evidence) (49,50). If multidisciplinary care is the standard and if palliative care is part of multidisciplinary lung cancer care, are the benefits associated with its initiation associated with early referral as routine practice or based on individual patient and caregiver needs?
Although studies have shown that a delayed initiation of palliative care results in poorer outcomes for people with cancer (when compared to early initiation after diagnosis) (30), studies comparing systematic vs. needs-based palliative care show no significant differences in survival (42), quality of life, psychological symptoms and family satisfaction with care (41). This suggest that providing palliative care when required might achieve the same outcomes for patients and caregivers. On-demand palliative care may also be a more cost-effective option.
A key element of providing multidisciplinary palliative care is the inclusion of primary care providers as part of the multidisciplinary care teams. Of the included trials in this review, only one trial (29) involved general practitioners in the provision of palliative care and only as part of standard oncology care (i.e., the control arm, where specialist palliative care was the intervention arm). Perhaps significantly, providing early specialist palliative care in this trial showed no significant effect on the primary outcome (which was the patient’s identified need), and no differences between the groups in relation to other patient-identified symptoms, including survivorship. None of the included trials in this review included primary care providers in their palliative care intervention. Facilitating the engagement of general practitioners in multidisciplinary care teams may provide benefit to patients and their caregivers as their palliative care needs would be supported in the environment in which they live (i.e., at home as opposed to the hospital). Supporting their involvement through evidence-based methods, such as case conferencing (51), could have the potential to add to the quality of clinical decision making within multidisciplinary teams (52), ultimately resulting in improved patient and caregiver outcomes.
Strengths and limitations
There are a number of strengths and limitations with this systematic review. Its strengths are having examined all of the data from the early palliative care trails and synthesized the results, a number of recommendations are made about current practice and future research. Its major limitation is that only two of the included trials had ≥50% of participants with lung cancer, while the others included participants with various cancers. The latter studies did not report the lung cancer data separately. Therefore, the results reported here reflect outcomes reported for all cancers making it difficult to know to what extend those findings can be generalized for lung cancer specifically. However, cancers with poor outcomes such as gastrointestinal were included, which makes for comparable outcomes. Variability in outcome measures and reporting reduced our capacity to synthesize results quantitatively.
Conclusions
Mixed results were found in the literature for the effectiveness of early referral and for specific components of supportive care for people with lung cancer and their caregivers, mirroring findings for other types of cancer. Combined with evidence that on-demand palliative care is equally, if not more effective than systematic palliative care, this raises the question of whether initiation and provision of palliative care as part of multidisciplinary lung cancer care should be guided by an early or need-based referral. Better understanding of the “active ingredients” of palliative care when delivered to people with lung cancer and their caregivers will help delineate the correlation with reported outcomes.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to thank Ms. Maja Garcia, a Research Assistant with IMPACCT, University of Technology Sydney, for her kind assistance with the data extraction and in preparing this manuscript for publication.
Funding: This work was supported by discretionary funds held by the IMPACCT team.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emily Stone) for the series “Lung Cancer Multidisciplinary Care” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2019.12.18). The series “Lung Cancer Multidisciplinary Care” was commissioned by the editorial office without any funding or sponsorship. DC reports and is an unpaid advisory board member for Helsinn Pharmaceuticals. He is a paid consultant and receives payment for intellectual property with Mayne Pharma and is a consultant with Specialised Therapeutics Australia Pty. Ltd. The authors have no other conflicts of interest to declare.
References
- 1.WHO. Cancer, https://www.who.int/news-room/fact-sheets/detail/cancer (2018, accessed 8 September 2019).
- 2.Australian Institute of Health and Welfare. Cancer in Australia 2019. Canberra: AIHW, 2019. [Google Scholar]
- 3.Cancer Council. Lung Cancer. Available online: https://www.cancer.org.au/about-cancer/types-of-cancer/lung-cancer.html (2019, accessed 8 September 2019).
- 4.Shin J, Temel J. Integrating palliative care: when and how? Curr Opin Pulm Med 2013;19:344-9. [DOI] [PubMed] [Google Scholar]
- 5.Iyer S, Roughley A, Rider A, et al. The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer 2014;22:181-7. 10.1007/s00520-013-1959-4 [DOI] [PubMed] [Google Scholar]
- 6.Lutz S, Norrell R, Bertucio C, et al. Symptom frequency and severity in patients with metastatic or locally recurrent lung cancer: a prospective study using the Lung Cancer Symptom Scale in a community hospital. J Palliat Med 2001;4:157-65. 10.1089/109662101750290191 [DOI] [PubMed] [Google Scholar]
- 7.Milbury K, Badr H, Fossella F, et al. Longitudinal associations between caregiver burden and patient and spouse distress in couples coping with lung cancer. Support Care Cancer 2013;21:2371-9. 10.1007/s00520-013-1795-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families? The Cancer Journal 2010;16:423-35. 10.1097/PPO.0b013e3181f684e5 [DOI] [PubMed] [Google Scholar]
- 9.Abernethy AP, Currow DC, Fazekas BS, et al. Specialized palliative care services are associated with improved short-and long-term caregiver outcomes. Support Care Cancer 2008;16:585-97. 10.1007/s00520-007-0342-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann C, Riechelmann R, Krzyzanowska M, et al. Effectiveness of specialized palliative care: a systematic review. JAMA 2008;299:1698-709. 10.1001/jama.299.14.1698 [DOI] [PubMed] [Google Scholar]
- 11.Greer JA, Jackson VA, Meier DE, et al. Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin 2013;63:349-63. 10.3322/caac.21192 [DOI] [PubMed] [Google Scholar]
- 12.Bauman JR, Temel JS. The integration of early palliative care with oncology care: the time has come for a new tradition. J Natl Compr Canc Netw 2014;12:1763-71. 10.6004/jnccn.2014.0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. 10.1056/NEJMoa1000678 [DOI] [PubMed] [Google Scholar]
- 14.Kaasa S, Loge JH, Aapro M, et al. Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol 2018;19:e588-653. 10.1016/S1470-2045(18)30415-7 [DOI] [PubMed] [Google Scholar]
- 15.Knaul FM, Farmer PE, Krakauer EL, et al. Alleviating the access abyss in palliative care and pain relief—an imperative of universal health coverage: the Lancet Commission report. Lancet 2018;391:1391-454. 10.1016/S0140-6736(17)32513-8 [DOI] [PubMed] [Google Scholar]
- 16.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880-7. 10.1200/JCO.2011.38.5161 [DOI] [PubMed] [Google Scholar]
- 17.Wood DE. National Comprehensive Cancer Network (NCCN) clinical practice guidelines for lung cancer screening. Thorac Surg Clin 2015;25:185-97. 10.1016/j.thorsurg.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 18.Davis MP, Temel JS, Balboni T, et al. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med 2015;4:99-121. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 20.Sladek R, Tieman J, Fazekas BS, et al. Development of a subject search filter to find information relevant to palliative care in the general medical literature. J Med Libr Assoc 2006;94:394-401. [PMC free article] [PubMed] [Google Scholar]
- 21.Sladek RM, Tieman J. Applying evidence in the real world: a case study in library and information practice. Health Info Libr J 2008;25:295-301. 10.1111/j.1471-1842.2008.00778.x [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 23.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT and Green S. Cochrane handbook for systematic reviews of interventions Version 5.1.0. Available online: www.handbook.cochrane.org. The Cochrane Collaboration, 2011.
- 25.Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A Product from the ESRC Methods Programme 2006; Version 1: b92.
- 26.McCorkle R, Jeon S, Ercolano E, et al. An Advanced Practice Nurse Coordinated Multidisciplinary Intervention for Patients with Late-Stage Cancer: A Cluster Randomized Trial. J Palliat Med 2015;18:962-9. 10.1089/jpm.2015.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol 2017;35:834-41. 10.1200/JCO.2016.70.5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Jawahri A, Greer JA, Pirl WF, et al. Effects of early integrated palliative care on caregivers of patients with lung and gastrointestinal cancer: a randomized clinical trial. Oncologist 2017;22:1528-34. 10.1634/theoncologist.2017-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groenvold M, Petersen MA, Damkier A, et al. Randomised clinical trial of early specialist palliative care plus standard care versus standard care alone in patients with advanced cancer: The Danish Palliative Care Trial. Palliat Med 2017;31:814-824. 10.1177/0269216317705100 [DOI] [PubMed] [Google Scholar]
- 30.Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1438-45. 10.1200/JCO.2014.58.6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dionne-Odom JN, Azuero A, Lyons KD, et al. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1446-52. 10.1200/JCO.2014.58.7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383:1721-30. 10.1016/S0140-6736(13)62416-2 [DOI] [PubMed] [Google Scholar]
- 33.Uster A, Ruehlin M, Mey S, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: A randomized controlled trial. Clin Nutr 2018;37:1202-9. 10.1016/j.clnu.2017.05.027 [DOI] [PubMed] [Google Scholar]
- 34.Yang GM, Teo I, Neo SH, et al. Pilot Randomized Phase II Trial of the Enhancing Quality of Life in Patients (EQUIP) Intervention for Patients With Advanced Lung Cancer. Am J Hosp Palliat Care 2018;35:1050-6. 10.1177/1049909118756095 [DOI] [PubMed] [Google Scholar]
- 35.Schellekens MPJ, van den Hurk DGM, Prins JB, et al. Mindfulness-based stress reduction added to care as usual for lung cancer patients and/or their partners: A multicentre randomized controlled trial. Psychooncology 2017;26:2118-26. 10.1002/pon.4430 [DOI] [PubMed] [Google Scholar]
- 36.Mosher CE, Winger JG, Hanna N, et al. Randomized pilot trial of a telephone symptom management intervention for symptomatic lung cancer patients and their family caregivers. J Pain Symptom Manage 2016;52:469-82. 10.1016/j.jpainsymman.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schofield P, Ugalde A, Gough K, et al. A tailored, supportive care intervention using systematic assessment designed for people with inoperable lung cancer: a randomised controlled trial. Psychooncology 2013;22:2445-53. 10.1002/pon.3306 [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamal AH, Currow DC, Ritchie CS, et al. Community-based palliative care: the natural evolution for palliative care delivery in the US. J Pain Symptom Manage 2013;46:254-64. 10.1016/j.jpainsymman.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 40.Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014;2:979-87. 10.1016/S2213-2600(14)70226-7 [DOI] [PubMed] [Google Scholar]
- 41.Scarpi E, Dall’Agata M, Zagonel V, et al. Systematic vs. on-demand early palliative care in gastric cancer patients: a randomized clinical trial assessing patient and healthcare service outcomes. Support Care Cancer 2019;27:2425-34. 10.1007/s00520-018-4517-2 [DOI] [PubMed] [Google Scholar]
- 42.Maltoni M, Scarpi E, Dall'Agata M, et al. Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer 2016;65:61-8. 10.1016/j.ejca.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 43.Brims F, Gunatilake S, Lawrie I, et al. Early specialist palliative care on quality of life for malignant pleural mesothelioma: a randomised controlled trial. Thorax 2019;74:354-61. 10.1136/thoraxjnl-2018-212380 [DOI] [PubMed] [Google Scholar]
- 44.Currow DC, Foley K, Zafar SY, et al. The need for a re-evaluation of best supportive care studies reported to date. Br J Cancer 2011;104:390-1. 10.1038/sj.bjc.6606081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zainal NZ, Booth S, Huppert FA. The efficacy of mindfulness-based stress reduction on mental health of breast cancer patients: A meta-analysis. Psychooncology 2013;22:1457-65. 10.1002/pon.3171 [DOI] [PubMed] [Google Scholar]
- 46.Rueda JR, Solà I, Pascual A, et al. Non-invasive interventions for improving well-being and quality of life in patients with lung cancer. Cochrane Database Syst Rev 2011;(9):CD004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nipp RD, Currow DC, Cherny NI, et al. Best supportive care in clinical trials: review of the inconsistency in control arm design. Br J Cancer 2015;113:6. 10.1038/bjc.2015.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zafar SY, Currow DC, Cherny N, et al. Consensus-based standards for best supportive care in clinical trials in advanced cancer. Lancet Oncol 2012;13:e77-82. 10.1016/S1470-2045(11)70215-7 [DOI] [PubMed] [Google Scholar]
- 49.Coory M, Gkolia P, Yang IA, et al. Systematic review of multidisciplinary teams in the management of lung cancer. Lung Cancer 2008;60:14-21. 10.1016/j.lungcan.2008.01.008 [DOI] [PubMed] [Google Scholar]
- 50.Hong NJL, Wright FC, Gagliardi AR, et al. Examining the potential relationship between multidisciplinary cancer care and patient survival: an international literature review. J Surg Oncol 2010;102:125-34. 10.1002/jso.21589 [DOI] [PubMed] [Google Scholar]
- 51.Abernethy AP, Currow DC, Hunt R, et al. A pragmatic 2× 2× 2 factorial cluster randomized controlled trial of educational outreach visiting and case conferencing in palliative care—methodology of the Palliative Care Trial [ISRCTN 81117481]. Contemp Clin Trials 2006;27:83-100. 10.1016/j.cct.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 52.Lamb BW, Brown KF, Nagpal K, et al. Quality of care management decisions by multidisciplinary cancer teams: a systematic review. Ann Surg Oncol 2011;18:2116-25. 10.1245/s10434-011-1675-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as