Abstract
Background
Radiological manifestations of coronavirus disease 2019 (COVID-19) featured ground-glass opacities (GGOs), especially in the early stage, which might create confusion in differential diagnosis with early lung cancer. We aimed to specify the radiological characteristics of COVID-19 and early lung cancer and to unveil the discrepancy between them.
Methods
One hundred and fifty-seven COVID-19 patients and 374 early lung cancer patients from four hospitals in China were retrospectively enrolled. Epidemiological, clinical, radiological, and pathological characteristics were compared between the two groups using propensity score-matched (PSM) analysis.
Results
COVID-19 patients had more distinct symptoms, tended to be younger (P<0.0001), male (P<0.0001), and had a higher body mass index (P=0.014). After 1:1 PSM, 121 matched pairs were identified. Regarding radiological characteristics, patients with a single lesion accounted for 17% in COVID-19 and 89% in lung cancer (P<0.0001). Most lesions were peripherally found in both groups. Lesions in COVID-19 involved more lobes (median 3.5 vs. 1; P<0.0001) and segments (median 6 vs. 1; P<0.0001) and tended to have multiple types (67%) with patchy form (54%). Early lung cancer was more likely to have a single type (92%) with oval form (66%). Also, COVID-19 and early lung cancer either had some distinctive features on computed tomography (CT) images.
Conclusions
Both COVID-19 and early lung cancers showed GGOs, with similar but independent features. The imaging characteristics should be fully understood and combined with epidemiological history, pathogen detection, laboratory tests, short-term CT reexamination, and pathological results to aid differential diagnosis.
Keywords: Coronavirus disease 2019 (COVID-19), lung neoplasms, ground glass opacity, radiology, propensity score
Introduction
Coronavirus disease 2019 (COVID-19) was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the epidemic was designated as a global health emergency by WHO on January 30, 2020 (1). As of June 20, 2020, there were more than 8,525,000 reported cases with over 456,000 deaths globally (2). The pathogenesis of COVID-19 infection generally includes three stages. In the early stage, the virus infects the nasal cells through binding to angiotensin-converting enzyme 2 (ACE-2) and mounts a minor inflammatory response. Later, the virus propagates to the conducting respiratory airways, and the COVID-19 disease is clinically manifested at this time. Finally, the virus reaches the gas exchange units of the lung and infects alveolar type II cells. About 20% of the infected patients progresses to this stage and develops pulmonary infiltrates, with some of them developing very severe disease including frank lung injury and acute respiratory distress syndrome (ARDS) (3).With the rapid transmission of COVID-19, consensus guidelines should be steadily established and updated to prevent transmission and facilitate diagnosis and treatment (4).
Real-time polymerase chain reaction (RT-PCR) for SARS-CoV-2 nucleic acid test and virus gene sequencing is the current gold standard of diagnosis, but the time-consuming procedures result in a prolonged diagnostic cycle (5). Remarkably, chest high-resolution computed tomography (HRCT) demonstrated a multiple-stage imaging evolution of COVID-19 (6). Most cases featured patchy/oval ground-glass opacities (GGOs) or consolidative pulmonary opacities on unenhanced computed tomography (CT) scans, while the enlargement and consolidation of GGO usually indicated the progression of pneumonia (7,8). Aside from its pivotal role in evaluating the severity of pulmonary involvement in COVID-19, thoracic CT also serves as a significant supplement to discern false-negative patients undergoing the SARS-CoV-2 nucleic acid test, as these patients are likely to have typical CT findings (9).
Notably, low-dose CT (LDCT) is currently applied as an effective tool for screening early lung cancer, on which GGOs usually indicate potential malignant lesions (10,11). The similarity of GGOs on CT images can create confusion in distinguishing COVID-19 from early lung cancer, especially in asymptomatic or nucleic acid negative patients. Also, there were reports on patients with early stage lung cancer combined COVID-19, presenting no symptoms of pneumonia. Some of them received surgery but experienced severe pneumonia and other serious complications or even death postoperatively (12). Thus, it is essential to distinguish these two diseases, which are both manifested as GGOs.
Herein we describe and characterize the essential radiological findings in a group of 157 patients with COVID-19 compared with a group of 374 patients with early stage lung cancer. To the best of our knowledge, this is the first analysis aiming to compare these two groups by accounting for confounding variables in attempt to minimize bias. Their epidemiological, clinical, and pathological characteristics are also evaluated in the hope of unveiling the discrepancy between these two diseases. We present the following article in accordance with the STROBE reporting checklist (http://dx.doi.org/10.21037/tlcr-20-892).
Methods
Ethical approval
The Ethics Commission of Ruijin Hospital approved this study (KY2020-26) and following the Helsinki Declaration (as revised in 2013). Written informed consent for this retrospective study was waived by the Ethics Commission of the designated hospital for emerging infectious diseases.
Clinical specimen and data collection
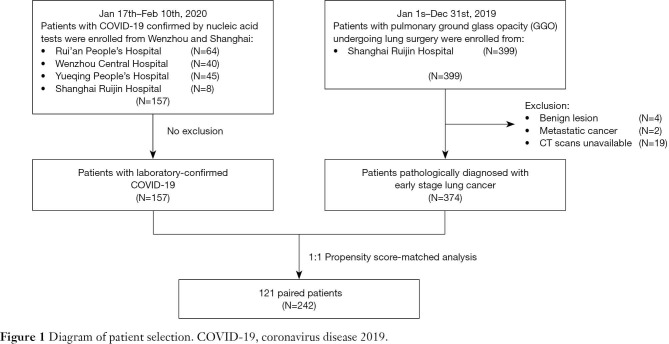
A total of 157 patients with COVID-19 confirmed by RT-PCR from January 17 through February 10, 2020, were enrolled in this study, including eight patients from Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai, China) and 149 patients from three tertiary hospitals in Wenzhou (Wenzhou, China). Meanwhile, we analyzed 374 patients admitted to Ruijin Hospital from January 1 through December 31, 2019, whose CT manifestations featured GGOs and whose postoperative pathology confirmed early stage lung cancer, as a control—the early lung cancer group (Figure 1).
Figure 1.
Diagram of patient selection. COVID-19, coronavirus disease 2019.
The demographic data, epidemiological history, medical symptoms, laboratory tests, CT findings, and pathologic results were obtained from patients’ medical records. For epidemiological and symptom data inaccessible from electronic medical records, the researchers communicated directly with patients or their families to substantiate.
CT image review
All CT examinations adhered to the common chest protocol: the patient was installed in a supine position with arms raised and held his/her breath during acquisition. For each patient, the following characteristics were evaluated (8): (I) type of lung lesions (pure GGO; mixed GGO; consolidation) (11); (II) form of lesions (oval; patchy); (III) distribution of lesions (unilateral lung; bilateral lung); (IV) number of involved lobes; (V) frequency of lobe involvement (right upper lobe; right middle lobe; right lower lobe; left upper lobe; left lower lobe); (VI) number of involved segments; (VII) frequency of segment involvement (L S1+2; L S3; L S4; L S5; L S6; L S7+8; L S9; L S10; R S1; R S2; R S3; R S4; R S5; R S6; R S7; R S8; R S9; R S10); (VIII) other findings: air bronchogram, centrilobular nodules, tree-in-bud, reticular pattern, subpleural linear opacity, bronchial dilation, cystic change, lobulated sign, pleural retraction, vessel convergence sign, lymphadenopathy, and pleural effusion.
Two fellowship-trained cardiothoracic radiologists with five years of experience reviewed all the CT scans through a viewing console independently. A third fellowship-trained cardiothoracic radiologist with 15 years of experience adjudicated a final decision when the two radiologists did not reach consensus.
Statistical analysis
To minimize the bias caused by the non-randomized selection of patients, we performed a propensity score-matched (PSM) analysis between two groups. Each patient’s propensity score was calculated under a multivariable logistic regression model with covariates that showed significant difference at baseline (Table 1), including sex, age, body mass index (BMI), comorbidity of cardio-cerebrovascular disease, digestive disease, and tumor history. Patients in the COVID-19 group were matched 1:1 with patients in the early lung cancer group using the nearest-neighbor method with a caliper of 0.02.
Table 1. Demographic and clinical characteristics of patients.
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| COVID-19 (n=157) | Lung cancer (n=374) | P value | COVID-19 (n=121) | Lung cancer (n=121) | P value | ||
| Sex, n (%) | <0.0001 | 1.00 | |||||
| Male | 86 (55%) | 131 (35%) | 58 (48%) | 57 (47%) | |||
| Female | 71 (45%) | 243 (65%) | 63 (52%) | 64 (53%) | |||
| Age (years), median (IQR) | 46 [36–55] | 58 [49–65] | <0.0001 | 50 [41–57] | 52 [41–60] | 0.77 | |
| BMI (kg/m2), median (IQR) | 24 [21–26] | 23 [21–25] | 0.014 | 23 [21–25] | 23 [22–26] | 0.71 | |
| Exposure history, n (%) | <0.0001† | <0.0001† | |||||
| Exposure to infected patient | 49 (31%) | 0 (0%) | 36 (30%) | 0 (0%) | |||
| Recent travel to Wuhan | 84 (54%) | 0 (0%) | 69 (57%) | 0 (0%) | |||
| Recent travel to other cities in Hubei | 5 (3%) | 0 (0%) | 2 (2%) | 0 (0%) | |||
| No exposure history | 19 (12%) | 374 (100%) | 14 (12%) | 121 (100%) | |||
| Comorbidity, n (%) | |||||||
| Cardio-cerebrovascular disease | 29 (18%) | 105 (28%) | 0.027 | 25 (21%) | 27 (22%) | 0.88 | |
| Digestive disease | 8 (5%) | 43 (12%) | 0.034 | 8 (7%) | 7 (6%) | 1.00 | |
| Endocrine disease | 9 (6%) | 32 (9%) | 0.35 | 9 (7%) | 5 (4%) | 0.41 | |
| Neuropathy | 0 (0%) | 6 (2%) | 0.19 | 0 (0%) | 2 (2%) | 0.50 | |
| Respiratory disease | 3 (2%) | 15 (4%) | 0.34 | 2 (2%) | 4 (3%) | 0.68 | |
| Tumor history | 2 (1%) | 28 (7%) | 0.0087 | 2 (2%) | 2 (2%) | 1.00 | |
| Symptoms, n (%) | |||||||
| Fever | 122 (78%) | 2 (1%) | <0.0001 | 99 (82%) | 0 (0%) | <0.0001 | |
| Cough | 92 (59%) | 19 (5%) | <0.0001 | 70 (58%) | 8 (7%) | <0.0001 | |
| Sputum | 50 (32%) | 8 (2%) | <0.0001 | 35 (29%) | 3 (2%) | <0.0001 | |
| Dyspnea | 3 (2%) | 0 (0%) | 0.026 | 2 (2%) | 0 (0%) | 0.50 | |
| Snot | 6 (4%) | 0 (0%) | 0.00062 | 5 (4%) | 0 (0%) | 0.060 | |
| Chest pain | 5 (3%) | 9 (2%) | 0.57 | 4 (3%) | 2 (2%) | 0.68 | |
| Chest distress | 16 (10%) | 3 (1%) | <0.0001 | 13 (11%) | 3 (2%) | 0.020 | |
| Muscle soreness | 8 (5%) | 0 (0%) | <0.0001 | 4 (3%) | 0 (0%) | 0.12 | |
| Diarrhea | 12 (8%) | 0 (0%) | <0.0001 | 7 (6%) | 0 (0%) | 0.014 | |
| No obvious symptoms | 5 (3%) | 344 (92%) | <0.0001 | 2 (2%) | 110 (91%) | <0.0001 | |
| Laboratory findings at admission, median (IQR) | |||||||
| White blood cell (G/L) | 4.6 (3.6–6.0) | 5.1 (4.3–6.0) | 0.00027 | 4.5 (3.3–5.7) | 5.4 (4.5–6.4) | <0.0001 | |
| Neutrophil (G/L) | 2.64 (1.99–3.90) | 2.81 (2.30–3.60) | 0.16 | 2.60 (1.80–3.90) | 3.07 (2.40–3.60) | 0.032 | |
| Lymphocyte (G/L) | 1.29 (0.98–1.65) | 1.70 (1.39–2.00) | <0.0001 | 1.19 (0.90–1.50) | 1.74 (1.50–2.10) | <0.0001 | |
| Alanine aminotransferase (U/L) | 20.0 (14.0–33.0) | 19.0 (14.0–26.0) | 0.11 | 18.5 (13.3–32.0) | 19.0 (14.0–29.0) | 0.85 | |
| Aspartate aminotransferase (U/L) | 23.0 (19.0–34.5) | 20.0 (17.0–25.0) | <0.0001 | 23.0 (19.0–33.5) | 20.0 (16.0–24.0) | <0.0001 | |
| D-dimer (mg/L) | 0.25 (0.18–0.52) | 0.24 (0.19–0.37) | 0.29 | 0.27 (0.19–0.63) | 0.21 (0.17–0.32) | 0.017 | |
| Viral nucleic acid detection, n (%) | 157 (100%) | NA§ | NA | 121 (100%) | NA§ | NA | |
†, Fisher’s exact test for overall distribution; §, viral nucleic acid detection was not applicable (NA) in patients with lung cancer. PSM, propensity score-matched; COVID-19, coronavirus disease 2019; IQR, interquartile range.
Continuous variables were presented as median [interquartile range (IQR)] and compared by Wilcoxon rank-sum test between the groups. Pearson’s Chi-squared test or Fisher’s exact test was applied for comparing categorical variables. PSM and statistical analyses were performed by using R software (version 3.5.3, R Foundation for Statistical Computing, Vienna, Austria). A P value less than 0.05 was considered statistically significant.
Role of the funding source
The funders of this study had no role in study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit the article for publication.
Results
Demographic and clinical characteristics
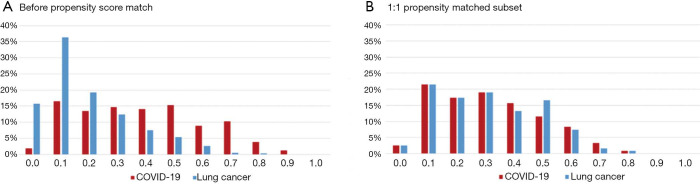
A total of 157 patients with confirmed COVID-19 and 374 early stage lung cancer patients were included in this study. Patients in the COVID-19 group tended to be younger [median 46 years (IQR 36–55 years) vs. 58 years (IQR 49–65 years); P<0.0001], male sex (55% vs. 35%; P<0.0001), and with higher BMI [median 24 kg/m2 (IQR 21–26 kg/m2) vs. 23 kg/m2 (IQR 21–25 kg/m2); P=0.014]. The incidence of comorbidities including cardio-cerebrovascular disease (18% vs. 28%; P=0.027), digestive disease (5% vs. 12%; P=0.034), and tumor history (1% vs. 7%; P=0.0087) were significantly lower in COVID-19 patients. After PSM analysis controlling for sex, age, BMI, and comorbidities, 121 cases from each group were matched (Figure 1). As expected, this resulted in similar distributions of propensity scores (Figure S1). The demographic and clinical data of two groups before and after PSM analysis were presented in Table 1.
Figure S1.
Distribution of propensity score.
Before PSM, most of the patients (138/157, 88%) with COVID-19 had obvious exposure history, either exposed to infected patient or having recent travel to Hubei Province. Patients with COVID-19 had significantly higher incidences of various pneumonia symptoms than lung cancer patients, including fever (78% vs. 1%; P<0.0001), cough (59% vs. 5%; P<0.0001), sputum (32% vs. 2%; P<0.0001), dyspnea (2% vs. 0%; P=0.026), snot (4% vs. 0%; P=0.00062), chest distress (10% vs. 1%; P<0.0001), muscle soreness (5% vs. 0%; P<0.0001), and diarrhea (8% vs. 0%; P<0.0001). For laboratory findings at admission, COVID-19 patients had significantly lower counts of white blood cell [median 4.6 G/L (IQR 3.6–6.0 G/L) vs. 5.1 G/L (IQR 4.3–6.0 G/L); P=0.00027] and lymphocyte count [median 1.29 G/L (IQR 0.98–1.65 G/L) vs. 1.70 G/L (IQR 1.39–2.00 G/L); P<0.0001], and significantly higher aspartate aminotransferase [median 23.0 U/L (IQR 19.0–34.5 U/L) vs. 20.0 U/L (IQR 17.0–25.0 U/L); P<0.0001] compared with lung cancer patients. Neutrophil count, alanine aminotransferase, and D-dimer revealed no significant difference between two groups before PSM.
After PSM, the difference of exposure history, incidence of symptoms, and laboratory findings were consistent. However, the incidence of dyspnea (P=0.50), snot (P=0.060), and muscle soreness (P=0.12) between two groups were no longer significantly different, whereas neutrophil count [median 2.60 G/L (IQR 1.80–3.90 G/L) vs. 3.07 G/L (IQR 2.40–3.60 G/L); P=0.032] and D-dimer [median 0.27 mg/L (IQR 0.19–0.63 mg/L) vs. 0.21 mg/L (IQR 0.17–0.32 mg/L); P=0.017] reached statistical difference.
Radiological findings
By reviewing CT scans, 17 COVID-19 patients had negative radiological results. A total of 1,207 lung lesions were found in the 140 COVID-19 patients, and 448 lesions were found in the 374 lung cancer patients. Peripheral lesions predominated the location in both groups (COVID-19, 923/1,207 lesions, 76%; lung cancer, 333/448 lesions, 74%). In the lesions of COVID-19 patients, the proportion of pure GGO, mixed GGO, and consolidation were 27%, 58%, and 14%, respectively. In the lesions of lung cancer patients, the correspondent proportions were 46%, 47%, and 5% (Table S1).
Table S1. Location and type of lung lesions.
| Variables | COVID-19 (n=1,207 lesions) | Lung cancer (n=448 lesions) | P value |
|---|---|---|---|
| Location of lesions, n (% of all lesions) | 0.014* | ||
| Peripheral | 923 (76%) | 333 (74%) | 0.40 |
| Central | 55 (5%) | 29 (6%) | 0.15 |
| Both | 209 (17%) | 86 (19%) | 0.42 |
| Unknown | 20 (2%) | 0 (0%) | 0.013 |
| Type of lesions, n (% of all lesions) | <0.0001* | ||
| Pure GGO | 321(27%) | 204 (46%) | <0.0001 |
| Mixed GGO | 695 (58%) | 210 (47%) | 0.00013 |
| Consolidation | 170 (14%) | 24 (5%) | <0.0001 |
| Unknown | 21 (2%) | 10 (2%) | 0.65 |
*, Pearson’s chi-squared test for overall distribution. GGO, ground-glass opacity.
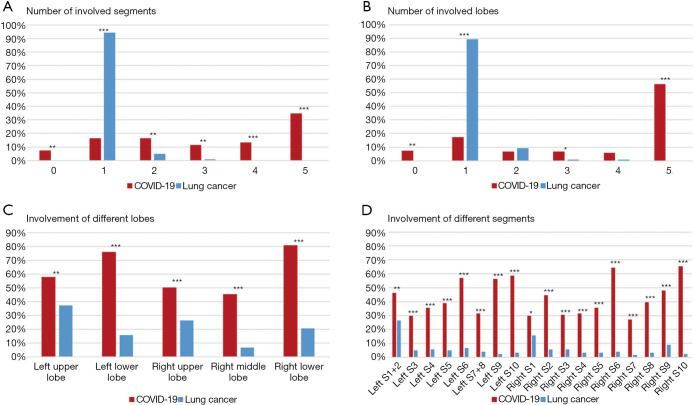
Table 2 presented the quantitative comparison of CT findings between two groups. The number, type, form, distribution of lesions, and its specific features achieved comparable results before and after PSM. Among the matched groups, 21/121 (17%) of the COVID-19 patients and 108/121 (89%) of the lung cancer patients presented with a single lesion (P<0.0001). Patients with COVID-19 had more involved lobes [median 3.5 (IQR 2–5) vs. 1 (IQR 1–1); P<0.0001; Figure 2A] and more involved segments [median 6 (IQR 2–13) vs. 1 (IQR 1–1); P<0.0001; Figure 2B] compared with lung cancer patients. The specific distribution of involved lobes and segments after PSM was showed in Figure 2C,D. Most patients with COVID-19 (81/121, 67%) had more than one type of lung lesions, while lung cancer patients tended to have either pure GGO (61/121, 50%) or mixed GGO (49/121, 40%). Regarding the form, COVID-19 presented more patchy lesions (65/121, 54%), while lung cancer presented more oval lesions (80/121, 66%). Most patients with COVID-19 had lesions bilaterally (89/121, 74%), while most cancer patients had unilateral lung lesions (117/121, 97%). Lymphadenopathy and pleural effusion were observed in 5/121 (4%) and 10/121 (8%) patients with COVID-19, respectively. Among the matched cancer patients, no one presented with lymphadenopathy, and only one patient (1/121, 1%) presented with pleural effusion on the CT scan. Air bronchogram and cystic change were observed in both groups. Air bronchogram was significantly more frequent in COVID-19 patients (60% vs. 11%; P<0.0001), while the cystic change was less frequent in the COVID-19 group (7% vs. 17%; P=0.027). COVID-19 was also featured by reticular pattern (73/121, 60%), subpleural linear opacity (29/121, 24%), bronchial dilatation (23/121, 19%), centrilobular nodules (5/121, 4%), and tree-in-bud (1/121, 1%), whereas none of these signs were observed on the CT scans of matched cancer patients. On the contrary, lobulated sign (25/121, 21%), pleural retraction (24/121, 20%), and vessel convergence sign (3/121, 2%) were present in lung cancer patients but absent in those with COVID-19.
Table 2. Radiological findings of patients.
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| COVID-19 (n=157) | Lung cancer (n=374) | P value | COVID-19 (n=121) | Lung cancer (n=121) | P value | ||
| Case of single lesion, n (%) | 26 (17%) | 326 (87%) | <0.0001 | 21 (17%) | 108 (89%) | <0.0001 | |
| Number of involved lobes, median [IQR] | 4 [2–5] | 1 [1–1] | <0.0001 | 3.5 [2–5] | 1 [1–1] | <0.0001 | |
| Number of involved segments, median [IQR] | 6 [2–13] | 1 [1–1] | <0.0001 | 6 [2–13] | 1 [1–1] | <0.0001 | |
| Type of lesions, n (%) | 0.00050† | 0.00050† | |||||
| Pure GGO | 6 (4%) | 156 (42%) | <0.0001 | 1 (1%) | 61 (50%) | <0.0001 | |
| Mixed GGO | 27 (17%) | 175 (47%) | <0.0001 | 25 (21%) | 49 (40%) | 0.0013 | |
| Consolidation | 5 (3%) | 14 (4%) | 0.95 | 5 (4%) | 2 (2%) | 0.45 | |
| Pure & mixed GGO | 53 (34%) | 21 (6%) | <0.0001 | 39 (32%) | 6 (5%) | <0.0001 | |
| Pure GGO & consolidation | 1 (1%) | 3 (1%) | 1.00 | 1 (1%) | 1 (1%) | 1.00 | |
| Mixed GGO & consolidation | 16 (10%) | 5 (1%) | <0.0001 | 14 (12%) | 2 (2%) | 0.0044 | |
| Pure & Mixed & consolidation | 32 (20%) | 0 (0%) | <0.0001 | 27 (22%) | 0 (0%) | <0.0001 | |
| No lesions | 17 (11%) | 0 (0%) | <0.0001 | 9 (7%) | 0 (0%) | 0.0033 | |
| Form of lesions, n (%) | <0.0001§ | <0.0001† | |||||
| Oval | 7 (4%) | 236 (63%) | <0.0001 | 6 (5%) | 80 (66%) | <0.0001 | |
| Patchy | 77 (49%) | 112 (30%) | <0.0001 | 65 (54%) | 36 (30%) | 0.00026 | |
| Oval & patchy | 56 (36%) | 26 (7%) | <0.0001 | 41 (34%) | 5 (4%) | <0.0001 | |
| No lesions | 17 (11%) | 0 (0%) | <0.0001 | 9 (7%) | 0 (0%) | 0.0033 | |
| Distribution of lesions, n (%) | <0.0001§ | <0.0001† | |||||
| Unilateral lung | 27 (17%) | 356 (95%) | <0.0001 | 23 (19%) | 117 (97%) | <0.0001 | |
| Bilateral lung | 113 (72%) | 18 (5%) | <0.0001 | 89 (74%) | 4 (3%) | <0.0001 | |
| No lesions | 17 (11%) | 0 (0%) | <0.0001 | 9 (7%) | 0 (0%) | 0.0033 | |
| Lymphadenopathy, n (%) | 7 (4%) | 1 (0%) | 0.0011 | 5 (4%) | 0 (0%) | 0.060 | |
| Pleural effusion, n (%) | 11 (7%) | 1 (0%) | <0.0001 | 10 (8%) | 1 (1%) | 0.014 | |
| Other features, n (%) | |||||||
| Air bronchogram | 88 (56%) | 52 (14%) | <0.0001 | 73 (60%) | 13 (11%) | <0.0001 | |
| Centrilobular nodules | 6 (4%) | 0 (0%) | 0.00062 | 5 (4%) | 0 (0%) | 0.060 | |
| Tree-in-bud | 2 (1%) | 0 (0%) | 0.087 | 1 (1%) | 0 (0%) | 1.00 | |
| Reticular pattern | 86 (55%) | 0 (0%) | <0.0001 | 73 (60%) | 0 (0%) | <0.0001 | |
| Subpleural linear opacity | 32 (20%) | 0 (0%) | <0.0001 | 29 (24%) | 0 (0%) | <0.0001 | |
| Bronchial dilatation | 27 (17%) | 5 (1%) | <0.0001 | 23 (19%) | 0 (0%) | <0.0001 | |
| Cystic change | 12 (8%) | 80 (21%) | 0.00022 | 8 (7%) | 20 (17%) | 0.027 | |
| Lobulated sign | 0 (0%) | 90 (24%) | <0.0001 | 0 (0%) | 25 (21%) | <0.0001 | |
| Pleural retraction | 0 (0%) | 86 (23%) | <0.0001 | 0 (0%) | 24 (20%) | <0.0001 | |
| Vessel convergence sign | 0 (0%) | 16 (4%) | 0.0045 | 0 (0%) | 3 (2%) | 0.25 | |
†, Fisher’s exact test for overall distribution; §, Pearson’s chi-squared test for overall distribution. PSM, propensity score-matched; COVID-19, coronavirus disease 2019; IQR, interquartile range; GGO, ground-glass opacity.
Figure 2.
Involvement of different lobes and segments. COVID-19, coronavirus disease 2019.
Pathological results
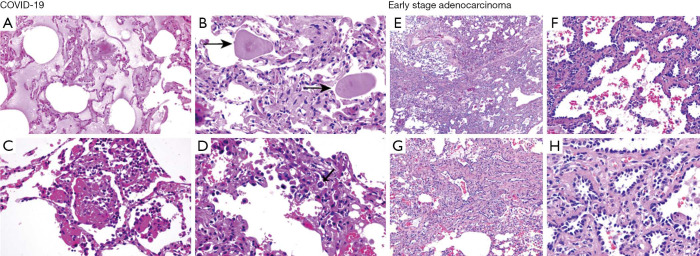
There is currently insufficient pathologic data on the COVID-19 from autopsy or biopsy, especially its comparison with early lung cancer presenting as GGO. However, a patient with an irregular solid nodule in the right middle lobe and bilateral ground-glass lesions underwent right middle lobectomy, and the specimen was found to have been infected by SARS-CoV-2 (12). The pathology indicated that the early phase of pulmonary pathological change of COVID-19 might be presented as edema, prominent globular proteinaceous exudate (Figure S2A), scattered spherical secretions or globules (Figure S2B), patchy inflammatory infiltration of mononuclear cells and multinucleated giant cells (Figure S2C), and focal hyperplasia of pneumocytes with interstitial thickening (Figure S2D). Meanwhile, neutrophil infiltration and hyaline membranes were not prominent. Occasionally, suspected viral inclusions might also be noted in some of these cells (Figure S2D).
Regarding early stage lung cancer, adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and surrounding pulmonary tissue could coexist (Figure S2E). Yet to COVID-19, the morphological feature of AIS was usually presented by tumor cells growing along the alveolar wall without cellular space, and most cells were arranged in a single layer with moderate density (Figure S2F). MIA glands intruded into the stroma at acute angles and triggered focal connective tissue reactive hyperplasia (Figure S2G). Another feature of adenocarcinoma was the increased cellular dysplasia, including enlarged nuclei, thicker chromatin, and pseudo-nuclear inclusions (Figure S2H).
Discussion
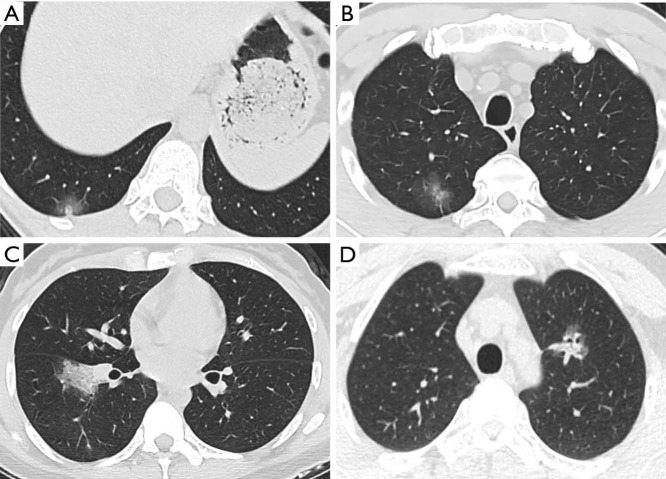
The ongoing outbreak of novel coronavirus has infected hundreds of thousands of people in China and other countries worldwide. One of the most challenging problems is to ensure diagnostic sensitivity and specificity. In addition to the gold standard of nucleic acid test, CT is a practical and indispensable auxiliary examination for the diagnosis of COVID-19. Moreover, it is possible to discover COVID-19 despite negative nucleic acid levels (13,14). Meanwhile, lung cancer is the most common malignancy around the world, mostly at the early stage (15). Enormous demand is called for to distinguish COVID-19 from early stage lung cancer during this public health emergency. Among the CT scans featured as GGO, attenuation was increased without covering up bronchial blood vessels, which showed focal damage of alveolar. The special imaging manifestation was paralleled with the pathological findings such as alveolar edema and proteinaceous exudates, where prominent inspissated spherical secretions, vascular congestion and multinucleated giant cells within the airspaces were seen. Moreover, proliferation of pneumocyte and interstitial fibroblasts decreased alveolar cavity (12). GGO predominates the radiological features of both COVID-19 (16) and early stage lung cancer (17,18). Both diseases could show unilateral or bilateral, oval, or patchy GGO as single or multiple lesions on CT scans, which poses challenges to the discrimination, especially for clinically suspected COVID-19 cases with negative nucleic acid results. As shown in Figure 3, the two exemplified GGO lesions could hardly tell the difference between COVID-19 and early stage lung cancer on CT images. Unfortunately, the similarity could cheat the surgeons by GGO-featured COVID-19 lesions and result in inappropriate operations, where pathology eventually confirmed adenocarcinoma combined with SARS-CoV-2 infection.
Figure 3.
CT scans with similar manifestations between COVID-19 and lung cancer. Lesions in (A) and (B) were both presented as GGO: (A) CT scan of a patient diagnosed with COVID-19; (B) CT scan of a patient diagnosed with lung cancer. Lesions in (C) and (D) were both presented as patchy opacification: (C) CT scan of a patient diagnosed with COVID-19; (D) CT scan of a patient diagnosed with lung cancer. COVID-19, coronavirus disease 2019; GGO, ground-glass opacity.
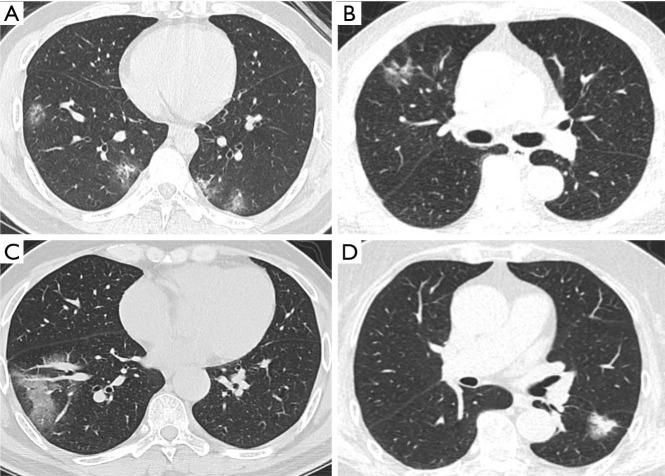
Difficulties do exist, but we have found some characteristics of differentiation. This study reveals that COVID-19 predominates bilateral (74%), patchy (54%) GGOs, while early stage lung cancer predominates unilateral (97%), oval (66%) GGOs. The tendency of shape and distribution is consistent with previous reports (19,20). More lobes are involved in the COVID-19 group than in the lung cancer group, like the results from other papers (7,19). Specific radiographic patterns might help with distinction. COVID-19 is more featured by air bronchogram, reticular pattern, subpleural linear opacity, bronchial dilatation, centrilobular nodules, and tree-in-bud; on the contrary, lobulated sign, pleural retraction, cystic change, and vessel convergence sign are mostly present in lung cancer patients (Figure 4). Also, the rapid progression of COVID-19 on CT offers a dynamic reference for diagnosis. The initial phase of COVID-19, i.e., zero to four or five days, witnesses a classical GGO characteristic with occasional consolidation (6,19). The lesion grows in size as the disease progresses around five days later (Figure 5), and additional manifestations appear including crazy-paving pattern, increased consolidation, enlarged fibrous stripe, and reverse halo sign (7,19). In lung cancer patients, the growth of GGOs is associated with increasing malignancies (21). However, these changes are preferably indolent with an extremely long doubling time, particularly in pure GGOs (median, 1,200 days) (22). Therefore, yet to COVID-19, the dynamic evolution of early lung cancer on CT is merely expected during a brief follow-up period (Figure 5).
Figure 4.
CT scans with distinct manifestations between COVID-19 and lung cancer. Lesions in (A) and (B) were both presented as GGO: (A) CT scan of a patient diagnosed with COVID-19, without pleural retraction or vessel convergence sign; (B) CT scan of a patient diagnosed with lung cancer, with pleural retraction and cystic change. Lesions in (C) and (D) were both presented as patchy opacification: (C) CT scan of a patient diagnosed with COVID-19 presenting irregular shape on CT, without pleural retraction; (D) CT scan of a patient diagnosed with lung cancer, with pleural retraction, lobulated sign, and spiculate protuberance. GGO, ground-glass opacity; COVID-19, coronavirus disease 2019.
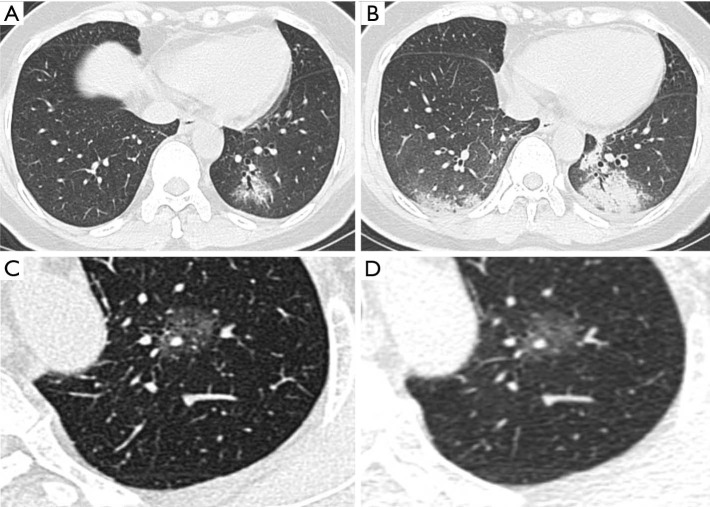
Figure 5.
Progression of GGOs in COVID-19 and lung cancer. Panels (A) and (B) showed typical evolution on CT scans of a patient diagnosed with COVID-19 who presented with the only cough as a symptom: (A) on day 4; a mixed GGO was demonstrated in the left lower lobe with air bronchogram; (B) on day 11, the primary lesion was significantly enlarged with consolidation. What is more, there was a new area of subpleural patchy opacification in the right lower lobe. Panels (C) and (D) showed the chest CT of a patient diagnosed with lung cancer: (C) a mixed GGO was found in the left upper lobe; (D) no visible change was detected one month later. GGOs, ground-glass opacities; COVID-19, coronavirus disease 2019.
The above differences in CT images are subtle but of excellent value to differentiate the two entities. However, COVID-19 diagnosis requires a comprehensive evaluation combined with clinical symptoms, epidemiological investigation, and laboratory tests. Patients with manifestations of fever, cough, shortness of breath, and myalgia should be emphasized (23). Travel history to Wuhan or other epidemic areas and close contacts with COVID-19 patients raise suspicion of infection. Above all, real-time RT-PCR analysis of nucleic acid remains the key to a definite diagnosis (24).
It is necessary but also clinically significant to illustrate the fine distinction of CT imaging between COVID-19 and early lung cancer. Identification of GGO indicating COVID-19 by chest CT could discover those asymptomatic (25) or nucleic acid negative pneumonia patients (9) and therefore help reduce the missed diagnosis rate and limit the transmission. GGO patients with highly suspected lung cancer should undergo an overall evaluation to exclude COVID-19 infection before the operation. On the one hand, we need to minimize the possibility of COVID-19 being misdiagnosed as lung cancer; alternatively, it has been reported patients with cancer experienced a higher risk of COVID-19 infection and had a poorer prognosis (26). The imprudent decision might expose patients to the attack of surgical trauma and COVID-19 and increase the risk of medical workers as well. Once the diagnosis is confirmed, the priority must be assigned to the treatment of COVID-19 rather than surgery.
Thanks to the rapid and comprehensive response of the Chinese government, public health, medical, and scientific communities, the epidemic control over COVID-19 has been successfully achieved (27). Quarantine measures are gradually relaxed, and more chest CT scans will be performed on regular patients. The importance of discrimination of other pulmonary diseases from COVID-19, particularly early lung cancer, by CT imaging will be highlighted. In conclusion, the distinctive radiological characteristics of GGOs between COVID-19 and early stage lung cancer, combined with pathogen detection, epidemiological history, laboratory tests, short-term CT reexamination, and pathological results, will benefit the differential diagnosis and lower the rate of missed diagnosis.
Figure S2.
Pathological results of COVID-19 (A,B,C,D)1 and early stage lung cancer (E,F,G,H). Scale: 1:200. COVID-19, coronavirus disease 2019. 1, reproduced by permission of the owner of the publishing rights of Reference 12.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by National Natural Science Foundation of China (grant numbers: 81871882, 81902951), Shanghai Municipal Commission of Health and Family Planning Outstanding Academic Leaders Training Program (grant number: 2017BR055), and Shanghai Municipal Education Commission - Gaofeng Clinical Medicine Grant (grant number 20172005).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Commission of Ruijin Hospital approved this study (KY2020-26) following the Helsinki Declaration (as revised in 2013). Written informed consent for this retrospective study was waived by the Ethics Commission of the designated hospital for emerging infectious diseases.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-892
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-892
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-892). Dr. YJZ reports grants from National Natural Science Foundation of China, during the conduct of the study. Dr. HCL reports grants from National Natural Science Foundation of China, grants from Shanghai Municipal Commission of Health and Family Planning Outstanding Academic Leaders Training Program, grants from Shanghai Municipal Education Commission - Gaofeng Clinical Medicine Grant, during the conduct of the study. The other authors have no conflicts of interest to declare.
References
- 1.World Health Organization. WHO Director-General’s statement on IHR Emergency Committee on Novel Coronavirus (2019-nCoV). 2020 [cited 2020 Jun 21]. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov)
- 2.World Health Organization. Coronavirus Disease (COVID-19) Situation Reports. 2020 [cited 2020 Jun 21]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 3.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J 2020;55:2000607. 10.1183/13993003.00607-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pneumonia diagnosis and treatment plan for SARS-CoV-2 infection (trial version 7). 2020 [cited 2020 Jun 21]. Available online: http://www.gov.cn/zhengce/zhengceku/2020-03/04/5486705/files/ae61004f930d47598711a0d4cbf874a9.pdf
- 5.Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan F, Ye T, Sun P, et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020;295:715-21. 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020;30:3306-9. 10.1007/s00330-020-06731-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung M, Bernheim A, Mei X, et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020;295:202-7. 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie X, Zhong Z, Zhao W, et al. Chest CT for Typical Coronavirus Disease 2019 (COVID-19) Pneumonia: Relationship to Negative RT-PCR Testing. Radiology 2020;296:E41-5. 10.1148/radiol.2020200343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Lung Screening Trial Research Team , Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. 10.1056/NEJMoa1209120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson FL, Jaklitsch MT. Computed Tomography Scanning for Early Detection of Lung Cancer. Annu Rev Med 2018;69:235-45. 10.1146/annurev-med-020917-053556 [DOI] [PubMed] [Google Scholar]
- 12.Tian S, Hu W, Niu L, et al. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol 2020;15:700-4. 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang P, Liu T, Huang L, et al. Use of Chest CT in Combination with Negative RT-PCR Assay for the 2019 Novel Coronavirus but High Clinical Suspicion. Radiology 2020;295:22-3. 10.1148/radiol.2020200330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Zhang H, Xie J, et al. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020;296:E115-7. 10.1148/radiol.2020200432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J Thorac Oncol 2016;11:1653-71. 10.1016/j.jtho.2016.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanne JP, Chest CT. Findings in 2019 Novel Coronavirus (2019-nCoV) Infections from Wuhan, China: Key Points for the Radiologist. Radiology 2020;295:16-7. 10.1148/radiol.2020200241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim TJ, Goo JM, Lee KW, et al. Clinical, pathological and thin-section CT features of persistent multiple ground-glass opacity nodules: comparison with solitary ground-glass opacity nodule. Lung Cancer 2009;64:171-8. 10.1016/j.lungcan.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 18.MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. 10.1148/radiol.2017161659 [DOI] [PubMed] [Google Scholar]
- 19.Bernheim A, Mei X, Huang M, et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020;295:200463. 10.1148/radiol.2020200463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinsey CM, Estepar RS, Zhao Y, et al. Invasive adenocarcinoma of the lung is associated with the upper lung regions. Lung Cancer 2014;84:145-50. 10.1016/j.lungcan.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907-16. 10.1016/S1470-2045(16)30069-9 [DOI] [PubMed] [Google Scholar]
- 22.Song YS, Park CM, Park SJ, et al. Volume and mass doubling times of persistent pulmonary subsolid nodules detected in patients without known malignancy. Radiology 2014;273:276-84. 10.1148/radiol.14132324 [DOI] [PubMed] [Google Scholar]
- 23.Chang D, Lin M, Wei L, et al. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA 2020;323:1092-3. 10.1001/jama.2020.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng MY, Lee EY, Yang J, et al. Imaging Profile of the COVID-19 Infection: Radiologic Findings and Literature Review. Radiol Cardiothorac Imaging 2020;2:e200034. 10.1148/ryct.2020200034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335-7. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.China National Health Commission. Chinese mainland sees 20 provincial regions clear of COVID-19 for over 28 days. 2020 [cited 2020 Jun 21]. Available online: http://en.nhc.gov.cn/2020-03/29/c_78448.htm
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as