Abstract
Increasing contributions of prymnesiophytes such as Phaeocystis pouchetii and Emiliania huxleyi to Barents Sea (BS) phytoplankton production have been suggested based on in situ observations of phytoplankton community composition, but the scattered and discontinuous nature of these records confounds simple inference of community change or its relationship to salient environmental variables. However, provided that meaningful assessments of phytoplankton community composition can be inferred based on their optical characteristics, ocean-colour records offer a potential means to develop a synthesis between sporadic in situ observations. Existing remote-sensing algorithms to retrieve phytoplankton functional types based on chlorophyll-a (chl-a) concentration or indices of pigment packaging may, however, fail to distinguish Phaeocystis from other blooms of phytoplankton with high pigment packaging, such as diatoms. We develop a novel algorithm to distinguish major phytoplankton functional types in the BS and apply it to the MODIS-Aqua ocean-colour record, to study changes in the composition of BS phytoplankton blooms in July, between 2002 and 2018, creating time series of the spatial distribution and intensity of coccolithophore, diatom and Phaeocystis blooms. We confirm a north-eastward expansion in coccolithophore bloom distribution, identified in previous studies, and suggest an inferred increase in chl-a concentrations, reported by previous researchers, may be partly explained by increasing frequencies of Phaeocystis blooms.
This article is part of the theme issue ‘The changing Arctic Ocean: consequences for biological communities, biogeochemical processes and ecosystem functioning’.
Keywords: phaeocystis, Arctic, phytoplankton, climate, ocean-colour, remote-sensing
1. Introduction
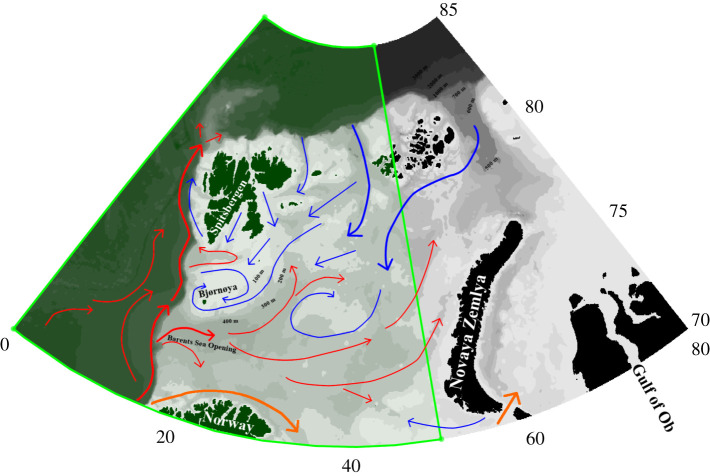
The Barents Sea (BS) extends north of mainland Arctic Norway, spanning the continental shelf region between the Svalbard Archipelago, Franz Josef land and Novaya Zemlya, occupying some 1.4 million km2, with a complex hydrography that results from the convergence of warmer, more saline Atlantic and fresher, cooler Arctic waters [1]. The BS is predominantly shallow, averaging only approximately 230 m depth. This shallow topography means that the movements of water-masses are guided by bathymetry such as the Barents Sea Opening (approx. 470 m deep), which serves as a gateway for the delivery of warmer, more saline and nutrient-rich Atlantic water onto the shelf [2]. These ocean dynamics are illustrated in figure 1.
Figure 1.
A bathymetric map of the Barents Sea, with inflowing Atlantic (red), Norwegian coastal current (orange) and Arctic (blue) water masses. A front forms between the warm and cold water masses. The area of interest in this study is outlined in green. Bathymetric data were sourced using the ETOPO1 NOAA global relief database (https://www.ngdc.noaa.gov/mgg/global/) with the R marmap package [3]. (Online version in colour.)
Bathymetric features stabilize the position of the boundary between Atlantic and Arctic water-masses, known as the Polar Front (PF), which means that the front’s position remains relatively constant year-on-year, insensitive to inter-annual variation in the flux of Atlantic water caused by climatological variability, such as the Arctic Oscillation [2]. Over the last 30 years, however, a sustained increase in the baseline rate of Atlantic water flux into the Arctic has doubled the volume occupied by Atlantic water in the BS, causing the northward displacement of the PF [2,4]. As the PF delimits the maximum extent of sea-ice cover [5], its retreat has resulted in the recession of average sea-ice extent in the BS, which has occurred at a disproportionately rapid rate compared with the rest of the Arctic [6].
The hydrography and climatology of the BS create an environment conducive to massive phytoplankton blooms, and the inter-annual variation in these environmental properties determine which phytoplankton groups will be most successful from year to year. The surface ocean is charged with nutrients in the winter when dense Atlantic waters are brought to the surface by vertical mixing [7] and this nutrient complement is further supplemented in spring by mineral-rich ice-rafted debris that is released by melting sea-ice imported through the Fram Strait region [8]. Thermal stratification of the water-column in spring, and salinity-based stratification associated with melting sea-ice, create a nutrient-replete sunlit surface layer that can support phytoplankton blooms in which chlorophyll-a (chl-a) concentrations exceed 10 mg m−3 [9]. Historically, these blooms have usually been dominated by large-celled diatom genera such as Chaetoceros, Fragilariopsis and Thalassiosira that can reproduce very rapidly when resources are abundant [10] and have been observed to form ice-edge blooms that follow the receding Marginal Ice Zone through summer [11]. Outside of the Seasonal Ice Zone, in waters where perennial freezing does not occur, summer mixing tends to be deeper and periodic recharging of the surface nutrient reservoir may occur, favouring smaller phytoplankton species with larger proportional surface areas, such as Emiliania huxleyi and Phaeocystis pouchetii [10].
We should, therefore, expect that environmental changes in the BS, caused by human-induced warming, will influence the community composition of phytoplankton blooms, with ramifications for overall new production, phenology and hence the supply of food to higher trophic levels in this great northern fishery, and influencing the ecosystem services that are rendered. Indeed, in situ investigations in the Eurasian basin during the summer 2012 sea-ice minimum revealed a rare mass-sedimentation of diatomaceous ice-algae filaments, consistent with the expectation of authors such as Krumpen et al. [8] that thinner sea-ice, carrying lower levels of exogenous sediment, should transmit more sunlight and hence permit greater under-ice primary production. This carries potential implications for nutrient cycling mechanisms and carbon sequestration in a future Arctic Ocean that may be more conducive to perennial ice-algal sedimentation [12]. In the pelagic realm, some authors have reported that coccolithophore blooms are migrating eastward, further into historically more Arctic-influenced areas [13], and sampling in the Fram Strait has revealed an increasing predominance of Phaeocystis pouchetii and Emiliania huxleyi [14]. Average annual primary production, inferred from remotely sensed chl-a, has increased by 30% between 1998 and 2012 [15], but this picture of increasing primary production is difficult to reconcile with observations of declining end-winter silica inventories in the BS [16], critical for diatoms to build their frustules, and with studies of biochemical proxies in Greenlandic ice-cores that imply a centennial scale decline in the productivity of subarctic seas [17]. Remote-sensing inference of a decadal increase in Arctic dimethyl sulphide (DMS) production [18]—often associated with prymnesiophyte species that do not require silica, such as Phaeocystis pouchetii or Emiliania huxleyi—suggests that change in community composition may help to explain these seemingly conflicting findings, although the algorithm of Galí et al. [18] is based on comparisons of estimated productivity and inferred mixed-layer-depth, rather than the recognition of optical signatures of community composition. Time series of phytoplankton samples in the BS necessary to test the hypothesis of community compositional change are, however, sporadic and usually do not exceed the required decadal scales [19]. We, therefore, may ask whether a taxonomically informed study of changes in ocean-colour over the twenty-first century might help elucidate the matter.
There already exist several approaches towards this end. Remote sensing of ocean-colour has successfully detected coccolithophore blooms [13,20,21], owing to the strong scattering properties of calcite liths. However, the detection of other phytoplankton functional types has proved more challenging, although numerous attempts have been made; such as semi-analytical approaches based on forward-modelling from the optical properties of different phytoplankton groups to arrive at ocean-colour signatures that may distinguish them [22,23] or the inversion of systems of equations relating ocean-colour and phytoplankton size classes retrieved with high performance liquid chromatography (HPLC) [24]. Some authors have explored linear unmixing models of phytoplankton light absorption [25] to infer community composition, but these have not yet been applied to remotely sensed ocean-colour. There have also been attempts to detect optical signatures of specific phytoplankton pigments [26], which have been demonstrated to be successful with in situ radiometry, but have not yet been extended to remotely sensed observations.
Some existing remote-sensing algorithms for the inference of phytoplankton functional types initially assume some chl-a dependences in their construction [24], which confounds the task of recognizing shifts in BS bloom composition, given that the inferred chl-a in this region has been increasing, and chl-a and community compositional change may therefore be convolved [13,15]. We also recognized that other algorithms to distinguish diatoms in high-latitude waters are predicated on differences in specific absorption coefficient between diatoms and mixed populations [22]. Pigment packaging is the phenomenon of the flattening of absorption spectra of suspensions of pigment as distributions change from dispersed to tightly packaged aggregates [27,28]. Phaeocystis forms aggregated colonies and therefore exhibits a normalized absorption spectrum that is indicative of levels of pigment packaging similar to diatoms [26], and we, therefore, may expect its optical properties to resemble those of diatoms—indeed this is indicated in fig. 2 of Alvain et al. [29], who recognized a Phaeocystis-like group in ocean-colour measurements in their development upon the PHYSAT algorithm for the inference of different phytoplankton functional types. Given diatoms and Phaeocystis blooms are typically associated with different styles of production (with Phaeocystis associated with greater regeneration [30]), remote-sensing approaches which treat these two groups as synonymous may produce results that provide misleading conclusions about ecosystem change—a salient observation that bears special consideration in the BS where gradients in remotely sensed production anti-correlate with the mass of benthic megafauna [31], suggesting that the direct interpretation of remotely sensed production as an indicator for export production is fraught. We reviewed the global PHYSAT algorithm and found that, while extensive Phaeocystis-like blooms are identified in the Southern Ocean, PHYSAT describes the Barents as nanophytoplankton-dominated [29], suggesting that the global PHYSAT algorithm struggles to detect different Barents phytoplankton communities. The ‘Phaeocystis-like’ group identified in PHYSAT is based on ocean-colour observations from the Southern Ocean, where Phaeocystis antarctica predominates, as opposed to Phaeocystis pouchetii in the Arctic, so differences in ocean-colour between these two species may explain more pervasive identification of Phaeocystis in the Southern Ocean.
We hence opted to construct our own experimental bio-optical algorithm tuned to the bespoke phytoplankton functional types (coccolithophores, nanophytoplankton, diatoms and Phaeocystis) and conditions prevailing in the BS, to classify ocean-colour observations (see electronic supplementary material for a detailed description).
In the near future NASA’s PACE (Plankton, Aerosol, Cloud, Ecosystem) mission aims to measure ocean-colour at a 5 nm spectral resolution [32], which may offer researchers the capacity to detect subtle hyper-spectral features in ocean-colour attributable to key taxon-specific phytoplankton pigments, and may therefore facilitate increased confidence in the identification of distinct phytoplankton groups from ocean-colour. However, the legacy of ocean-colour measurements already collected will still represent a rich historic dataset and we should, therefore, retain an interest in exploring the detection of distinct phytoplankton groups in existing satellite records and assessing the extent to which bio-optical algorithms can be marshalled to describe ecosystem baselines or detect incipient signals of ecosystem change in our warming climate, especially as it is this historical record that will place future observations in context. Here we develop a bio-optical algorithm in the mould of Alvain et al. [29]. We constructed a training dataset of ocean-colour, in order to inform a linear discriminant analysis to identify blooms of phytoplankton with a high degree of pigment packaging (hereafter referred to as ‘highly packaged phytoplankton’), such as diatom or Phaeocystis blooms. We employ an adaptation of the experimental chlorophyll-c3 algorithm of Astoreca et al. [26] to test whether it might also be possible to distinguish Phaeocystis from diatom blooms in the BS and assess whether it is possible to confirm isolated reports of changing phytoplankton community composition and extend our understanding by using ocean-colour to construct a regional synthesis.
2. Methods
Level-3 daily binned ocean-colour observations during the month of July 2002–2018 were downloaded from the MODIS-Aqua website, for λ = 412, 443, 469, 488, 531, 547, 555, 645, 667 and 678 nm [33] between 0-50 degrees east and 70–85 degrees north. We chose to investigate only ocean-colour observations from July, because while annual net primary production has been increasing [15] lower ice extents are associated with decreased chl-a biomass in spring [13]. We hence chose to study summer blooms alone to avoid the convolution of these antithetical changes.
Our experimental algorithm is a stack of 3 individual algorithm modules, which is depicted as a flow-chart in electronic supplementary material, figure S1. The first module masks ocean-colour observations that are classified as being influenced by sediment, coloured dissolved organic matter (CDOM)-rich river plumes or coccolithophore blooms. These masks were based on MODIS-Aqua ocean-colour observations from July 2014 between 0–80 degrees east and 70-85 degrees north. Modes of ocean-colour variance associated with the Ob river plume—a feature of high CDOM output, the Svalbard coast—high sediment load, and coccolithophore blooms in the central BS were identified and used to inform criteria to establish masks (electronic supplementary material, figures S2 and S3). Only un-masked ocean-colour observations are passed on to the second module.
The second module uses a linear discriminant—trained on a slew of Level-3 daily binned MODIS-Aqua ocean-colour observations, measured on the same day and within 1 degree latitude or longitude as a series of natural phytoplankton samples collected in 2017–2018—to distinguish whether ocean-colour properties are compatible with blooms of highly packaged phytoplankton (either diatom or colonial Phaeocystis cells). The use of Level-3 daily data for the purpose of identifying distinct phytoplankton groups follows from the example set by the development of the PHYSAT algorithm, and our 1-degree criterion follows from that used in PHYSAT validation [29,34]. Hold-p-out cross-validation on our training dataset demonstrated that highly packaged phytoplankton could be distinguished from nanophytoplankton effectively (97 and 80% of diatom and Phaeocystis-affiliated ocean-colour observations were assessed to be highly packaged, and some 68% of nanophytoplankton were correctly identified).
After ocean-colour observations are classified as highly packaged phytoplankton or as unicellular nanophytoplankton populations, chl-a estimates are recalculated using the Lab-OC4L diatom algorithm for all highly packaged phytoplankton and the Lab-OC4L prymn relationship for all nanophytoplankton populations. We justified our choice to use the Lab-OC4L diatom algorithm to estimate chl-a for all highly packaged phytoplankton (whether they would subsequently go on to be classified as diatoms or as Phaeocystis) because Cota et al. had described remote-sensing algorithms developed by Arrigo et al. for the inference of diatom, Phaeocystis and (diatom + Phaeocystis) communities as ‘very similar, perhaps indistinguishable’ [35,36].
The final module takes all observations classified as blooms of highly packaged phytoplankton and uses the algorithm developed by Astoreca et al. [26] to distinguish between Phaeocystis and diatom blooms—on the basis that Stuart et al. [37] had used chlorophyll-c3 as a marker pigment to distinguish diatom and prymnesiophyte-dominated samples. Discrimination is informed by a suite of hydrolight simulated ocean-colour reflectances based on optical properties of in situ samples of diatom and Phaeocystis communities. It was found the ratio of retrieved chl-a to inferred chlorophyll-c3 absorption separated these simulations. We recognized that MODIS-Aqua ocean-colour reflectance may not be amenable to the detection of subtle hyperspectral features, so we performed a sensitivity analysis to evaluate the effects of noise and variable bandwidth on the resolution of this feature, and diatom and Phaeocystis hydrolight simulations remained distinct. We found that our discriminant for Phaeocystis from diatoms, when applied to our training dataset, corresponded to a bimodal feature in the optical properties of highly packaged phytoplankton (electronic supplementary material, figure S6).
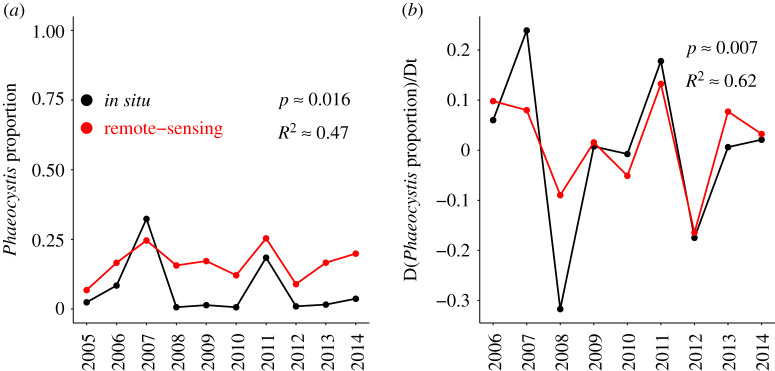
We found that a significant shift in mean retrieved chlorophyll-c3 signal (We use the term ‘signal’ rather than ‘absorption’ here because this metric sometimes takes negative values, for example Astoreca et al. [26] recorded a diatom bloom with an apparent negative chlorophyll-c3 absorption) occurred after 2009 in disparate parts of the global ocean (see electronic supplementary material, figures S7–S8) and adapted the method of Taylor et al. [38], who corrected for drift in the output of the IASI (infrared atmospheric sounding interferometer). Corrected chlorophyll-c3 absorption signal was then used in module 3 of our algorithm to infer the presence or absence of Phaeocystis blooms. The bio-optical algorithm was validated against a yearly time series of spring phytoplankton samples in the Labrador Sea, reported by Fragoso et al. [39] (dataset accessible at https://doi.pangaea.de/10.1594/PANGAEA.871872). A linear regression between the yearly Phaeocystis proportion of in situ samples, collected by Fragoso et al. [39] (defined as the portion of chl-a associated with Phaeocystis divided by the sum of chl-a associated with either Phaeocystis or diatoms) shows a highly significant correlation with the yearly Phaeocystis proportion in ocean-colour classifications (defined as the ratio of Phaeocystis to the sum of Phaeocystis and diatom classifications) (figure 2). The relationship between the inter-annual differences of these two curves is also highly significant. None of the curves show a significant linear trend over the time period 2005–2014.
Figure 2.
(a) Year-to-year variation in the dominance of Phaeocystis blooms in the in situ observations of Fragoso et al. [39] (black line) and from ocean-colour classifications across the Labrador Sea (red line). (b) Year-to-year variation in the derivative of Phaeocystis dominance. (Online version in colour.)
We noted that the Phaeocystis dominance estimated across the Labrador Sea from remote-sensing was often biased high, compared to the in situ samples (figure 2a). This may be because the dataset of Fragoso et al. [39] does not contain observations along the East-Greenland coast, which we found was often pervaded by Phaeocystis classifications (see electronic supplementary material, figure S9, years 2007–2008, 2010–2012).
We believe we have demonstrated that our bio-optical algorithm facilitates the construction of time series of diatom and Phaeocystis blooms that show biogeographical patterns consistent with in situ observations in another Subarctic Atlantic system.
3. Results
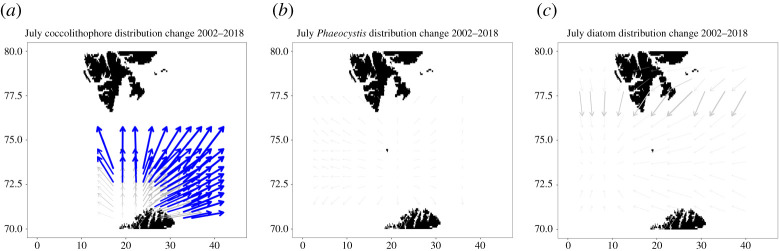
We first tested for changes in the geographical distribution of phytoplankton blooms with time. We discuss the example of coccolithophores to clarify our approach. We controlled for changes in the geography of the sampled area, as a result of the inter-annual recession in sea-ice extents for example, by excluding pixels poleward of 80 degrees north—the approximate limit of ice at the start of our time series. We also excluded observations in the box between 10–30 degrees east and 76–80 degrees north, because the coastal regions of Svalbard are often identified as coccolithophore-dominated (electronic supplementary material, figure S10). It is not clear whether these classifications are spurious, but even if they are genuine their association with the coast means that they do not reflect any biogeographic changes in open-ocean coccolithophore blooms. We took all the unique pixels in our time series and computed the relative frequency of coccolithophores in each pixel for a given year—such that a pixel observed once as coccolithophore-dominated is 100% coccolithophore-dominated and a pixel observed four times, but classified as coccolithophore only twice, is 50% coccolithophore-dominated. The accuracy of the frequency estimate increases with greater observations. We then calculated the longitudes and latitudes of the quantiles of the pixel distribution, weighted by the coccolithophore frequencies in each pixel, for 10 equally-spaced values of τ between 0.1 and 0.9, corresponding to the 10th and 90th quantiles of the distribution. We performed this exercise for each year in the time series. We then took our time series of latitudes and longitudes for each quantile value and performed a linear regression with time, weighted by the number of coccolithophore observations in each year, so that a year with a strange geographical distribution but few observations would not exert undue influence. Finally, we inverted the linear models to predict changes over time in latitude and longitude for each value of τ. We built multiple linear regressions for all latitude-longitude combinations of varying τ against time, and assessed their significance by calculating p-values. The result is a field of vectors which visually describe the change in coccolithophore distribution with time (figure 3). The most north-eastern vector corresponds to the model for change in geography of the 90th quantiles for both latitude and longitude, while the most south-western vector corresponds to the model for change in the geography of the 10th quantiles for both latitude and longitude. Coccolithophores were the only phytoplankton community which demonstrated significant changes in biogeography between 2002 and 2018.
Figure 3.
(a) Changing distributions of coccolithophore ocean-colour observations between 2002 and 2018. Significant (p-value < 0.05) vectors are coloured blue. (b) Vector field for Phaeocystis distribution change, no significant vectors. (c) Vector field for diatom distribution change, no significant vectors. (Online version in colour.)
The eastern limit of coccolithophore blooms has shifted some 7 degrees eastward over the study window, while the northern limit has expanded about 1.5 degrees poleward, in accord with the findings of previous studies [13,21]. Interestingly, figure 3 also shows that the western limit of coccolithophore blooms has expanded westward by 4 degrees. The vector field of coccolithophore distribution change in figure 3 implies that there are distinct eastern and western coccolithophore blooms. It appears that the eastern bloom dominates the distribution and is migrating to the northeast, while a minor branch of western blooms is migrating towards the Fram Strait region. These two pelagic blooms may be diverging as they migrate north, because the Svalbard archipelago separates the western and eastern portions of the BS, and consequently warm ocean currents bifurcate as they head north, steered by bathymetry (figure 1).
Coccolithophore blooms identified by Smyth et al. [20] between 1987 and 2002 were typically centred between 30 and 40 degrees east and rarely extended beyond 40 degrees east.
By contrast, this study shows that it has become routine in recent years for coccolithophore blooms to extend past 40 degrees.
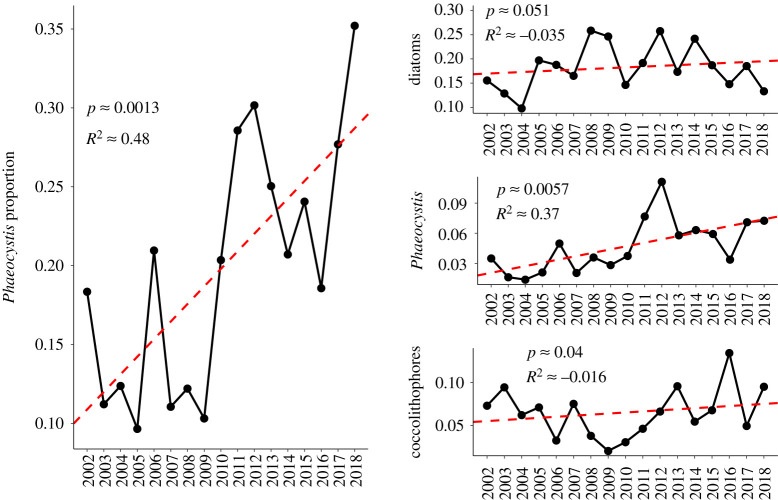
We also constructed time series of the proportion of all pixels in the study area dominated by different phytoplankton communities (figure 4). Hovland et al. [21] commented that a large mass of Atlantic water penetrated far into the east of the BS during 2007, compared with 2010, which was a year with relatively less Atlantic influence. Figure 4 shows that 2007 has a greater proportion of coccolithophore detections than 2010, conforming to the results of these earlier studies. Although inter-annual variations in diatom frequency are large—varying between 10% and 25% of observations—no discernible pattern of biogeographic change prevails over the study window and there is no significant linear trend in their occurrence between 2002 and 2018 (figure 4). Sub-significant patterns in the vector field of figure 3 imply a slight gravitation of diatoms towards the Atlantic inflow region and this may reflect a local increase in diatom identifications here (figure 5). By contrast, Phaeocystis frequency is low at the start of the study window, but climbs rapidly in 2010 (figure 4). We find this particularly interesting, given that previous authors found a massive increase in the occurrence of Phaeocystis and associated marine gels in the Fram Strait in 2010 [40]. We also note a positive anomaly in Phaeocystis detections in 2006, coinciding with reports of an increase in Phaeocystis in the Fram Strait by Nöthig et al. [14]. We consider increasing Phaeocystis predominance compatible with the predictions that Atlantification of the Barents should be expected to increase Phaeocystis frequency and result in a northward expansion of more Atlantic-associated species such as E. huxleyi [41].
Figure 4.
Evolution of the dominance of different phytoplankton groups in the Barents Sea in July. Phaeocystis proportion is defined as the number of Phaeocystis classifications divided by the sum of diatom and Phaeocystis classifcations. All other subplots record the number of classifications of a specific phytoplankton group as a proportion of the total number of observations. Linear regressions with time are represented by red dashed lines. (Online version in colour.)
Figure 5.
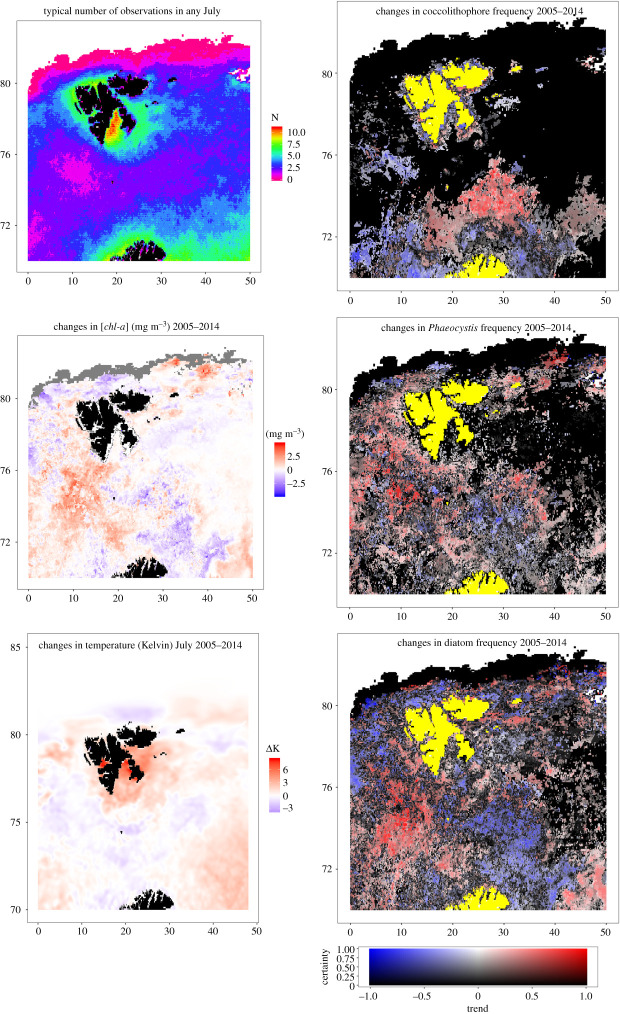
Maps of the changes of phytoplankton chl-a, temperature and community composition 2005–2014. The top-left sub-plot is a map of the average number of ocean-colour observations in a typical July. (Online version in colour.)
Given that a pronounced change in phytoplankton community structure was found over the study period, difference maps showing the change in the frequency of occurrence of different phytoplankton groups were created, so that we could identify the geographical regions driving these patterns (figure 5). We limited the temporal extent to within 2005–2014 to match the period of our validation scheme in the Labrador Sea (figure 2), so that we could have confidence in the results. The difference maps were derived by computing linear models of the variable of interest—such as chl-a concentration or relative frequency of coccolithophores—in each unique pixel location as a function of time. We calculated the average change in each pixel that the linear models predicted and inferred significance of plankton community shifts from their p-values (expressed in figure 5 as ‘Certainty’, defined as 1– p-value).
Figure 5 shows that there have been increases in the mean July chl-a concentration in the western BS, southern Fram Strait and far south-eastern BS. The largest increases exceed 1 mg m−3, west of Bjørnøya. There is an equivocal pattern of change in the frequency of diatom occurrence; while there are strong increases in the Atlantic inflow region west of Bjørnøya there are decreases further down-stream in the northern Fram Strait or over the Sentralbanken. Phaeocystis increases are also achieved throughout much of the western and southern BS, but are absent beyond the typical position of the PF in the Arctic-derived waters of the northeast. Changes in patterns of coccolithophore occurrence are consonant with figure 3; the frequency of coccolithophores has declined in the southwest of their geographic range and increased in the northeast. The migration of coccolithophores into the Sentralbanken region may offer an explanation for declines in the frequency of diatom detections here. Curiously, patterns of change in sea surface temperature data from the ESA SST CCI reprocessed sea surface temperature analyses (temperature maps for the 15th of July in each year downloaded from CMEMS; Copernicus Marine Envrionment Monitoring Service: http://marine.copernicus.eu/) did not correspond clearly to changes in phytoplankton community composition. The pattern of change reproduces the regional change described for 2005–2016 in Barton et al. figure 2C [5]. We might tentatively suggest that a broad region of declining sea surface temperature in the Atlantic inflow region aligns with increasing chl-a concentrations, while warming regions of the northern Fram Strait align with declines in diatoms and increases in Phaeocystis. Interestingly patterns of temperature change in the Fugløya Atlantic inflow section (corresponding to the BS Opening: https://ocean.ices.dk/iroc/) show that there has been a broad increase in sea temperatures between 50 and 200 m between 2002 and 2018, whereas surface temperature data from CMEMS implies a decline over this same period. This emphasizes that consideration of sea surface temperature in isolation may provide misleading explanations for variation in the state of ecosystems.
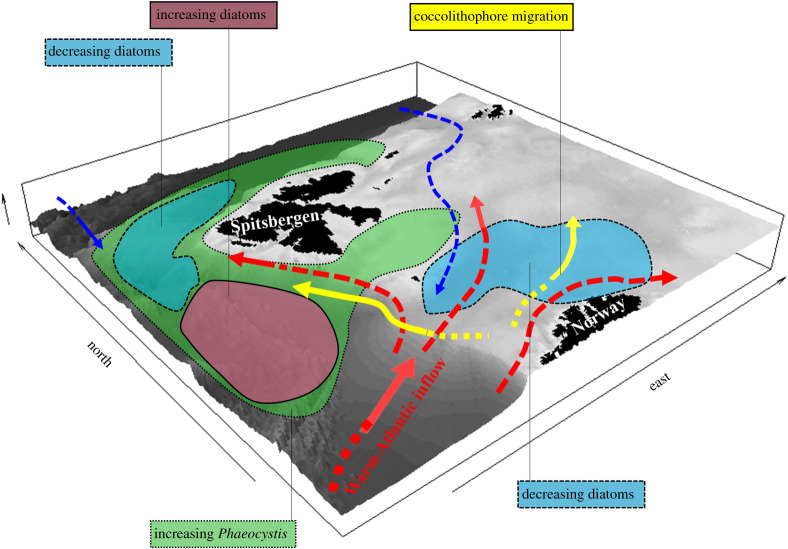
The sum of all the changes to phytoplankon community composition that have been found in this study are presented in a synthesis as a single cartoon in figure 6.
Figure 6.
A synthesis of all the changes to phytoplankton community composition found in this study. Warm inflowing currents are represented in red, while cold waters flowing from the Nansen basin are represented in blue. (Online version in colour.)
4. Discussion
(a). Phytoplankton community shift in the context of ‘Atlantification’
The most significant trend we have uncovered is an inferred increasing predominance of Phaeocystis in midsummer in the BS. The distribution of change illustrated in figure 6 suggests that the findings of ship-board sampling by Nöthig et al. [14] apply generally to the more Atlantic-influenced portions of the BS, consistent with expectations expressed in Reigstad et al. [1]. This may well be consistent with predictions by Randelhoff et al. [42] that enhanced Atlantic water input onto the western shelves of the BS would alleviate late summer nutrient limitation and hence support increased pelagic primary production. It is also compatible with the report of Oziel et al. [13] of increasing chl-a concentrations in the BS in late summer, and the observation of Arrigo & Van Dijken [15] of increasing annual pelagic primary production in the region. The Fram Strait region has also experienced a significant increase in Phaeocystis occurrence, consonant with the existing literature [14]. Given the association of Phaeocystis blooms with the influx of warmer Atlantic derived waters into the Fram Strait [14], it is perhaps no surprise that the least Atlantified waters of the BS prove the most resistant to increases in Phaeocystis predominance. These findings are complementary to our investigations of coccolithophore biogeography, that expand upon the work of authors such as Oziel et al. [13], Smyth et al. [20] and Hovland et al. [21], showing that coccolithophore blooms have dispersed downstream in Atlantic inflow currents. The co-association of these changes is strongly suggestive of a signal of ‘Atlantification’ in midsummer Barents phytoplankton communities—especially as patterns in change appear to be delimited by the position of major fronts—implying a bottom-up control of inter-annual variation in phytoplankton community structure. We think it is curious that no significant change in biogeography for Phaeocystis was recovered, but this may be a result of this genus already having a cosmopolitan distribution in the BS [19]. We note that increases in Phaeocystis incidence illustrated in figure 6 generally occur downstream of the sites of intense coccolithophore blooms and beyond the ‘Southern Front’ identified in Oziel et al. [2] but not beyond the PF, perhaps implying that phytoplankton communities of different Barents water-masses exhibit subtly different responses to environmental change. Diatom detections exhibited a complex change between 2005 and 2014, increasing their incidence in inflow regions but declining further downstream. This might result from the dual changes of increasing Atlantic inflow and decreasing Atlantic inflow silica inventories [16]. Proximity to the inflow silica may not be limiting, so an increased flux may justify higher diatom growth, but further downstream, the declining silica content of Atlantic water may mean that phytoplankton groups less reliant on this nutrient are favoured. We stress that simple studies of chl-a concentration or the use of bio-optical algorithms that do not subdivide highly packaged phytoplankton into diatom and Phaeocystis dominated assemblages would provide a much coarser impression of ecosystem change (for example, see figure 5 chl-a change). Coarser products such as these might be interpreted as being concomitant with suggestions of increasing new production [15]. However, our finding of increasing Phaeocystis predominance suggests that the style of production is likely becoming increasingly regenerated in character. This was explicitly considered by Arrigo & Van Dijken [15] as a possibility, and might be inferred from isolated in situ records [14,40], but we have here provided the first bio-optical evidence of this. Other authors have also pointed out that deepening mixed layers in midsummer could explain greater regenerated production [13], a hypothesis compatible with increasing Phaeocystis, which is a shade-tolerant genus that prospers under conditions of deep mixing [39]. These interpretations are consistent with observations that remotely sensed chl-a increases are concentrated in mid and late summer—when episodic mixing prevails—and that chl-a concentrations detected in spring—when production depends on the nutrient inventory stocked over winter—have declined in years with lower sea-ice [10,13]. This shows that the retrieval of dominant phytoplankton groups from ocean-colour can inform a more nuanced understanding of incipient ecosystem change in the Arctic in the face of human-induced climate change. We anticipate that the launch of hyper-spectral sensors (PACE) into space will further invigorate taxonomically informed approaches in the study of ocean-colour.
(b). Implications
The ecological significance of increasing Phaeocystis predominance is difficult to ascertain. Previous authors have argued that zooplankton may modify their diets, buffering any substantial effects on higher trophic levels [43]. By contrast, other authors have suggested that the efficiency of the biological pump should decline under Phaeocystis predominance [44], because descending Phaeocystis aggregates are remineralized faster than diatoms [30]—an observation consistent with the faster sinking rates reported for more massive diatom cells [45,46]. Suggestions of declining biological pump efficiency can be contrasted in turn with rare reports of mass-sedimentation of Phaeocystis [47], when colonies are ballasted by gypsum crystals rejected from sea-ice [48], or the frustules of senescent diatom blooms [49,50]. Phaeocystis blooms frequently succeed diatoms in the BS and the co-occurrence of diatoms ballasting Phaeocystis colonies has been frequently recorded [51,52]. Hence, there are credible reasons in the literature to believe that increasing Phaeocystis should decrease carbon export, perhaps increase it or have little effect at all.
Empirical studies of benthic carbon flux and trawls of benthic organisms also yield conflicting results. Lalande et al. [53] reports greater biogenic and faecal pellet export under high sea-ice conditions—suggesting a warmer less diatom-dominated BS should have a weaker biological pump—while Cochrane et al. [54] report lower macrobenthic animal biomass and sediment carbon content north of the oceanic PF, implying that Atlantification and the northward recession of the PF ought to increase nourishment to the benthos. These conflicting reviews can perhaps be resolved by Jørgensen et al. [55], who notes that the benthic expression of the PF extends much further south than the ocean surface outcrop. Jørgensen et al. found that megafaunal (greater than 1 cm body length) biomass distribution differed from macrofaunal (greater than 1 mm body length, but less than 1 cm), with greater mass north of the benthic PF. They hypothesized that a longer equilibration time-scale for larger benthic animals may mean changes in the benthos lag environmental change and subsequent research showed that gradients in benthic megafaunal biomass are decoupled from surface production and therefore that carbon flux must be controlled to a first order by biological pump efficiency—perhaps mediated by bloom composition, dynamics or ice-edge physics [31].
We may, therefore, synthesize these findings to suggest that a history of frequent fast-sinking diatom blooms justifies greater megafaunal biomass in northern sites, and that the Atlantification of these waters, and a switch to Phaeocystis predominance, will result in a poorer nourishment of the benthos and a shift in the long term to lower megafaunal biomass. We must also consider that, during mid and late summer, phytoplankton blooms in the BS descend toward the nutricline to form subsurface chlorophyll maxima (SCMs) [56], meaning some production is missed by remote-sensing approaches. In this study, this may have the effect of giving us the false impression that phytoplankton communities north of the PF are resistant to environmental change (figure 5), when communities residing at depth here may, in fact, be changing. As there is currently much debate in the literature as to the effects of neglecting the SCM on production estimates (e.g. [57–59]), Arctic shelf SCMs clearly warrant further investigation.
(c). Limitations to our experimental bio-optical algorithm
Overall, much greater confidence can be invested in the detection of coccolithophore blooms, which are clearly defined by the characteristic light-scattering properties of their shed coccoliths. Our approach is also necessarily limited by the available spectral resolution of the MODIS-Aqua sensor and by the underlying assumption in our bio-optical algorithm’s construction that CDOM is invariant. This assumption is fair in the BS, where CDOM absorption remains constant even across major hydrographic features [60], but is not true outside of the BS, limiting the wider application of our algorithm to other Arctic regions. We must also acknowledge that Astoreca et al. [26] caution that their reflectance algorithm for the retrieval of chlorophyll-c3 may require modification in turbid waters and that accurate quantification of chlorophyll-c3 concentrations may not be possible even under ideal conditions—only the recognition of high concentrations indicative of Phaeocystis blooms. We believe that we have taken care to use Astoreca’s algorithm within these limitations. We might also regard the requirement to use the MODIS landbands (such as 469 nm) as a limitation, but we note that previous authors such as Hu et al. have made successful use of these bands for the inference of phytoplankton functional types [61]. We caution that our approach to correct for temporal drift in the inferred signal of chlorophyll-c3 absorption is specific to the MODIS-Aqua r2018.1 ocean-colour reprocessing, and that this approach may need to be modified for future reprocessings, which may subtly vary the values of different ocean-colour bands. The results from our bio-optical algorithm suggest that it is possible to construct regional algorithms for the retrieval of major phytoplankton functional types in the Arctic, and that the output from such algorithms can form a useful supplement to incomplete in situ records of phytoplankton community composition.
5. Conclusion
We have developed a bio-optical algorithm to retrieve dominant phytoplankton groups in the BS and probed its applicability to investigate incipient changes in community composition. We have presented evidence that an expansion of coccolithophore range further down-stream in Atlantic inflow currents is ongoing, consistent with the expectations of previous authors. We have suggested that Phaeocystis predominance in the BS in midsummer is likely increasing in more Atlantic-influenced regions, which is consistent with a signal of Atlantification in the Barents phytoplankton community and the expectations of several previous authors. The combination of these changes may mean that reported increases in remotely sensed production in the BS are driven in part by higher standing stocks of phytoplankton associated with regenerated styles of production increasingly mediated by the microbial loop—a change with potential significance for higher trophic levels. The retrieval of these signals of Atlantification by our bio-optical algorithm emphasizes that existing records of ocean-colour will remain useful for interpretation alongside newer ocean-colour products from up-coming missions such as PACE.
Supplementary Material
Acknowledgements
We acknowledge D. McKee of the University of Strathclyde 16 Richmond St, Glasgow G1 1XQ, for assisting in the configuration of Hydrolight. We acknowledge S. Sathyendranath of the Plymouth Marine Laboratory, Prospect Place, Plymouth, PL1 3DH, for assessing the validity of our correction scheme for temporal drift.
Data accessibility
The datasets of optical properties used in the construction of the phytoplankton community composition algorithm used in this study are available at [doi:10.5285/97daa7ea-8792-6cff-e053-6c86abc0dd46] [doi:10.5285/982b6da2-7e11-060a-e053-6c86abc09389] [doi:10.5285/982b6da2-7e12-060a-e053-6c86abc09389], accompanying datasets for chlorophyll-a concentration are available at [doi:10.5285/97daa7ea-8793-6cff-e053-6c86abc0dd46] [doi:10.5285/982b6da2-7e13-060a-e053-6c86abc09389] [doi:10.5285/982b6da2-7e14-060a-e053-6c86abc09389] MODIS-A data is available from NASA’s website for ocean-colour. [https://oceancolor.gsfc.nasa.gov/data/10.5067/AQUA/MODIS/L3M/RRS/2018/]
Authors' contributions
A.O. carried out analyses and drafted the manuscript. H.A.B. supervised analysis and the drafting of the manuscript. T.P. edited the manuscript. B.N. assisted in the interpretation of the significance of our results for the structure of benthic communities. I.K. set up Hydrolight simulations. All authors read and approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
This research was funded by the Natural Environment Research Council (NERC)grant no. NE/P006507/1.
Reference
- 1.Reigstad M, Wassmann P, Riser CW, Øygarden S, Rey F. 2002. Variations in hydrography, nutrients and chlorophyll a in the marginal ice-zone and the central Barents Sea. J. Mar. Sys. 38, 9–29. ( 10.1016/S0924-7963(02)00167-7) [DOI] [Google Scholar]
- 2.Oziel L, Sirven J, Gascard JC. 2016. The Barents Sea frontal zones and water masses variability (1980–2011). Ocean Sci. 12, 169–184. ( 10.5194/os-12-169-2016) [DOI] [Google Scholar]
- 3.Pante E, Simon-Bouhet B. 2013. marmap: a package for importing, plotting and analyzing bathymetric and topographic data in R. PLoS ONE 8, e73051 ( 10.1371/journal.pone.0073051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis JA, Hunter E. 2007. Drivers of declining sea ice in the Arctic winter: a tale of two seas. Geophys. Res. Lett. 34, L17503 ( 10.1029/2007GL030995) [DOI] [Google Scholar]
- 5.Barton BI, Lenn YD, Lique C. 2020. Observed atlantification of the Barents Sea causes the polar front to limit. JPO 500, 18–0003. [Google Scholar]
- 6.Cavalieri DJ, Parkinson CL. 2012. Arctic sea ice variability and trends, 1979–2010. The Cryosphere 6, 881–889. ( 10.5194/tc-6-881-2012) [DOI] [Google Scholar]
- 7.Loeng H. 1991. Features of the physical oceanographic conditions of the Barents Sea. Polar Res. 10, 5–18. ( 10.3402/polar.v10i1.6723) [DOI] [Google Scholar]
- 8.Krumpen T. et al. 2019. Arctic warming interrupts the transpolar drift and affects long-range transport of sea ice and ice-rafted matter. Sci. Rep. 9, 5459 ( 10.1038/s41598-019-41456-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Båmstedt U, Eilertsen HC, Tande KS, Slagstad D, Skjoldal HR. 1991. Copepod grazing and its potential impact on the phytoplankton development in the Barents Sea. Polar Res. 10, 339–354. ( 10.1111/j.1751-8369.1991.tb00658.x) [DOI] [Google Scholar]
- 10.Wassmann P, Ratkova T, Andreassen I, Vernet M, Pedersen G, Rey F. 1999. Spring bloom development in the marginal ice zone and the central Barents Sea. Mar. Ecol. 20, 321–346. ( 10.1046/j.1439-0485.1999.2034081.x) [DOI] [Google Scholar]
- 11.Carmack E, Wassmann P. 2006. Food webs and physical–biological coupling on pan-Arctic shelves: unifying concepts and comprehensive perspectives. Prog. Oceanogr. 71, 446–477. ( 10.1016/j.pocean.2006.10.004) [DOI] [Google Scholar]
- 12.Boetius A. et al. 2013. Export of algal biomass from the melting Arctic sea ice. Science 339, 1430–1432. ( 10.1126/science.1231346) [DOI] [PubMed] [Google Scholar]
- 13.Oziel L, Neukermans G, Ardyna M, Lancelot C, Tison JL, Wassmann P, Sirven J, Ruiz-Pino D, Gascard JC. 2017. Role for Atlantic inflows and sea ice loss on shifting phytoplankton blooms in the Barents Sea. J. Geophys. Res.: Oceans 122, 5121–5139. ( 10.1002/2016JC012582) [DOI] [Google Scholar]
- 14.Nöthig EM. et al. 2015. Summertime plankton ecology in Fram Strait- a compilation of long and short-term observations. Polar Res. 34, 23349 ( 10.3402/polar.v34.23349) [DOI] [Google Scholar]
- 15.Arrigo KR, van Dijken GL. 2015. Continued increases in Arctic Ocean primary production. Prog. Oceanogr. 136, 60–70. ( 10.1016/j.pocean.2015.05.002) [DOI] [Google Scholar]
- 16.Rey F. 2012. Declining silicate concentrations in the Norwegian and Barents Seas. ICES J. Mar. Sci. 69, 208–212. ( 10.1093/icesjms/fss007) [DOI] [Google Scholar]
- 17.Osman MB, Das SB, Trusel LD, Evans MJ, Fischer H, Grieman MM, Kipfstuhl S, McConnell JR, Saltzman ES. 2019. Industrial-era decline in subarctic Atlantic productivity. Nature 569, 551–555. ( 10.1038/s41586-019-1181-8) [DOI] [PubMed] [Google Scholar]
- 18.Galí M, Devred E, Babin M, Levasseur M. 2019. Decadal increase in Arctic dimethylsulfide emission. Proc. Natl Acad. Sci. USA 116, 19 311–19 317. ( 10.1073/pnas.1904378116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degerlund M, Eilertsen HC. 2010. Main species characteristics of phytoplankton spring blooms in NE Atlantic and Arctic waters (68–80 N). Estuaries Coasts 33, 242–269. ( 10.1007/s12237-009-9167-7) [DOI] [Google Scholar]
- 20.Smyth T, Tyrrell T, Tarrant B. 2004. Time series of coccolithophore activity in the Barents Sea, from twenty years of satellite imagery. Geophys. Res. Lett. 31, L11302 ( 10.1029/2004GL019735) [DOI] [Google Scholar]
- 21.Hovland EK, Dierssen HM, Ferreira AS, Johnsen G. 2013. Dynamics regulating major trends in Barents Sea temperatures and subsequent effect on remotely sensed particulate inorganic carbon. Mar. Ecol. Progress Ser. 484, 17–32. ( 10.3354/meps10277) [DOI] [Google Scholar]
- 22.Sathyendranath S, Watts L, Devred E, Platt T, Caverhill C, Maass H. 2004. Discrimination of diatoms from other phytoplankton using ocean-colour data. Mar. Ecol. Progress Ser. 272, 59–68. ( 10.3354/meps272059) [DOI] [Google Scholar]
- 23.Jackson T, Bouman HA, Sathyendranath S, Devred E. 2010. Regional-scale changes in diatom distribution in the Humboldt upwelling system as revealed by remote sensing: implications for fisheries. ICES J. Mar. Sci. 68, 729–736. ( 10.1093/icesjms/fsq181) [DOI] [Google Scholar]
- 24.Devred E, Sathyendranath S, Stuart V, Platt T. 2011. A three component classification of phytoplankton absorption spectra: application to ocean-color data. Remote Sens. Environ. 115, 2255–2266. ( 10.1016/j.rse.2011.04.025) [DOI] [Google Scholar]
- 25.Zhang H, Devred E, Fujiwara A, Qiu Z, Liu X. 2018. Estimation of phytoplankton taxonomic groups in the Arctic Ocean using phytoplankton absorption properties: implication for ocean-color remote sensing. Opt. Express 26, 32 280–32 301. ( 10.1364/OE.26.032280) [DOI] [PubMed] [Google Scholar]
- 26.Astoreca R, Rousseau V, Ruddick K, Knechciak C, Van Mol B, Parent JY, Lancelot C. 2008. Development and application of an algorithm for detecting Phaeocystis globosa blooms in the case 2 Southern North Sea waters. J. Plankton Res. 31, 287–300. ( 10.1093/plankt/fbn116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duyens L. 1956. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim. Biophys. Acta 19, 1–12. ( 10.1016/0006-3002(56)90380-8) [DOI] [PubMed] [Google Scholar]
- 28.Kirk JT. 1994. Light and photosynthesis in aquatic ecosystems. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 29.Alvain S, Moulin C, Dandonneau Y, Loisel H. 2008. Seasonal distribution and succession of dominant phytoplankton groups in the Global Ocean: a satellite view. Global Biogeochem. Cycles 22, GB3001 ( 10.1029/2007GB003154) [DOI] [Google Scholar]
- 30.Reigstad M, Wassmann P. 2007. Does Phaeocystis spp. contribute significantly to vertical export of organic carbon? In: Phaeocystis, major link in the biogeochemical cycling of climate-relevant elements, p. 217–234. Springer.
- 31.Degen R, Jørgensen LL, Ljubin P, Ellingsen IH, Pehlke H, Brey T. 2016. Patterns and drivers of megabenthic secondary production on the Barents Sea shelf. Mar. Ecol. Progress Ser. 546, 1–16. ( 10.3354/meps11662) [DOI] [Google Scholar]
- 32.Petro S, Pham K, Hilton G. 2020. Plankton, Aerosol, Cloud, Ocean Ecosystem (PACE) Mission Integration and Testing.
- 33.OBPG. 2019. Moderate-resolution Imaging Spectroradiometer (MODIS) Aqua Daily L3SMI Ocean Colour Data. NASA Goddard Space Flight Center, Ocean Ecology Laboratory, Ocean Biology Processing Group Moderate-resolution Imaging Spectroradiometer (MODIS) Aqua Daily L3SMI Ocean Colour Data Data; NASA OBDAAC, Greenbelt, MD, USA.
- 34.Alvain S, Moulin C, Dandonneau Y, Bréon FM. 2005. Remote sensing of phytoplankton groups in case 1 waters from global SeaWiFS imagery. Deep Sea Res. Part I 52, 1989–2004. ( 10.1016/j.dsr.2005.06.015) [DOI] [Google Scholar]
- 35.Cota GF, Harrison WG, Platt T, Sathyendranath S, Stuart V. 2003. Bio-optical properties of the Labrador Sea. J. Geophys. Res.: Oceans 108, C7, 3228 ( 10.1029/2000JC000597) [DOI] [Google Scholar]
- 36.Arrigo KR, Robinson DH, Worthen DL, Schieber B, Lizotte MP. 1998. Bio-optical properties of the southwestern Ross Sea. J. Geophys. Res.: Oceans 103, 21 683–21 695. ( 10.1029/98JC02157) [DOI] [Google Scholar]
- 37.Stuart V, Sathyendranath S, Head EJH, Platt T, Irwin B, Maass H. 2000. Bio-optical characteristics of diatom and prymnesiophyte populations in the Labrador Sea. Mar. Ecol. Prog. Ser. 201, 91–106. [Google Scholar]
- 38.Taylor IA, Preston J, Carboni E, Mather TA, Grainger RG, Theys N, Hidalgo S, Kilbride BM. 2018. Exploring the utility of IASI for monitoring volcanic SO2 emissions. J. Geophys. Res.: Atmos. 123, 5588–5606. ( 10.1002/2017JD027109) [DOI] [Google Scholar]
- 39.Fragoso GM, Poulton AJ, Yashayaev IM, Head EJ, Purdie DA. 2017. Spring phytoplankton communities of the Labrador Sea (2005–2014): pigment signatures, photophysiology and elemental ratios. Biogeosciences 14, 1235–1259. ( 10.5194/bg-14-1235-2017) [DOI] [Google Scholar]
- 40.Engel A, Piontek J, Metfies K, Endres S, Sprong P, Peeken I, Gäbler-Schwarz S, Nöthig EM. 2017. Inter-annual variability of transparent exopolymer particles in the Arctic Ocean reveals high sensitivity to ecosystem changes. Sci. Rep. 7, 4129 ( 10.1038/s41598-017-04106-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rat’kova TN, Wassmann P. 2002. Seasonal variation and spatial distribution of phyto-and protozooplankton in the central Barents Sea. J. Mar. Sys. 38, 47–75. ( 10.1016/S0924-7963(02)00169-0) [DOI] [Google Scholar]
- 42.Randelhoff A. et al. 2018. Seasonality of the physical and biogeochemical hydrography in the inflow to the Arctic Ocean through Fram Strait. Front. Mar. Sci. 5, 224 ( 10.3389/fmars.2018.00224) [DOI] [Google Scholar]
- 43.Vernet M, Richardson TL, Metfies K, Nöthig EM, Peeken I. 2017. Models of plankton community changes during a warm water anomaly in Arctic waters show altered trophic pathways with minimal changes in carbon export. Front. Mar. Sci. 4, 160 ( 10.3389/fmars.2017.00160) [DOI] [Google Scholar]
- 44.Wassmann P. 1994. Significance of sedimentation for the termination of Phaeocystis blooms. J. Mar. Sys. 5, 81–100. ( 10.1016/0924-7963(94)90018-3) [DOI] [Google Scholar]
- 45.Falkowski PG, Barber RT, Smetacek V. 1998. Biogeochemical controls and feedbacks on ocean primary production. Science 281, 200–206. ( 10.1126/science.281.5374.200) [DOI] [PubMed] [Google Scholar]
- 46.Jiang L, Schofield OM, Falkowski PG. 2005. Adaptive evolution of phytoplankton cell size. Am. Nat. 166, 496–505. ( 10.1086/444442) [DOI] [PubMed] [Google Scholar]
- 47.Wassmann P, Vernet M, Mitchell BG, Rey F. 1990. Mass sedimentation of Phaeocystis pouchetii in the Barents Sea. Mar. Ecol. Progress Ser. 66, 183–195. ( 10.3354/meps066183) [DOI] [Google Scholar]
- 48.Wollenburg J. 2018. et al. Ballasting by cryogenic gypsum enhances carbon export in a Phaeocystis under-ice bloom. Sci. Rep. 8, 7703 ( 10.1038/s41598-018-26016-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peperzak L, Colijn F, Vrieling E, Gieskes W, Peeters J. 2000. Observations of flagellates in colonies of Phaeocystis globosa (Prymnesiophyceae); a hypothesis for their position in the life cycle. J. Plankton Res. 22, 2181–2203. ( 10.1093/plankt/22.12.2181) [DOI] [Google Scholar]
- 50.Cadée GC, Hegeman J. 1991. Phytoplankton primary production, chlorophyll and species composition, organic carbon and turbidity in the Marsdiep in 1990, compared with foregoing years. Hydrobiol. Bull. 25, 29–35. ( 10.1007/BF02259586) [DOI] [Google Scholar]
- 51.Sazhin AF, Artigas LF, Nejstgaard JC, Frischer ME. 2007. The colonization of two Phaeocystis species (Prymnesiophyceae) by pennate diatoms and other protists: a significant contribution to colony biomass. In: Phaeocystis, major link in the biogeochemical cycling of climate-relevant elements, pp. 137–145. Springer.
- 52.Verity PG, Brussaard CP, Nejstgaard JC, van Leeuwe MA, Lancelot C, Medlin LK. 2007. Current understanding of Phaeocystis ecology and biogeochemistry, and perspectives for future research. Biogeochemistry 83, 311–330. ( 10.1007/s10533-007-9090-6) [DOI] [Google Scholar]
- 53.Lalande C, Bauerfeind E, Nöthig EM, Beszczynska-Möller A. 2013. Impact of a warm anomaly on export fluxes of biogenic matter in the eastern Fram Strait. Prog. Oceanogr. 109, 70–77. ( 10.1016/j.pocean.2012.09.006) [DOI] [Google Scholar]
- 54.Cochrane SK, Denisenko SG, Renaud PE, Emblow CS, Ambrose WG Jr, Ellingsen IH, Skard. h. hamar J. 2009. Benthic macrofauna and productivity regimes in the Barents Sea- ecological implications in a changing Arctic. J. Sea Res. 61, 222–233. ( 10.1016/j.seares.2009.01.003) [DOI] [Google Scholar]
- 55.Jørgensen LL, Ljubin P, Skjoldal HR, Ingvaldsen RB, Anisimova N, Manushin I. 2014. Distribution of benthic megafauna in the Barents Sea: baseline for an ecosystem approach to management. ICES J. Mar. Sci. 72, 595–613. ( 10.1093/icesjms/fsu106) [DOI] [Google Scholar]
- 56.Slagstad D, Støle-Hansen K. 1991. Dynamics of plankton growth in the Barents Sea: model studies. Polar Res. 10, 173–186. ( 10.1111/j.1751-8369.1991.tb00643.x) [DOI] [Google Scholar]
- 57.Hill VJ, Matrai PA, Olson E, Suttles S, Steele M, Codispoti LA, Zimmerman RC. 2013. Synthesis of integrated primary production in the Arctic Ocean: II. In situ and remotely sensed estimates. Progress Oceanogr. 110, 107–125. ( 10.1016/j.pocean.2012.11.005) [DOI] [Google Scholar]
- 58.Arrigo KR, Matrai PA, Van Dijken GL. 2011. Primary productivity in the Arctic Ocean: impacts of complex optical properties and subsurface chlorophyll maxima on large-scale estimates. J. Geophys. Res.: Oceans 116, C11022 ( 10.1029/2011JC007273) [DOI] [Google Scholar]
- 59.Bouman HA, Jackson T, Sathyendranath S, Platt T. 2020. Vertical structure in chlorophyll profiles: influence on primary production in the Arctic Ocean. Phil. Trans. R. Soc. A 378, 20190351 ( 10.1098/rsta.2019.0351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hancke K, Hovland EK, Volent Z, Pettersen R, Johnsen G, Moline M, Sakshaug E. 2014. Optical properties of CDOM across the Polar Front in the Barents Sea: origin, distribution and significance. J. Mar. Sys. 130, 219–227. ( 10.1016/j.jmarsys.2012.06.006) [DOI] [Google Scholar]
- 61.Hu C, Cannizzaro J, Carder KL, Muller-Karger FE, Hardy R. 2010. Remote detection of Trichodesmium blooms in optically complex coastal waters: examples with MODIS full-spectral data. Remote Sens. Environ. 114, 2048–2058. ( 10.1016/j.rse.2010.04.011) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets of optical properties used in the construction of the phytoplankton community composition algorithm used in this study are available at [doi:10.5285/97daa7ea-8792-6cff-e053-6c86abc0dd46] [doi:10.5285/982b6da2-7e11-060a-e053-6c86abc09389] [doi:10.5285/982b6da2-7e12-060a-e053-6c86abc09389], accompanying datasets for chlorophyll-a concentration are available at [doi:10.5285/97daa7ea-8793-6cff-e053-6c86abc0dd46] [doi:10.5285/982b6da2-7e13-060a-e053-6c86abc09389] [doi:10.5285/982b6da2-7e14-060a-e053-6c86abc09389] MODIS-A data is available from NASA’s website for ocean-colour. [https://oceancolor.gsfc.nasa.gov/data/10.5067/AQUA/MODIS/L3M/RRS/2018/]