Abstract
Ethylene (ET), salicylic acid (SA) and indole-3-acetic acid (IAA) are important phytohormones regulating plant growth and development, as well as plant-microbe interactions. Plant growth-promoting bacteria (PGPB) naturally associate with plants and facilitate plant growth through a variety of mechanisms, including the ability to modulate the concentrations of these phytohormones in planta. Importantly, the wide presence of phytohormone degradation mechanisms amongst symbiotic and other soil- and plant-associated bacteria indicates that the ability to modulate phytohormone concentrations plays an important role in bacterial colonization and plant-growth promotion abilities. Obtaining phytohormone-degrading bacteria is therefore key for the development of novel solutions aiming to increase plant growth and protection. In this paper, we report an optimized targeted methodology and the consequent isolation of novel soil- and plant-associated bacteria, including rhizospheric, endophytic and phyllospheric strains, with the ability to degrade the phytohormones, SA and IAA, as well as the ET precursor, 1-aminocyclopropane-1-carboxylic acid (ACC). By using an optimized targeted methodology, we rapidly isolated diverse soil- and plant-associated bacteria presenting phytohormone-degrading abilities from several plants, plant tissues and environments, without the need for prior extensive and laborious isolation and maintenance of large numbers of isolates. The developed methodology facilitates PGPB research, especially in developing countries. Here, we also report, for the first time, the isolation of bacterial strains able to concomitantly catabolize three phytohormones (SA, IAA and ACC). Ultimately, the described targeted methodology and the novel phytohormone-degrading bacteria obtained in this work may be useful tools for future plant-microbe interaction studies, and in the development of new inoculant formulations for agriculture and biotechnology.
Keywords: 1-aminocyclopropane-1-carboxylic acid, salicylic acid, indole-3-acetic acid, plant growth-promoting bacteria, plant–microbe interactions
Introduction
Plant growth and development is tightly regulated by internal cues such as phytohormone biosynthesis and signalling [1]. Phytohormones not only regulate plant development and stress responses, but also plant microbiome assembly and general plant-microbe interactions, including beneficial interactions with symbionts (rhizobia) and other plant growth-promoting bacteria (PGPB) that support plant growth under a variety of limiting conditions [2]. In this regard, the phytohormones ethylene (ET), indole-3-acetic acid (IAA) (an auxin) and salicylic acid (SA) are major regulators and central players in plant developmental programmes, the plant microbiome assembly process and general plant-microbe interactions [3–7].
Plant-associated bacteria evolved the ability to modulate the levels of these important hormones (ET, IAA, SA) in soils and plant tissues, and consequently, affect plant growth, development and stress resistance. The bacterial modulation of plant hormone levels can occur in an additive manner, by direct production of phytohormones (e.g. IAA and other auxins), or in a subtractive manner, by the direct catabolism of phytohormones (e.g. IAA and SA) or their precursors (e.g. 1-aminocyclopropane-1-carboxylatic acid, ACC, the ET precursor). While many aspects of bacterial phytohormone production (mainly IAA biosynthesis) have been described and studied in detail [6, 7], much less is understood about the impact of bacterial phytohormone catabolism in beneficial plant-microbe interactions. However, several studies have indicated the important role of bacterial phytohormone catabolism in positively modulating plant growth and symbiotic processes, as well as resistance to biotic and abiotic stresses. For example, many PGPB can decrease both plant ACC and ET levels by producing the enzyme ACC deaminase, which cleaves ACC into ammonia and ⍺-ketobutyrate [8]. PGPB expressing the enzyme ACC deaminase have been shown to promote the growth and nodulation of several plant species under a variety of stress conditions [9, 10]. Some soil- and plant-associated bacteria, such as the Bradyrhizobium , Azoarcus , Paraburkholderia , Pseudomonas , Acinetobacter , Arthrobacter and Rhodococcus strains [11–17], can present the ability to catabolize IAA through a variety of biochemical pathways. Moreover, IAA catabolism plays an important role in the plant growth-promoting traits of PGPB such as Pseudomonas putida 1290 and Paraburkolderia phytofirmans PsJN [12, 16]. The catabolism of SA is a trait found in various soil- and plant-associated bacteria [4, 18, 19] and results from the action of SA hydroxylase and accessory enzymes [18, 20, 21]. The degradation of SA is intimately involved in the colonization abilities of plant-associated bacteria [4].
The presence of ACC, IAA and SA degradation mechanisms amongst symbiotic and plant-associated bacteria predicts a close evolutionary relationship with a plant host and plant growth promotion activities [9]. As such, obtaining ACC-, IAA- and SA-degrading bacteria is key for the study and development of novel solutions aiming to increase plant growth and protection. Nonetheless, obtaining diverse PGPB strains able to catabolize these phytohormones is a difficult task, since using traditional isolation methods usually involves laborious and time-consuming screening of bacteria isolates, which is often unsuccessful.
In order to isolate novel phytohormone-degrading bacteria with agricultural and biotechnological potential, and to overcome previous isolation limitations, we describe and demonstrate an optimized targeted methodology that allows the simple and fast isolation and characterization of soil- and plant-associated bacteria (including rhizospheric, endophytic and phyllospheric strains) with the ability to directly degrade phytohormones (SA and IAA) or their precursors (e.g. ACC, the ET precursor).
Methods
Overview of the targeted methodology
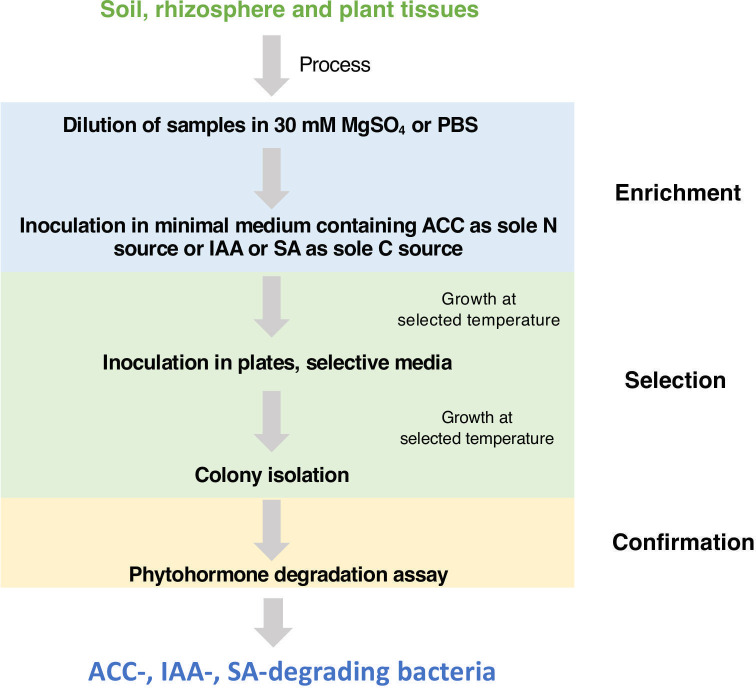
The optimized targeted methodology (Fig. 1) is based on direct bacterial enrichment in minimal medium containing the selected phytohormone as the sole carbon (IAA and SA) or nitrogen (ACC) source, and posterior isolation in selected growth media for the targeting of specific bacterial groups (e.g. Pseudomonas Agar F for the isolation of fluorescent Pseudomonas , or Yeast Mannitol Agar supplemented with Congo red for the isolation of rhizobia). Phytohormone-degrading abilities are then confirmed based on optimized, simple and affordable methods, using Salkowski’s reagent (IAA determination), Trinder reagent and/or UV fluorescence (SA determination) and an optimized ACC deaminase activity determination protocol. A detailed protocol of the methodology, containing all the necessary steps, reagents and materials, is presented in File S1 in the Supplementary Information file (available in the online version of this article).
Fig. 1.
Schematic representation of the developed methodology employed in order to obtain phytohormone-degrading bacteria.
Isolation of phytohormone-degrading bacteria from diverse plant and soil samples
Samples and preparation
In order to obtain various bacteria presenting phytohormone-degrading activities, several plant and soil samples obtained from diverse environments and countries were used (Table 1). For this purpose, plants such as Solanum capsicoides, Mimosa scabrella, Mimosa bimucronata, Mimosa pudica and Sesbania virgata; lower plants, such as an Sanionia uncinata, obtained in Antarctica; and soil samples obtained from agricultural and environmental areas (including stress-inducing soils, such as those of mining areas) were exploited (Table 1).
Table 1.
Bacteria isolated using a targeted methodology and their characterization (identification by 16S rRNA sequencing and analysis, and phytohormone-degrading activities)
|
Bacteria ID |
Accession no.# |
Source |
Sample collection site |
Degradation |
ACD activity* |
||
|---|---|---|---|---|---|---|---|
|
ACC |
IAA |
SA |
|||||
|
Achromobacter sp. AB2 |
Antarctic soil |
King George Island, Antarctica (62° 16' 48.2" S, 58° 26' 36.5" W) |
+ |
+ |
+ |
1.164 |
|
|
Achromobacter xylosoxidans SOLR10 |
Solanacea rhizosphere |
Florianópolis, Brazil (27° 35' 49.7" S 48° 30' 54.7" W) |
+ |
+ |
+ |
1.392 |
|
|
Arthrobacter sp. PM3 |
Bermuda grass rhizosphere |
Florianópolis, Brazil (27° 26' 55.7"S 48°28'08.3"W) |
+ |
− |
− |
1.467 |
|
|
Sesbania virgata root nodules (interior) |
Florianópolis, Brazil (27° 30' 16.4" S 48° 30' 49.7" W) |
+ |
− |
− |
3.908 |
||
|
Burkholderia sp. OPX |
Unknown tree trunk/fungi-fruit body |
Florianópolis, Brazil (27° 35' 57.5" S 48° 31' 03.9" W) |
+ |
− |
− |
13.125 |
|
|
Burkholderia sp. TRE3 |
Acid mine – drainage soil |
Criciúma, Brazil (28° 34' 31.0" S 49° 27' 21.8" W) |
− |
+ |
+ |
− |
|
|
Lelliottia sp. AC1 |
Pinus pinea leaf |
Setúbal, Portugal (38° 29' 06.2" N 8° 56' 38.4" W) |
− |
+ |
− |
− |
|
|
Microbacterium sp. PM5 |
Bermuda grass rhizosphere |
Florianópolis, Brazil (27° 26' 55.7" S 48° 28' 08.3" W) |
+ |
− |
− |
0.439 |
|
|
Pantoea sp. NE1 |
Sesbania sp. root nodule (interior) |
Florianópolis, Brazil (27° 26' 55.9" S 48° 28' 07.4" W) |
+ |
− |
− |
5.748 |
|
|
Pantoea sp. MSR2 |
Mimosa scabrella rhizosphere |
Florianópolis, Brazil (27° 26' 55.1" S 48° 28' 07.4" W) |
+ |
− |
− |
4.793 |
|
|
Paraburkholderia sp. MBD1 |
Mimosa bimucronata root nodule (interior) |
Florianópolis, Brazil (27° 26' 56.7" S 48° 32' 15.8" W) |
+ |
− |
− |
13.601 |
|
|
Paraburkholderia sp. MBS1 |
M. bimucronata root nodule (interior) |
Florianópolis, Brazil (27° 30' 31.1" S 48° 30' 48.9" W) |
+ |
− |
− |
4.041 |
|
|
Paraburkholderia sp. MP1 |
Mimosa pudica root nodule (interior) |
Florianópolis, Brazil (27° 30' 16.4" S 48° 30' 49.7" W) |
+ |
− |
− |
4.179 |
|
|
Pseudomonas sp. ACR2 |
Cactaceae rhizosphere |
Setúbal, Portugal (38° 29' 02.0" N 8° 58' 09.4" W) |
+ |
− |
− |
7.679 |
|
|
Pseudomonas sp. MS8 |
Shoot of M. scabrella (interior) |
Florianópolis, Brazil (27° 35' 54.3" S 48° 30' 56.5" W) |
+ |
− |
− |
3.756 |
|
|
Pseudomonas sp. NFX1 |
Eucalyptus sp. rhizosphere |
Setúbal, Portugal (38° 30' 27.9" N 8°55'44.4"W) |
− |
+ |
− |
− |
|
|
Agricultural soil |
Palmela, Portugal (38° 32' 36.9" N 8° 56' 11.0" W) |
+ |
− |
− |
15.322 |
||
|
Interior of a Solanum capsicoides fruit |
Florianópolis, Brazil (27° 34' 22.7" S 48° 25' 21.1" W) |
+ |
− |
− |
18.592 |
||
|
Pseudomonas sp. ACM7 |
Moss rhizosphere |
Collins Glacier, Antarctica (62° 09.752' S; 58° 55.825' W) |
− |
+ |
− |
− |
|
|
Pseudomonas sp. PLM1 |
Agricultural soil |
Palmela, Portugal (38° 32' 36.9" N 8° 56' 11.0" W) |
+ |
− |
− |
10.511 |
|
|
Pseudomonas sp. PLMAX |
Agricultural soil |
Palmela, Portugal (38° 32' 36.9" N 8° 56' 11.0" W) |
− |
+ |
+ |
− |
|
|
Serratia marcescens DAMR1 |
Acid mine – drainage soil |
Criciúma, Brazil (28° 34' 31.0" S 49° 27' 21.8" W) |
− |
− |
+ |
− |
#16S rRNA GenBank accession number.
*ACC deaminase activity in μmol α-ketobutyrate/mg protein/h.
†+, positive.
‡−, negative.
Soil bacteria
Approximately 200 mg of soil was stored in a sterile 50 ml Falcon tube containing a 10 ml solution of 30 mM MgSO4 and gently mixed for 30 s using a vortex. The obtained solution was diluted (10−3) using sterile 30 mM MgSO4 and stored at 4 °C for several days, until further use.
Rhizospheric bacteria
The selected plants were removed from soil, the shoot was cut with a sterile scalpel or a similar cutting instrument, and the root stored in a sterile 50 ml Falcon tube using disinfected forceps. A small section of the root (i.e. 5–10 cm) was directly dipped several times in a sterile 10 ml solution of 30 mM MgSO4. This solution was diluted (10−3) using sterile 30 mM MgSO4 and stored at 4 °C for several days, until further use.
Root, root nodule, shoot and fruit endophytes
Small sections of plant tissues and root nodules were disinfected by rinsing with 70 % ethanol (1.5 min) and then with 1 % bleach (10 min), followed by five consecutive washes with sterile distilled water. A small section of disinfected plant tissues (2×2 cm), as well as root nodules, was transferred to a sterile microcentrifuge tube containing 1 ml of sterile 30 mM MgSO4 and crushed several times with the help of a sterile micropestle. This solution was diluted (10−3) using sterile 30 mM MgSO4 and stored at 4 °C for several days, until further use.
Enrichment and isolation of phytohormone-degrading bacteria from diverse plant and soil samples
For the isolation of ACC deaminase-producing bacteria, 50 µl of the solutions obtained in the sample preparation section was inoculated in 5 ml of liquid Dworkin and Foster (DF) or M9 minimal medium (described File S1 in the Supplementary Information file) containing ACC (in a final concentration of 3 mM) as the sole nitrogen source, and incubated at 28 °C in an orbital shaker (150 r.p.m.) for 4–12 days. After observing increased bacterial growth (typically ~5 days), 10 to 20 µl of the bacterial suspension was reinoculated in 5 ml minimal medium containing 3 mM ACC and incubated at 28 °C in an orbital shaker (150 r.p.m.) for 4–12 days. Finally, 10 to 20 µl of the bacterial suspension was plated onto Tryptic Soy Agar, Pseudomonas Agar F and Actinomycetes Agar (HiMedia), and colonies were isolated.
In order to isolate SA- and IAA-degrading bacteria, 50 µl of the solutions obtained in the sample preparation section was inoculated in 5 ml of liquid DF or M9 minimal medium containing SA or IAA (in a final concentration of 1 mM) as the sole carbon source, and incubated at 28 °C in an orbital shaker (150 r.p.m.) for 4–12 days. The procedure was repeated as described above. After observing increased bacterial growth (typically ~7 days), 10 to 20 µl of the bacterial suspension was plated onto Tryptic Soy Agar, Pseudomonas Agar F and Actinomycetes Agar (HiMedia), and colonies were isolated.
Confirmation of bacterial phytohormone-degradation abilities
Determination of ACC degradation
Qualitative ACC degradation was confirmed by testing the bacteria isolated (pure cultures) for their ability to grow in minimal medium containing ACC as a sole nitrogen source. Additionally, ACC deaminase activity was tested using a simplified version (described in detail in the Supplementary Information fileFile S1) of the method described by Penrose and Glick [22].
Qualitative determination of SA and IAA degradation
Qualitative IAA or SA degradation was confirmed by testing the isolated bacterial cells (pure cultures) for their ability to grow in minimal medium containing IAA or SA as a sole carbon source. Additionally, a SA and IAA degradation test was performed in 24-well plates containing minimal medium supplemented with 1 mM SA or IAA and 0.8 % agar. In this case, 5 µl of an overnight-grown culture (grown in Tryptic Soy Broth medium) was inoculated in the centre of the well. The plate was then incubated for 24 h at 28 °C. SA degradation was identified by examining plates under UV radiation, where wells inoculated with strains unable to degrade SA had a fluorescent appearance, and wells inoculated with strains able to degrade SA did not fluoresce (Supplementary Information fileFile S1). IAA degradation was determined based on the use of the Salkowski’s reagent [23]. The wells containing bacteria unable to degrade IAA changed to a pink colour (negative), while IAA-degrading bacteria removed IAA from the medium and no pink colour developed (positive) (Supplementary Information file File S1).
Bacterial identification by 16S rRNA sequencing
The 16S rRNA gene sequencing was conducted following genomic DNA extraction from an overnight culture using the GenElute Bacterial Genomic DNA kit (Sigma Aldrich) according to the manufacturer’s instructions. The obtained DNA was sent to the Macrogen company (Republic of Korea), and amplified by PCR using primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) following the Macrogen PCR amplification and sequencing protocol. The obtained sequences were analysed using Geneious software v 9.0 and submitted to GenBank (the accession numbers can be found in Table 1).
Results and Discussion
By using a targeted methodology, several ACC-, IAA- and SA-degrading bacterial strains were successfully isolated and characterized in this study (Table 1). These bacteria were obtained from different plants (e.g. Mimosa, Sesbania and Solanum), plant tissues (roots/rhizospheres, root nodules, shoots, leaves and fruits), as well as lower plants (e.g. Antarctic moss) and soils (agricultural, environmental and polluted) obtained from different countries and continents, (including Antarctica), indicating that phytohormone-degrading bacteria are ubiquitous to many environments and are common members of the plant and soil microbiome.
Moreover, the isolated bacteria were identified based on the partial 16S rRNA gene sequence (~1346 bp). Phytohormone-degrading bacteria belonging to different genera (and phyla) were identified, such as Azorhizobium ( Alphaproteobacteria ); Achromobacter , Burkholderia and Paraburkholderia ( Betaproteobacteria ); Lelliottia , Pantoea , Serratia and Pseudomonas ( Gammaproteobacteria ); and Arthrobacter and Microbacterium ( Actinobacteria ) (Table 1). This indicated that phytohormone catabolism can be found in a wide range of bacterial genera and species. These results are consistent with the important role of bacterial phytohormone catabolism in general plant-microbe interactions.
Interestingly, several of the isolated bacteria possessed the ability to degrade more than one phytohormone (Table 1). For example, Achromobacter sp. strain AB2 isolated from Antarctic soil, and Achromobacter xylosoxidans SOLR10, isolated from the rhizosphere of a Solanaceae plant in Brazil, possessed the ability to use ACC, SA and IAA as nitrogen and carbon sources, respectively (Table 1). This is the first report of bacteria that are able to degrade three phytohormones. In addition, Burkholderia sp. TRE3 (isolated from acid mine drainage soil in Brazil) and Pseudomonas sp. PLMAX (isolated from agricultural soil in Portugal) presented the ability to degrade both IAA and SA (Table 1). These results are in agreement with previous reports of IAA and SA degradation by Pseudomonas and Burkholderia (sensu lato), as well as other studies demonstrating the aromatic compound degradation abilities of Achromobacter , Burkholderia and Pseudomonas , and their important role in soil and promoting plant growth [11, 12, 16, 24].
In addition, several rhizobial strains, including both Alpha proteobacteria ( Azorhizobium ) and Betaproteobacteria ( Paraburkholderia ), presenting ACC deaminase activity were isolated (Table 1). ACC deaminase expression increases the nodulation abilities of several rhizobia [25–28] by decreasing ethylene levels, which inhibit the symbiotic nodulation process [3, 29]. Hence, obtaining rhizobia and other free-living bacteria presenting ACC deaminase activity is important for increased nodulation and biological nitrogen fixation in selected leguminous plants. Curiously, Pantoea sp. NE1, a root nodule endophyte presenting ACC deaminase activity, was also isolated from a disinfected root nodule of a Sesbania plant in Florianópolis, Brazil (Table 1). Recent studies demonstrated that bacterial endophytes presenting ACC deaminase activity increased the nodulation abilities of rhizobia [30, 31], and thus it is likely that Pantoea sp. NE1 presenting ACC deaminase activity facilitates the nodulation and nitrogen fixation process.
Some studies have reported the isolation of phytohormone-degrading bacteria (mainly those degrading ACC); however, these were laborious and expensive to conduct. For instance, in an effort to isolate endophytic ACC deaminase-producing bacteria from tomato, Rashid et al. [32] isolated 174 bacterial strains, yet only 25 of these (13 %) were able to degrade ACC. Similarly, in a survey in 30 different sites across southern Saskatchewan, Canada, Duan et al. [33] found that only 27 out of 233 (11.6 %) isolated rhizobia presented ACC deaminase activity. The fact that only a small portion of the cultivable bacterial community presents ACC deaminase activity makes the isolation of these bacteria a challenging process. On the other hand, the targeted methodology employed in this study allowed the simple, fast and reproducible isolation of various ACC deaminase-producing bacteria from a wide range of plant species, tissues and environments, without the need for extensive and laborious isolation of bacterial isolates. This methodology is based on readily accessible materials that can be used in most microbiology laboratories, and therefore facilitates PGPB research, especially in developing countries.
While the prevalence of ACC deaminase activity has been studied in some plant-associated bacterial communities, not much is understood about the prevalence of IAA- and SA-degrading bacteria or their impact on the microbiome of plants. The methodology developed in this work may also potentiate novel studies on the role of bacterial IAA and SA catabolism in plant growth and stress resistance.
Ultimately, the novel ACC-, IAA- and SA-degrading bacteria obtained in this work can be used in future studies focusing on phytohormone modulation and plant-microbe interactions, as well as for the development of a new generation of inoculants that are suitable for both agricultural and biotechnological applications. New studies regarding the functional and genomic characterization of these new phytohormone-degrading bacteria are being conducted and will unveil the mechanisms behind the phytohormone catabolism and the plant growth promotion abilities of the isolated strains.
Supplementary Data
Funding information
This work received no specific grant from any funding agency.
Acknowledgements
We thank Professor Rubens Duarte (UFSC) for providing the Antarctic soil and Sanionia uncinata samples. F. X. N. acknowledges receiving a fellowship (SFRH/BD/86954/2012) from the Fundação para a Ciência e a Tecnologia (FCT), Portugal. M. J. R. acknowledges receiving a fellowship (DT 306167/2015–8) from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: ACC, 1-aminocyclopropane-1-carboxylic acid; ET, Ethylene; IAA, Indole-3-acetic acid; PGPB, Plant-growth-promoting bacteria; SA, Salicyclic acid.
Supplementary material is available with the online version of this article.
References
- 1.Gray WM. Hormonal regulation of plant growth and development. PLoS Biol. 2004;2:e311. doi: 10.1371/journal.pbio.0020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nascimento FX, Rossi MJ, Glick BR, Ethylene GBR. Ethylene and 1-aminocyclopropane-1-carboxylate (ACC) in Plant–Bacterial interactions. Front Plant Sci. 2018;9:1–17. doi: 10.3389/fpls.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebeis SL, Paredes SH, Lundberg DS, Breakfield N, Gehring J, et al. Plant microbiome. salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science. 2015;349:860–864. doi: 10.1126/science.aaa8764. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010;61:49–64. doi: 10.1146/annurev-arplant-042809-112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duca D, Lorv J, Patten CL, Rose D, Glick BR. Indole-3-Acetic acid in plant-microbe interactions. Antonie Van Leeuwenhoek. 2014;106:85–125. doi: 10.1007/s10482-013-0095-y. [DOI] [PubMed] [Google Scholar]
- 7.Spaepen S, Vanderleyden J. Auxin and plant-microbe interactions. Cold Spring Harb Perspect Biol. 2011;3:a001438. doi: 10.1101/cshperspect.a001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169:30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Nascimento FX, Rossi MJ, Soares CRFS, McConkey BJ, Glick BR. New insights into 1-aminocyclopropane-1-carboxylate (ACC) deaminase phylogeny, evolution and ecological significance. PLoS One. 2014;9:e99168. doi: 10.1371/journal.pone.0099168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glick BR, Cheng Z, Czarny J, Duan J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol. 2007;119:329–339. doi: 10.1007/s10658-007-9162-4. [DOI] [Google Scholar]
- 11.Leveau JHJ, Lindow SE. Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl Environ Microbiol. 2005;71:2365–2371. doi: 10.1128/AEM.71.5.2365-2371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leveau JHJ, Gerards S. Discovery of a bacterial gene cluster for catabolism of the plant hormone indole 3-acetic acid. FEMS Microbiol Ecol. 2008;65:238–250. doi: 10.1111/j.1574-6941.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 13.Scott JC, Greenhut IV, Leveau JHJ. Functional characterization of the bacterial IAC genes for degradation of the plant hormone indole-3-acetic acid. J Chem Ecol. 2013;39:942–951. doi: 10.1007/s10886-013-0324-x. [DOI] [PubMed] [Google Scholar]
- 14.Egebo LA, Nielsen SV, Jochimsen BU. Oxygen-dependent catabolism of indole-3-acetic acid in Bradyrhizobium japonicum . J Bacteriol. 1991;173:4897–4901. doi: 10.1128/jb.173.15.4897-4901.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen JB, Egsgaard H, Van Onckelen H, Jochimsen BU. Catabolism of indole-3-acetic acid and 4- and 5-chloroindole-3-acetic acid in Bradyrhizobium japonicum . J Bacteriol. 1995;177:5762–5766. doi: 10.1128/jb.177.20.5762-5766.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zúñiga A, Poupin MJ, Donoso R, Ledger T, Guiliani N, et al. Quorum sensing and indole-3-acetic acid degradation play a role in colonization and plant growth promotion of Arabidopsis thaliana by Burkholderia phytofirmans PsJN. Mol Plant Microbe Interact. 2013;26:546–553. doi: 10.1094/MPMI-10-12-0241-R. [DOI] [PubMed] [Google Scholar]
- 17.Ebenau-Jehle C, Thomas M, Scharf G, Kockelkorn D, Knapp B, et al. Anaerobic metabolism of indoleacetate. J Bacteriol. 2012;194:2894–2903. doi: 10.1128/JB.00250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuenmayor SL, Wild M, Boyes AL, Williams PA. A gene cluster encoding steps in conversion of naphthalene to gentisate in Pseudomonas sp. strain U2. J Bacteriol. 1998;180:2522–2530. doi: 10.1128/jb.180.9.2522-2530.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch R, Moore ER, García-Valdés E, Pieper DH, NahW PDH. NahW, a novel, inducible salicylate hydroxylase involved in mineralization of naphthalene by Pseudomonas stutzeri AN10. J Bacteriol. 1999;181:2315–2322. doi: 10.1128/jb.181.8.2315-2322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto S, Katagiri M, Maeno H, Hayaishi O. Salicylate a monooxygenase requiring flavin adenine dinucleotide. Joural Biol Chem. 1965;240:3408–3413. [PubMed] [Google Scholar]
- 21.Ishiyama D, Vujaklija D, Davies J. Novel pathway of salicylate degradation by Streptomyces sp. strain WA46. Appl Environ Microbiol. 2004;70:1297–1306. doi: 10.1128/AEM.70.3.1297-1306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 23.Glickmann E, Dessaux Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61:793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colla TS, Andreazza R, Bücker F, de Souza MM, Tramontini L, et al. Bioremediation assessment of diesel–biodiesel-contaminated soil using an alternative bioaugmentation strategy. Environ Sci Pollut Res. 2014;21:2592–2602. doi: 10.1007/s11356-013-2139-2. [DOI] [PubMed] [Google Scholar]
- 25.Ma W, Guinel FC, Glick BR. Rhizobium leguminosarum biovar viciae 1-aminocyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Appl Environ Microbiol. 2003;69:4396–4402. doi: 10.1128/AEM.69.8.4396-4402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nascimento F, Brígido C, Alho L, Glick BR, Oliveira S. Enhanced chickpea growth-promotion ability of a Mesorhizobium strain expressing an exogenous ACC deaminase gene. Plant Soil. 2012;353:221–230. doi: 10.1007/s11104-011-1025-2. [DOI] [Google Scholar]
- 27.Nascimento FX, Tavares MJ, Glick BR, Rossi MJ. Improvement of Cupriavidus taiwanensis nodulation and plant growth promoting abilities by the expression of an exogenous ACC deaminase gene. Curr Microbiol. 2018;75:961–965. doi: 10.1007/s00284-018-1474-4. [DOI] [PubMed] [Google Scholar]
- 28.Kong Z, Glick BR, Duan J, Ding S, Tian J, et al. Effects of 1-aminocyclopropane-1-carboxylate (ACC) deaminase-overproducing Sinorhizobium meliloti on plant growth and copper tolerance of Medicago lupulina . Plant Soil. 2015;391:383–398. doi: 10.1007/s11104-015-2434-4. [DOI] [Google Scholar]
- 29.Guinel FC, Ethylene GFC. Ethylene, a hormone at the of nodulation. Front Plant Sci. 2015;6:1121. doi: 10.3389/fpls.2015.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavares MJ, Nascimento FX, Glick BR, Rossi MJ. The expression of an exogenous ACC deaminase by the endophyte Serratia grimesii BXF1 promotes the early nodulation and growth of common bean. Lett Appl Microbiol. 2018;66:252–259. doi: 10.1111/lam.12847. [DOI] [PubMed] [Google Scholar]
- 31.Nascimento FX, Tavares MJ, Franck J, Ali S, Glick BR, et al. ACC deaminase plays a major role in Pseudomonas fluorescens YsS6 ability to promote the nodulation of Alpha- and Betaproteobacteria rhizobial strains. Arch Microbiol. 2019;201:817–822. doi: 10.1007/s00203-019-01649-5. [DOI] [PubMed] [Google Scholar]
- 32.Rashid S, Charles TC, Glick BR. Isolation and characterization of new plant growth-promoting bacterial endophytes. Applied Soil Ecology. 2012;61:217–224. doi: 10.1016/j.apsoil.2011.09.011. [DOI] [Google Scholar]
- 33.Duan J, Müller KM, Charles TC, Vesely S, Glick BR. 1-Aminocyclopropane-1-Carboxylate (ACC) deaminase genes in rhizobia from southern Saskatchewan. Microb Ecol. 2009;57:423–436. doi: 10.1007/s00248-008-9407-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.