Abstract
Dysregulation of histone methyltransferase enhancer of zeste homolog 2 (EZH2) has been implicated in the pathogenesis of many cancers. However, the role of EZH2 in peritoneal fibrosis remains unknown. We investigated EZH2 expression in peritoneal dialysis (PD) patients and assessed its role in peritoneal fibrosis in cultured human peritoneal mesothelial cells (HPMCs) and murine models of peritoneal fibrosis induced by chlorhexidine gluconate (CG) or high glucose peritoneal dialysis fluid (PDF) by using 3-deazaneplanocin A (3-DZNeP), and EZH2 conditional knockout mice. An abundance of EZH2 was detected in the peritoneum of patients with PD associated peritonitis and the dialysis effluent of long-term PD patients, which was positively correlated with expression of transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor, interleukin-6. EZH2 was also highly expressed in the peritoneum of mice after injury by CG or PDF. In both mouse models, treatment with 3-DZNeP attenuated peritoneal fibrosis and inhibited activation of several pro-fibrotic signaling pathways, including TGF-β1/Smad3, Notch1, epidermal growth factor receptor and Src. EZH2 inhibition also inhibited STAT3 and nuclear factor-κB phosphorylation, reduced lymphocyte and macrophage infiltration and angiogenesis in the injured peritoneum. 3-DZNeP effectively improved high glucose PDF-associated peritoneal dysfunction by decreasing D/P ratio of BUN and increasing D/D0 ratio of glucose. Moreover, delayed administration of 3-DZNeP inhibited peritoneal fibrosis progression, reversed established peritoneal fibrosis and reduced tissue inhibitors of metalloproteinase 2, matrix metalloproteinases-2 and −9 expression. Finally, EZH2-KO mice exhibited less peritoneal fibrosis compared with EZH2-WT mice. In HPMCs, treatment with EZH2 siRNA or 3-DZNeP suppressed TGF-β1-induced upregulation of α-SMA and Collagen I and preserved E-cadherin. These results indicate that EZH2 is a key epigenetic regulator that promotes peritoneal fibrosis. Targeting EZH2 may have the potential to prevent and treat peritoneal fibrosis.
Keywords: Enhancer of zeste homolog 2, peritoneal fibrosis, pro-fibrotic signaling pathways, angiogenesis, epithelial-to-mesenchymal transition
Introduction
Continuous ambulatory peritoneal dialysis (CAPD) has been recognized as a renal replacement therapy since 1970s. It has become a major alternative treatment for patients with end-stage renal disease (ESRD) worldwide [1]. However, persistent exposure of peritoneum to bio-incompatible peritoneal dialysis fluid (PDF) leads to peritoneal fibrosis, which decreases ultrafiltration capacity of peritoneum and limits its clinic application [2,3]. To date, there is no available treatment to prevent peritoneal fibrosis and halt its progression. Therefore, understanding the mechanisms leading to peritoneal fibrosis and discovery of new agents to block its progression holds great significance for continuing PD long term in patients.
Peritoneal fibrosis is characterized by epithelial-to-mesenchymal transition (EMT) of mesothelial cells, activation of fibroblasts and deposition of extracellular matrix (ECM) components as well as angiogenesis [4]. Many growth factors/cytokines, in particular, transforming growth factor-1 (TGF-β1), mediate activation of peritoneal fibroblasts and EMT, and play a key role in the pathogenesis of peritoneal fibrosis [5,6]. Activation of epidermal growth factor receptor (EGFR), Src and Notch signaling pathway also contributes to these processes [7–9]. Moreover, in the case of peritonitis, nuclear factor-kappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT3) are activated and induce expression and production of multiple pro-inflammatory cytokines/chemokines, including interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) [5–13]. MCP1 and other chemoattractants can induce accumulation of inflammatory cells, such as macrophages in the peritoneum. Macrophages can also produce TGF-β and connective tissue growth factor (CTGF), vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), to promote progression of peritoneal fibrosis [13,14]. Vascular endothelial growth factor (VEGF) stimulates angiogenesis and vasculopathy, eventually leading to peritoneal ultrafiltration failure [4].
Increasing evidence indicates that epigenetic modification of gene expression mediates expression and activation of many transcriptional factors and signaling molecules associated with peritoneal fibrosis [15]. DNA methylation and histone modifications are two major types of epigenetic modifications. Among the possible ways of histone modification, mono-, di- or tri-methylation can occur on lysine or arginine amine acids of histone H3 and some non-histone proteins [16,17]. Histone methylation can alter chromatin structure and affect transcriptional factor accessibility to DNA promoters, thereby regulating gene expression. In addition, methylation of a non-histone can also affect its activation and expression [17,18]. Protein methylation is catalyzed by histone lysine and arginine methyltransferases. Enhancer of zeste homolog 2 (EZH2) is one of the histone lysine methyltransferase that catalyzes trimethylation of histone H3 at lysine 27 (H3K27me3) with methyltransferase activity, and promotes transcriptional silencing of many genes, including E-cadherin [19,20]. EZH2 can also directly methylate some intracellular signaling molecules, such as STAT3, and regulate their phosphorylation and activation [21].
Numerous studies have shown that EZH2 is highly expressed in many cancers and closely related to tumor invasion, metastasis, and poor prognosis [19,22]. Inhibition of EZH2 activity has become a new strategy for anti-tumor therapy. A variety of EZH2 inhibitors have been used for antitumor preclinical studies and have achieved encouraging results [23,24]. 3-deazaneplanocin A (3-DZNeP) is an inhibitor of S-adenosylhomocysteine (SAH) hydrolase that inhibits the activity of EZH2 and down-regulates the methylation level of H3K27 [25]. It has been reported that 3-DZNeP can inhibit activation of hepatic stellate cells (HSCs), and prevent the progression of liver fibrosis [26]. Our recent studies also showed that EZH2 is highly expressed in the diseased human kidney with fibrosis and the mouse kidney after unilateral ureteral obstruction (UUO) and that inhibition of EZH2 with 3-DZNeP blocks renal myofibroblast activation, EMT and relieves UUO-induced renal fibrosis [11,27]. However, it remains unknown whether EZH2 is involved in peritoneal fibrosis.
In this study, we examined the expression of EZH2 in the peritoneum of patients with PD-related peritonitis and in murine models of peritoneal fibrosis induced by 4.25% peritoneal dialysis fluid (PDF) and 0.1% chlorhexidine gluconate (CG); we also evaluated the effect on peritoneal fibrosis of early or late administration of 3-DZNeP and specific deletion of EZH2 in peritoneal fibrosis animal model to learn the mechanisms involved. Furthermore, we examined pharmacological and genetic inhibition of EZH2 on TGF-β1-induced EMT in cultured human peritoneal mesothelial cells (HPMCs).
Materials and methods
Chemicals and antibodies
3-Deazaneplanocin A (3-DZNeP) was purchased from Selleckchem (Houston, TX). Antibodies to EZH2, H3K27me3, p-EGFR, p-Src, Src, p-ERK1/2, ERK1/2, p-STAT3, STAT3, p-Smad3, Smad3, Notch1, MMP2, MMP9, Histone H3 and Cleaved caspase 3 were purchased from Cell Signaling Technology (Dancers, MA). Antibodies to GAPDH, E-cadherin, p-NF-κB (p65), NF-κB (p65), Collagen I (A2), TGF-βRI, Smad7, CD68, CD31, VEGF and EGFR were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). CD3, H3K9me3, TIMP2 and Bcl-2 were purchased from Abcam (Cambridge, MA). Antibody to Bax was purchased from BD Biosciences (San Jose, CA). Antibody to β-actin was purchased from TransGen Biotech (Beijing, China). TNF-α, IL-1β, TGF-β1, MCP-1, IL-6, EZH2, VEGF, CA125 enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN). Peritoneal dialysis solution with 4.25% glucose was purchased from Baxter Healthcare (Guangzhou, China). EZH2 siRNA was purchased from GenePharma (Shanghai, China). Lipofectamine 2000 was purchased from Invitrogen (Grand Island, NY). α-SMA, DMSO, CG, Tamoxifen and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Blood urea nitrogen (BUN) and glucose biochemical reagent kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Cell culture and treatments
Human peritoneal mesothelial cells (HPMCs) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with F12 containing 10% fetal bovine serum (FBS), 1% penicillin and streptomycin in an atmosphere of 5% CO2, and 95% air at 37℃. To examine the anti-fibrotic effect of 3-DZNeP in TGF-β1-induced EMT in vitro, HPMCs were starved for 24 hours with DMEM/F12 containing 0.5% FBS and then exposed to TGF-β1 (2 ng/ml) in the presence of 3-DZNeP (0, 1, 5, 10 μM) for 36 hours. In addition, serum-starved HPMCs were cultured in peritoneal dialysis fluid containing 4.25% glucose for 36 hours with different concentrations of 3-DZNeP (0, 1, 5, 10 μM), in order to verify the anti-apoptotic effect of 3-DZNeP. Then, cells were harvested for further immunoblot analysis. All of the in vitro experiments were repeated for at least three times.
siRNA transfection
The small interfering (si) RNA oligonucleotides targeted to EZH2 were used to down-regulate EZH2 more specifically in vitro. HPMCs were seeded to 30–40% confluence in antibiotic-free medium and grown for 24 hours, and then transfected with EZH2 siRNA (100 pmol) with lipofectamine 2000 according to the manufacturer’s instructions. In parallel, scrambled siRNA (100 pmol) was used as control for off-target changes in HPMCs. After 24h transfection, the original antibiotic-free medium was changed and cells were further treated with TGF-β1 (2 ng/ml) for an additional 36h before being harvested for the experiments.
Wound-healing assay
HPMCs were seeded in a 6-well plate and allowed to reach 90% confluence. A scratch wound was created on the cell surface using a micropipette tip. Then, cells were washed with PBS in three times and incubated in serum-free DMEM/F12 with TGF-β1 (2 ng/ml) in the presence or absence of 3-DZNeP (10 μM). Photomicrographs (40X) of migrating cells were taken at 0h and 24h. The width of the wound was measured by ImageJ software. Migratory rate was calculated as (A-B)/A*100%, where A and B reflect the width of the wound at 0h and 24h respectively.
Animal models and experimental design
Male C57/black mice (Shanghai Super-B&K Laboratory Animal Corp. Ltd) that weighed 20–25g were housed under a 12:12-h light-dark cycle with food and water supplied ad libitum. Two mouse models of peritoneal fibrosis were established. The first peritoneal fibrosis model was created by daily intraperitoneal injection of 100ml/kg peritoneal dialysis solution with 4.25% glucose for 28 days [10]. The second peritoneal fibrosis model was established by intraperitoneally injection of 0.1% chlorhexidine gluconate (CG) dissolved in saline every other day for 21 days [28,29]. To investigate the effect of 3-DZNeP in peritoneal fibrosis, mice were injected intraperitoneally with a single dose of 3-DZNeP (1 mg/kg) in DMSO every day. Mice were randomly divided the mice into four groups in each model: (1) mice injected with an equivalent amount of saline intraperitoneally and DMSO (n=6), defined as the sham group; (2) mice injected an equivalent amount of saline intraperitoneally and 3-DZNeP (n=6), defined as sham + 3-DZNeP group; (3) mice injected PDF or CG intraperitoneally and DMSO (n=6), defined as PDF/CG group; and (4) mice injected PDF or CG intraperitoneally and 3-DZNeP, defined as PDF/CG + 3-DZNeP group (n=6). All animals were sacrificed, and parietal peritoneum was collected from each mouse for further protein analysis and histological examination at the end of 28 days in the PDF model or 21 days in the CG model. To examine the therapeutic its effect on established peritoneal fibrosis, 3-DZNeP was administered starting at 28 days in mice after injection with PDF and then given daily for 14 days. At the end of 42 days, all mice were euthanized for collection of peritoneal tissue. All the experiments were conducted in accordance with the animal experimentation guidelines of Tongji University School of Medicine, China.
Peritoneal equilibration test
Mice in each group received a peritoneal equilibration test (PET) before euthanasia in the last day. Mice were injected with 2 ml 4.25% PDF for 2 hours and then euthanized for collection of blood and dialysate. Glucose in dialysate and BUN in plasma and dialysate were determined by biochemical reagent kits according to the manufacturer’s instructions. Functional alteration of peritoneal membrane was evaluated by the urea nitrogen transport rate from plasma with the dialysate-to-plasma (D/P) ratio of blood urea nitrogen, and the glucose absorption rate from dialysate with the ratio of dialysate glucose at 2 hour after PDF injection to dialysate glucose at 0 hour (D/D0).
EZH2 knockout mouse model
EZH2loxP/loxP and tamoxifen-inducible Col1a2-Cre mice (Col1a2-CreER+/−) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). EZH2 knockout mice were generated by breeding EZH2loxP/loxP mice with Col1a2-CreER+/− and then backcrossing progeny to EZH2loxP/loxP mice to obtain Col1a2-Cre+: EZH2loxP/loxP mice (EZH2-KO) and Col1a2-Cre-: EZH2loxP/loxP mice (EZH2-WT). Both of EZH2-KO and EZH2-WT mice received a daily intraperitoneal injection of 100 ml/kg peritoneal dialysis solution with 4.25% glucose for 28 days to establish peritoneal fibrosis model and administered tamoxifen (75 mg/kg body weight) by intraperitoneal injection for seven consecutive days.
Immunoblot analysis
Immunoblot analysis of peritoneum tissue samples was conducted as described previously [11]. Densitometry analysis of immunoblot results was conducted by using ImageJ software (National Institutes of Health, Bethesda, MD).
Morphologic studies of peritoneum
Formalin-fixed peritoneum was embedded in paraffin and prepared in 3-μm-thick sections. For evaluation of peritoneal fibrosis, Masson trichrome staining was performed according to the protocol provided by the manufacturer (Sigma-Aldrich). The thickness of the submesothelial tissue was evaluated (in micrometers), and the average of ten independent measurements was calculated for each section (original magnification, ×200).
Immunofluorescent staining
Immunofluorescent staining was carried out according to the procedure described in our previous studies [30]. Briefly, peritoneal tissue sections were fixed in 4.5% buffered formalin, dehydrated, and embedded in paraffin. For immunofluorescent staining, the tissue sections were rehydrated and labeled with antibodies, including primary antibodies α-SMA, EZH2 and then exposed to Texas red-labeled or FITC green-labeled secondary antibodies (Invitrogen).
Immunohistochemical staining
The 3μm thick sections were de-paraffinized and rehydrated, quenched with 3% H2O2, immersed in citrate buffer and heated in a microwave. Immunohistochemical staining was carried out according to the procedure described in our previous studies [30].
ELISA analysis
ELISA detection of TNF-α, IL-1β, TGF-β1, MCP-1, IL-6, EZH2, VEGF, CA125 protein was performed in accordance with the manufacturer’s instructions.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed by using a ChIP assay Kit (Millipore, MA) according to the manufacturer’s instructions and ChIP antibody against H3K27me3 was purchased from Cell Signaling Technology (Dancers, MA). The precipitated DNA fragments were quantified by real-time PCR and normalized using the internal control IgG. The ChIP data was presented as a percentage relative to the input DNA.
Clinical sample collection and ethics statement
To detect EZH2 expression in the peritoneum samples of PD patients, we collected peritoneal tissue during operations to insert (n=10) or remove (n=6) PD catheters at Shanghai East Hospital affiliated with Tongji university and conducted immunohistrochemistry. We also collected the human PD effluents in diverse PD time, respectively, 1 month (n=9), 24 months (n=8) and 48 months (n=8) at Shanghai East Hospital, Shanghai Wusong Central Hospital and Shanghai Songjiang District Central Hospital from January 2017 to February 2019. This study was approved by the Medical Ethics Committee of Shanghai East Hospital and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient. And we have obtained the registration number from Chinese Clinical Trial Register (ChiCTR): ChiCTR1800015272.
Statistical analysis
All the experiments were conducted at least three times. Data depicted in graphs represent the means ± SD. for each group. Intergroup comparison was made using one-way analysis of variance. Multiple means were compared using Tukey’s test. The differences between two groups were determined by Student’s T-test. Statistical significant difference between mean values was marked in each graph. P<0.05 is considered significant. The statistical analyses were conducted by using IBM SPSS Statistics 20.0 (Beijing, China).
Results
EZH2 is highly expressed in the peritoneum of patients with PD associated peritonitis
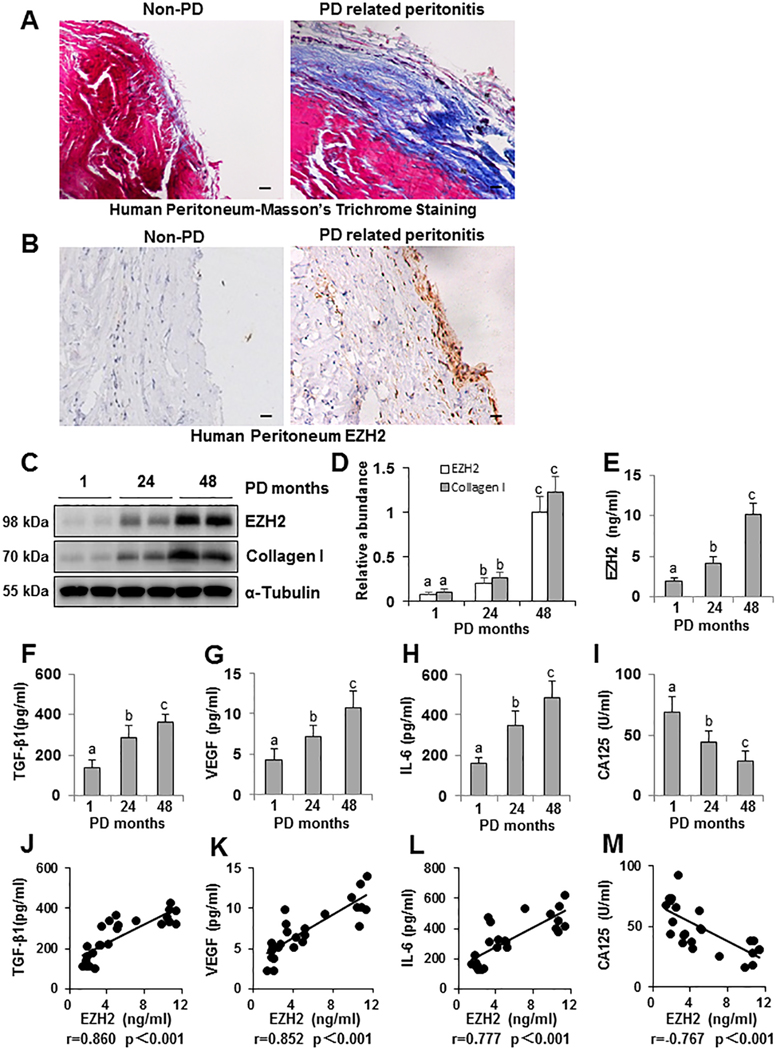
To examine EZH2 expression in patients with PD, we collected the peritoneum from both non-PD patients (n=10) and PD patients associated with peritonitis (n=6). As shown in Figure 1A, Masson trichrome staining illustrated that the peritoneum of patients with PD associated peritonitis had severe peritoneal fibrosis as characterized with the increased thickness of the submesothelial area, a layer of mature fibrous tissue containing collagen and elastin fibers. The thickness and positive area of the submesothelial area in patients’ peritoneum with PD associated peritonitis was much greater than in non-PD patients. A high expression level of EZH2 was also observed in the peritoneum of patients with PD associated peritonitis compared with non-PD (Figure 1B).
Figure 1. High expression of EZH2 is detected in peritoneum of patients with PD associated peritonitis and EZH2 also positively correlates with enhanced expression of TGF-β1, VEGF, IL-6 and negatively with CA125 in human PD effluents.
(A) Photomicrographs (200X) show Masson trichrome staining of the peritoneum in both non-PD patients and patients with PD associated peritonitis. (B) Photomicrographs (200X) illustrate EZH2 staining of the peritoneal tissues in both non-PD patients and patients with PD associated peritonitis patients. (C) Human PD effluent was subjected to immunoblot analysis with antibodies against EZH2, Collagen I or α-Tubulin. (D) Expression levels of EZH2 and Collagen I were quantified by densitometry and normalized with α-Tubulin. Human PD effluent was subjected to the ELISA, as described under Materials and Methods, at different PD time points, respectively, 1 month (n=9), 24 months (n=8) and 48months (n=8). The expression levels of EZH2 (E), TGF-β1 (F), VEGF (G), IL-6 (H), and CA125 (I) are indicated in each group. Correlation analysis was conducted between EZH2 and TGF-β1 (J), VEGF (K), IL-6 (L) as well as CA125 (M). Data are represented as the mean ± S.E.M. Means with different superscript letters are significantly different from one another (P< 0.05). All scale bars = 20 μm.
Dialysate CA125 and IL-6 has been reported to be a marker for evaluating the peritoneal membrane in non-infected patients to predict peritoneal fibrosis [31,32]. Thus, we also collected PD effluent from patients on PD for different lengths of time: 1 month (n=9), 24 months (n=8), and 48 months (n=8), respectively. After centrifugation, the pallet of PD effluent was submitted for immunoblot analysis. We found elevated expression levels of EZH2 and Collagen I in samples collected from PD samples from patients on PD for 24 months and 48 months compared to effluent from patients on PD for only 1 month (Figure 1C, 1D). In line with this observation, ELISA assays of PD effluent showed an increase in the expression of EZH2 and multiple growth factors and cytokines, including TGF-β1, VEGF, IL-6, and a decrease in the expression of CA125. Further analysis indicated that EZH2 expression was positively correlated with expression of TGF-β1, VEGF, IL-6 and negatively with CA125 in human PD effluent (Figure 1E–M). Therefore, EZH2 may be used as a biomarker of peritoneal fibrosis in PD patients.
3-DZNeP attenuates development of peritoneal fibrosis induced by high glucose peritoneal dialysis fluid (PDF) or chlorhexidine gluconate (CG) in mice
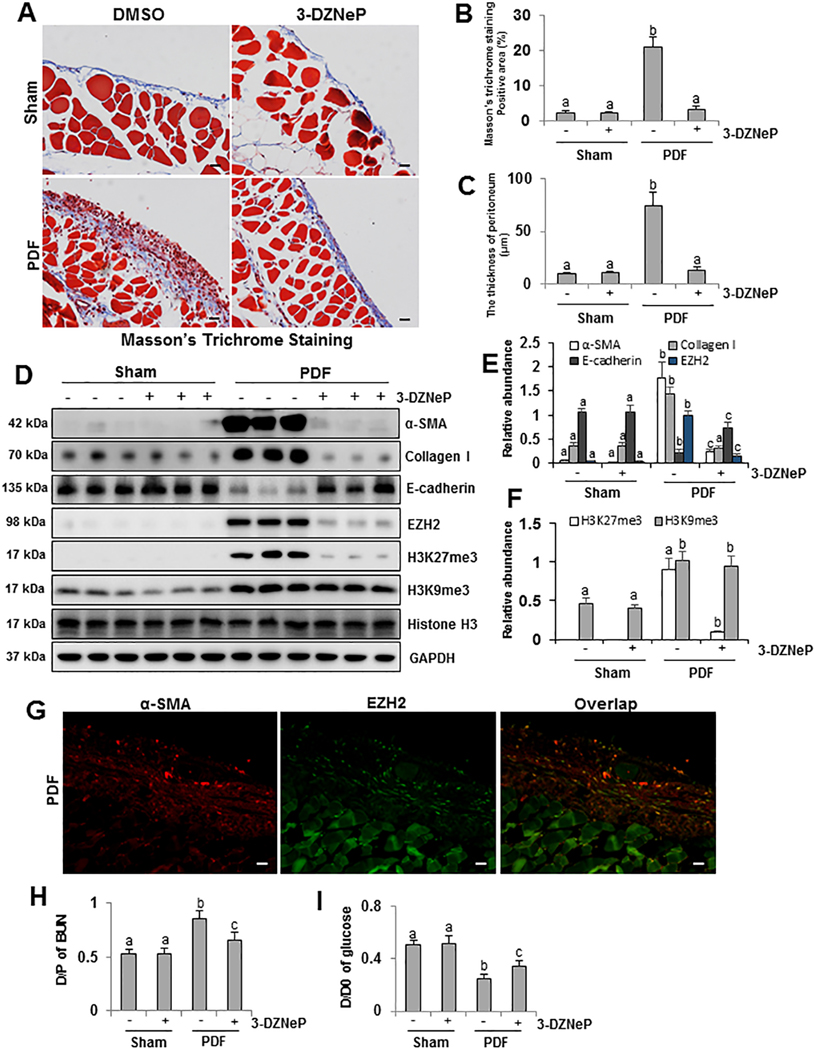
To elucidate the role of EZH2 in mediating peritoneal fibrosis, we examined the effect of 3-DZNeP on the development of peritoneal fibrosis induced by high glucose peritoneal dialysate or chlorhexidine gluconate. As shown in Figure 2A–C and Supplemental Figure 1A–C, Masson trichrome staining illustrated that we successfully established two mouse models of peritoneal fibrosis induced by 4.25% glucose PDF or 0.1% CG, as characterized by increased thickness of the sub-mesothelial area and a layer of mature fibrous tissue containing collagen and elastin fibers. The thickness and positive area of the sub-mesothelial area in PDF- or CG- injured mice were evidently greater than sham peritoneum with/without administration of 3-DZNeP. Administration of 3-DZNeP attenuated these pathological changes. These data indicate that exposure to high glucose PDF or CG contributes to peritoneal fibrosis, while 3-DZNeP is a potent agent for preventing development of peritoneal fibrosis.
Figure 2. Administration of 3-DZNeP inhibits EMT and improves functional impairments of peritoneal membrane in the high glucose PDF-injured peritoneum.
(A) Photomicrographs (200X) show Masson trichrome staining of the peritoneum in each group. (B) The graph shows the positive area of the Masson-positive submesothelial area (blue) from ten random fields of six mice peritoneal samples. (C) The graph shows the thickness of the compact zone measured from ten random fields of six mice peritoneal samples. (D) Peritoneum tissue lysates were prepared and subjected to immunoblot analysis with antibodies against α-SMA, Collagen I, E-cadherin, EZH2, H3K27me3, H3K9me3, Histone H3 or GAPDH. (E) Expression levels of α-SMA, Collagen I, E-cadherin and EZH2 were quantified by densitometry and normalized with GAPDH. (F) Expression levels of H3K27me3 and H3K9me3 were quantified by densitometry and normalized with Histone H3. (G) Co-immunofluorescence photomicrographs (200X) illustrate co-staining of α-SMA and EZH2 in the peritoneum from peritoneal fibrosis mice induced by high glucose PDF injection. (H) The dialysate-to-plasma (D/P) ratio of blood urea nitrogen (BUN). (I) Ratio of dialysate glucose at 2 hour after PDF injection to dialysate glucose at 0 hour (D/D0). Data are represented as the mean ± S.E.M (n = 6). Means with different superscript letters are significantly different from one another (P< 0.05). All scale bars = 20 μm.
Low levels of EZH2 and H3K27me3 were detected in sham peritoneum with/without administration of 3-DZNeP, and expression of each was predominantly increased after 4.25% glucose PDF or CG injection as indicated in Figure 2D–F and Supplemental Figure 1D, 1F, 1G. Administration of 3-DZNeP remarkably inhibited PDF or CG induced upregulation of EZH2 and H3K27me3. However, 3-DZNeP had no inhibitory effect on trimethylation of histone H3 at lysine 9 (H3K9me3) (Figure 2D, 2F). Furthermore, immunofluorescent co-staining of α-SMA and EZH2 showed that EZH2 is abundantly expressed in the α-SMA-positive cells (Figure 2G). Thus, these results suggest that EZH2-induced histone methylation is associated with peritoneal fibrosis.
Administration of 3-DZNeP suppresses EMT and improves dysfunction of peritoneal membrane in the fibrotic peritoneum of mice
Peritoneal fibrosis is characterized by EMT and pathological changes in submesothelial area with an increment of ECM proteins and myofibroblasts [33]. To assess the anti-fibrotic effect and mechanisms of 3-DZNeP, we examined the expression levels of α-SMA and Collagen I, two hallmarks of EMT and myofibroblasts, in two peritoneal fibrosis mouse models. Western blot and immunohistochemistry analysis showed that α-SMA was barely detected in sham peritoneum with/without 3-DZNeP treatment; its expression level was dramatically increased in the peritoneum after continuous exposure to PDF or CG. Administration of 3-DZNeP decreased its upregulation (Figure 2D, 2E and Supplemental Figure 1D, 1E). 3-DZNeP also inhibited excessive expression of Collagen I in injured peritoneum (Figure 2D, 2E and Supplemental Figure 1D, 1E).
Loss of E-cadherin, one of the selected adhesion molecules, contributes to separation of intercellular junctions of mesothelial cell (MCs), resulting in reorganization and migration of MCs [34,35]. In normal peritoneum, high level of E-cadherin was detected by Western blot analysis, while injury to the peritoneum reduced E-cadherin expression. Inhibition of EZH2 with 3-DZNeP restored E-cadherin expression to normal levels (Figure 2D, 2E and Supplemental Figure 1D, 1E). Taken together, these results suggest that 3-DZNeP may inhibit progression of peritoneal fibrosis by suppressing EMT. Furthermore, we also evaluated the functional alteration of peritoneal membrane by the urea nitrogen transport rate from plasma with the D/P ratio of BUN and the glucose absorption rate from dialysate with the ratio of D/D0. 3-DZNeP effectively improved high glucose PDF-associated peritoneal functional impairments by decreasing D/P ratio of BUN and increasing D/D0 ratio of glucose (Figure 2H, 2I).
Blockade of EZH2 inhibits activation of TGF-β/Smad, EGFR/Src and Notch1/Jagged-1 signaling pathways in the peritoneum exposed to high glucose peritoneal dialysate
TGF-β1/Smad, EGFR/Src and Notch1/Jagged-1 are three momentous pro-fibrotic signaling pathways involved in almost all of fibrosis diseases, including peritoneal fibrosis [7,9,10,36–39]. We thus examined whether EZH2 plays a role in the regulation of these signaling pathways in peritoneal fibrosis. We first examined the expression levels of TGF-β1 in each group by ELISA. Figure 3B showed that TGF-β1 level in peritoneal fibrosis was higher than in the control group and that 3-DZNeP treatment decreased this response. Immunoblotting results showed that 3-DZNeP suppressed TGF-βRI and p-Smad3 levels, and preserved Smad7 expression (Figure 3A, 3C, 3D). Inhibition of EZH2 with 3-DZNeP also dramatically reduced EGFR and Src phosphorylation as well as the ratio of p-EGFR/EGFR and p-Src/Src (Figure 3E, 3F). Furthermore, as shown in Figure 3G and 3H, the base levels of Notch1 and Jagged-1 were negligible in the peritoneum of sham operated animals. However, their expression levels were remarkably up-regulated in the injured peritoneum following 4.25% glucose PDF injection. Treatment with 3-DZNeP suppressed expression of Notch1 and Jagged-1. These data suggest that 3-DZNeP may suppress peritoneal fibrosis by inhibiting activation of several pro-fibrotic signaling pathways, including TGF-β1/Smad, EGFR/Src and Notch1/Jagged-1.
Figure 3. Inhibition of EZH2 suppresses TGF-β1/Smad3 and Notch1/Jagged-1 signaling pathway and inhibits phosphorylation of EGFR as well as Src in the fibrotic peritoneum.
(A) Peritoneum tissue lysates were prepared and subjected to immunoblot analysis with antibodies against TGF-βRI, p-Smad3, Smad3, Smad7, or GAPDH. (B) Peritoneal tissue lysates were subjected to the determination of TGF-β1 levels by the ELISA. (C) Expression levels of TGF-βRI and p-Smad3 were quantified by densitometry and normalized respectively with GAPDH and Smad3. (D) Expression level of Smad7 was quantified by densitometry and normalized with GAPDH. (E) The prepared peritoneum tissue lysates were subjected to immunoblot analysis with antibodies against p-EGFR, EGFR, p-Src, Src, or GAPDH. These proteins were quantified by densitometry. (F) p-EGFR and p-Src levels were normalized with their total protein levels. (G) Peritoneum tissue lysates were prepared and subjected to immunoblot analysis with antibodies against Notch1, Jagged-1 or GAPDH. (H) Expression levels of Notch1 and Jagged-1 were quantified by densitometry and normalized with GAPDH. Data are represented as the mean ± S.E.M (n = 6). Means with different superscript letters are significantly different from one another (P< 0.05).
Blockade of EZH2 ameliorates peritoneal fibrosis by abrogating inflammation, reducing lymphocyte and macrophage infiltration, and inhibiting angiogenesis in the injured peritoneum
NF-κB is a classic and fundamental signaling pathway in the regulation of inflammation, which leads to a number of fibrotic diseases, such as hepatic fibrosis [40], pulmonary fibrosis [41], periprosthetic osteolysis [42], renal interstitial fibrosis [12], and peritoneal fibrosis [8]. In vivo experiments showed that p-STAT3 was highly expressed in the peritoneum after PDF exposure, and treatment with 3-DZNeP reduced its expression level (Figure 4A, 4C). Figure 4B and 4C demonstrated that phosphorylated NF-κB level remained at a high standard in PDF-induced peritoneal fibrosis group compared with sham group. The ratio between p-NF-κB and total NF-κB was further decreased with 3-DZNeP treatment, supporting the conclusion that 3-DZNeP effectively prevents the development of peritoneal fibrosis by inhibiting pro-inflammatory signaling pathways. NF-κB signaling pathway activation is also essential for the presentation of multiple pro-inflammatory cytokines and chemokines such as IL-6, MCP-1, TNF-α and IL-1β. In Figure 4D, we examined the levels of those inflammatory factors by ELISA. Levels of four ELISA targets in the peritoneal fibrosis group were higher than in the control group, especially for MCP-1 (Figure 4D). Their levels in peritoneal tissue trended down after treatment with 3-DZNeP in 4.25% glucose PDF model of peritoneal fibrosis. These data suggest that inflammation plays an important role in the initiation and progression of peritoneal fibrosis, and 3-DZNeP can inhibit inflammation responses and prevent peritoneal fibrosis.
Figure 4. 3-DZNeP inhibits inflammation and angiogenesis as well as lymphocyte and macrophage infiltration in the model of PDF-induced peritoneal fibrosis.
(A, B) Peritoneum tissue lysates were subjected to immunoblot analysis with antibodies against p-STAT3, STAT3, p-NF-κB, NF-κB, or GAPDH. These proteins were quantified by densitometry. (C) p-STAT3 and p-NF-κB levels were normalized with their total protein levels. Peritoneal lysates were subjected to the ELISA as described under Materials and Methods. (D) The expression levels of IL-6, TNF-α, IL-1β and MCP-1 are indicated in each group. (E) Photomicrographs (200X) illustrate CD3 staining of the peritoneal tissues from sham-operated or 4.25% PDF-treated mice with/without 3-DZNeP administration. The count of CD3-positive cells was calculated from ten random fields of six mice peritoneal samples. (F) Photomicrographs (200X) illustrate CD68 staining of the peritoneal tissues. The count of CD68-positive cells was calculated from ten random fields of six mice peritoneal samples. (G) Photomicrographs (200X) illustrate VEGF staining of the peritoneal tissues. The count of VEGF-positive cells was calculated from ten random fields of six mice peritoneal samples. (H) Photomicrographs (200X) illustrate CD31 staining of the peritoneal tissues. The count of CD31-positive vessels was calculated from ten random fields of six mice peritoneal samples. Data are represented as the mean ± S.E.M (n = 6). Means with different superscript letters are significantly different from one another (P< 0.05). All scale bars = 20 μm.
T cells are shown to participate in the inflammatory response through the secretion of cytokines/chemokines and association with macrophages, which affects fibrosis by activating fibroblasts and fibrocytes [43–47]. Immune regulation has been shown to promote peritoneal fibrosis though inflammation [43,48]. In this study, we focused on the relationship between CD3+ T cells and peritoneal fibrosis and the impact of EZH2 inhibition on this immune regulatory mechanism. As shown in Figure 4E, CD3+ T cells were rarely expressed in sham peritoneum; PDF-induced peritoneal fibrosis led to an increase in the number of CD3+ T cells in the thickened submesothelial area. Blockade of EZH2 with 3-DZNeP mainly reduced the number of CD3 positive cells in the peritoneum (Figure 4E), suggesting that 3-DZNeP may prevent peritoneal fibrosis by reducing lymphocyte infiltration. We then used immunohistochemistry to examine the expression level of CD68, a bio-marker of macrophages, in each group. CD68 positive cells were rarely expressed in sham peritoneum with/without inhibitor. PDF-induced peritoneal fibrosis resulted in enhanced expression of CD68 positive cells in the thickened submesothelial area. Administration of 3-DZNeP reduced the number of CD68 positive cells in the injured peritoneum group (Figure 4F).
Peritoneal angiogenesis is a common and vital pathological change in long-term peritoneal dialysis and critically associated with peritoneal ultrafiltration failure [49,50]. Therefore, we evaluated the effect of 3-DZNeP administration on angiogenesis during peritoneal fibrosis by immunohistochemical staining of two angiogenetic markers, CD31 and VEGF. The number of CD31-positive vessels and VEGF-positive enothelial cells increased in fibrotic peritoneum. Inhibition of EZH2 with 3-DZNeP suppressed these populations of vessels and cells (Figure 4G, 4H). In summary, 3-DZNeP significantly reduces CD31-positive vessels and VEGF-positive cells in the fibrotic peritoneum, which provides the evidence that EZH2 is predominantly involved in peritoneal angiogenesis.
Inhibition of EZH2 with 3-DZNeP or siRNA suppresses TGF-β1-induced epithelial-to-mesenchymal transition in cultured human peritoneal mesothelial cells
Several studies have reported that TGF-β1 induced EMT plays a key role in the development of peritoneal fibrosis [8,10,51]. To further understand the role of EZH2 in peritoneal fibrosis, we examined the effect of 3-DZNeP or siRNA specifically targeting EZH2 on TGF-β1-induced EMT in cultured human peritoneal mesothelial cells (HPMCs). As shown in Supplemental Figure 2A, 2B and 2E, 2F, TGF-β1 significantly increased the expression of α-SMA and Collagen I and suppressed E-cadherin expression levels. Administration of 3-DZNeP inhibited upregulation of α-SMA and Collagen I and blocked downregulation of E-cadherin in a dose-dependent manner (Supplemental Figure 2A, 2B). EZH2 siRNA also had the similar inhibitory effects (Supplemental Figure 2E, 2F). ChIP assay results in Supplemental Figure 3A further demonstrated that EZH2 directly suppressed E-cadherin expression by trimethylation of histone H3 at lysine 27. In parallel, TGF-β1 exposure resulted in increased expression of EZH2 and H3K27me3, which was blocked by 3-DZNeP or EZH2 siRNA (Supplemental Figure 2C, 2D, 2G, 2H). Collectively, these data suggest that EZH2 activation is critically involved in the EMT of HPMCs.
Blockade of EZH2 with 3-DZNeP inhibits apoptosis and migration of HPMCs and down-regulates several pro-fibrotic signaling pathways in vitro
In order to examine the anti-apoptotic ability of 3-DZNeP, serum-starved HPMCs were exposed to peritoneal dialysis fluid containing 4.25% glucose for 36 hours in the presence or absence of different concentrations of 3-DZNeP. High glucose exposure induced cell apoptosis of HPMCs, while co-treatment with 3-DZNeP significantly decreased Cleaved caspase 3 and Bax expression levels and increased Bcl-2, an important apoptosis suppression gene (Supplemental Figure 3B–D). Wound-healing assay showed that 3-DZNeP has the ability to prevent cell migration and reduce the migratory rate of HPMCs (Supplemental Figure 3E, 3F). Moreover, treatment with 3-DZNeP or EZH2 siRNA also suppressed phosphorylation of Smad3 and EGFR, preserved Smad7 level and inhibited expression of Notch1 (Supplemental Figure 4 and Supplemental Figure 5). Collectively, these data suggest that EZH2 activation is critically involved in cell apoptosis and migration and above-mentioned pro-fibrotic signaling pathways of HPMCs.
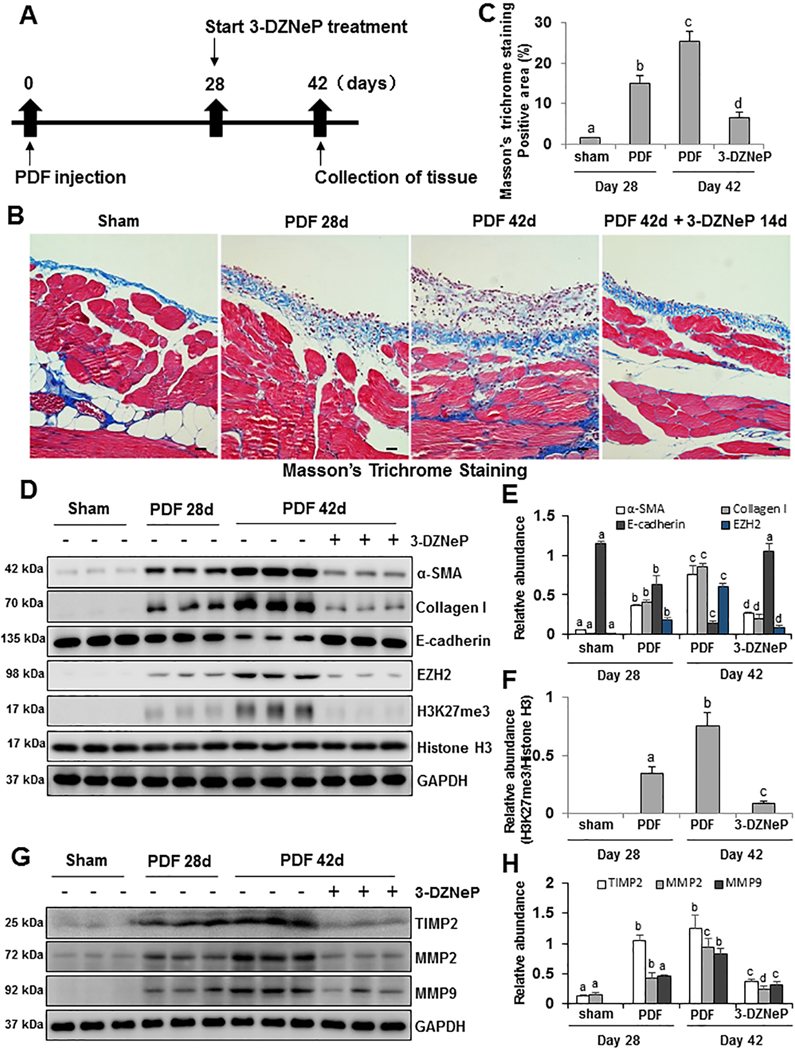
Delayed administration of 3-DZNeP attenuates and partially reverses the progression of peritoneal fibrosis induced by 4.25% glucose PDF
To explore the therapeutic effect of 3-DZNeP on the progression of peritoneal fibrosis, we designed an animal experiment with delayed treatment using 3-DZNeP (Figure 5A). 4.25% glucose PDF was injected daily for 42 days to establish a mouse model of peritoneal fibrosis, and 3-DZNeP was used at the beginning of 28 days, when peritoneal fibrosis had been formed with obvious thickened peritoneum tissue. At 42 days, animals were euthanized for collection of peritoneum and further analysis. The thickness of the submesothelial compact zone and Masson trichrome-positive area increased within 28 days and were further elevated at 42 days after continuous high glucose dialysate exposure. In comparison, peritoneal injury in mice treated with 3-DZNeP from day 28 onward was much milder than that of other two groups (Figure 5B, 5C).
Figure 5. Delayed treatment with 3-DZNeP attenuates and reverses progression of peritoneal fibrosis and suppresses EMT.
(A) Schematic of the experimental design for delayed treatment with 3-DZNeP. To investigate the therapeutic effect of 3-DZNeP on peritoneal fibrosis mice models, 3-DZNeP was administered starting at 28 days after injection with 4.25% high glucose dialysate and then given daily for 14 days. At 42 days, animals were euthanized for collection of peritoneum. (B) Photomicrographs (200X) show Masson trichrome staining of the peritoneum in each group. (C) The graph shows the positive area of the Masson-positive submesothelial area (blue) from ten random fields of six mice peritoneal samples. (D) Peritoneum tissue lysates were subjected to immunoblot analysis with specific antibodies against α-SMA, Collagen I, E-cadherin, EZH2, H3K27me3, Histone H3 or GAPDH. (E) Expression levels of α-SMA, Collagen I, E-cadherin and EZH2 were quantified by densitometry and normalized with GAPDH. (F) Expression level of H3K27me3 was quantified by densitometry and normalized with Histone H3. (G) Peritoneum tissue lysates were prepared and subjected to immunoblot analysis with antibodies against TIMP2, MMP2, MMP9, or GAPDH. (H) Expression levels of TIMP2, MMP2 and MMP9 were quantified by densitometry and normalized with GAPDH. Data are represented as the mean ± S.E.M (n = 6). Means with different superscript letters are significantly different from one another (P< 0.05). All scale bars = 20 μm.
To confirm this observation, we further examined the expression levels of α-SMA, Collagen I, E-cadherin, EZH2, H3K27me3 and Histone H3 by immunoblot analysis. 4.25% glucose PDF injection for the first 28 days led to increased expression of α-SMA, Collagen I, EZH2 and H3K27me3; continuous daily injection of PDF until 42 days led to further increased expression of α-SMA, Collagen I, EZH2 and H3K27me3. In contrast, late treatment with 3-DZNeP from day 28 to day 42 reduced their expression levels below their levels at day 28 (Figure 5D–F). Conversely, 4.25% glucose PDF injection decreased E-cadherin expression over time, whereas delayed administration of 3-DZNeP not only prevented further reduction of E-cadherin expression from day 28 to day 42, but also restored its expression to the normal level (Figure 5D, 5E).
Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that hydrolyze ECM components. Among them, MMP2 and MMP9, two specific type IV collagenases, have the ability to induce degradation of collagen IV and destroy the integrity of the basement membrane [52–54]. A recent study reported that absence of MMP2 in MMP2 deficient mice protects obstructed kidney against hydronephrosis and renal fibrosis during UUO [55]. As demonstrated, the presence of Tissue inhibitors of metalloproteinase 2 (TIMP2) is essential for the cell surface activation of pro-MMP2 in the kidney upon injury [56]. TIMP2 can interact with membrane type 1 MMP and pro-MMP2 to its active form [57]. TIMP2 increases the MMP2/9 expression and regulates the degradation of ECM protein in fibrotic diseases [56,58,59]. Continuous daily injection of PDF until day 42 induced higher expression levels of TIMP2, MMP2 and MMP9 than at day 28. Delayed administration of 3-DZNeP attenuated TIMP2, MMP2 and MMP9 levels below those seen at day 28 (Figure 5G, 5H). Taken together, our results illustrate that inhibition of EZH2 with 3-DZNeP slows progression of peritoneal fibrosis, and down-regulation of TIMP2, MMP2 and MMP9 may become a key mechanism for 3-DZNeP to ameliorate peritoneal fibrosis.
EZH2-KO mice develop less peritoneal fibrosis compared with EZH2-WT mice
EZH2 knockout mice were generated by breeding our EZH2loxP/loxP mice with tamoxifen-inducible Col1a2-Cre mice (Col1a2-CreER+/−) and then backcrossing progeny to EZH2loxP/loxP mice to obtain Col1a2-Cre+: EZH2loxP/loxP mice (EZH2-KO) and Col1a2-Cre-: EZH2loxP/loxP mice (EZH2-WT). After daily intraperitoneal injection of 100ml/kg peritoneal dialysis solution with 4.25% glucose for 28 days, we found that the thickness and positive area of the submesothelial area in EZH2-WT mice were evidently greater than EZH2-KO mice as shown in Masson trichrome staining (Figure 6A–C). Western blot analysis showed that α-SMA and Collagen I were barely detected in sham peritoneum of EZH2-WT and EZH2-KO mice, while their expression levels were dramatically increased in the EZH2-WT mice after continuous exposure to high glucose peritoneal dialysate compared with EZH2-KO (Figure 6D, 6E). Furthermore, we also detected the levels of EZH2 and H3K27me3 in both EZH2-WT or KO mice with/without PDF injection. Results in Figure 6D–F showed that low levels of EZH2 and H3K27me3 were detected in sham peritoneum with/without EZH2 gene knockout, and their levels were remarkably increased after 4.25% PDF injection in EZH2-WT mice, but not obvious in EZH2-KO mice. Similarly, EZH2-KO mice showed lower expression levels of TIMP2, MMP2 and MMP9 than EZH2-WT group after 4.25% PDF injection for 28 days (Figure 6G, 6H). Taken together, these results further reiterate the importance of EZH2 in peritoneal fibrosis, and genetic blockage of EZH2 attenuates the development of peritoneal fibrosis.
Figure 6. Comparison of peritoneal fibrosis in EZH2-WT and EZH2-KO mice after high glucose PDF injury.
EZH2 knockout mice were generated by breeding our EZH2loxP/loxP mice with tamoxifen-inducible Col1a2-Cre mice (Col1a2-CreER+/−) and then backcrossing progeny to EZH2loxP/loxP mice to obtain Col1a2-Cre+: EZH2loxP/loxP mice (EZH2-KO) and Col1a2-Cre-: EZH2loxP/loxP mice (EZH2-WT). Both of EZH2-KO and EZH2-WT mice were received daily intraperitoneal injection of 100ml/kg peritoneal dialysis solution with 4.25% glucose for 28 days to establish peritoneal fibrosis model. (A) Photomicrographs (200X) show Masson trichrome staining of the peritoneum in each group. (B) The graph shows the positive area of the Masson-positive submesothelial area (blue) from ten random fields of six mice peritoneal samples. (C) The graph shows the thickness of the compact zone measured from ten random fields of six mice peritoneal samples. (D) Peritoneum tissue lysates were prepared and subjected to immunoblot analysis with antibodies against α-SMA, Collagen I, EZH2, H3K27me3, Histone H3 or GAPDH. (E) Expression levels of α-SMA, Collagen I and EZH2 were quantified by densitometry and normalized with GAPDH. (F) Expression level of H3K27me3 was quantified by densitometry and normalized with Histone H3. (G) Peritoneum tissue lysates were prepared and subjected to immunoblot analysis with antibodies against TIMP2, MMP2, MMP9, or GAPDH. (H) Expression levels of TIMP2, MMP2 and MMP9 were quantified by densitometry and normalized with GAPDH. Data are represented as the mean ± S.E.M (n = 6). Means with different superscript letters are significantly different from one another (P< 0.05). All scale bars = 20 μm.
Discussion
Peritoneal fibrosis is one of the most significant complications for CAPD patients, and yet there targeted solution so far [2]. Here we demonstrated that pharmacological inhibition of EZH2 with 3-DZNeP or genetic deletion of EZH2 attenuated peritoneal fibrosis. 3-DZNeP effectively improved high glucose PDF-associated peritoneal functional impairments, as evidenced by decreasing D/P ratio of BUN and increasing D/D0 ratio of glucose. The antifibrotic effect of EZH2 inhibition is associated with blocking several profibrotic signaling pathways, including, TGF-β/Smad, Notch, EGFR, Src. Delayed administration of 3-DZNeP 14 days subsequent to injury also inhibits peritoneal fibrosis progression and partially reverses the established peritoneal fibrosis. Thus, to our knowledge, this is the first study showing that EZH2 plays a pivotal role in the development and progression of peritoneal fibrosis, suggesting that inhibition of EZH2 may be a potential therapeutic strategy for prevention and treatment of peritoneal fibrosis in patients with long term PD.
EZH2 has been recognized as a critical mediator of carcinogenesis [22]. Its overexpression was observed in many epithelial cancers [60,61], and mediated poor prognosis in several cancers including renal cell carcinoma [60]. This implicates that EZH2 is not only a therapeutic target, but also a tumor biomarker. In this study, we demonstrated that EZH2 was also highly present in the dialysis effluent of PD patients associated with inflammation and elevated over dialysis time. Meanwhile, its expression levels were positively correlated with expression of several profibrotic growth factors, including TGF-β1, VEGF, IL-6. In addition, EZH2 was also overexpressed in the peritoneum of peritoneal fibrosis induced by CG or PDF in mice. These data suggest that EZH2 may be a biomarker of peritoneal fibrosis, but further investigation is needed. Currently, the source of EZH2 in the dialysis effluents remains unclear. We assume that it may come from detached peritoneal mesothelial and inflammatory cells. In support of this hypothesis, our immunological staining showed that EZH2 is highly expressed in the mesothelial layer of the injured peritoneum in two animal models and increased expression of EZH2 is detected in cultured human peritoneal mesothelial cells.
EZH2 may contribute to peritoneal fibrosis through induction of peritoneal EMT and activation of fibroblasts. Our data show that an abundance of EZH2 is not only expressed in the mesothelial layer, but also in submesothelial zone in two models of peritoneal fibrosis. In support to the role of EZH2 in mediating renal fibroblast activation, we found that EZH2 is co-stained with α-SMA, a hallmark of myofibroblasts in the submesothelial zone, and administration of 3-DZNeP and specific depletion of EZH2 inhibited expression of α-SMA and deposition of ECM proteins in the peritoneum of those two models. In addition, we found that treatment with 3-DZNeP effectively restored E-cadherin levels toward the baseline in injured peritoneum as well as in cultured human peritoneal mesothelial cells exposed to TGF-β1. ChIP assay results also illustrated that EZH2 directly suppresses E-cadherin expression by trimethylation of histone H3 at lysine 27. This provides evidence for the role of EZH2 in mediating peritoneal EMT. Since fibroblast activation and EMT are two primary cellular events leading to production of ECM protein, their effective inhibition by pharmacological and genetic inhibition of EZH2 suggest that EZH2 is a key epigenetic regulator promoting peritoneal fibrosis.
How does EZH2 mediate activation of fibroblasts and EMT in peritoneal fibrosis? A number of studies demonstrated that TGF-β1 is a prototypical inducer of EMT and a key molecule in PD fluid-induced peritoneal membrane deterioration [4,62]. In this study, we demonstrated that TGF-βRI expression is subject to the epigenetic regulation of EZH2. EZH2-mediated H3K27me3 may not directly bind to the TGF-βRI promoter and increase its expression [11]. Because H3K27me3 tends to form a compact chromatin structure and leads to suppression rather than facilitation of gene expression [63]. Thus, we speculated that EZH2 may regulate the profibrotic gene expression through a histone methyltransferase independent mechanism or silencing of antifibrotic genes, such as peroxisome proliferator-activated receptor γ (PPARγ) [64]. On the other hand, the translocation of phosphorylated Smads from the cytoplasm into the nucleus is considered as a rate-limiting step in TGF-β signal transduction. It was reported that EZH2 interacted with nucleoporins would increase Smads accumulation in the nucleus, which promotes TGF-β signaling reversely [65]. Furthermore, EZH2 inhibition preserves Smad7 expression. Smad7 not only inhibits Smad3 phosphorylation [11], but also acts as a scaffold protein to recruit Smad ubiquitin regulatory factor 2 (Smurf2), an E3 ubiquitin ligase, to the TGF-β receptor complex to facilitate its ubiquitination and change into a degradable state [66]. Given the important role of EZH2 in the regulation of TGF-β/Smad signaling, we suggest a potential benefit from the clinical trial of treatment of peritoneal fibrosis by EZH2 inhibition.
Furthermore, attenuation of peritoneal fibrosis by EZH2 suppression may also be associated with inhibition of EGFR signaling [8]. This is evidenced by our observations that inhibition of EZH2 by 3-DZNeP reduces EGFR and Src phosphorylation. Since EZH2 inhibition increases the expression of phosphatase and tensin homolog (PTEN), a protein previously associated with dephosphorylation of tyrosine kinase receptors [11], it is speculated that EZH2 inhibition also suppresses EGFR signaling by facilitating PTEN expression. In addition, it has been documented that peritoneal fibrosis is regulated by Notch signaling pathways [7], and EZH2 can regulate Notch1 transcriptional activation by directly binding to the Notch1 promoter and inhibiting Notch suppressor [67,68]. In line with this observation, we found that EZH2 inhibition suppressed expression of Notch1 and its ligand, Jagged-1 in the injured peritoneum.
Inflammation is a driving force for progression of peritoneal fibrosis [8]. Activation of transcriptional factors such as STAT3 and NF-κB promotes inflammatory response during the pathogenesis of peritoneal fibrosis [69,70]. Our study indicated that EZH2 activity is required for phosphorylation of STAT3. In support of this notion, Kim et al. have reported that EZH2 can bind to and methylate STAT3, leading to enhanced STAT3 activity by increasing its tyrosine phosphorylation [21]. However, the mechanism involved STAT3 methylation-induced phosphorylation increment is still obscure. Given the observation that STAT3 acetylation can prevent its dephosphorylation [71]. We speculated that methylation may protect STAT3 from dephosphorylation. EZH2 is also associated with activation of NF-κB signaling and expression of its target genes (IL-6, IL-8, TNF-α) [72]. EZH2 can also control macrophage inflammatory polarization via PAK1-dependent NF-κB signaling [73]. Furthermore, EZH2 is required for lymphocyte development and promotion of T cell differentiation by inhibiting corresponding transcription factors [74,75]. In support of the role of EZH2 in the inflammatory response, we found that inhibition of EZH2 with 3-DZNeP suppresses phosphorylation of STAT3 and NF-κB brought on by high glucose dialysate, infiltration of macrophages and lymphocytes and increased expression of multiple pro-inflammatory cytokines and chemokines. In line with these results, a high level of EZH2 was detected in the dialysis effluent of patients with long-term PD and positively correlated with expression of IL-6, a cytokine that links peritoneal inflammation to angiogenesis in the peritoneal membrane [76]. This suggests the importance of EZH2 in regulating peritoneal inflammation and angiogenesis.
Angiogenesis is also an important mediator in the process of peritoneal fibrosis and associated with peritoneal ultrafiltration failure [49,50]. Our study demonstrated that EZH2 inhibition significantly reduced angiogenesis, as evidenced by decrease of CD31 and VEGF positive cells in the fibrotic peritoneum treated with 3-DZNeP. Mechanistically, EZH2 has been reported to increase H3K27me3 activity on regulatory regions of endothelial nitric oxide synthase (eNOS) and brain-derived neurotrophic factor (BDNF) promoters. Both eNOS and BDNF are highly expressed in endothelial cells and promote vascular development and endothelial cell proliferation [77]. In addition, since IL-6 is able to promote VEGF expression and new vessel formation, EZH2 inhibition may also inhibit peritoneal angiogenesis through suppression of IL-6 production. In this regard, we observed that EZH2 expression is positively correlated with IL-6 levels in the dialysis effluents of long-term PD patients. Thus, EZH2 blockade with 3-DZNeP could be a potential therapeutic strategy for inhibiting vascular proliferation and protecting the filtration function of peritoneum in patients with long term PD.
Our data showed that delayed administration of 3-DZNeP attenuates the progression of peritoneal fibrosis and partially reverses the established peritoneal fibrosis. The delayed administration of 3-DZNeP may elicit a peritoneal protective effect through its effects on MMPs. Of the MMPs, MMP2 and MMP9 are best known for their ability to degrade basement membrane components, laminin and type Ⅳ collagen, disrupt cell-cell or cell-matrix attachment, facilitate the EMT and aggravate fibrosis [52,78]. Numerous investigations have proved that STAT3 is the upstream signaling molecule for MMP2 [79,80]. Intriguingly, we and other group have found that inhibition of EZH2 activity can suppress STAT3 phosphorylation [60]. Therefore, we speculated that EZH2 blockade suppressed the MMP2 by inhibiting the EZH2-STAT3 signaling axis. In addition, genome wide approaches suggest that EZH2 targets Fosl1 and Klf5, two activators of MMP9 [54]. In accordance with this observation, we found that EZH2 inhibition decreases MMP9 level. As such, down-regulation of MMP2 and MMP9 caused by the delayed administration of 3-DZNeP would partially reverse peritoneal fibrosis
Increasing evidence has indicated that EZH2 is a critical mediator in tumorgenesis and has become an effective target for the treatment of some tumors [19,24,61,81]. We and others have shown in previous studies that EZH2 plays an essential role in mediating tissue fibrosis in several organs including liver, lung and kidney [11,65,82]. In the current study, we provided strong evidence that EZH2 is critically involved in the development and progression of peritoneal fibrosis. Although 3-DZNeP was used to inhibit tissue fibrosis in most studies, other EZH2 inhibitors have also been synthesized and used in the treatment of tumors in either preclinical studies or clinical trials [83,84]. We look forward to a possible benefit from a clinical trial of treatment of PD related to peritoneal fibrosis by EZH2 inhibition.
In conclusion, we demonstrated that genetic or pharmacologic blockade of EZH2 can prevent and reverse peritoneal fibrosis. Inhibition of EZH2 with 3-DZNeP effectively improved high glucose PDF-associated peritoneal functional impairments. Mechanistically, EZH2 inhibition protected the peritoneal membrane from fibrosis by blocking EMT, fibroblast activation, inflammatory responses and angiogenesis though inactivation of multiple pro-fibrogenic signaling pathways. As such, blocking EZH2 may have a potential therapeutic benefit in preventing and treating patients with peritoneal fibrosis on long term PD.
Supplementary Material
Acknowledgements
This study was supported by the National Nature Science Foundation of China grants (81670690, 81470991 and 81200492 to N.L., 81270778, 81470920, 81670623 and 81830021 to S.Z.), the Key Discipline Construction Project of Pudong Health Bureau of Shanghai (PWZxk2017-05 to N.L.), the Branch grant of National key grants of Ministry of Science and Technology (2018YFA0108802 to S.Z.), the US National Institutes of Health (2R01DK08506505A1 to S.Z.), the Shanghai Scientific Committee of China (13PJ1406900 to N.L.).
We thank Dr. George Bayliss for critically reading and editing this manuscript.
References
- 1.Liu FX, Gao X, Inglese G, et al. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit Dial Int 2015; 35: 406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakayama M, Zhu WJ, Watanabe K, et al. Dissolved molecular hydrogen (H2) in Peritoneal Dialysis (PD) solutions preserves mesothelial cells and peritoneal membrane integrity. BMC Nephrol 2017; 18: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxena AB. Recent advances in the management of peritoneal dialysis patients. F1000Prime Rep 2015; 7: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Q, Bajo MA, Del Peso G, et al. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int 2016; 90: 515–524. [DOI] [PubMed] [Google Scholar]
- 5.Yao Q, Pawlaczyk K, Ayala ER, et al. The role of the TGF/Smad signaling pathway in peritoneal fibrosis induced by peritoneal dialysis solutions. Nephron Exp Nephrol 2008; 109: e71–78. [DOI] [PubMed] [Google Scholar]
- 6.Padwal M, Margetts PJ. Experimental systems to study the origin of the myofibroblast in peritoneal fibrosis. Kidney Res Clin Pract 2016; 35: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu F, Li T, Qiu F, et al. Preventive effect of Notch signaling inhibition by a gamma-secretase inhibitor on peritoneal dialysis fluid-induced peritoneal fibrosis in rats. Am J Pathol 2010; 176: 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Liu N, Xiong C, et al. Inhibition of EGF Receptor Blocks the Development and Progression of Peritoneal Fibrosis. J Am Soc Nephrol 2016; 27: 2631–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Wang L, Xu L, et al. Targeting Src attenuates peritoneal fibrosis and inhibits the epithelial to mesenchymal transition. Oncotarget 2017; 8: 83872–83889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, Liu N, Gu H, et al. Histone deacetylase 6 inhibition counteracts the epithelial–mesenchymal transition of peritoneal mesothelial cells and prevents peritoneal fibrosis. Oncotarget 2017; 8: 88730–88750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou X, Zang X, Ponnusamy M, et al. Enhancer of Zeste Homolog 2 Inhibition Attenuates Renal Fibrosis by Maintaining Smad7 and Phosphatase and Tensin Homolog Expression. J Am Soc Nephrol 2016; 27: 2092–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu N, Wang L, Yang T, et al. EGF Receptor Inhibition Alleviates Hyperuricemic Nephropathy. J Am Soc Nephrol 2015; 26: 2716–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016; 44: 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinashi H, Ito Y, Mizuno M, et al. TGF-beta1 promotes lymphangiogenesis during peritoneal fibrosis. J Am Soc Nephrol 2013; 24: 1627–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KH, Ryu HM, Oh SH, et al. Effect of DNA demethylation in experimental encapsulating peritoneal sclerosis. Ther Apher Dial 2014; 18: 628–636. [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Fang J. Epigenetic regulation of epithelial-mesenchymal transition. Cell Mol Life Sci 2016; 73: 4493–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fardi M, Solali S, Farshdousti Hagh M. Epigenetic mechanisms as a new approach in cancer treatment: An updated review. Genes Dis 2018; 5: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crews D, Gillette R, Miller-Crews I, et al. Nature, nurture and epigenetics. Mol Cell Endocrinol 2014; 398: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Wu Q, Li L. Functional and therapeutic significance of EZH2 in urological cancers. Oncotarget 2017; 8: 38044–38055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Q, Yu J, Dhanasekaran SM, et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 2008; 27: 7274–7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim E, Kim M, Woo DH, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell 2013; 23: 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi H, Hung MC. Regulation and Role of EZH2 in Cancer. Cancer Res Treat 2014; 46: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan W, Herman JG, Guo M. Epigenome-based personalized medicine in human cancer. Epigenomics 2016; 8: 119–133. [DOI] [PubMed] [Google Scholar]
- 24.Kondo Y. Targeting histone methyltransferase EZH2 as cancer treatment. J Biochem 2014; 156: 249–257. [DOI] [PubMed] [Google Scholar]
- 25.Uchiyama N, Tanaka Y, Kawamoto T. Aristeromycin and DZNeP cause growth inhibition of prostate cancer via induction of mir-26a. Eur J Pharmacol 2017; 812: 138–146. [DOI] [PubMed] [Google Scholar]
- 26.Zeybel M, Luli S, Sabater L, et al. A Proof-of-Concept for Epigenetic Therapy of Tissue Fibrosis: Inhibition of Liver Fibrosis Progression by 3-Deazaneplanocin A. Mol Ther 2017; 25: 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Xiong C, Tolbert E, et al. Targeting histone methyltransferase enhancer of zeste homolog-2 inhibits renal epithelial-mesenchymal transition and attenuates renal fibrosis. Faseb j 2018: fj201800237R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokubo S, Sakai N, Furuichi K, et al. Activation of p38 Mitogen-Activated Protein Kinase Promotes Peritoneal Fibrosis by Regulating Fibrocytes. Perit Dial Int 2012; 32: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Io K, Nishino T, Obata Y, et al. SAHA Suppresses Peritoneal Fibrosis in Mice. Perit Dial Int 2015; 35: 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang M, Kothapally J, Mao H, et al. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 2009; 297: F996–f1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ditsawanon P, Supasyndh O, Aramwit P. Dialysate cancer antigen 125 in long-term peritoneal dialysis patients. Clin Exp Nephrol 2014; 18: 10–15. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y, Yan H, Yuan J, et al. Different patterns of inflammatory and angiogenic factors are associated with peritoneal small solute transport and peritoneal protein clearance in peritoneal dialysis patients. BMC Nephrol 2018; 19: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Dong Z, Liu H, et al. Transition of Mesothelial Cell to Fibroblast in Peritoneal Dialysis: EMT, Stem Cell or Bystander? Perit Dial Int 2015; 35: 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aroeira LS, Aguilera A, Sanchez-Tomero JA, et al. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol 2007; 18: 2004–2013. [DOI] [PubMed] [Google Scholar]
- 35.Lee YC, Hung SY, Liou HH, et al. Vitamin D Can Ameliorate Chlorhexidine Gluconate-Induced Peritoneal Fibrosis and Functional Deterioration through the Inhibition of Epithelial-to-Mesenchymal Transition of Mesothelial Cells. Biomed Res Int 2015; 2015: 595030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu FX, Gao X, Inglese G, et al. A Global Overview of the Impact of Peritoneal Dialysis First or Favored Policies: An Opinion. Perit Dial Int 2015; 35: 406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Chen JK, Nagai K, et al. EGFR signaling promotes TGFbeta-dependent renal fibrosis. J Am Soc Nephrol 2012; 23: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Lee JY, Lee CM, et al. Amphiregulin, an Epidermal Growth Factor Receptor Ligand, Plays an Essential Role in the Pathogenesis of Transforming Growth Factor-β-induced Pulmonary Fibrosis. J Biol Chem 2012; 287: 41991–42000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komposch K, Sibilia M. EGFR Signaling in Liver Diseases. Int J Mol Sci 2016; 17: e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luedde T, Schwabe RF. NF-κB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011; 8: 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian B, Zhao Y, Sun H, et al. BRD4 mediates NF-κB-dependent epithelial-mesenchymal transition and pulmonary fibrosis via transcriptional elongation. Am J Physiol Lung Cell Mol Physiol 2016; 311: L1183–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin TH, Tamaki Y, Pajarinen J, et al. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Biomater 2014; 10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González-Mateo GT, Fernández-Míllara V, Bellón T, et al. Paricalcitol reduces peritoneal fibrosis in mice through the activation of regulatory T cells and reduction in IL-17 production. PLoS One 2014; 9: e108477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi N, Kopec AK, Cline-Fedewa H, et al. Lymphocytes contribute to biliary injury and fibrosis in experimental xenobiotic-induced cholestasis. Toxicology 2017; 377: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wirsdörfer F, Jendrossek V. The Role of Lymphocytes in Radiotherapy-Induced Adverse Late Effects in the Lung. Front Immunol 2016; 7: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nogueira A, Pires MJ, Oliveira PA. Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. In Vivo 2017; 31: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008; 214: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liappas G, González-Mateo GT, Sánchez-Díaz R, et al. Immune-Regulatory Molecule CD69 Controls Peritoneal Fibrosis. J Am Soc Nephrol 2016; 27: 3561–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanabe K, Maeshima Y, Ichinose K, et al. Endostatin peptide, an inhibitor of angiogenesis, prevents the progression of peritoneal sclerosis in a mouse experimental model. Kidney Int 2007; 71: 227–238. [DOI] [PubMed] [Google Scholar]
- 50.Yoshio Y, Miyazaki M, Abe K, et al. TNP-470, an angiogenesis inhibitor, suppresses the progression of peritoneal fibrosis in mouse experimental model. Kidney Int 2004; 66: 1677–1685. [DOI] [PubMed] [Google Scholar]
- 51.Ueno T, Nakashima A, Doi S, et al. Mesenchymal stem cells ameliorate experimental peritoneal fibrosis by suppressing inflammation and inhibiting TGF-beta1 signaling. Kidney Int 2013; 84: 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chien YC, Liu LC, Ye HY, et al. EZH2 promotes migration and invasion of triple-negative breast cancer cells via regulating TIMP2-MMP-2/−9 pathway. Am J Cancer Res 2018; 8: 422–434. [PMC free article] [PubMed] [Google Scholar]
- 53.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta 2012; 1825: 29–36. [DOI] [PubMed] [Google Scholar]
- 54.Delgado-Olguin P, Dang LT, He D, et al. Ezh2-mediated repression of a transcriptional pathway upstream of Mmp9 maintains integrity of the developing vasculature. Development 2014; 141: 4610–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tveitarås MK, Skogstrand T, Leh S, et al. Matrix Metalloproteinase-2 Knockout and Heterozygote Mice Are Protected from Hydronephrosis and Kidney Fibrosis after Unilateral Ureteral Obstruction. PLoS One 2015; 10: e0143390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Famulski K, Lee J, et al. TIMP2 and TIMP3 have divergent roles in early renal tubulointerstitial injury. Kidney Int 2014; 85: 82–93. [DOI] [PubMed] [Google Scholar]
- 57.Hernandez-Barrantes S, Toth M, Bernardo MM, et al. Binding of active (57 kDa) membrane type 1-matrix metalloproteinase (MT1-MMP) to tissue inhibitor of metalloproteinase (TIMP)-2 regulates MT1-MMP processing and pro-MMP-2 activation. J Biol Chem 2000; 275: 12080–12089. [DOI] [PubMed] [Google Scholar]
- 58.Li XM, Peng JH, Sun ZL, et al. Chinese medicine CGA formula ameliorates DMN-induced liver fibrosis in rats via inhibiting MMP2/9, TIMP1/2 and the TGF-beta/Smad signaling pathways. Acta Pharmacol Sin 2016; 37: 783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pitiyage GN, Lim KP, Gemenitzidis E, et al. Increased secretion of tissue inhibitors of metalloproteinases 1 and 2 (TIMPs −1 and −2) in fibroblasts are early indicators of oral sub-mucous fibrosis and ageing. J Oral Pathol Med 2012; 41: 454–462. [DOI] [PubMed] [Google Scholar]
- 60.Zhang D, Yang XJ, Luo QD, et al. EZH2 enhances the invasive capability of renal cell carcinoma cells via activation of STAT3. Mol Med Rep 2018; 17: 3621–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Völkel P, Dupret B, Le Bourhis X, et al. Diverse involvement of EZH2 in cancer epigenetics. Am J Transl Res 2015; 7: 175–193. [PMC free article] [PubMed] [Google Scholar]
- 62.Loureiro J, Aguilera A, Selgas R, et al. Blocking TGF-beta1 protects the peritoneal membrane from dialysate-induced damage. J Am Soc Nephrol 2011; 22: 1682–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laugesen A, Hojfeldt JW, Helin K. Molecular Mechanisms Directing PRC2 Recruitment and H3K27 Methylation. Mol Cell 2019; 74: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mann J, Chu DC, Maxwell A, et al. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology 2010; 138: 705–714, 714.e701–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao X, Senavirathna LK, Gou X, et al. EZH2 enhances the differentiation of fibroblasts into myofibroblasts in idiopathic pulmonary fibrosis. Physiol Rep 2016; 4: e12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eichhorn PJ, Rodon L, Gonzalez-Junca A, et al. USP15 stabilizes TGF-beta receptor I and promotes oncogenesis through the activation of TGF-beta signaling in glioblastoma. Nat Med 2012; 18: 429–435. [DOI] [PubMed] [Google Scholar]
- 67.Zheng X, Pang B, Gu G, et al. Melatonin Inhibits Glioblastoma Stem-like cells through Suppression of EZH2-NOTCH1 Signaling Axis. Int J Biol Sci 2017; 13: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez ME, Moore HM, Li X, et al. EZH2 expands breast stem cells through activation of NOTCH1 signaling. Proc Natl Acad Sci U S A 2014; 111: 3098–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li PK, Ng JK, McIntyre CW. Inflammation and Peritoneal Dialysis. Semin Nephrol 2017; 37: 54–65. [DOI] [PubMed] [Google Scholar]
- 70.Dai T, Wang Y, Nayak A, et al. Janus kinase signaling activation mediates peritoneal inflammation and injury in vitro and in vivo in response to dialysate. Kidney Int 2014; 86: 1187–1196. [DOI] [PubMed] [Google Scholar]
- 71.Kramer OH, Knauer SK, Greiner G, et al. A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev 2009; 23: 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee ST, Li Z, Wu Z, et al. Context-specific regulation of NF-kappaB target gene expression by EZH2 in breast cancers. Mol Cell 2011; 43: 798–810. [DOI] [PubMed] [Google Scholar]
- 73.Zhang W, Liu H, Liu W, et al. Polycomb-mediated loss of microRNA let-7c determines inflammatory macrophage polarization via PAK1-dependent NF-kappaB pathway. Cell Death Differ 2015; 22: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen G, Subedi K, Chakraborty S, et al. Ezh2 Regulates Activation-Induced CD8(+) T Cell Cycle Progression via Repressing Cdkn2a and Cdkn1c Expression. Front Immunol 2018; 9: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacobsen JA, Woodard J, Mandal M, et al. EZH2 Regulates the Developmental Timing of Effectors of the Pre-Antigen Receptor Checkpoints. J Immunol 2017; 198: 4682–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Catar R, Witowski J, Zhu N, et al. IL-6 Trans-Signaling Links Inflammation with Angiogenesis in the Peritoneal Membrane. J Am Soc Nephrol 2017; 28: 1188–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitic T, Caporali A, Floris I, et al. EZH2 modulates angiogenesis in vitro and in a mouse model of limb ischemia. Mol Ther 2015; 23: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol 2011; 12: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim DJ, Chan KS, Sano S, et al. Signal transducer and activator of transcription 3 (Stat3) in epithelial carcinogenesis. Mol Carcinog 2007; 46: 725–731. [DOI] [PubMed] [Google Scholar]
- 80.Qiu Z, Huang C, Sun J, et al. RNA interference-mediated signal transducers and activators of transcription 3 gene silencing inhibits invasion and metastasis of human pancreatic cancer cells. Cancer Sci 2007; 98: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christofides A, Karantanos T, Bardhan K, et al. Epigenetic regulation of cancer biology and anti-tumor immunity by EZH2. Oncotarget 2016; 7: 85624–85640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin-Mateos R, De Assuncao TM, Arab JP, et al. Enhancer of Zeste Homologue 2 Inhibition Attenuates TGF-beta Dependent Hepatic Stellate Cell Activation and Liver Fibrosis. Cell Mol Gastroenterol Hepatol 2019; 7: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurmasheva RT, Sammons M, Favours E, et al. Initial testing (stage 1) of tazemetostat (EPZ-6438), a novel EZH2 inhibitor, by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer 2017; 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Italiano A, Soria JC, Toulmonde M, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 2018; 19: 649–659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.