Abstract
In around 10% of SARS-CoV-2 infected patients, coronavirus disease-2019 (Covid-19) symptoms are complicated with a severe lung damage called Acute Respiratory Distress Syndrome (ARDS), which is often lethal. ARDS is mainly associated with an uncontrolled overproduction of immune cells and cytokines, called “cytokine storm syndrome”; it appears 7–15 days following the onset of symptoms, leading to systemic inflammation and multiple organ failure. Because they are well-known metabolic precursors of specialized pro-resolving lipid mediators (SPMs), omega-3 long-chain polyunsaturated fatty acids (omega-3 LC-PUFAs) could help improve the resolution of the inflammatory balance, limiting therefore the level and duration of the critical inflammatory period. Omega-3 LC-PUFAs may also interact at different stages of the viral infection, notably on the virus entry and replication. In the absence of demonstrated treatment and while waiting for vaccine possibility, the use of omega-3 LC-PUFAs deserve therefore to be considered, based on previous clinical studies suggesting that omega-3 supplementation could improve clinical outcomes of critically ill patients at the acute phase of ARDS. In this context, it is crucial to remind that the omega-3 PUFA dietary intake levels in Western countries remains largely below the current recommendations, considering both the omega-3 precursor α-linolenic acid (ALA) and long chain derivatives such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). An optimized omega-3 PUFAs status could be helpful to prevent infectious diseases, including Covid-19.
Keywords: α-linolenic acid, Docosopentaenoic acid, Eicosapentaenoic acid, Immunity, Lipid, Virus
Abbreviations: ALA, α-linolenic acid; ARA, arachidonic acid; ARDS, Acute Respiratory Distress Syndrome; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; LC-PUFA, long-chain polyunsaturated fatty acid; SPM, specialized pro-resolving lipid mediators; SREBP, Sterol Regulatory Element Binding Protein
Highlights
-
•
Covid-19 disease is responsible for cytokine storm and systemic inflammation.
-
•
Omega-3 PUFA are precursors of specialized pro-resolving lipid mediators.
-
•
Omega-3 PUFA could modulate the Covid-19 anti-inflammatory response.
-
•
Clinical trials should evaluate the effect of omega-3 PUFA in Covid-19 patients.
1. Introduction
The viral epidemic caused by the new Coronavirus SARS-CoV-2 is responsible for the Coronavirus-2019 disease (Covid-19). Upon binding via its Spike (S) protein, SARS-CoV-2 uses the angiotensin-converting enzyme 2 (ACE2) as an entry receptor in several cell types, including lung alveolar epithelial cells (type 1 and type 2 pneumocytes). This receptor-mediated endocytosis is followed by the activation of the S protein in the viral envelope by the transmembrane serine protease 2 (TMPRSS2), a membrane-bound enzyme localized near the ACE2 receptor [1]. Alternatively, direct proteolytic cleavage of the viral S protein by TMPRSS2 on the surface of host cells has been described to induce the fusion of the viral and plasma membranes, leading to the release of the viral single stranded-RNA into the cytoplasm [2]. Once in endosomes or cytoplasm, the viral RNA activate nucleic acid sensing systems like Toll-like receptors (TLR7 and 9) which initiate the anti-viral response of the innate immune system by triggering first the expression of type 1 interferons, followed by activation of NF-kB and proinflammatory cytokines.
In 10–15% of all cases, Covid-19 is complicated by severe pneumonia requiring hospitalization, with a high risk of developing an Acute Respiratory Distress Syndrome (ARDS), which can lead the patient to the intensive care unit (ICU) and is often lethal. ARDS is mainly associated with an uncontrolled overproduction of immune cells and cytokines, called “cytokine storm syndrome”, which appears 7–15 days following the onset of symptoms, leading to systemic inflammation and multiple organ failure. Covid-19 general mortality rate is about 2% and hospital mortality rate is about 10% [3]. To date, there is no vaccine and only the antiviral prodrug Remdesivir has been approved as an effective drug therapy to treat the disease [4,5]. Additionally, little is known to prevent its progression to a serious condition. The global burden of infection being high and worldwide, all kind of treatments, including dietary supplementation and pharmaco nutrition trials, deserve to be tested [6]. Background from other coronaviruses, like SARS-CoV-1 and MERS-CoV are also important to remind.
In this context, it is well admitted that adequate supply and balance of nutrients are required for proper functioning of both the innate and adaptive immune system. Essential PUFAs coming from the diet have a very special role in this process, because they participate to control chronic and acute inflammations. Because they are well-known metabolic precursors of specialized pro-resolving lipid mediators (SPMs), omega-3 long-chain polyunsaturated fatty acids (omega-3 LC-PUFAs) could notably help improve the resolution of the inflammatory balance, limiting therefore the level and duration of the critical inflammatory period [7]. Omega-3 LC-PUFAs may also interact at different stages of the viral infection, notably on the virus entry and replication. Therefore, the nutritional status for PUFAs is particularly important in tissue inflammatory status and overall immune response [7,8]. In this context, it is crucial to remind that the omega-3 PUFA dietary intake levels in Western countries remain largely below the current recommendations, considering both the omega-3 precursor α-linolenic acid (ALA) and long chain derivatives such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [9,10].
In this Hypothesis article, we suggest that omega-3 PUFA dietary supplementation may be beneficial to reduce the risk of coronavirus complications, progressing to serious outcomes like ARDS, with the need for artificial ventilation in ICU. The rational for such Hypothesis is based on the following data. An increased intake level (long-term supplementation for prevention against infection or short-term supplementation in acute inflammation phase) of omega-3 PUFAs, including both α-linolenic acid (ALA) as precursor and long-chain derivatives (EPA, DPA docosapentaenoic acid and DHA) may increase their overall tissue storage. This higher cell and tissue omega-3 status could lead to a better conversion to SPMs, leading in turn to a more rapid resolution of inflammation, improving the severity of the cytokine storm, and improving finally the outcome of Covid-19 infected patients.
To support these hypotheses, we first present the demonstrated and putative molecular mechanisms involving omega-3 PUFAs in the prevention from viral infection and in the inflammation resolution. Then, we review the data obtained in animal experiments, human clinical trials and epidemiological studies supporting an interesting role of omega-3 PUFA supplementation against viral infection-associated inflammation.
2. Omega-3 PUFAs may interfere with virus entry and replication
2.1. Omega-3 PUFAs may modulate membrane rafts where ACE2 and TMPRSS2 are mainly expressed
ACE2 and TMPRSS2 are described to be mainly present in lipids rafts [1], which are cholesterol and sphingolipid-enriched membrane microdomains within the lipid bilayer. It was previously shown that lipid rafts were involved in SARS-CoV entry into Vero E6 cells [11]. In addition, Glende et al. [12] suggested that lipid raft modulation may be an option to reduce ACE2-mediated virus infection.
As constitutive part of the membrane phospholipids, omega-3 PUFAs can regulate membrane properties, such as membrane fluidity and protein complex assembly in lipid rafts. The number and size of raft and non-raft domains may also modulate the expression, stability and enzymatic activities of ACE2 and TMPRSS2. Among omega-3 LC-PUFAs, DHA is described to directly regulate the formation of lipid rafts [13].
2.2. Omega-3 PUFAs may counteract the viral activation of SREBP1/2
Viruses are known to manipulate cell metabolism to facilitate their replication cycle. A described virus strategy is to reprogram host lipid metabolism, in order to provide enough lipid molecules required for the synthesis of the virion replication membranes. The isomers of the transcription factor SREBP (Sterol Regulatory Element Binding Protein) are involved in this metabolic reprogramming. For instance, MERS-CoV has been shown to manipulate host cellular lipid metabolism and reprogram the de novo SREBP-dependent lipogenesis pathway, to ensure its replication [14]. SREBPs has also been shown to be activated during flavivirus infection [15]. Therefore, SREBP1/2 was defined as a potential broad-spectrum antiviral target for therapeutic intervention.
In this context, one can speculate that, since omega-3 LC-PUFAs are well-known intracellular inhibitors of SREBP transcription and maturation, a higher cellular impregnation level may counteract the activating effect of the virus and lower its replication duration. Indeed, EPA and DHA inhibit SREBP1 conversion from its inactive form to its active mature form and increase the degradation of SREBP-1c mRNA. This inhibition results in overall decrease in cellular concentration of the mature form of SREBP and less transcription of lipogenic genes coding notably for Acetyl-CoA Carboxylase, Fatty Acid Synthase and Stearoyl-CoA Desaturase [16]. It was suggested that the cholesterol and fatty acid biosynthesis pathways play a role in Hepatitis C Virus (HCV) RNA replication and infection [17]. In connection with the previous hypothesis on lipid rafts, omega-3 LC-PUFAs may also inhibit SREBP2-induced cholesterol synthesis, mainly via the transcription level of HMG-CoA reductase.
3. Omega-3 PUFAs may help improve the resolution phase of inflammation
LC-PUFAs play a critical role in inflammatory processes and innate immune response, thereby affecting human health and diseases. As a first step of the immune response, inflammation is a biological defense against physical, biological or infectious injury to body cells. It involves a series of processes, like leucocyte recruitment, whose first purpose is to eliminate the causes and aggressors. This first phase is called the “promotion” phase. Then, the second phase tries to restore the affected cell and tissue homeostasis and is called the “resolution” phase.
Inflammation begins in particular with the release of PUFAs in phospholipid sn-2 position from the cell membranes, under the action of the enzyme phospholipase A2 (PLA2) [18]. Arachidonic acid (ARA) is the main precursor for this synthesis, leading to the production of cellular mediators promoting inflammation, like leukotrienes (LTB4 and LTC4) and prostaglandins (PGE2 and PGD2), through the action of cyclooxygenases (COX1 and 2), lipoxygenases (LOX) and cytochrome P450 [19,20]. Therefore, PUFAs of the omega-6 series are described to activate the promotion phase of inflammation.
Omega-3 PUFAs are now described to activate the resolving phase, directly in the site of inflammation [[21], [22], [23]]. EPA and DHA are the main precursors for those resolving inflammation [21]. Indeed, the specialized pro-resolving mediators (SPMs) issued from EPA and DHA, known as resolvins, maresins and protectins inhibit the synthesis of pro-inflammatory cytokines through the down-regulation of the NF-κB pathway [24]. So, they play an efficient role for inflammation resolution processes. E-series resolvins from EPA, D-series resolvins and protectins from DHA have a significant anti-inflammatory effect by limiting leucocytes infiltrations in damaged tissues [23,25]. Maresins derived from DHA can also induce macrophages phagocytosis of neutrophils to resolve inflammation [26]. Recently, another minor omega-3 LC-PUFA, the docosapentaenoic acid (C22:5 n- 3, omega-3-DPA) has also been suggested to be important in inflammatory resolution in the lung [27].
An optimal resolution of acute inflammation, as well as the control of chronic low-grade inflammation [28], is therefore based on the cellular availability of these omega-3 fatty acids exclusively provided by the diet and in particular on the dietary intake level of ALA, EPA and DHA.
4. Omega-3 PUFA dietary intake levels are largely below the recommendations
In this context, it is crucial to remind that the omega-3 PUFA dietary intake levels in Western countries remains largely below the current recommendations, considering both the omega-3 precursor α-linolenic acid (ALA) and long chain derivatives such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [29]. Linked to great changes in agricultural production and consumption habits these last 50 years, today’s consumption of omega-6 PUFAs is at least equal to the current recommendation and often excessive (depending on the countries) while the omega-3 PUFA’s intake is almost always in deficit in western countries [30,31]. This leads to a high (10 and more) omega-6/omega-3 ratio since the 80s [30].
In France for instance, the advised dietary intake recommends 2.2 g of ALA daily intake (1% of energy intake per day) and 500 mg for EPA + DHA (in which 250 mg of DHA minimum) [10]. However, the French INCA 3 consumption study reported in 2017 the mean omega-3 consumption as follows: 1 g/day of ALA instead of the 2.2 recommended, and 286 mg/day of EPA + DHA instead of 500 recommended. Therefore, only 15% of the French population meet DHA intake recommendation, only 8% for EPA and only 1.6% for ALA [9,32]. Breast milk composition is also a good biomarker for PUFAs intake levels. As expected, it exhibits a lack of omega-3 in most of the western countries [33].
In a large range of other countries (38 countries) [34], the omega-3 LC-PUFA intake varied from 0.02% of total energy in Bulgaria (corresponding to about 40 mg/day) to 0.44% of total energy in Iceland (corresponding to 900 mg/day). For the authors of this study, only a few number of countries have an optimal intake of omega-3 LC-PUFAs over 0.2% of total energy (corresponding to about 410 mg/day): Portugal (0.203%, i.e. 420 mg/day), South Korea (0.218%, i.e. 450 mg/day), Finland (0.228%, i.e. 470 mg/day), Norway (0.244%, i.e. 500 mg/day), Malaysia (0.341%, i.e. 700 mg/day), Japan (0.374%, i.e. 760 mg/day) and Iceland (0.435%, i.e. 900 mg/day).
These abundant and consensual deficit data deserve our attention in the context of this pandemic and its inflammatory complications. Indeed, countries with a high omega-3 LC-PUFA intake (0,2% of total energy intake) have a very low number of victims related to the population: more than 4 times lower than the average of the 31 other countries (36 versus 175 deaths per million in June 2020) [35].
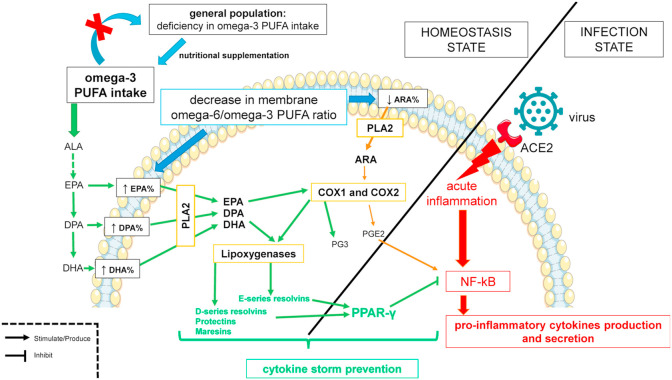
Taking into consideration (i) the role of omega-3 LC-PUFAs in the virus entry, replication and in the resolution phase of inflammation; (ii) the inflammatory processes leading to ARDS; (iii) the large and general deficit in omega-3 LC-PUFAs in the western population, it is possible to raise the hypothesis of a link between omega-3 LC-PUFA consumption deficiency and Covid-19 excessive inflammatory complication (Fig. 1 ).
Fig. 1.
– Expected mechanisms of the anti-inflammatory effect of omega-3 long chain polyunsaturated fatty acids (omega-3 LC-PUFAs) and cytokine storm prevention during the Coronavirus disease-2019. General population has deficiency in Omega-3 LC-PUFAs that increases proportion of arachidonic acid (ARA) from Omega-6 PUFA family in phospholipid membranes of the cells. ARA is liberated from its sn-2 position by the phospholipase A2 (PLA2) and become a substrate for cyclooxygenase enzymes (COX1 and COX2). Prostaglandins E2 (PGE2) is synthesized from ARA and is pro-inflammatory by activating NF-κB and lead to systemic chronic low-grade inflammation and a higher response to severe acute inflammation. A nutritional supplementation in omega-3 fatty acids may decrease or even remove the deficiency in population. This supplementation will increase eicosapentaenoic acid (EPA), omega-3 DPA and docosahexaenoic acid (DHA) proportion in phospholipid membranes of the cells. EPA, DPA and DHA are, like ARA, liberated by PLA2. It is a limiting step for the synthesis of lipid mediators from PUFAs. EPA is a substrate for COX enzymes and also lipoxygenases (LOX). PGE3 and E-series resolvins are produced from EPA and have anti-inflammatory properties. DHA is a substrate for LOX and produces D-series resolvins, protectins and maresins that have anti-inflammatory properties. These specialized pro-resolving mediators from EPA and DHA, in a case of acute inflammation by activation of NF-κB, will activate peroxisome proliferator-activated receptor (PPAR)-γ. PPAR-γ inhibits NF-κB. Consequently, the production of pro-inflammatory cytokines is reduced and may prevent cytokine storm.
5. Animal data, clinical trials and epidemiological studies supporting an interesting role of omega-3 LC-PUFA supplementation in ARDS
5.1. Omega-3 PUFA supplementation in animal models
In animal studies, omega-3 LC-PUFA supplementation clearly led to increased levels of tissue omega-3, including the lung. For instance, rats fed semi-synthetic diets supplemented with 1% total lipids of EPA, DPA and DHA in ethyl esters form from weaning to 6 weeks clearly increased the omega-3 LC-PUFA level in all studied tissues, including the lung [36]. Similarly, maternal omega-3 LC-PUFA (ALA, EPA and DHA) supplementation during the perinatal period (from embryonic day 15 to day 20 after parturition) increased the level of total omega-3 in breast milk as well as the lung tissue of the rat pups and protected them against hyperoxia-induced pulmonary hypertension [37].
Animal experiments have also shown a beneficial impact of high omega-3 diets against acute pneumonia when animals were exposed to microbial infection. Sharma et al. [38] used mice infected by Klebsiella pneumoniae with control or omega-3 PUFA enriched diets. They reported that the dietary supplementation of omega-3 LC-PUFAs can exert an overall beneficial effect through the upregulation of the host’s specific and nonspecific immune defenses. It is interesting to underline that the beneficial effect exists whatever the type of omega-3 LC-PUFA supplementation (ALA, EPA or DHA diets). Hinojosa et al. [39] used mice intranasally infected with Streptococcus pneumoniae. Survival rate increased significantly in omega-3 diet’s group when bacterial burden decreased. In omega-3 LC-PUFA-fed animal tissues, it was possible to measure a significant decrease in the levels of pro-inflammatory cytokines.
5.2. Omega-3 PUFA supplementation in humans
In 2018, the European Society for clinical nutrition and metabolism (ESPEN) expert group recommended the use of omega-3 rich fish oil in enteral and parenteral nutrition, for its general clinical benefits due to its anti-inflammatory and immune-modulating effects [40]: reduction in infection rate, and length of hospital stay in medical and surgical patients admitted to the ICU.
Indeed, in humans, supplementation with EPA and DHA was shown to lead to the incorporation of both omega-3 LC-PUFA in body lipid pools [41]. The rate of incorporation varied between sample types, with the time to maximal incorporation ranging from days (plasma phosphatidylcholine) to months (mononuclear cells), and higher for adipose tissue [41]. In addition, several plasma EPA- and DHA-derived SPMs were shown to respond linearly with the increased intake of EPA and DHA [42].
In connection with these interesting results, various SPMs synthesized from EPA and DHA have already shown protection and resolution of acute lung lesions in ARDS [43]. Omega-3 PUFA, in the form of fish oil, mostly rich in EPA and DHA, have also been used as a treatment in several clinical trials in enteral and parenteral nutrition with ARDS patients. Recent meta-analyses [[44], [45], [46]] demonstrated a beneficial impact of these omega-3 supplementation, especially when the supplementation was limited to enteral nutrition [44]. Even if the doses were different and if various other supplementation (such as antioxidants) occurred in the different trials, the authors highlighted significant benefits obtained in only a few days (28 days) on mortality (−36%), as well as on the duration of ventilation, the length of stay in ICU [44] with enteral supplementation of omega-3 [21,44,45]. In these trials, omega-3 PUFA was provided at high doses to patients with ARDS (from 5 to 20 times the recommended nutritional intake for EPA and DHA).
Considering all these results, administering omega-3 PUFAs appears a reasonable strategy in ARDS. A recent review suggested the systematic use of omega-3 LC-PUFAs if enteral or parenteral nutrition is indicated in the Covid-19 patients admitted in the ICU [47]. In addition to EPA and DHA, recent epidemiological and mechanistic data also suggest a specific role for omega-3 DPA in lung tissue [27,36,48]. Indeed, little studied so far, omega-3 DPA seems to be the starting point for specific routes of synthesis of pro-resolution mediators at the level of the lung [27].
6. Conclusion: on the way to clinical trials
Although scientific research on drug treatment are progressing, the ARDS experts recommend now, not only for Covid-19 derived ARDS, to give more attention on prevention in addition to treatment [49]. In this context, we can raise the hypothesis that increasing the level of omega-3 LC-PUFAs in the diet or as a pharmaco nutrient in addition to artificial nutrition, could reduce the impact of inflammation caused by viral infectious diseases, such as the Covid-19. The biochemical mechanisms described here, reinforced by clinical, animal and epidemiological data suggest that the effect of nutritional supplementation could accelerate recovery, reduce hospitalization, duration of stay in ICU and finally reduce mortality due to the new Coronavirus SARS-Cov-2. Omega-3 LC-PUFAs could play a central role in prevention of the cytokine storm (Fig. 1), at least maybe by decreasing the intensity of inflammation and risk of mortality for patients with Covid-19.
Some questions are still opened. What should be the quantity of total omega-3 and LC-PUFA supplementation/intake? For the general population and in primary prevention, the data presented in this article, in addition to all the arguments previously developed concerning the prevention of other pathologies, support the crucial and urgent need to meet (at least) the nutritional recommendations (2.2 g/day for ALA and 500 mg/day for EPA+ DHA). In secondary prevention, and for patients with symptoms and/or which are tested positive for Covid-19, the optimal doses for both ALA and omega-3 LC-PUFAs are not known. Meeting the nutritional recommendation of 2.2 g/day of ALA appears crucial, as well. Concerning omega-3 LC-PUFAs, it has been suggested that enteral nutrition enriched with 3.5 g/day EPA and DHA can be administered in Covid-19 patients, not in a bolus [47]. Higher amounts of omega-3 LC-PUFAs, up to 9 g/day in the form of fish oil, have been administered safely [50].
What is the relative impact of the different fatty acids of the omega-3 family? Probably all the fatty acids of the omega-3 family seem have a role to play. EPA, omega-3 DPA and DHA are direct substrates for pro-resolution mediator synthesis. ALA is the initial dietary precursor of these LC-PUFAs by successive elongation and desaturation. Although it is well known that the overall conversion of ALA to DHA is weak [51], ALA may have its own effect on inflammation resolution, as seen above, in ARDS animal trial in case of microbial infection [38]. Other authors compared the effect of ALA or DHA diets on heart function [52,53] and found no differences. They additionally observed an accumulation of omega-3 DPA in the membranes of the cardiac cells in the ALA-fed rats.
To conclude, two clinical trials are on their way in Covid-19 patients and in older people with the aims to evaluate whether omega-3 PUFA diet enrichment could protect patients against severe forms of Covid-19, and to increase the awareness of the scientific community towards the beneficial role of good nutrition as a “barrier diet” against future pandemic.
Author contribution
PW, CP, PL, VR, RT contributed to literature search, analysis of data, writing and editing the manuscript. PW, PL, RT contributed to the conception of the clinical trial. All authors have approved the final version of the article.
Conflict of interest
Pierre Weill was former president of Valorex, a feed and food company dedicated to protein and oleaginous seeds processing.
Ronan Thibault received consulting and conference fees: Aguettant, Baxter, BBraun, Fresenius-Kabi, Nutricia, Roche; conference fees: Astra-Zeneca, Homeperf, Lactalis, Nestlé, Shire; research grants: Valorex, Bleu-Blanc-Coeur.
The other authors declare no conflict of interest.
References
- 1.Ballout R.A., Sviridov D., Bukrinsky M.I., Remaley A.T. The lysosome: a potential juncture between SARS-CoV-2 infectivity and Niemann-Pick disease type C, with therapeutic implications. Faseb. J. 2020;34:7253–7264. doi: 10.1096/fj.202000654R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmoud I.S., Jarrar Y.B., Alshaer W., Ismail S. SARS-CoV-2 entry in host cells-multiple targets for treatment and prevention. Biochimie. 2020;175:93–98. doi: 10.1016/j.biochi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Sen Tan K., Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- A n update on the status. Mil. Med. Res. 2020;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus (Covid-19) Update: FDA issues emergency use authorization for potential COVID-19 treatment. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment FDA, (n.d.) accessed August 24, 2020.
- 5.First Covid-19 treatment recommended for EU authorisation European medicines agency. https://www.ema.europa.eu/en/news/first-covid-19-treatment-recommended-eu-authorisation (n.d.) accessed August 24, 2020.
- 6.Rogero M.M., de Leão M.C., Santana T.M., de M.V., Pimentel M.B., Carlini G.C.G., da Silveira T.F.F., Gonçalves R.C., Castro I.A. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic. Biol. Med. 2020;156:190–199. doi: 10.1016/j.freeradbiomed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messina G., Polito R., Monda V., Cipolloni L., Di Nunno N., Di Mizio G., Murabito P., Carotenuto M., Messina A., Pisanelli D., Valenzano A., Cibelli G., Scarinci A., Monda M., Sessa F. Functional role of dietary intervention to improve the outcome of COVID-19: a hypothesis of work. Int. J. Mol. Sci. 2020;21:3104. doi: 10.3390/ijms21093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ANSES . Etude INCA3); 2017. Avis relatif à la 3ème étude individuelle nationale des consommations alimentaires. [Google Scholar]
- 10.ANSES . Rapport d’expertise collective; 2011. Actualisation des apports nutritionnels conseillés pour les acides gras. [Google Scholar]
- 11.Lu Y., Liu D.X., Tam J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008;369:344–349. doi: 10.1016/j.bbrc.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glende J., Schwegmann-Wessels C., Al-Falah M., Pfefferle S., Qu X., Deng H., Drosten C., Naim H.Y., Herrler G. Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2. Virology. 2008;381:215–221. doi: 10.1016/j.virol.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassall S.R., Leng X., Canner S.W., Pennington E.R., Kinnun J.J., Cavazos A.T., Dadoo S., Johnson D., Heberle F.A., Katsaras J., Shaikh S.R. Docosahexaenoic acid regulates the formation of lipid rafts: a unified view from experiment and simulation. Biochim. Biophys. Acta Biomembr. 2018;1860:1985–1993. doi: 10.1016/j.bbamem.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan S., Chu H., Chan J.F.W., Ye Z.W., Wen L., Yan B., Lai P.M., Tee K.M., Huang J., Chen D., Li C., Zhao X., Yang D., Chiu M.C., Yip C., Poon V.K.M., Chan C.C.S., Sze K.H., Zhou J., Chan I.H.Y., Kok K.H., To K.K.W., Kao R.Y.T., Lau J.Y.N., Jin D.Y., Perlman S., Yuen K.Y. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 2019;10:120. doi: 10.1038/s41467-018-08015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pombo J.P., Sanyal S. Perturbation of intracellular cholesterol and fatty acid homeostasis during flavivirus infections. Front. Immunol. 2018;9:1276. doi: 10.3389/fimmu.2018.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deckelbaum R., Worgall T., Seo T. n−3 Fatty acids and gene expression. Am. J. Clin. Nutr. 2006;83:1520S–1525S. doi: 10.1093/ajcn/83.6.1520S. https://academic.oup.com/ajcn/article/83/6/1520S/4633297 accessed July 16, 2020. [DOI] [PubMed] [Google Scholar]
- 17.Kapadia S.B., Chisari F.V. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami M., Nakatani Y., Atsumi G.I., Inoue K., Kudo I. Regulatory functions of phospholipase A2. Crit. Rev. Immunol. 2017;37:121–180. doi: 10.1615/CritRevImmunol.v37.i2-6.20. [DOI] [PubMed] [Google Scholar]
- 19.Choque B., Catheline D., Rioux V., Legrand P. Linoleic acid: between doubts and certainties. Biochimie. 2014;96:14–21. doi: 10.1016/j.biochi.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Serhan C.N., Petasis N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calder P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortega-Gómez A., Perretti M., Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol. Med. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basil M.C., Levy B.D. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serhan C.N., Arita M., Hong S., Gotlinger K. Lipids, Lipids. 2004. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers; pp. 1125–1132. [DOI] [PubMed] [Google Scholar]
- 26.Rius B., López-Vicario C., González-Périz A., Morán-Salvador E., García-Alonso V., Clària J., Titos E. Resolution of inflammation in obesity-induced liver disease. Front. Immunol. 2012;3:257. doi: 10.3389/fimmu.2012.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morin C., Hiram R., Rousseau E., Blier P.U., Fortin S. Docosapentaenoic acid monoacylglyceride reduces inflammation and vascular remodeling in experimental pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H574–H586. doi: 10.1152/ajpheart.00814.2013. [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 29.Legrand P., Morise A., Kalonji E. Update of French nutritional recommendations for fatty acids. World Rev. Nutr. Diet. 2011;102:137–143. doi: 10.1159/000327800. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21865827 [DOI] [PubMed] [Google Scholar]
- 30.Ailhaud G., Massiera F., Weill P., Legrand P., Alessandri J.M., Guesnet P. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog. Lipid Res. 2006;45:203–236. doi: 10.1016/j.plipres.2006.01.003. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16516300 [DOI] [PubMed] [Google Scholar]
- 31.Blasbalg T.L., Hibbeln J.R., Ramsden C.E., Majchrzak S.F., Rawlings R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21367944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubuisson C., Dufour A., Carrillo S., Drouillet-Pinard P., Havard S., Volatier J.L. The third French individual and national food consumption (INCA3) survey 2014-2015: method, design and participation rate in the framework of a European harmonization process. Publ. Health Nutr. 2019;22:584–600. doi: 10.1017/S1368980018002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tressou J., Buaud B., Simon N., Pasteau S., Guesnet P. Very low inadequate dietary intakes of essential n-3 polyunsaturated fatty acids (PUFA) in pregnant and lactating French women: the INCA2 survey. Prostaglandins Leukot. Essent. Fatty Acids. 2019;140:3–10. doi: 10.1016/j.plefa.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Hibbeln J.R., Nieminen L., Blasbalg T.L., Riggs J., Lands W. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- 35.Coronavirus COVID-19 (2019-nCoV) https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (n.d.) accessed July 17, 2020.
- 36.Drouin G., Catheline D., Guillocheau E., Gueret P., Baudry C., Le Ruyet P., Rioux V., Legrand P. Comparative effects of dietary n-3 docosapentaenoic acid (DPA), DHA and EPA on plasma lipid parameters, oxidative status and fatty acid tissue composition. J. Nutr. Biochem. 2019;63:186–196. doi: 10.1016/j.jnutbio.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Zhong Y., Catheline D., Houeijeh A., Sharma D., Du L., Besengez C., Deruelle P., Legrand P., Storme L. Maternal omega-3 PUFA supplementation prevents hyperoxia-induced pulmonary hypertension in the offspring. Am. J. Physiol. Cell. Mol. Physiol. 2018;315:L116–L132. doi: 10.1152/ajplung.00527.2017. [DOI] [PubMed] [Google Scholar]
- 38.Sharma S., Chhibber S., Mohan H., Sharma S. Dietary supplementation with omega-3 polyunsaturated fatty acids ameliorates acute pneumonia induced by Klebsiella pneumoniae in BALB/c mice. Can. J. Microbiol. 2013;59:503–510. doi: 10.1139/cjm-2012-0521. [DOI] [PubMed] [Google Scholar]
- 39.Hinojosa C.A., Gonzalez-Juarbe N., Rahman M.M., Fernandes G., Orihuela C.J., Restrepo M.I. Omega-3 fatty acids in contrast to omega-6 protect against pneumococcal pneumonia. Microb. Pathog. 2020;141:103979. doi: 10.1016/j.micpath.2020.103979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calder P.C., Adolph M., Deutz N.E., Grau T., Innes J.K., Klek S., Lev S., Mayer K., Michael-Titus A.T., Pradelli L., Puder M., Vlaardingerbroek H., Singer P. Lipids in the intensive care unit: recommendations from the ESPEN expert group. Clin. Nutr. 2018;37:1–18. doi: 10.1016/j.clnu.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 41.Browning L.M., Walker C.G., Mander A.P., West A.L., Madden J., Gambell J.M., Young S., Wang L., Jebb S.A., Calder P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish 1-4. Am. J. Clin. Nutr. 2012;96:748–758. doi: 10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostermann A., West A., Schoenfeld K., Browning L., Walker C., Jebb S., Calder P., Schebb N. Plasma oxylipins respond in a linear dose-response manner with increased intake of EPA and DHA: results from a randomized controlled trial in healthy humans. Am. J. Clin. Nutr. 2019;109:1251–1263. doi: 10.1093/ajcn/nqz016. https://academic.oup.com/ajcn/article-abstract/109/5/1251/5475740?redirectedFrom=fulltext accessed July 21, 2020. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton B., Ware L.B., Matthay M.A. Lipid mediators in the pathogenesis and resolution of sepsis and ARDS. In: Vincent J.L., editor. Annu. Updat. Intensive Care Emerg. Med. 2018. 3–11. [DOI] [Google Scholar]
- 44.Langlois P.L., D’Aragon F., Hardy G., Manzanares W. Omega-3 polyunsaturated fatty acids in critically ill patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Nutrition. 2019;61:84–92. doi: 10.1016/j.nut.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dushianthan A., Cusack R., Burgess V.A., Grocott M.P.W., Calder P.C. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database Syst. Rev. 2019;2019:CD012041. doi: 10.1002/14651858.CD012041.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dushianthan A., Cusack R., Burgess V.A., Grocott M.P.W., Calder P. Immunonutrition for adults with ARDS: results from a cochrane systematic review and meta-analysis. Respir. Care. 2020;65:99–110. doi: 10.4187/respcare.06965. [DOI] [PubMed] [Google Scholar]
- 47.Thibault R., Seguin P., Tamion F., Pichard C., Singer P. Nutrition of the COVID-19 patient in the intensive care unit (ICU): a practical guidance. Crit. Care. 2020;24:447. doi: 10.1186/s13054-020-03159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leng S., Picchi M.A., Tesfaigzi Y., Wu G., James Gauderman W., Xu F., Gilliland F.D., Belinsky S.A. Dietary nutrients associated with preservation of lung function in hispanic and Non-Hispanic white smokers from New Mexico. Int. J. COPD. 2017;12:3171–3181. doi: 10.2147/COPD.S142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spragg R.G., Bernard G.R., Checkley W., Curtis J.R., Gajic O., Guyatt G., Hall J., Israel E., Jain M., Needham D.M., Randolph A.G., Rubenfeld G.D., Schoenfeld D., Thompson B.T., Ware L.B., Young D., Harabin A.L. Am. J. Respir. Crit. Care Med. Am J Respir Crit Care Med; 2010. Beyond mortality: future clinical research in acute lung injury; pp. 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koekkoek W. Kristine, Panteleon V., van Zanten A.R. Current evidence on ω-3 fatty acids in enteral nutrition in the critically ill: a systematic review and meta-analysis. Nutrition. 2019;59:56–68. doi: 10.1016/j.nut.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Burdge G.C., Calder P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005;45:581–597. doi: 10.1051/rnd:2005047. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16188209 [DOI] [PubMed] [Google Scholar]
- 52.Ayalew-Pervanchon A., Rousseau D., Moreau D., Assayag P., Weill P., Grynberg A. Long-term effect of dietary α-linolenic acid or decosahexaenoic acid on incorporation of decosahexaenoic acid in membranes and its influence on rat heart in vivo. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H2296–H2304. doi: 10.1152/ajpheart.00194.2007. [DOI] [PubMed] [Google Scholar]
- 53.Brochot A., Guinot M., Auchere D., MacAire J.P., Weill P., Grynberg A. Effects of alpha-linolenic acid vs. docosahexaenoic acid supply on the distribution of fatty acids among the rat cardiac subcellular membranes after a short- or long-term dietary exposure. Nutr. Metab. 2009;6:14. doi: 10.1186/1743-7075-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]