Abstract
Primary lateral sclerosis (PLS) is an adult-onset upper motor neuron disease manifesting in progressive spasticity and gradually resulting in considerably motor disability. In the absence of early disease-specific diagnostic indicators, the majority of patients with PLS face a circuitous diagnostic journey. Until the recent publication of consensus diagnostic criteria, 4-year symptom duration was required to establish the diagnosis. The new diagnostic criteria introduced the category of ‘probable PLS’ for patients with a symptom duration of 2–4 years. “Evolving diagnostic criteria in primary lateral sclerosis: The clinical and radiological basis of "probable PLS" [1]. This dataset provides radiological metrics in a cohort of ‘probable PLS’ patients, ‘definite PLS’ patients and age-matched healthy controls. Region-of-interest radiological data include diffusivity metrics in the corticospinal tracts and corpus callosum as well as mean cortical thickness values in the pre- and para-central gyri in each hemisphere. Our data indicate considerable grey matter and relatively limited white matter involvement in ‘probable PLS’ which supports the rationale for this diagnostic category as a clinically useful entity. The introduction of this diagnostic category will likely facilitate the timely recruitment of PLS patients into research studies and pharmacological trials before widespread neurodegenerative change ensues.
Keywords: Primary lateral sclerosis, Neuroimaging, MRI, Upper motor neuron
Specifications Table
| Subject | Primary Lateral Sclerosis, Radiology, Neuroimaging |
| Specific subject area | Neurology, Radiology, Motor neuron disease, Primary Lateral Sclerosis |
| Type of data | Magnetic resonance imaging: quantitative neuroimaging metrics data form a standardised acquisition protocol |
| How data were acquired | In a population-based prospective neuroimaging study, MRI data were acquired on a Philips Achieva 3T MRI scanner with an 8-channel head coil. Standardised clinical assessment took place on the day of the MRI scan. |
| Data format | Axial diffusivity (AD), mean diffusivity (MD), radial diffusivity (RD), fractional anisotropy (FA) values are presented in the left corticospinal tract, right corticospinal tract and corpus callosum. Cortical thickness values are presented in the precentral and para-central gyri in each hemisphere. |
| Parameters for data collection | 3D T1-weighted sequence: spatial resolution: 1 × 1 × 1 mm, Field of view: 256 × 256 × 160 mm, repetition time= 8.5 ms, Echo time = 3.9 ms, Inversion time =1060 ms, flip angle = 8°, SENSE factor = 1.5, sagittal acquisition; 256 slices. DTI images were acquired using a 32-direction SE-EPI sequence. Field of view: 245 × 245 × 150 mm, spatial resolution = 2.5 mm3, 60 slices, repetition time / Echo time = 7639/59 ms, |
| Description of data collection | Data were collected on a 3 Tesla MRI system. Demographic variables were recorded before the MRI scan, and a standardised neurological examination was also performed on the day of the MRI. |
| Data source location | Institution: Computational neuroimaging group, Trinity Biomedical Sciences Institute, Trinity College Dublin City/Town/Region: Dublin Country: Ireland |
| Data accessibility | Raw imaging metrics are available online at Mendeley Data; http://dx.doi.org/10.17632/kbzmcydjzw.2 |
| Related research article | Evolving diagnostic criteria in primary lateral sclerosis: The clinical and radiological basis of "probable PLS". Finegan E, Li Hi Shing S, Siah WF, Chipika RH, Chang KM, McKenna MC, Doherty MA, Hengeveld JC, Vajda A, Donaghy C, Hutchinson S, McLaughlin RL, Hardiman O, Bede P. J Neurol Sci. 2020 Jul 24;417:117052. doi: 10.1016/j.jns.2020.117052. Online ahead of print. PMID: 32731060 |
Value of the Data
-
•
Radiological data of ‘Probable PLS’ supports the introduction of this new diagnostic category.
-
•
Data of ‘Probable’ and ‘Definite’ PLS patients help the categorisation of individual patients.
-
•
Data from ‘Probable’ and ‘Definite’ PLS demonstrate that pharmacological studies should include patients with ‘probable PLS’.
-
•
Radiological PLS data helps to validate observations from other motor neuron disease phenotypes.
-
•
Radiological data from 100 healthy controls may help the interpretation of imaging data from other neurological conditions.
1. Data Description
In the absence of disease-specific indicators and resemblance to other neurological conditions that early diagnosis of PLS is notoriously challenging [2, 3]. Previous diagnostic criteria required a symptom duration of 4-years to establish the diagnosis [4]. The protracted diagnostic uncertainty in suspected patients is a source of distress which is often coupled with the fear of transitioning to ALS. The recent diagnostic criteria [5] introduced the category of ‘probable PLS’ for patients with 2–4 years symptom duration to stimulate research into early phase PLS. Existing imaging studies in PLS invariably focus on established PLS cohorts with long symptom duration [6], [7], [8], [9], [10] and disease burden in early PLS is poorly characterised [2, 11, 12]. In this dataset (Table 1) we present grey and white matter imaging metrics in 32 patients with ‘definite PLS’, 7 patients with ‘probable PLS’ and 100 healthy controls. Raw imaging metrics are available online at Mendeley Data; http://dx.doi.org/10.17632/kbzmcydjzw.2. Corticospinal spinal tract and corpus callosum integrity was interrogated using multiple white matter diffusivity parameters. The demographic, genetic and clinical characteristics of study participants are reported in the companion article [1]. Supplementary clinical information is presented Fig. 1., where the ALS functional rating scale (ALSFRS-r) and Penn UMN sub-scale profile of each PLS group are additionally presented [13, 14]. Raw grey and white matter data are also presented in box plots for each cohort in Fig. 2. Based on estimated marginal means, adjusted for age and gender, the comparative imaging profiles of the two patient groups are depicted in Fig. 3, Fig. 4 with reference to healthy controls.
Table 1.
Data categories and measures PLS = amyotrophic lateral sclerosis; ALSFRS-r = amyotrophic lateral sclerosis functional rating scale-revised; PLS = Primary lateral sclerosis; FA=Fractional Anisotropy; MD= Mean Diffusivity; AD= Axial Diffusivity; RD=Radial Diffusivity.
| Data categories | Specific measures |
|---|---|
| Demographic variables | Age - years(SD) |
| Sex- (male%) | |
| Handedness- (Right%) | |
| Education-years | |
| Clinical data for PLS Patients | Symptom Duration- months |
| ALSFRS-r bulbar sub-score (max 12) | |
| ALSFRS-r upper limb sub-score (max 12) | |
| ALSFRS-r lower limb sub-score (max 12) | |
| ALSFRS-r respiratory sub-score (max 12) | |
| PUMNS: Penn UMN score | |
| Cortical Regions Evaluated | Precentral (Left)-mm |
| Precentral (Left)-mm | |
| Paracentral (Right)-mm | |
| Paracentral (Left)-mm | |
| Cortical metrics | Region-of-interest cortical thickness (avareged) |
| White Matter Tracts evaluated | Left Corticospinal Tract (Left) |
| Right Corticospinal Tract (Right) | |
| Body of the Corpus Callosum | |
| Diffusivity metrics evaluated | Mean Diffusivity (MD) |
| Fractional Anisotropy (FA) | |
| Axial Diffusivity (AD) | |
| Radial Diffusivity (RD) |
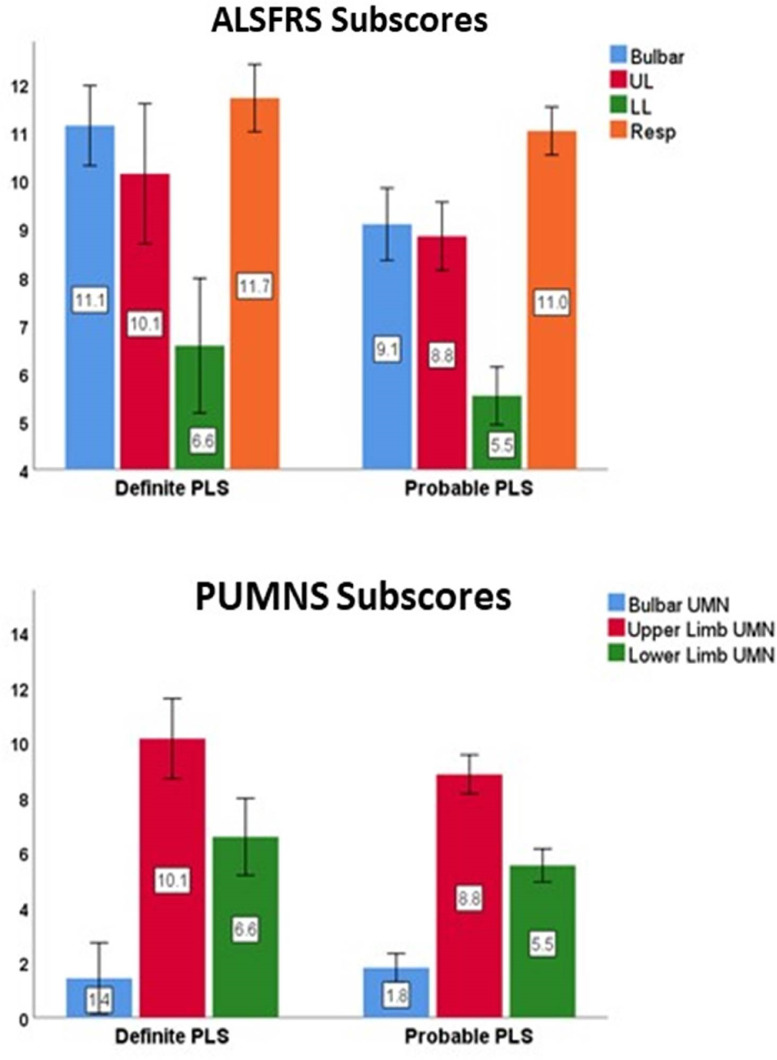
Fig. 1.
Bar charts displaying group means and 95% confidence intervals for ALSFRS-R and UMN sub-scales. ALSFRS: ALS functional rating scale, UL: Upper limb; LL: Lower Limb; Resp: Respiratory; UMN: Upper motor neuron; PUMNS: Penn UMN score.
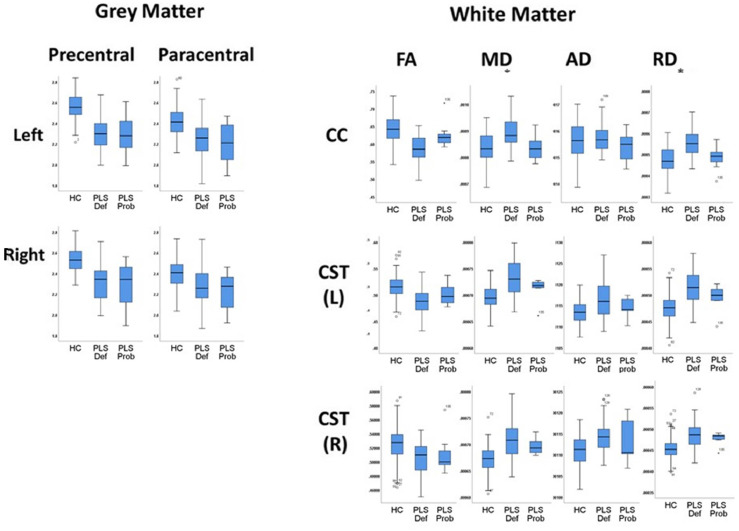
Fig. 2.
Boxplots depicting raw cortical thickness and white matter profiles in ‘probable PLS’ (PLS Prob), ‘definite PLS’ (PLS Def) and healthy controls (HC) CST: Corticospinal Tract; CC: Corpus Callosum; FA: Fractional Anisotropy; MD= Mean Diffusivity; AD= Axial Diffusivity; RD=Radial Diffusivity, L: Left, R: Right.
Fig. 3.
The diffusivity profile of ‘probable PLS’ (Prob PLS) and ‘definite PLS’ (Def PLS) with reference to healthy controls. Estimated marginal means of the relevant metrics were calculated for each structure with the following values age =58.66, gender=1.45, The estimated marginal means of healthy controls represent 100%.
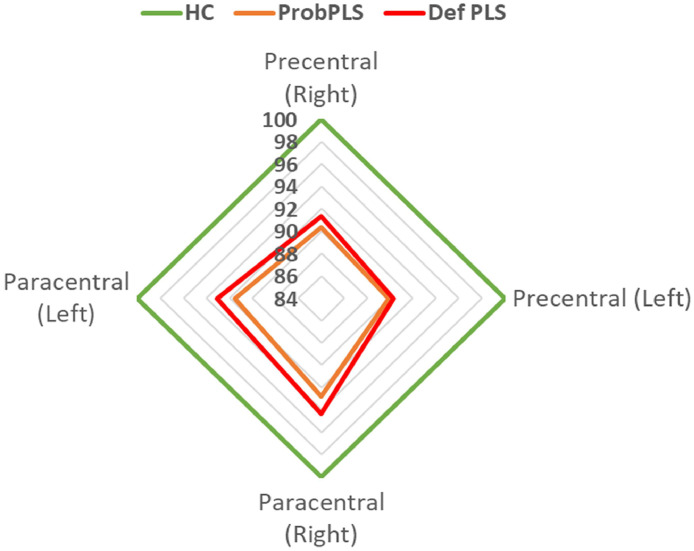
Fig. 4.
The cortical thickness profile of ‘probable PLS’ (Prob PLS) and ‘definite PLS’ (Def PLS) with reference to healthy controls. Estimated marginal means were calculated for each metric with the following values age =58.66, gender=1.45. The estimated marginal means of healthy controls represent 100%.
2. Experimental Design, Materials, and Methods
This prospective neuroimaging study was approved Ethics Committee of Beaumont Hospital, Dublin, and participants gave informed consent prior to participation. Patients underwent standardised clinical assessment by the same experienced neurologist on the day of magnetic resonance imaging which included the recording of functional rating scale sub-sores (ALSFRS-r) and the Penn Upper Motor Neuron Score (PUMNS). PLS patients were stratified by the 2020 consensus diagnostic criteria [5] into ‘probable PLS’ (n = 7) and ‘definite PLS’ (n = 32). A standardised imaging protocol was implemented which included T1-wieghted (T1w), T2-weighted (T2w), Diffusion tensor imaging (DTI), Fluid-attenuated inversion recovery (Flair) pulse sequences. T1-weighted images were acquired with a spatial resolution of 1 × 1 × 1 mm and DTI images with a resolution of 2.5 mm3. FLAIR images were acquired in axial orientation using an Inversion Recovery Turbo Spin Echo (IR-TSE) sequence: FOV = 230 × 183 × 150 mm, spatial resolution = 0.65 × 0.87 × 4 mm, 30 slices with 1 mm gap, TR/TE = 11,000 / 125 ms, TI = 2800 ms, 120° refocusing pulse, with flow compensation and motion smoothing and a saturation slab covering the neck region. FLAIR images were acquired to assess for vascular white matter lesion load and images were individually reviewed by the same experienced neurologist.
Raw MRI data underwent thorough quality control before pre-processing T1w and DTI images in standard pipelines [15], [16], [17]. Following registration of native T1w and DTI images to common space (MNI) and atlas based approach was used to retrieve imaging metrics from relevant anatomical regions [18], [19], [20].
Declaration of Competing Interest
None.
Acknowledgments
We thank all the patients with ALS and PLS for participating in this research study and we are also grateful for the participation of the healthy controls. This study was supported by the Spastic Paraplegia Foundation, Inc. (SPF). Professor Peter Bede is also supported by the Health Research Board (HRB EIA-2017-019), the EU Joint Programme – Neurodegenerative Disease Research (JPND), the Andrew Lydon scholarship, the Irish Institute of Clinical Neuroscience (IICN), and the Iris O'Brien Foundation; he is the patron of the Irish Motor Neuron Disease Association (IMNDA). Doctor Foteini Christidi is supported by the EU-IKY Scholarship Program (European Social Fund-ESF), the Greek “Reinforcement of Postdoctoral Researchers” grant (5033021) of the “Human Resources Development Program, Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF 2014–2020). Professor Russell L McLaughlin is supported by the Motor Neurone Disease Association (957–799) and Science Foundation Ireland (17/CDA/4737). Mr Mark A Doherty is supported by Science Foundation Ireland (15/SPP/3244). The sponsors had no role in the design of the study, data analyses, or the decision to submit these findings for publication.
References
- 1.Finegan E. Evolving diagnostic criteria in primary lateral sclerosis: the clinical and radiological basis of “probable PLS”. J. Neurol. Sci. 2020;417 doi: 10.1016/j.jns.2020.117052. [DOI] [PubMed] [Google Scholar]
- 2.Clark M.G. Loss of functional connectivity is an early imaging marker in primary lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19(7–8):562–569. doi: 10.1080/21678421.2018.1517180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finegan E. Primary lateral sclerosis: a distinct entity or part of the ALS spectrum? Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(3–4):133–145. doi: 10.1080/21678421.2018.1550518. [DOI] [PubMed] [Google Scholar]
- 4.Gordon P.H. The natural history of primary lateral sclerosis. Neurology. 2006;66(5):647–653. doi: 10.1212/01.wnl.0000200962.94777.71. [DOI] [PubMed] [Google Scholar]
- 5.Turner M.R. Primary lateral sclerosis: consensus diagnostic criteria. J. Neurol. Neurosurg. Psychiatry. 2020;91(4):373–377. doi: 10.1136/jnnp-2019-322541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finegan E. Widespread subcortical grey matter degeneration in primary lateral sclerosis: a multimodal imaging study with genetic profiling. Neuroimage Clin. 2019;24 doi: 10.1016/j.nicl.2019.102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finegan E. The clinical and radiological profile of primary lateral sclerosis: a population-based study. J. Neurol. 2019;266(11):2718–2733. doi: 10.1007/s00415-019-09473-z. [DOI] [PubMed] [Google Scholar]
- 8.Meoded A. Cerebro-cerebellar connectivity is increased in primary lateral sclerosis. NeuroImage Clin. 2015;7:288–296. doi: 10.1016/j.nicl.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Floeter M.K., Mills R. Progression in primary lateral sclerosis: a prospective analysis. Amyotr. Lateral Scler. 2009;10(5–6):339–346. doi: 10.3109/17482960903171136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bede P. Brainstem pathology in amyotrophic lateral sclerosis and primary lateral sclerosis: a longitudinal neuroimaging study. Neuroimage Clin. 2019;24 doi: 10.1016/j.nicl.2019.102054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bede P., Querin G., Pradat P.F. The changing landscape of motor neuron disease imaging: the transition from descriptive studies to precision clinical tools. Curr. Opin. Neurol. 2018;31(4):431–438. doi: 10.1097/WCO.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 12.Chipika R.H. Tracking a fast-moving disease: longitudinal markers, monitoring, and clinical trial endpoints in ALS. Front. Neurol. 2019;10:229. doi: 10.3389/fneur.2019.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cedarbaum J.M. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J. Neurol. Sci. 1999;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 14.Quinn C. Reliable and efficient scale to assess upper motor neuron disease burden in amyotrophic lateral sclerosis. Muscle Nerve. 2020;61(4):508–511. doi: 10.1002/mus.26764. [DOI] [PubMed] [Google Scholar]
- 15.Omer T. Neuroimaging patterns along the ALS-FTD spectrum: a multiparametric imaging study. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(7–8):611–623. doi: 10.1080/21678421.2017.1332077. [DOI] [PubMed] [Google Scholar]
- 16.Schuster C., Hardiman O., Bede P. Development of an automated MRI-based diagnostic protocol for amyotrophic lateral sclerosis using disease-specific pathognomonic features: a quantitative disease-state classification study. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bede P. Virtual brain biopsies in amyotrophic lateral sclerosis: diagnostic classification based on in vivo pathological patterns. Neuroimage Clin. 2017;15:653–658. doi: 10.1016/j.nicl.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuster C. The segmental diffusivity profile of amyotrophic lateral sclerosis associated white matter degeneration. Eur. J. Neurol. 2016;23(8):1361–1371. doi: 10.1111/ene.13038. [DOI] [PubMed] [Google Scholar]
- 19.Bede P. Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: a multimodal neuroimaging study. Brain Imaging Behav. 2018;12(6):1696–1707. doi: 10.1007/s11682-018-9837-9. [DOI] [PubMed] [Google Scholar]
- 20.Nasseroleslami B. Characteristic increases in EEG connectivity correlate with changes of structural MRI in amyotrophic lateral sclerosis. Cereb. Cortex. 2019;29(1):27–41. doi: 10.1093/cercor/bhx301. [DOI] [PubMed] [Google Scholar]