ABSTRACT
Severe COVID-19 infection results in bilateral interstitial pneumonia, often leading to acute respiratory distress syndrome (ARDS) and pulmonary fibrosis in survivors. Most patients with severe COVID-19 infections who died had developed ARDS. Currently, ARDS is treated with supportive measures, but regenerative medicine approaches including extracellular vesicle (EV)-based therapies have shown promise. Herein, we aimed to analyse whether EV-based therapies could be effective in treating severe pulmonary conditions that affect COVID-19 patients and to understand their relevance for an eventual therapeutic application to human patients. Using a defined search strategy, we conducted a systematic review of the literature and found 39 articles (2014–2020) that reported effects of EVs, mainly derived from stem cells, in lung injury models (one large animal study, none in human). EV treatment resulted in: (1) attenuation of inflammation (reduction of pro-inflammatory cytokines and neutrophil infiltration, M2 macrophage polarization); (2) regeneration of alveolar epithelium (decreased apoptosis and stimulation of surfactant production); (3) repair of microvascular permeability (increased endothelial cell junction proteins); (4) prevention of fibrosis (reduced fibrin production). These effects were mediated by the release of EV cargo and identified factors including miRs-126, −30b-3p, −145, −27a-3p, syndecan-1, hepatocyte growth factor and angiopoietin-1. This review indicates that EV-based therapies hold great potential for COVID-19 related lung injuries as they target multiple pathways and enhance tissue regeneration. However, before translating EV therapies into human clinical trials, efforts should be directed at developing good manufacturing practice solutions for EVs and testing optimal dosage and administration route in large animal models.
KEYWORDS: Exosome, acute lung injury, ali, sars-CoV-2, coronavirus, regenerative medicine, pandemic, cell-free, microRNA, miRNA
Introduction
On 11 March 2020, the World Health Organization declared the coronavirus disease (COVID-19) outbreak a pandemic [1]. COVID-19 is a new disease in humans that is caused by the severe acute respiratory syndrome (SARS) CoV-2 virus, a positive-sense single-stranded RNA virus [2]. This viral outbreak started at the end of 2019 in Wuhan, China, and has quickly been spreading across the world [3,4]. In humans, transmission of this new coronavirus occurs primarily via respiratory droplets and often results in a respiratory tract infection. Although we are still learning about the epidemiology of this viral illness, COVID-19 seems to affect patients of different age and sex with varying degrees of virulence [5,6]. Some individuals are either asymptomatic or have a mild disease with fever, cough and fatigue, whereas others develop bilateral interstitial pneumonia with abnormal findings on chest computed tomography [7–9]. Moreover, some subjects have a severe form of COVID-19 that rapidly progresses to acute respiratory distress syndrome (ARDS) and might result in sepsis and multiple organ failure [3,4]. ARDS is a life-threatening condition characterized by an acute onset (within a week of a known insult) of respiratory failure not fully explained by cardiac function or volume overload, with diffuse opacities on lung imaging, and severe hypoxaemia requiring mechanical ventilation, as summarized in the 2012 Berlin definition [10,11]. In ARDS, lungs have diffused alveolar and endothelial cell damage with severe inflammation, increased vascular permeability and poor pulmonary oxygenation (Figure 1). Further, pulmonary interstitial fibrosis caused by excessive fibrin deposition is observed at autopsy, as well as in ARDS survivors with healing pneumonia (Figure 1) [12]. In COVID-19 patients, ARDS appears to be even more severe. According to the first available data, 1 in 4 patients with COVID-19 (26%) develops ARDS, which has been shown to be a negative prognostic factor for survival, as more than 90% of non-survivors developed ARDS [3]. Moreover, it has been anticipated that even if the virus is fully eradicated, a proportion of COVID-19 survivors will develop pulmonary fibrosis [13]. This would be in line with similar coronavirus outbreaks, such as the Middle East Respiratory Syndrome (MERS), where a third of survivors had lung fibrosis after hospital discharge [14].
Figure 1.
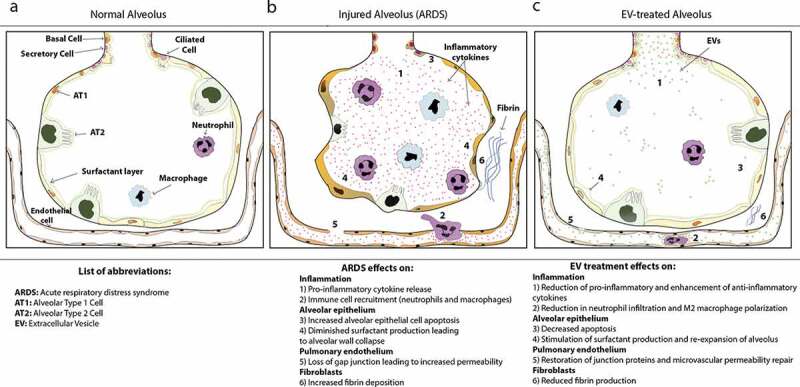
Restorative effects of extracellular vesicle (EV) therapies in acute respiratory distress syndrome (ARDS). Compared to the normal alveolus (A), ARDS (B) is characterized by increased pulmonary inflammation [1], increased immune cell recruitment including neutrophils and macrophages [2], increased alveolar epithelial cell apoptosis [3], inactivated surfactant from degradation of alveolar surfactant layer and alveolar wall collapse [4], as well as increased endothelial cell permeability and gap junction formation [5], fibrin deposition [6] and increased platelet formation. EV treatments (C) can ameliorate the majority of these ARDS features by resolving inflammation and angiogenesis [1], altering local immune cell recruitment [2], decreasing apoptosis in alveolar epithelial cells [3], stimulating surfactant proteins leading to re-expansion of the alveolus [4], restoring endothelial junction proteins and decreasing endothelial barrier permeability [5], and reducing fibrin levels [6].
During this global pandemic, we are witnessing an incredible amount of efforts directed at stopping the viral spread, with public health measures such as social distancing, as well as a rush to develop a new vaccine or an effective antiviral drug to combat COVID-19. Whilst awaiting these more direct anti-viral measures, efforts are being aimed at testing new strategies that would attenuate the overactive response of the infected lungs and prevent or treat the complications of the viral pneumonia. At present, there is no effective treatment for ARDS beyond supportive measures. A regenerative medicine approach with the use of mesenchymal stromal cells (MSCs) has shown promise as it targets multiple pathways [15,16]. However, the use of cell-based therapies still has hurdles to pass, including the potential cell variability, the large-scale production and the reconstitution limitations of cryopreserved cells [17]. Extracellular vesicles (EVs) could be an alternative treatment strategy in this context as they have several advantages: EVs are cell-free, immunologically innocuous, not teratogenic and contain no adventitious agents [18,19]. Moreover, EVs derived from various sources have been used to modulate the inflammatory response in sepsis and attenuate multiple organ failure [20,21]. In this regard, EVs could be addressing the plea for a multi-targeted therapy that has been made on the principle that a single drug or antiviral therapy would unlikely be able to improve the most severe forms of COVID-19 [22]. With these promising features and the urgent need for an effective therapy for COVID-19 patients, a phase I clinical trial has recently been registered in China (NCT04276987) for the investigation of MSC-EVs as a therapy for ARDS secondary to COVID-19 [23]. While this reflects the increasing interest in testing the therapeutic potential of EVs, it remains unclear whether we are ready to use EVs as a treatment to battle the COVID-19 pandemic.
The aim of this systematic review was to analyse whether EV-based therapies could be effective in treating severe pulmonary conditions that affect COVID-19 patients, namely severe pneumonia, ARDS, acute lung injury (ALI) and pulmonary fibrosis, and to understand their relevance for an eventual therapeutic application to human patients. This review will inform the international scientific community not only about the potential efficacy of EVs on the injured lung, but also about the optimal EV source, administration route, dosage and other factors that are critical to translate laboratory findings from the bench to the bedside.
Methods
A systematic review of the literature was conducted to search for all articles reporting the experimental or clinical use of any type of EVs as a treatment for lung conditions related to COVID-19, i.e. pneumonia, ARDS, ALI and pulmonary fibrosis. This review was performed following the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions [24], and the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [25]. This review was registered on the international prospective register of systematic reviews PROSPERO (registration #CRD42020176266) (National institute for Health Research) [26]. The systematic review was conducted using a defined search strategy by four investigators (KK, RF, LA and GL) using electronic databases (PubMed/MEDLINE, Scopus, Cochrane Collaboration and Web of Science) (Table 1).
Table 1.
Inclusion criteria of the systematic review.
| Publication | |
|---|---|
| Language | English |
| Time period | January 1950 – April 2020 |
| Subject | All species |
| Study type | Randomized Controlled
Trial Prospective Case-control Cohort Retrospective |
| Excluded | Case-reports Opinion articles Editorials Letters Grey Literature |
| Keywords | Exosomes Extracellular vesicles Lung Respiratory Pulmonary |
Inclusion/exclusion criteria
MeSH headings and terms used were “Exosomes OR Extracellular vesicles” and “Lung OR Respiratory OR Pulmonary” (Supplemental file 1). Studies published from 1950 until April 24th, 2020 were included. Reference lists were searched to identify relevant cross-references. Case reports, opinion articles, editorials and letters were excluded. All grey literature publications (i.e. reports, theses, conference proceedings, bibliographies, commercial documentations and official documents not published commercially) were excluded, except for abstracts published in the online accessible books of the International Society for Extracellular Vesicles annual meetings (ISEV; www.isev.org) held between 2016 and 2019 (Table 1). All studies that reported at least one outcome of interest (pneumonia, ARDS, ALI, or pulmonary fibrosis) were included in the analysis. After the selection of potential eligible papers using the title and the abstract, three reviewers (KK, RF and LA) independently retrieved the full-text articles to assess the final eligibility. Any disagreement over the eligibility of a specific study was resolved through the discussion with a fourth author (AZ). For the definition and classification of EVs, we followed the minimal information for studies of extracellular vesicles (MISEV2018) guidelines [27]. Synthetic-made nanoparticles were excluded by manual curation from the analysis as they did not meet the ISEV EV definition (Figure 2).
Figure 2.
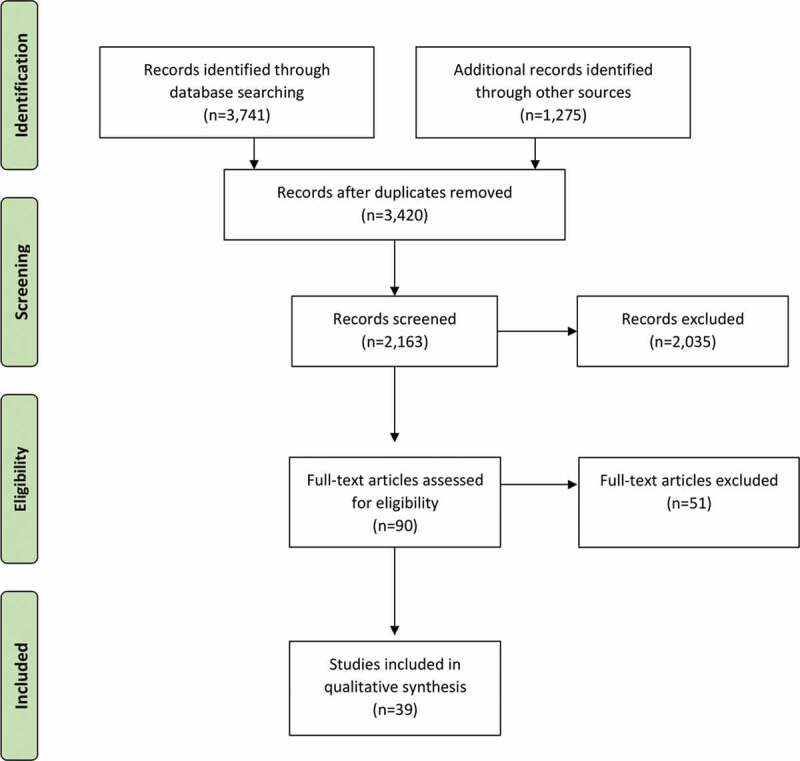
Diagram of workflow in the systematic review according to the PRISMA statement.
Outcome measures
We included the type of experimental model, EV source, EV separation and characterization techniques, details of EV administration including dosage and route, and EV biological effects. Additional evaluation of EV pathways/mechanisms of action was performed. Further analysis was conducted on comparative studies which were classified as having two or more different comparative EV groups, including but not limited to different sources of EVs or derivation/modification strategies on the same in vitro or in vivo model of administration.
Quality assessment
The risk of bias for each study was evaluated in duplicate (RF and GL) using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias tool [28]. The Confidence in the Evidence from Reviews of Qualitative research (CERQual) tool was also utilized to assess evidence quality of each outcome in the systematic review [29]. The aim of this tool is to evaluate the extent to which the results of animal studies can be generalized to clinical trials or application, as determined by the results of evidence quality. Differences between the two reviewers (RF and GL) were resolved through consensus and discussion with a third author (AZ). Moreover, the present study was assessed in duplicate by two investigators (RF and GL) using A Measurement Tool to Assess Systematic Reviews, AMSTAR 2 [30]. A PRISMA figure following PRISMA checklist criteria was created [25].
Results
Of the 4,925 titles and abstracts screened, 233 articles were reviewed, 90 were assessed for eligibility and 39 were included in the final analysis (Figure 2) [31–69]. The selected studies were published between 2014 and 2020 and included in vitro, ex vivo, and in vivo studies (Table 2). None of the studies selected was conducted on human subjects. The most frequently used source of EVs was from MSCs derived from the bone-marrow or the umbilical cord of animal or human origin. EVs were also isolated from other stem cell sources such as adipose tissue, urine (urine-derived induced pluripotent stem cells) and menstrual blood (endometrial stem cells). Other sources of EVs included: fibroblasts, blood (serum and whole blood), placenta, lung spheroids, pulmonary endothelial cells and endothelial progenitor cells, primary adipose tissue, amnion epithelial cells, neutrophils and Staphylococcus aureus. The majority of the in vivo studies were performed in mouse models, with only one reporting data of EV therapy in a large animal model (pigs) [59]. The selected studies addressed the effects of EVs as a therapy for ALI/ARDS and pneumonia, as well as for prevention or treatment of pulmonary fibrosis. To model ALI/ARDS, most studies used administration of either lipopolysaccharide (LPS), bleomycin or Escherichia coli, in isolation or in association with mechanical ventilation injury, trauma, influenza virus injection or carbonyl dichloride inhalation.
Table 2.
Articles reporting the effects of EV therapy on lung injury models.
| Study | Source of EVs | Model | Sample | EV effects | EV treatment outcome summary |
|---|---|---|---|---|---|
| Wang et al. 2020 [31] | Adipose MSCs | ALI: in vivo mouse (LPS) | Lung tissue BAL |
↓TNF-α, ↓IL-1β, ↓IL-6, ↑IL-10, ↓iNOS, ↓NF-κB | Reduction of inflammation (decreased pro-inflammatory cytokines, increased anti-inflammatory cytokines, and reduced neutrophils in alveolar fluid), pulmonary endothelial barrier permeability, and alveolar septal thickness |
| ALI: in vitro mouse BMDMs (LPS) | BMDMs | ↓TNF-α, ↓IL-1β, ↓iNOS, ↑YM-1, ↑MRC-1, ↑miR-27a-3p | |||
| Dinh et al. 2020 [32] | Lung spheroid cells | Fibrosis: in vivo mouse (BLM) | Lung tissue | ↑AQP5, ↑vWF, ↓αSMA, ↓SMAD3, ↓Hydroxyproline | Promotion of alveolar repair (increased aquaporin), attenuation of vascular injury and reduction of collagen deposition |
| Gao et al. 2020 [33] | Adipose MSCs | ALI: in vivo rat (PM2.5) | Lung tissue BAL |
↓TNF-α, ↓ROS | Reduction of inflammation (decreased pro-inflammatory cytokines and oxidative stress), alveolar epithelial apoptosis and necrosis, and alveolar wall oedema and collapse |
| ALI: in vitro rat AEC2 (PM2.5) | AEC2 | ↓Apoptosis | |||
| Yu et al. 2020 [34] | Adipose tissue, Adipose MSCs, Serum | ALI: in vivo mouse (Ventilator-induced lung injury) | Lung tissue BAL |
↓IL-6, ↓TRPV4, ↑β-Catenin, ↑VE-Cadherin ↓MPO |
Reduction of inflammation (decreased pro-inflammatory cytokines) and pulmonary endothelial barrier permeability |
| ALI: in vitro PMVECs (Cyclic stretching) | PMVECs | ↓TNF-α, ↓IL-6, ↓TRPV4, ↑β-Catenin, ↑VE-Cadherin |
|||
| Huang et al. 2019 [35] | Adipose MSCs | ALI: in vivo mouse (LPS) | Lung tissue BAL |
↓IL-1β and ↑IL-10 | Reduction of inflammation (lower neutrophil and macrophage recruitment in alveolar fluid) and alveolar wall thickness |
| ALI: in vitro mouse BMDMs (LPS) | BMDMs | ↓IL-6, ↓IL-1β, ↓TNF-α, ↓iNOS, ↑TGF-β1, ↑YM-1 | |||
| Silva et al. 2019 [36] | Bone marrow MSCs | ARDS: in vivo mouse (LPS) | Lung tissue | ↓TNF-α, ↓IL-6, ↓KC, ↓VEGF, ↓TGF- β | Reduction of inflammation (lower neutrophils and macrophages in alveolar fluid) and alveolar wall collapse |
| ARDS: in vitro mouse alveolar macrophages (LPS) | Serum | ↓iNOS, ↓IL-1β, ↓IL-6, ↑Arginase, ↑TGF-β | |||
| Zhang et al. 2019 [37] | PMVECs with high levels of Syndecan-1 (SDC1) | ALI: in vivo mouse (LPS) | Lung tissue | ↓IL-6, ↓IL-1β, ↓TNF-α | Reduction of inflammation (decreased pro-inflammatory cytokines), preservation of pulmonary endothelial function, and decrease in alveolar wall thickness |
| ALI: in vitro mouse PMVECs (LPS) | PMVECs | ↓F-actin, ↓MLC, ↓MYPT1, ↓NF-κB |
|||
| Hao et al. 2019 [38] | Bone marrow MSCs | ALI: in vivo mouse (E. coli) | BAL | ↓MIP-2, ↓TNF-α, ↑LTB4 | Antimicrobial effect (increased monocyte phagocytosis and decreased bacterial levels) and reduction of inflammation (decreased leukocytes and neutrophils in alveolar fluid) |
| ALI: in vitro mouse RAW267.4 cells (LPS) | RAW267.4 | ↓MRP1-protein, ↑miR-145 | |||
| Kim et al. 2019 [39] | Placental chorionic and decidual MSCs | ALI: in vitro human BEAS-2B and THP-1 cells (LPS) | THP-1 BEAS-2B |
↓TNF-α | Reduction of inflammation (decreased oxidative stress and pro-inflammatory cytokines) and restoration of bronchiolar epithelial cell migration and proliferation |
| Yi et al. 2019 [40] | Bone marrow MSCs | ALI: in vivo mouse (LPS) | Lung tissue | ↓MPO, ↓IL-1β, ↓TNF-α, ↓IL-6, ↑KGF, ↑IL-10, ↓SAA3 | Reduction of inflammation (decreased pro-inflammatory cytokines), alveolar epithelial apoptosis, and lung interstitial vessel and alveolar septal thickness |
| ALI: in vitro mouse AEC2 (LPS) | BAL AEC2 |
↓SAA3, ↓NF-κB, ↓ERK1/2, ↓p38MAPK, ↓MEK1/2, ↓JNK, ↑miR-30b-3p | |||
| Chen et al. 2019 [41] | Umbilical cord MSCs | ALI: in vivo rat (BLM) | Lung tissue BAL |
↓TNF-α, ↓IL-6, ↑HGF, ↑c-Met, ↑Akt, ↑mTOR | Reduction of inflammation (reduced leukocytes and neutrophils in alveolar fluid and pro-inflammatory cytokines), alveolar epithelial apoptosis, alveolar wall thickness, pulmonary hyaline membrane, and collagen deposition |
| ALI: in vitro rat AEC2 and rat PMVECs (BLM) | AEC2 PMVECs |
↑c-Met, ↑Akt, ↑mTOR, ↑HGF | |||
| Xu et al. 2019 [42] | Bone marrow MSCs | ALI: in vivo rat (Phosgene-induced) | Lung tissue BAL Plasma |
↓MMP-9 ↑SP-C ↓TNF-α, ↓IL-1β, ↓IL-6, ↑IL- 10 | Restoration of respiratory function (increased peak of inspiratory and expiratory flow, decreased lung resistance), reduction of inflammation (decreased oedema, pro-inflammatory cytokines), promotion of alveolar epithelial surfactant synthesis |
| Mansouri et al. 2019 [43] |
Bone marrow MSCs |
Fibrosis: in vivo mouse
(BLM) |
Lung tissue BAL |
↓Arg-1, ↓CCL2 |
Reduction of inflammation (decreased pro-inflammatory
cytokine and Arg-1), alveolar epithelial apoptosis, septal thickness, and
pulmonary collagen deposition |
| Loy et al. 2019 [44] | Umbilical cord MSCs | ALI: in vitro human AECs (H5N1) | AECs | No particular mechanism studied | Restoration of alveolar fluid clearance and reduction of alveolar protein permeability |
| Li et al. 2019 [45] | Bone marrow MSCs | ALI: in vivo rat (Traumatic) | Lung tissue BAL |
↓P2X7, ↓MDA, ↓H2O2, ↑GSH, ↑SOD,
↓TNF-α, ↓IL-6, ↓IL-8, ↑IL-10 |
Reduction of inflammation (decreased oedema and pro-inflammatory cytokines), capillary hyperaemia, and alveolar wall thickness |
| Varkouhi et al. 2019 [46] | γ–primed umbilical cord MSCs | ALI: in vivo rat (E. coli) | Lung tissue BAL |
↑eNOS, ↓TNF-α | Increase of animal survival, reduction of inflammation (decreased pro-inflammatory cytokine, increase of macrophage bacterial phagocytosis), restoration of alveolar epithelial surfactant synthesis and pulmonary endothelial cell permeability, and reduction of alveolar wall thickness |
| Zhou et al. 2019 [47] | Umbilical cord EPC (rich in miR-126) | ALI: in vivo mouse (LPS) | Lung tissue BAL |
↓TNF-α, ↓IL-6, ↓IL-1β, ↓IFN-γ, ↓MIP-1, ↓MIP-2, ↓MIG, ↓IP-10, ↓MPO | Reduction of inflammation (reduced pro-inflammatory cytokines and neutrophils in alveolar space), alveolar wall thickness, and hyaline membrane formation |
| ALI: in vitro human AECs (LPS) | AECs | ↑Claudin1, ↑Claudin4, ↑Occludin | |||
| Park et al. 2019 [48] | Bone marrow MSCs | ALI: ex vivo perfused human lung (E. coli) | Lung tissue BAL |
TNF-α (no difference) | Antimicrobial effects (increased macrophages phagocytosis and decreased bacterial levels), restoration of alveolar epithelial surfactant synthesis and alveolar fluid clearance, reduction of pulmonary endothelial permeability and alveolar wall thickness |
| Royce et al. 2019 [49] | Amnion epithelial cells | Fibrosis: in vivo mouse (BLM) | Lung tissue | ↓TGF- β | Reduction of tissue inflammation and myofibroblast accumulation |
| Sun et al. 2019 [50] | Menstrual blood-derived endometrial stem cells | Fibrosis: in vivo mouse (BLM) | Lung tissue | ↓Hydroxyproline, ↓MDA, ↑Let-7 | Reduction of inflammation (decreased inflammasome), DNA damage (decreased ROS) and collagen deposition |
| Fibrosis: in vitro mouse AECs (BLM) | AECs | ↓ROS, ↓LOX1, ↓NLRP3, ↓Hydroxyproline, ↓MDA, ↑Let-7 | |||
| Liu et al. 2019 [51] | Umbilical cord MSCs | ALI: in vivo rat (Burn) | Lung tissue Serum |
↓TNF-α, ↓IL-1β, ↓IL-6, ↑IL- 10, ↓MDA, ↓MPO, ↑SOD,
↓TLR4, ↓p-p65, ↑miR-451 |
Reduction of inflammation (lower pro-inflammatory cytokines and decreased immune cell recruitment) |
| Sun et al. 2018 [52] | Whole blood | Fibrosis: in vivo mouse (Zeocin) | Lung tissue | ↓Hydroxyproline | Reduction of inflammation (decreased immune cell recruitment), alveolar wall thickness and collagen deposition |
| Bandeira et al. 2018 [53] | Adipose MSCs | Fibrosis/Silicosis: in vivo mouse (Silica) | Lung tissue | ↓TGF-β, ↓TNF-α, ↓IL-1β | Reduction of inflammation (decreased pro-inflammatory cytokines and macrophages) and collagen deposition |
| Tan et al. 2018 [54] | Amnion epithelial cells | Fibrosis: in vivo mouse (BLM) | Lung tissue | ↑CTNNB1, ↑BMP4, ↑BMPR1, ↑FOXM1, ↑LEF1, ↑NFATC1, ↑PGK1, ↑PTN, ↑SCA1, ↑WLS, ↓cMYC | Prevention and reduction of inflammation (lower CD4 + T cells and pulmonary interstitial macrophages) and collagen deposition |
| Hu et al. 2018 [55] | Bone marrow MSCs | ALI: in vitro human LMVECs (Cytomix) | LMVECs | ↑VE-cadherin, ↑ZO-1, ↑p-myosin light chain 2, ↑Ang1, ↑S1PK | Decrease of microvascular permeability |
| Zhang et al. 2018 [56] | Pulmonary microvascular endothelial cells | ALI: in vivo mouse (LPS) | Lung tissue | ↓TNF-α, ↓IL-1β, ↓IL-6 | Reduction of inflammation (lower pro-inflammatory cytokines) and restoration of pulmonary endothelial function (rearranged cytoskeleton and decreased microvascular permeability) |
| BAL | ↓TNF-α, ↓IL-2, ↓IL-3, ↓IL-6, ↓GM-CSF, ↓CCL-2, ↓MCP-5, ↓CCL-5 | ||||
| Shah et al. 2018 [57] | Bone marrow MSCs | ARDS: in vivo mouse (LPS) | Lung tissue | ↑Runx1p66/p52, ↑TβRI/Alk5 | Reduction of perivascular area and interstitial thickness |
| Wu et al. 2018 [58] |
Endothelial progenitor cells |
ALI: in vivo rat
(LPS) |
Lung tissue |
↑RAF, ↑ERK, ↓SPRED-1, ↓MDA, ↑miR-126 |
Enhancement of gas exchange (improved
PaO2), reduction of inflammation (reduced oedema and neutrophil in
alveolar space), regeneration of pulmonary endothelial cells and reduction of
alveolar wall thickness |
| Khatri et al. 2018 [59] | Bone marrow MSCs | ALI: in vivo pig (Influenza) | Lung tissue BAL Nasal swabs |
↓TNF-α, ↓CXCL10, ↑ IL-10 | Reduction of inflammation (decreased pro-inflammatory cytokines and chemokines), lung endothelial cell apoptosis, alveolar wall thickness and collapse, and inhibition of influenza virus replication |
| ALI: in vitro pig LECs (Influenza) | LECs | ↓Apoptosis | |||
| Wang et al. 2017 [60] | Bone marrow MSCs | ALI: in vitro mouse PMVECs (LPS) | PMVECs | ↑VE-cadherin, ↑ZO-1,
↓IL-6, ↑IL-10 |
Reduction of inflammation (decreased pro-inflammatory cytokines) and pulmonary endothelial permeability |
| Morrison et al. 2017 [61] | Bone marrow MSCs | ARDS: in vivo mouse (LPS) | BAL | ↓TNF-α | Reduction of inflammation (lower pro-inflammatory cytokine and neutrophils in alveolar fluid) |
| Gao et al. 2017 [62] | Neutrophils loaded with piceatannol | ALI: in vivo mouse (LPS) | Lung tissue BAL Plasma |
↓TNF-α, ↓IL-6, ↓MPO | Increase in animal survival, reduction of inflammation (lower pro-inflammatory cytokines, leukocytes and neutrophils in alveolar fluid) and pulmonary endothelial permeability |
| ALI: in vitro human HUVECs (LPS) | HUVECs | ↓ICAM-1, ↓IκBα, ↓p65 | |||
| Tang et al. 2017 [63] | Bone marrow MSCs | ALI: in vivo mouse (LPS) | Lung tissue BAL | ↓MIP-2 | Reduction of inflammation (decreased pro-inflammatory cytokines, leukocytes and neutrophils in alveolar fluid), pulmonary endothelial permeability, and alveolar wall thickness |
| ALI: in vitro human LMVECs and mouse RAW264.7 cells (LPS) | LMVECs RAW264.7 |
↓TNF-α, ↑IL-10 | |||
| Ju et al. 2017 [64] | Urine-derived pluripotent stem cells | ALI: in vitro human MVECs (LPS) | MVECs | ↓ICAM-1, ↓MPO | Reduction of inflammation (attenuated neutrophils adhesion and blocked inflammatory activation in the pulmonary endothelium) |
| Shentu et al. 2017 [65] | Bone marrow MSCs | Fibrosis: in vitro human LFCs | LFCs | ↓αSMA, ↓Col IA1, ↓Col III A1 | Reduction of myofibroblast accumulation and collagen deposition |
| Li et al. 2015 [66] | Bone marrow MSCs | ALI: in vivo mouse (E. coli) | BAL | ↓MIP-2 | Reduction of inflammation (decreased pro-inflammatory cytokines, leukocytes and neutrophils and increased anti-inflammatory cytokines in the alveolar fluid) |
| ALI: in vitro mouse RAW264.7 cells (E. coli) | RAW264.7 | ↓TNF-α, ↑IL-10 | |||
| Choi et al. 2015 [67] | S. aureus | Pneumonia: in vivo mouse (Staphylococcus aureus) | Lung tissue Blood |
↓IL-1β, ↓IL-6, ↑IL-17, ↑IL-4, ↑IFN-γ | Increase in animal survival, reduction of inflammation (decreased pro-inflammatory cytokines) and induction of adaptative immunity (increased T cell response) |
| Monsel et al. 2015 [68] | Bone marrow MSCs | Pneumonia: in vivo
mouse (E. coli) |
Lung tissue BAL |
↓MIP-2, ↑KGF, ↓TNF-α | Increase in animal survival and antimicrobial effect (increased monocyte phagocytosis), reduction of inflammation (lower pro-inflammatory cytokines and neutrophil in alveolar space), pulmonary endothelial permeability, and alveolar wall thickness |
| Pneumonia: in vitro human AEC2 (LPS and Cytomix) | AEC2 | ||||
| Zhu et al. 2014 [69] | Bone marrow MSCs | ARDS: in vivo
mouse (E. coli) |
Lung tissue BAL |
↓MIP-2 | Reduction of inflammation (lower pro-inflammatory cytokines, leukocytes and neutrophils in alveolar fluid) and interstitial thickness |
| ARDS: in vitro mouse RAW264.7 cells (E. coli) | RAW264.7 | ↑IL-10, ↓TNF-α, ↓MIP-2 |
Abbreviations:
AEC2: Alveolar epithelial cells type 2; AECs: Alveolar epithelial cells; ALI: Acute lung injury; ANG1: Angiopoietin 1; AQP: Aquaporin; ARDS: Acute respiratory distress syndrome; BAL: Bronchioalveolar lavage; BEAS-2B: Bronchial epithelial cells; BLM: Bleomycin; BMDMs: Bone marrow-derived macrophages; BMP: Bone morphogenetic protein; BMPR1: Bone morphogenetic protein receptor 1; COL: Collagen; CTNNB1: Catenin beta 1; CXCL: Chemokine (C-X-C motif) ligand; EPC: Endothelial progenitor cell; ERK1/2: Extracellular regulated kinase ½; EVs: Extracellular vesicles; FOXM: Forkhead Box M1; GM-CSF: Granulocyte macrophage colony stimulating factor; HGF: Hepatocyte growth factor; HUVECs: Human umbilical vein endothelial cells; ICAM-1: Intercellular adhesion molecule-1; IFN: Interferon; IL: Interleukin; IP: Interferon gamma-induced protein; IкBα: Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; JNK: c-Jun N-terminal kinases; KC: Keratinocyte chemoattractant; KGF: Keratinocyte growth factor; LECs: Lymphatic endothelial cells; LEF1: Lymphoid enhancer-binding factor-1; LFCs: Lung fibroblast cells; LMVEC: Lung microvascular endothelial cell; LOX1: Lectin-like oxidized LDL receptor-1; LPS: Lipopolysaccharide; LTB4: Leukotriene B4; MCP: Monocyte chemotactic protein; MDA: Malondialdehyde; MEK1/2: P-dual specificity mitogen-activated protein kinase ½; MIG: Monokine induced by gamma interferon; MIP: Macrophage induced protein; MIR: MicroRNA; MMP-9: Matrix metalloproteinase-9; MPO: Myeloperoxidase; MRC-1: Mannose Receptor C-Type 1; MRP1: Multidrug resistance associated protein 1; MSC: Mesenchymal stem/stromal cell; MVECs: Microvascular endothelial cells; NFATC1: Nuclear factor of activated T-cells, cytoplasmic 1; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3: NLR Family pyrin domain containing 3; NOS: Nitric oxide synthase (iNOS – inducible, eNOS – endothelial); P38MAPK: p38 mitogen-activated protein kinase; PGK1: Phosphoglycerate kinase 1; PM2.5: fine particulate matter; PMVECs: Pulmonary microvascular endothelial cells; PTN: Pleiotrophin; RAW267.4: Monocyte/macrophage lineage; ROS: Reactive oxygen species; S1PK: Sphingosine kinase; SAA3: Serum amyloid A3; SCA1: Stem cell antigen-1; SMA: Smooth muscle actin; SMAD3: Mothers against decapentaplegic homolog 3; SOD: Superoxide dismutase; SP-C: Surfactant protein-C; SPRED-1: Sprouty-related, EVH1 domain-containing protein 1; TGF: Transforming growth factor; THP-1: Human monocyte cell line; TLR: Toll-like receptor; TNF: Tumour necrosis factor; TRPV4: Transient receptor potential vanilloid 4; TβRI: Transforming growth factor-beta receptor I; VE-Cadherin: Vascular endothelial cadherin; VEGF: Vascular endothelial growth factor; VWF: Von Willebrand factor; WLS: Wnt Ligand Secretion Mediator; YM-1: Chitinase 3-like 3, a macrophage protein; ZO-1: Tight junction protein
Overall, the effects of the different EV therapies on the experimental models were reported to recover lung injury, improve respiratory function and increase animal survival rate (Table 2). In ALI/ARDS, EV administration led to reduction of inflammation, alveolar epithelial regeneration and repair of the pulmonary endothelium [39,60,62] The EV modulation of the inflammatory response was primarily achieved by reduction of pro-inflammatory cytokines and neutrophil infiltration, enhancement of anti-inflammatory cytokines and macrophage polarization to the M2 reparative phenotype. Specifically, EV administration resulted in the downregulation of interleukin (IL)-1β, tumour necrosis factor-α (TNF-α), IL-6 [37,47,62], macrophage inflammatory protein 2 (MIP-2), neutrophil chemokine KC [36,47], and in the upregulation of IL-10 (anti-inflammatory) [35,45]. Moreover, EVs re-established alveolar epithelial cell homoeostasis, with prevention of apoptosis [40], increased epithelial cell migration [39] and stimulated surfactant production by upregulation of surfactant protein C expression that resulted in resolution of alveolar wall collapse [42]. EVs were also reported to restore endothelial cell junction protein expression including vascular endothelial cadherin and occludin [60], and endothelial cell-cell adhesion factors via beta-catenin [34] and intercellular adhesion molecule 1 (ICAM-1) pathways [62,64]. Further, EVs also preserved the alveolar-capillary barrier [37] and enhanced pulmonary endothelial cell proliferation [60] (Table 2). In studies modelling pneumonia, EVs induced adaptive immunity and reduced bacterial load [67]. Studies that tested EVs on models of pulmonary fibrosis reported that EV treatment reduced collagen deposition and density, halted fibrosis progression by inhibiting myofibroblast differentiation, and improved the Ashcroft score, a standard scoring system for pulmonary fibrosis [32,43,50,65].
Most studies reported that EVs exert their effects via a number of different mechanisms. Many studies included experimental conditioned medium groups, which demonstrated that EVs were responsible for the effects observed. MicroRNAs (miRNAs) were the molecules contained in the EV cargo that were most often investigated. More than one study reported that miR-126 was involved in the attenuation of inflammation via different pathways. Wu et al. demonstrated EV modulation in endothelial cells via sprouty-related EVH1 domain-containing protein 1 axis, which targets the RAF/ERK signalling pathway [58], and Zhou et al. showed that EVs increase expression of tight junction proteins via the phosphoinositide-3-kinase regulatory subunit 2 (PIK3R2), as well as high mobility group box 1 (HMGB1), and vascular endothelial growth factor-α (VEGFα) [47]. Interestingly, miR-126 has been reported to suppress inflammation in several conditions in other organs, is highly expressed in the lung of normal patients, and downregulated in the airway cells of patients with lung conditions, such as cystic fibrosis [70–73]. Yi and colleagues reported that EVs containing increased miR-30b-3p levels could increase alveolar epithelial cell proliferation and diminish apoptosis via decreased serum amyloid A3, a positive regulator of the inflammatory response [40]. This study is in line with another report showing that miR-30b-3p levels are decreased both in children with pneumonia and in mice with LPS-induced ALI [74]. Further, MSC-EVs suppressed multidrug resistance-associated protein 1 through transfer of miR-145, which positively signalled through the leukotriene (LTB4/BLT1) signalling pathway [38], and reduced TNF-α via increased M2 reparative macrophage polarization from miR-27a-3p transfer [31]. Both miR-145 and −27a-3p have been independently reported to modulate lung fibrosis by targeting myofibroblast differentiation [75,76]. All of the miRNAs identified in our systematic review (miRs-126, −30b-3p, −145, −27a-3p) have been reported by other groups to be present in their respective source EV cargo content [77–80].
In addition to these miRNAs, some studies showed that the EV effect on ARDS lungs was due to the release of proteins, such as syndecan-1 [37], hepatocyte growth factor [60] and angiopoietin-1 [63]. All these three proteins are known to attenuate lung injury in several models: syndecan-1 modulates the innate immune response to influenza infection via c-Met signalling [81]; hepatocyte growth factor mediates epithelial-mesenchymal interaction for lung regeneration following lung injury [82]; lastly, angiopoietin-1 plays a role in attenuating lung permeability and inflammation in experimental pneumonia and ALI [83,84]. All these three proteins have already been described to be present in their respective source cargo, as reported in Exocarta and Vesiclepedia [85,86].
To better understand EV mechanisms of action, some studies performed a comparative analysis on EV efficacy and regenerative capacity according to their source (Table 3). Different sources of MSC-EVs, namely chorionic and decidual, had the same capability in regenerating the injured lung [39] (Table 3). Huang and colleagues demonstrated that EVs derived from human adipose MSCs from a young patient (25 years old) decreased inflammation levels more efficiently than those isolated from an old patient (72 years old) [35].
Table 3.
Articles comparing the effects of different EV populations.
| Study | EV populations | EV treatment outcome summary |
|---|---|---|
| Dinh et al. 2020 [32] | LSC-EVs vs MSC-EVs | Both EV populations ameliorated lung fibrosis. LSC-EVs promoted more alveolar repair via increased aquaporins and had reduced SMAD3 levels compared to MSC-EVs |
| Yu et al. 2019 [34] | Mouse AT-EVs vs. S-EVs vs. ADSC-EVs | All EV populations restored adherens junction protein expression and attenuated inflammation. AT-EVs and ADSC-EVs were more efficient in suppressing endothelial inflammation than S-EVs |
| Huang et al. 2019 [35] | Human adipose MSC-EVs from young (25 years old) vs. old (72 years old) donors | Young (but not old) MSC-EVs alleviated ALI and altered macrophage phenotypes |
| Zhang et al. 2019 [37] | Mouse PMVECs with high vs. low syndecan-1 | SDC1-high-EVs (but not SDC1-low-EVs) ameliorated lung inflammation by reducing pro-inflammatory cytokines |
| Kim et al. 2019 [39] | Human DMSC23-EVs vs. CMSC29-EVs | Both EV populations reduced pro-inflammatory cytokines and increased the migration of human bronchial epithelial cells |
| Yi et al. 2019 [40] | Mouse MSC-EVs with high vs. low miR-30b-3p | Only MSC-EVs with high miR-30b-3p levels increased cell proliferation and reduced cell apoptosis and pro-inflammatory cytokines |
| Varkouhi et al. 2019 [46] | Human interferon-γ-primed MSC-EVs vs. naïve MSC-EVs | Both EV populations enhanced survival and bacterial phagocytosis in THP-1 cells and reduced the alveolar wall thickness. Only IFN-γ-primed MSC-EVs decreased pro-inflammatory cytokines |
| Zhou et al. 2019 [47] | Human EPC-EVs vs. NIH3T3-EVs | EPC-EVs (but not NIH3T3-EVs) reduced pro-inflammatory cytokine and chemokine production and lung tissue injury and restored alveolar barrier |
| Tan et al. 2018 [54] | Human AEC-EVs vs. HLF-EVs | AEC-EVs and HLF-EVs similarly reduced neutrophil infiltration and interstitial macrophages. AEC-EVs were more effective in attenuating lung fibrosis in aged mice compared to HLF-EVs |
| Wang et al. 2017 [60] | Mouse HGF knockdown MSC-EVs vs. MSC-EVs | MSCs-EVs (but not HGF knockdown MSC-EVs) reduced pro-inflammatory cytokines and restored endothelial permeability regulation |
| Gao et al. 2017 [62] | Human NS-EVs vs. NC-EVs | NS-EVs and NC-EVs similarly reduced pro-inflammatory cytokines and leukocytes and restored alveolar epithelial function |
| Tang et al. 2017 [63] | Human Ang-1 knockdown MSC-EVs vs. MSC-EVs | MSC-EVs (but not Ang-1 knockdown MSC-EVs) reduced lung inflammation and pulmonary capillary permeability |
| Li et al. 2015 [66] | Human MSCIPC-30-EVs vs. MSCIPC-60-EVs vs. MSCIPC-90-EVs | MSCIPC-60-EVs were more effective at reducing pro-inflammatory cytokines and increasing anti-inflammatory cytokines than MSCIPC-30-EVs and MSCIPC-90-EVs |
| Monsel et al. 2015 [68] | Human MSC-EVs vs. NHLF-EVs | MSC-EVs (but not NHLF-EVs) increased survival rate and decreased pro-inflammatory cytokines |
| Zhu et al. 2014 [69] | Human MSC-EVs vs. NHLF-EVs | MSC-EVs (but not NHLF-EVs) reduced inflammation and protein permeability and decreased lung injury |
Abbreviations
ADSC-EVs: Adipose-derived stem cell extracellular vesicles; AEC-EVs: Amnion epithelial cell extracellular vesicles; ALI: Acute lung injury; AT-EVs: Adipose tissue extracellular vesicles; BEAS-2B: Bronchial epithelial cell line; CMSC29-EVs: Chorionic-derived mesenchymal stem cell extracellular vesicles; DMSC23-EVs: Decidual-derived mesenchymal stem cell extracellular vesicles; EPC-EVs: Endothelial progenitor cell extracellular vesicles; EVs: Extracellular vesicles; HGF: Hepatocyte growth factor; HLF-EVs: Human lung fibroblast extracellular vesicles; LSC-EVs: Lung spheroid cell extracellular vesicles; MSC-EVs: Mesenchymal stem/stromal cell extracellular vesicles; MSCIPC-30-EVs: MSCs subjected to Ischaemic Pre-Conditioning for 30 minutes-derived extracellular vesicles; MSCIPC-60-EVs: MSCs subjected to Ischaemic Pre-Conditioning for 60 minutes-derived extracellular vesicles; MSCIPC-90-EVs: MSCs subjected to Ischaemic Pre-Conditioning for 90 minutes-derived extracellular vesicles; NC-EVs: Nitrogen cavitation generated extracellular vesicles; NHLF-EVs: Normal human lung fibroblasts; NIH3T3-EVs: fibroblast-derived extracellular vesicles; NS-EVs: Naturally secreted extracellular vesicles; PMVEC-EVs: Pulmonary microvascular endothelial cell extracellular vesicles; SDC1: Gene for encoding syndecan-1; S-EVs: Serum-derived extracellular vesicles
We also analysed EV separation and characterization methodology with a specific reference to ISEV guidelines, as well as dosage and administration route (Table 4). Ultracentrifugation was the most popular EV separation technique, followed by reagent-based commercially available kits. Only few studies followed ISEV recommendations and additional experimental details were not reported through online databases, such as EV-track [87]. The concentrations and dosage frequency were not consistent across studies, even when the same source of EVs was used. On average, the models were treated with one or two doses of EVs over a 1–10-day period. In in vivo animal models, the EV administration route was either intravenous or intratracheal or a combination of both. None of the studies included in this analysis isolated EVs following good manufacturing practices (GMP).
Table 4.
Details of EVs used as a therapy in lung injury models.
| Study | EV source | EV separation | EV characterization | EV dosage |
|---|---|---|---|---|
| Wang et al. 2020 [31] | Adipose MSCs | Pre-clearing cells/debris, UC (118,000 g for 16 h at 4°C) | NTA; DLS; TEM; Flow Cytometry (CD63); WB (CD40, CD44, CD63, CD81, CD105; GM130, Calnexin) | 1 dose; 100 μg/mL of EVs added to culture medium, or 50 µg/0.05 ml of EVs, administered via intratracheal injection (in vivo) |
| Dinh et al. 2020 [32] | Lung spheroid cells | Ultrafiltration, Centrifugation (5000 g for 10–15 min) | NTA; TEM; WB (CD63, CD81, TSG101) | 1 dose; 10 × 109 particles per kg of body weight, administered intranasally (aerosolized) |
| Gao et al. 2020 [33] | Adipose MSCs | Ultracentrifugation (details unspecified) | NTA; TEM; WB (CD63, TSG101, Alix, GM130) | 1 dose; 1 × 109 EVs added to culture medium, or 2.5 ~ 2.8 × 1010 EVs in 20 μL PBS, administered via intratracheal injection (in vivo) |
| Yu et al. 2020 [34] | Adipose tissue, Adipose MSCs, Serum | Pre-clearing cells/debris, TEIR | NTA; TEM; WB (CD63, HSP70, TSG101) | 1–2 doses; 0, 25, 50, and 100 μg/ml of EVs added to culture medium or injected intravenously 1 h before and immediately after mechanical ventilation |
| Huang et al. 2019 [35] | Adipose MSCs | Pre-clearing cells/debris, UC (118,000 g for 16 h at 4°C) | NTA; TEM; WB (CD63, CD81, CD105, CD44, GM130, Calnexin) | 1 dose; 100 μg/200 µl of EVs, administered via tail vein injection |
| Silva et al. 2019 [36] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C, twice) | NTA; SEM | 1 dose; EVs from 105 cells, administered via jugular vein injection |
| Zhang et al. 2019 [37] | PMVECs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C, twice) | NTA; TEM; WB (CD9, CD63, CD81) | 2 doses; 3 μg/g of EVs, administered via tail vein injection |
| Hao et al. 2019 [38] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C, twice) | NTA; SEM; Flow Cytometry (CD9, CD44) | 1–4 doses; 3, 6, and 12 × 108 particles added to culture medium, or 90 µl of EV per mouse (1x1010 particles), administered intravenously (in vivo) |
| Kim et al. 2019 [39] | Placental chorionic or decidual MSCs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C, twice) | NTA; TRPS; SEM; TEM; AFM | 1–2 doses; ranging 6 × 105–1.5 × 107 particles per ml, added to culture medium |
| Yi et al. 2019 [40] | Bone marrow MSCs | Pre-clearing cells/debris, centrifugation (10,000 g for 1 h at 4°C, twice) | NTA; Flow Cytometry (CD63) | 1 dose; 1 μg/100 μL or 100 μg/200 μL, administered via intravenous injection (caudal veins) |
| Chen et al. 2019 [41] | Umbilical cord MSCs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C, twice) | SEM; Flow Cytometry (CD34, CD44, CD45, CD73, CD105) | 1 dose; 4 mg/kg, administered via intratracheal injection |
| Xu et al. 2019 [42] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C, twice) | NTA; TEM; WB (CD9, CD63, CD81) | 1 dose; EVs from 5 × 106 cells, administered via intratracheal injection |
| Mansouri et al. 2019 [43] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 g for 3.5 h at 4°C) | NTA, TEM, WB (Alix, CD63, CD9, Flot-1) | 1 dose; diluted in PBS to correspond to 5 × 106 cell equivalent, administered via intravenous injection |
| Loy et al. 2019 [44] | Umbilical cord MSCs or bone marrow MSCs | Pre-clearing with filtration; ExoEasy Maxi Kit, miRCURY Exosome Isolation Kit | Not specified | 1 dose; not specified concentration of EVs, administered via intravenous injection |
| Li et al. 2019 [45] | Bone marrow MSCs | Pre-clearing cells/debris, centrifugation (12,000 g for 1 h at 4°C, twice) | NTA; TEM; WB (CD9, CD63, CD81) | 2 doses per day for 7 days; 25 μg of EVs, administered via tail vein injection |
| Varkouhi et al. 2019 [46] | γ–primed umbilical cord MSCs |
Pre-clearing cells/debris, UC (100,000 g for 1.5 h at 4°C) | TEM; Flow Cytometry for size | 1 dose; 10 × 108 EVs/kg, administered via intravenous injection |
| Zhou et al. 2019 [47] | Umbilical cord EPCs | Pre-clearing cells/debris, TEIR | NTA; WB (CD9, CD63, CD81) | 1 dose; 70 μg of EVs, administered via intratracheal injection |
| Park et al. 2019 [48] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C, twice) | NTA; SEM; Flow Cytometry (CD9, CD44); | 1 dose; 200 μl – 400 μl of EVs, administered via intravenous injection |
| Royce et al. 2019 [49] | Amnion epithelial cells | Not specified | Not specified | 1 dose; 5–25 μg of EVs, administered intranasally |
| Sun et al. 2019 [50] | Menstrual blood-derived endometrial stem cells | TEIR | NTA; TEM; WB (TSG101, CD9, CD63, Calnexin) | 1 dose; 0.5 mg/kg of EVs, administered via tail vein injection |
| Liu et al. 2019 [51] | Umbilical cord MSCs | Pre-clearing cells/debris, ExoQuick | NTA; TEM; WB (CD9, CD63) | 1 dose; 800 μg of EVs, added to culture medium, or administered via tail vein injection (in vivo) |
| Sun et al. 2018 [52] | Whole blood | Sonication, Ultrafiltration (9,000 g for 15 min) | TEM; AFM | 2 doses; 2.5 mg/kg and 0.25 mg/kg of EVs, administered intranasally (nasal drip) |
| Bandeira et al. 2018 [53] | Adipose MSCs | Pre-clearing cells/debris, UC (100,000 g for 2 h) | TEM; NTA, WB (CD63, CD81, LAMP-1, CD9) | 1 dose; 50 µl of EVs, administered via intratracheal injection |
| Tan et al. 2018 [54] |
Amnion epithelial cells |
Centrifugation (details unspecified) |
NTA; TEM; WB and Flow cytometry (CD9, CD81, Alix,
HLA-G) |
1 dose; not specified concentration of EVs, added to
culture medium |
| Hu et al. 2018 [55] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C, twice) | NTA; SEM; Flow Cytometry (CD9, CD44) | 1–2 doses; 30 µl or 60 µl of EVs, added to culture medium |
| Zhang et al. 2018 [56] | Serum | TEIR | NTA; TEM; WB (CD63, Flot-1, TSG101) | 1 dose; 10 μl – 500 μl of EVs, administered via intratracheal injection |
| Shah et al. 2018 [57] | Bone marrow MSCs | Pre-clearing cells/debris, UC (28,000 g for 2 h at 4°C, twice) | NTA; Flow Cytometry for size | 2 doses; 2.9 × 105 and 5.8 × 105 of EVs in 100 μl PBS, administered via retroorbital injection |
| Wu et al. 2018 [58] | Endothelial progenitor cells | Pre-clearing cells/debris, UC (100,000 g for 2 h at 4°C, twice) | NTA; TEM; WB (CD63, Alix, TSG101) | 1 dose; 100 μg, administered via tail vein injection |
| Khatri et al. 2018 [59] | Bone marrow MSCs | Pre-clearing cells/debris, UC (25,000 g for 70 min at 4°C) | TEM; WB (CD9, CD63, CD81) | 1 dose; 10 μg/mL of EVs added to culture medium, or 80 μg/kg of EVs, administered via intratracheal injection |
| Wang et al. 2017 [60] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C, twice) | Flow Cytometry (CD29, CD34, CD44, CD45, CD105) TEM; SEM; | 1 dose; unspecified concentration of EVs, added to culture medium |
| Morrison et al. 2017 [61] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 g for 2 h) | Flow Cytometry for size and CD44 | 1 dose; unspecified concentration of EVs added to different models, administered intranasally (in vivo) |
| Gao et al. 2017 [62] | Neutrophils | NS-EVs: pre-clearing cells/debris, UC (100,000 g for 1 h). NC-EVs: nitrogen cavitation chamber at a pressure of 400–500 psi for 20 min at 0°C | qNano and dynamic light scattering; cryo-TEM for NC-EVs; WB (IntegrinB2, LAMP-1, Calnexin, COXIV) | 1 dose; unspecified number of drug loaded EVs (piceatannol at 3 mg/kg), administered via intravenous injection |
| Tang et al. 2017 [63] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C, twice) | TEM and SEM | Not specified |
| Ju et al. 2017 [64] | Urine-derived pluripotent stem cells | Pre-clearing cells/debris, UC (120,000 g for 70 min at 4°C, twice) | NTA; TRPS; TEM; WB (CD63, TSG101, Alix) | 1 dose; 10 μg protein of Exo/siRNA (EV) compound or micropoly with siRNA at a final concentration of 100 nM, added to culture medium |
| Shentu et al. 2017 [65] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 for 1 h, twice) | NTA; TEM; WB (CD63, CD81, Calnexin) | 1 dose; 10 μg of EVs added to culture medium |
| Li et al. 2015 [66] | Bone marrow MSCs | ExoQuick | WB (CD63) | 1 dose; 1.5 μg/g of EVs, administered via tail vein injection |
| Choi et al. 2015 [67] | S. aureus | Pre-clearing cells/debris, UC (150,000 g for 3 h at 4°C) | Not specified | 1 dose; 10 μg/ml of EVs added to culture medium |
| Monsel et al. 2015 [68] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C) | TEM, WB (CD44) | 3 doses; EVs from 10 μL per 1 × 106 cells added to culture medium, or EVs administered intratracheal (30 µl or 60 µl) or intravenously (90 µl) (in vivo) |
| Zhu et al. 2014 [69] | Bone marrow MSCs | Pre-clearing cells/debris, UC (100,000 g for 1 h at 4°C, twice) | TEM and SEM | 1–2 doses; 30 µl or 60 µl of EVs, administered via intravenous or intratracheal injection |
Abbreviations: AFM: Atomic force microscopy; Alix: Apoptosis-Linked Gene 2-Interacting Protein X; CD: Cluster of differentiation; COXIV: Cytochrome c oxidase Complex IV; DLS: Dynamic light scattering; EV: Extracellular vesicle; Flot-1: Flotillin 1; GM: Golgi matrix; HLA: Human leukocyte antigen; HSP: Heat shock protein; IntegrinB2: Integrin beta 2; LAMP-1: Lysosome-associated member glycoprotein 1; MSC: Mesenchymal stromal/stem cell; NTA: Nanoparticle tracking analysis; PMVEC: Pulmonary microvascular endothelial cell; SEM: Scanning electron microscopy; TEIR: Total exosome isolation reagent; TEM: Transmission electron microscopy; TRPS: Tunable resistive pulse sensing; TSG: Tumour susceptibility; UC: Ultracentrifugation; WB: Western blot
The quality assessment using SYRCLE showed that the selected studies had a medium level of bias, as reported in the quantitative assessment (Supplemental file 2 and Supplemental file 3). The majority of the in vivo studies presented high or unclear risk of bias due to a lack of information regarding selection method, allocation concealment, animal housing and replacement of dropout animals. Moreover, both in vivo and in vitro studies did not commonly report information regarding randomization and blinding. The present systematic review was independently assessed using AMSTAR 2 [30] and received a “high” score (Supplemental file 4). A list of excluded articles is provided in Supplemental file 5. Articles were excluded if they did not match the outcome of interest (e.g. lung injury models not relevant to COVID-19, such as bronchopulmonary dysplasia, cystic fibrosis and asthma) or the treatment criteria (e.g. EVs employed as biomarkers and not for treatment). The CERQual tool revealed that the inflammatory response and lung injury recovery in the alveolar epithelium received “high” scores, whereas the other selected parameters had lower scores (Supplemental file 6). Lastly, the PRISMA checklist was completed which included further details for the review scoring (Supplemental file 7).
Discussion
This systematic review reveals that a number of studies demonstrate that EVs of various sources are able to recover lung injury, improve respiratory function and in some cases increase animal survival. The majority of these articles were published in the last 4 years, which is indicative of how active the EV community has been in this field. Two thirds of the studies included in our review utilized MSC-EVs as a therapy for lung injury. Likely, these studies have stemmed out of the vast literature on lung regeneration using stem cell-based therapies, which primarily reported the anti-inflammatory properties of MSCs in animal models of lung injury [17]. Moreover, MSCs have also been tested as a therapy for patients with moderate to severe ARDS in phase 1 and phase 2a clinical trials [15,16]. These trials concluded that MSCs were safe to administer to ARDS patients, but their efficacy still had to be determined in a larger phase 2b trial. With these promising human data, in the past months we have witnessed an increasing number of registered clinical trials testing MSCs in patients with COVID-19-related lung injury [88], as well as two scientific articles reporting beneficial effects of MSCs in patients [89,90]. Leng et al described that seven COVID-19 patients with pneumonia had symptom resolution 2–4 days following administration of clinical grade human MSCs [89]. Similarly, Liang et al reported the case of a 65-year-old patient, who was critically ill with COVID-19 and recovered following administration of human umbilical cord MSCs [90]. Although both studies lack details on cell source and characterization, enrolment criteria and patient clinical status pre- and post-treatment, it is encouraging that MSC-based therapies could improve the outcomes of patients with COVID-19-related lung injury. As EVs are considered to be one of the most important effectors of MSC paracrine mechanism of action [91], the results of studies employing MSCs for COVID-19-related lung injury could be useful in predicting the potential outcome of MSC-EV-based therapies in the same population.
Our review confirms that also EVs from different sources address multiple pathways and can be beneficial to treat lung injury. In the analysed studies, EVs had the capacity to attenuate the inflammatory response and switch it to a reparative phenotype, to arrest the injury to the alveolar epithelium and implement its regeneration, to repair the lung vascular damage, and to ameliorate and prevent pulmonary fibrosis. Furthermore, of relevance to COVID-19, EVs have been reported to exert some antiviral properties, as shown in the only large animal study where MSC-EV injection inhibited influenza virus replication in pigs and virus-induced apoptosis in porcine lung epithelial cells [59]. Moreover, as COVID-19 infection is frequently complicated by multiple organ failure, it is unlikely that a single drug will be able to improve the most severe forms of COVID-19 [22], whereas EV-based treatments could yield more promising outcomes. However, many key considerations remain to be addressed in this exciting and relatively new field.
Before testing these promising EV therapies on human subjects, a number of translational issues should be carefully considered as summarized by Rohde et al [92] and as recently recommended by the ISEV and International Society for Cell & Gene Therapy (ISCT) communities [93]. Firstly, the optimal EV source should be identified. In this regard, our review shows that EVs derived from stem cells hold great promise, as they have been reported to reduce inflammation and regenerate and repair the injured lung tissues. Moreover, to keep consistency of the EV production and to maintain homogeneity of the EV cargo, several approaches could be considered, such as immortalization of EV parent cells or cell banking [92]. Additional steps include upscaling conditions, type of culture medium to use for cell growth and expansion, need for cell preconditioning and conditioned medium production for EV separation. Once the ideal conditioned medium is obtained, it is key to establish the optimal EV separation technique. This step is critical as previous studies have shown that different EV separation methods would produce preparations with varying EV size, yield and protein content, which could possibly alter the EV functional potential [94,95]. In the present systematic review, the included studies used different separation techniques, with the most popular being differential ultracentrifugation. Disadvantages of this technique include the presence of contaminants, a pellet that can be difficult to resuspend, and aggregated EVs with lower functionality [96]. On the other hand, utilizing other EV separation techniques such as size exclusion chromatography or magnetic bead isolation might yield an EV preparation that is purer, but less effective and needing concentration [94,96]. The heterogeneity of separation techniques found in our systematic review mirrors the recognized lack of harmonization across laboratories, which inevitably impedes reproducibility and delays clinical translation [96]. Even studies that used the same separation technique, such as ultracentrifugation, employed different protocols and potentially yielded different EV preparations. Although batch-to-batch variation might be minimal if a harmonized approach is followed, quality control measures should be conducted along each step of the manufacturing process. Likewise, these steps should be conducted under GMP guidelines, to ensure compliance with the guidelines recommended by regulatory agencies for clinical application of pharmaceutical products. Although recommendations on the separation and use of GMP-grade EVs have been proposed [97–99], the process is relatively costly and none of the studies included in this review tested GMP-grade EVs on the lung injury models reported.
An additional challenge in using EV therapies in COVID-19-infected patients is the specific nature of the disease. Factors such as the determination of the optimal route of administration, dosage and patient selection should be further considered. Although EV therapies have been reported to be efficacious with both intravenous and intratracheal routes, both have advantages and disadvantages. The intratracheal route has the benefit of delivering the EV treatment topically, without the possible risk of diluting the EVs and their cargo throughout the body. However, if the EV treatment is delivered in an aerosolized form, there is a risk that EVs would localize primarily to the proximal airways without sufficiently reaching the lower airways, which are more affected. Moreover, disconnecting the ventilator to administer the EV aerosol would be challenging for COVID-19 patients, as they are severely hypoxaemic, and for their caregivers due to the potential risk of viral exposure. EVs administered intratracheally in a liquid form could be an additional burden for the “wet” ARDS lungs, especially if the volume of the bolus, calculated on the predicted body weight is substantial. On the other hand, the intravenous route offers the advantage of being easily reproducible in human patients, as it has already been tested for other types of regenerative medicine compounds, such as stem cells [16]. However, a potential challenge for EV intravenous administration in COVID-19 patients lies in the reported characteristic of EVs to enhance coagulability [100,101]. Recent reports have indicated that COVID-19 predisposes patients to thrombotic disease, with disseminated coagulation without bleeding, and contributes to poor outcome and prognosis [102–104]. Nonetheless, it remains unknown how EVs administered to COVID-19 patients would function in the context of a disseminated intravascular coagulation. Moreover, there is still little evidence that intravenously administered EVs would reach the inflamed lungs, as the EV ability to naturally home to specific regions of the body remains debated [105]. In order to produce EVs with organ-specific targeting ability, some groups have taken advantage of natural homing processes of EVs, i.e. EVs isolated from B cell specifically bind to follicular dendritic cells [106], and enhanced their ability to load therapeutic cargo through alterations in their biogenesis [107,108]. Conversely, groups have demonstrated that EV uptake and biodistribution is multidirectional between various cell types and do not display cell-specific homing mechanism [109–111]. Nonetheless, patients with severe COVID-19 infection often suffer from multiple organ failure and sepsis [20], and in this context EVs administered intravenously might be beneficial for the recovery of the other organs in addition to the lung. Moreover, it has become evident that patients with COVID-19 may develop myocardial dysfunction and damage, microvascular dysfunction, plaque instability and myocardial infarction [112]. Stem cell-derived EVs have been reported to exert cardioprotective effects and promote formation of new cardiomyocytes in the ischaemic heart [113]. Therefore, intravenous EV administration could have beneficial effects on the cardiovascular system of patients with COVID-19.
With regards to dosage, in the studies herein analysed, the majority of groups extrapolated their EV doses on the basis of cell equivalents described in stem cell-based therapies. Although this might not be the right approach as EVs have a different potency than their parental cells [68], it is reasonable as there has not yet been any trial testing EVs in human patients. Phase 1 trials should be designed to address the safety of EV treatment, specifically focusing on EV pharmacokinetic and pharmacodynamic characteristics. To study the safety of EVs as a drug, it is critical to establish the therapeutic index, i.e. the amount of EVs that causes the therapeutic effect without causing toxicity.
Lastly, patient selection is crucial and should be examined closely. Clinical parameters and possibly available biomarkers may be helpful to identify the inclusion and exclusion criteria. Ideally, patients should be enrolled in carefully designed clinical trials, where phase 1 would inform on the safety of EVs (escalating doses), phase 2 would test EV efficacy and side effects (therapeutic doses), and phase 3 would determine EV efficacy, effectiveness and safety [114]. Following this systematic approach, an EV-based therapy might eventually be approved by national regulatory authorities for use in the general population. Alternatively, especially during a crisis period like this pandemic, patients could be offered an EV therapy outside of a trial through compassionate or named patient use. Compassionate use is restricted to patients who are not responding to conventional treatment strategies and have developed an immediate life-threatening clinical status, who do not meet the inclusion criteria for a trial if one is available, and whose caregivers agree that the benefit justifies the potential risks of the treatment [115]. Named patient use refers to the treatment of a single individual with an unauthorized product, under the direct responsibility of their physician [116]. We anticipate that during these unprecedented times, every effort is made by ethical boards and regulatory agencies to streamline and expedite the process [117]. However, the high quality of research should be maintained by respecting regulatory standards.
We acknowledge that the results of this systematic review are limited by the quality of the studies reported in the literature. As a result, we were not able to compare the findings obtained in different studies through a meta-analysis due to the heterogeneity in the models, EV sources and doses, as well as outcome measures. Moreover, only approximately half of the studies investigated the EV mechanism of action, by addressing EV specificity or cargo content. Nonetheless, it is encouraging to see that in a relatively short period of time, several groups have demonstrated the efficacy of EV therapies in lung injury. Moreover, in the quality assessment of the selected articles using CERQual tool, the two parameters that were mostly associated with the recovery of COVID-19 related lung injury, namely inflammation and alveolar epithelial damage, received high scores. As CERQual is a tool to evaluate preclinical studies and assess their potential for clinical translation [118], our rating is indicative of the high potential to transfer the findings from the selected animal studies to clinical application.
In conclusion, EV-based therapies hold great potential as a novel treatment for COVID-19-related lung injury, as they are able to target and ameliorate the multifactorial aspects of pneumonia, pulmonary fibrosis and ALI/ARDS pathogenesis. The several studies included in this review showed that EVs can reduce the inflammatory response in the lung, regenerate and repair the damaged alveolar epithelium and endothelium, and prevent pulmonary fibrosis. The mechanism behind EV effects is ascribed to the release of their cargo, and miRNAs seem to play a role in the EV mechanism of action. Although there is a rush towards savings lives during this COVID-19 pandemic, the EV scientific community should direct their efforts to developing manufacturing solutions in a GMP fashion, testing optimal dosage and administration route in large animal models, and assessing the safety and efficacy of EV-based therapies in clinical trial protocols. Findings from these studies could prove immensely valuable in the post COVID-19 world, as EVs could also be a treatment strategy for non-COVID-19-related lung injury.
Supplementary Material
Disclosure of interest
The authors have no conflicts of interest to declare.
Supplementary material
Supplemental data for this article can be accessed here
References
- [1].Who.int. WHO Statement on COVID-19 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020
- [2].Satija N, Lal SK.. The molecular biology of SARS coronavirus. Ann N Y Acad Sci. 2007;1102:26–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:P1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guan W-J, Ni Z-Y, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020. Epub ahead of print DOI: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wenham C, Smith J, Morgan R. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020. Epub ahead of print DOI: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hosseiny M, Kooraki S, Gholamrezanezhad A, et al. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and middle east respiratory syndrome. AJR Am J Roentgenol. 2020;1–5. DOI: 10.2214/AJR.19.22455. [DOI] [PubMed] [Google Scholar]
- [9].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Prim. 2019;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. [DOI] [PubMed] [Google Scholar]
- [12].Yao XH, Li TY, He ZC, et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Chin J Pathol. 2020;49:E009. [DOI] [PubMed] [Google Scholar]
- [13].Spagnolo P, Balestro E, Aliberti S, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020. Epub ahead of print DOI: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Das KM, Lee EY, Singh R, et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Laffey JG, Matthay MA. Fifty years of research in ARDS. cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am J Respir Crit Care Med. 2017;196:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ong S-G, Wu JC. Exosomes as potential alternatives to stem cell therapy in mediating cardiac regeneration1. Circ Res. 2015;7–9. DOI: 10.1161/CIRCRESAHA.115.306593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhou Y, Li P, Goodwin AJ, et al. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol Ther. 2018;26:1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Eppensteiner J, Davis RP, Barbas AS, et al. Immunothrombotic activity of damage-associated molecular patterns and extracellular vesicles in secondary organ failure induced by trauma and sterile insults. Front Immunol. 2018;9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gaborit BJ, Bergmann J-F, Mussini C, et al. Plea for multitargeted interventions for severe COVID-19. Lancet Infect Dis. 2020. Epub ahead of print DOI: 10.1016/S1473-3099(20)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Clinicaltrials.gov A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe novel coronavirus pneumonia. 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04276987?term=NCT04276987&draw=2&rank=1
- [24].Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0; updated July Cochrane; 2019. Available from: www.training.cochrane.org/handbook.
- [25].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. [DOI] [PubMed] [Google Scholar]
- [26].National Institute for Health Research PROSPERO international prospective register of systematic reviews. 2011. Available from: https://www.crd.york.ac.uk/prospero
- [27].Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hooijmans CR, Rovers MM, de Vries RBM, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lewin S, Glenton C, Munthe-Kaas H, et al. Using qualitative evidence in decision making for health and social interventions: an approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual). PLoS Med. 2015;12:e1001895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang J, Huang R, Xu Q, et al. Mesenchymal stem cell-derived extracellular vesicles alleviate acute lung injury via transfer of miR-27a-3p. Crit Care Med. 2020. Epub ahead of print DOI: 10.1097/CCM.0000000000004315. [DOI] [PubMed] [Google Scholar]
- [32].Dinh P-UC, Paudel D, Brochu H, et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat Commun. 2020;11:1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gao Y, Sun J, Dong C, et al. Extracellular vesicles derived from adipose mesenchymal stem cells alleviate PM2.5-Induced lung injury and pulmonary fibrosis. Med Sci Monit. 2020;26:e922782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yu Q, Wang D, Wen X, et al. Adipose-derived exosomes protect the pulmonary endothelial barrier in ventilator-induced lung injury by inhibiting the TRPV4/Ca2+ signaling pathway. Am J Physiol Lung Cell Mol Physiol. 2020;318:L723–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang R, Qin C, Wang J, et al. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging (Albany NY). 2019;11:7996–8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Silva JD, de Castro LL, Braga CL, et al. Mesenchymal stromal cells are more effective than their extracellular vesicles at reducing lung injury regardless of acute respiratory distress syndrome etiology. Stem Cells Int. 2019;2019:8262849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang C, Guo F, Chang M, et al. Exosome-delivered syndecan-1 rescues acute lung injury via a FAK/p190RhoGAP/RhoA/ROCK/NF-kappaB signaling axis and glycocalyx enhancement. Exp Cell Res. 2019;384:111596. [DOI] [PubMed] [Google Scholar]
- [38].Hao Q, Gudapati V, Monsel A, et al. Mesenchymal stem cell-derived extracellular vesicles decrease lung injury in mice. J Immunol. 2019;203:1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim SY, Joglekar MV, Hardikar AA, et al. Placenta stem/stromal cell-derived extracellular vesicles for potential use in lung repair. Proteomics. 2019;19:e1800166. [DOI] [PubMed] [Google Scholar]
- [40].Yi X, Wei X, Lv H, et al. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp Cell Res. 2019;383:111454. [DOI] [PubMed] [Google Scholar]
- [41].Chen W, Wang S, Xiang H, et al. Microvesicles derived from human Wharton’s Jelly mesenchymal stem cells ameliorate acute lung injury partly mediated by hepatocyte growth factor. Int J Biochem Cell Biol. 2019;112:114–122. [DOI] [PubMed] [Google Scholar]
- [42].Xu N, Shao Y, Ye K, et al. Mesenchymal stem cell-derived exosomes attenuate phosgene-induced acute lung injury in rats. Inhal Toxicol. 2019;31:52–60. [DOI] [PubMed] [Google Scholar]
- [43].Mansouri N, Willis GR, Fernandez-Gonzalez A, et al. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 2019;4:e128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Loy H, Kuok DIT, Hui KPY, et al. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A(H5N1) virus-associated acute lung injury. J Infect Dis. 2019;219:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li Q-C, Liang Y, Su Z-B. Prophylactic treatment with MSC-derived exosomes attenuates traumatic acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2019;316:L1107–17. [DOI] [PubMed] [Google Scholar]
- [46].Varkouhi AK, Jerkic M, Ormesher L, et al. Extracellular vesicles from interferon-gamma-primed human umbilical cord mesenchymal stromal cells reduce escherichia coli-induced acute lung injury in rats. Anesthesiology. 2019;130:778–790. [DOI] [PubMed] [Google Scholar]
- [47].Zhou Y, Li P, Goodwin AJ, et al. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit Care. 2019;23:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Park J, Kim S, Lim H, et al. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019;74:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Royce SG, Patel KP, Mao W, et al. Serelaxin enhances the therapeutic effects of human amnion epithelial cell-derived exosomes in experimental models of lung disease. Br J Pharmacol. 2019;176:2195–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sun L, Zhu M, Feng W, et al. Exosomal miRNA Let-7 from menstrual blood-derived endometrial stem cells alleviates pulmonary fibrosis through regulating mitochondrial DNA damage. Oxid Med Cell Longev. 2019;2019:4506303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu J-S, Du J, Cheng X, et al. Exosomal miR-451 from human umbilical cord mesenchymal stem cells attenuates burn-induced acute lung injury. J Chin Med Assoc. 2019;82:895–901. [DOI] [PubMed] [Google Scholar]
- [52].Sun A, Lai Z, Zhao M, et al. Native nanodiscs from blood inhibit pulmonary fibrosis. Biomaterials. 2018;192:51–61. [DOI] [PubMed] [Google Scholar]
- [53].Bandeira E, Oliveira H, Silva JD, et al. Therapeutic effects of adipose-tissue-derived mesenchymal stromal cells and their extracellular vesicles in experimental silicosis. Respir Res. 2018;19:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tan JL, Lau SN, Leaw B, et al. Amnion epithelial cell-derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl Med. 2018;7:180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hu S, Park J, Liu A, et al. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl Med. 2018;7:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang D, Lee H, Wang X, et al. Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol Ther. 2018;26:2119–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shah T, Qin S, Vashi M, et al. Alk5/Runx1 signaling mediated by extracellular vesicles promotes vascular repair in acute respiratory distress syndrome. Clin Transl Med. 2018;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wu X, Liu Z, Hu L, et al. Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-126. Exp Cell Res. 2018;370:13–23. [DOI] [PubMed] [Google Scholar]
- [59].Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang H, Zheng R, Chen Q, et al. Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF). Stem Cell Res Ther. 2017;8:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196:1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gao J, Wang S, Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials. 2017;135:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tang X-D, Shi L, Monsel A, et al. Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang-1 mRNA. Stem Cells. 2017;35:1849–1859. [DOI] [PubMed] [Google Scholar]
- [64].Ju Z, Ma J, Wang C, et al. Exosomes from iPSCs delivering siRNA attenuate intracellular adhesion molecule-1 expression and neutrophils adhesion in pulmonary microvascular endothelial cells. Inflammation. 2017;40:486–496. [DOI] [PubMed] [Google Scholar]
- [65].Shentu T-P, Huang T-S, Cernelc-Kohan M, et al. Thy-1 dependent uptake of mesenchymal stem cell-derived extracellular vesicles blocks myofibroblastic differentiation. Sci Rep. 2017;7:18052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li L, Jin S, Zhang Y. Ischemic preconditioning potentiates the protective effect of mesenchymal stem cells on endotoxin-induced acute lung injury in mice through secretion of exosome. Int J Clin Exp Med. 2015;8:3825–3832. [PMC free article] [PubMed] [Google Scholar]
- [67].Choi SJ, Kim M-H, Jeon J, et al. Active immunization with extracellular vesicles derived from staphylococcus aureus effectively protects against staphylococcal lung infections, mainly via Th1 cell-mediated immunity. PLoS One. 2015;10:e0136021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Monsel A, Zhu Y, Gennai S, et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhu Y-G, Feng X-M, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Schober A, Nazari-Jahantingh M, Wei Y, et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hu J, Zeng L, Huang J, et al. miR-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Res. 2015;1608:191–202. [DOI] [PubMed] [Google Scholar]
- [72].Tang S-T, Wang F, Shao M, et al. MicroRNA-126 suppresses inflammation in endothelial cells under hyperglycemic condition by targeting HMGB1. Vascul Pharmacol. 2017;88:48–55. [DOI] [PubMed] [Google Scholar]
- [73].Oglesby IK, Bray IM, Chotirmall SH, et al. miR-126 is downregulated in cystic fibrosis airway epithelial cells and regulates TOM1 expression. J Immunol. 2010;184:1702–1709. [DOI] [PubMed] [Google Scholar]
- [74].Zhou T, Chen Y-L. The functional mechanisms of miR-30b-5p in acute lung injury in children. Med Sci Monit Int Med J Exp Clin Res. 2019;25:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Cui H, Banerjee S, Xie N, et al. MicroRNA-27a-3p is a negative regulator of lung fibrosis by targeting myofibroblast differentiation. Am J Respir Cell Mol Biol. 2016;54:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yang S, Cui H, Xie N, et al. miR-145 regulates myofibroblast differentiation and lung fibrosis. Faseb J. 2013;27:2382–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Balakrishnan I, Yang X, Brown J, et al. Genome-wide analysis of miRNA-mRNA interactions in marrow stromal cells. Stem Cells. 2014;32:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gong M, Yu B, Wang J, et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8:45200–45212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fang S, Xu C, Zhang Y, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal MicroRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 2016;5:1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].van Balkom BWM, Eisele AS, Pegtel DM, et al. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J Extracell Vesicles. 2015;4:26760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Brauer R, Ge L, Schlesinger SY, et al. Syndecan-1 attenuates lung injury during influenza infection by potentiating c-met signaling to suppress epithelial apoptosis. Am J Respir Crit Care Med. 2016;194:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mason RJ. Hepatocyte growth factor: the key to alveolar septation? Am J Respir Cell Mol Biol. 2002;26:517–520. [DOI] [PubMed] [Google Scholar]
- [83].McCarter SD, Mei SHJ, Lai PFH, et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med. 2007;175:1014–1026. [DOI] [PubMed] [Google Scholar]
- [84].Gutbier B, Neuhauß A-K, Reppe K, et al. Prognostic and pathogenic role of angiopoietin-1 and −2 in pneumonia. Am J Respir Crit Care Med. 2018;198:220–231. [DOI] [PubMed] [Google Scholar]
- [85].Simpson RJ, Kalra H, Mathivanan S. ExoCarta as a resource for exosomal research. J Extracell Vesicles. 2012;1:18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Van Deun J, Mestdagh P, Agostinis P, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14:228–232. [DOI] [PubMed] [Google Scholar]
- [88].Clinicaltrials.gov Registered trials for use of Mesenchymal stem cells for COVID-19. 2020. Available from: https://clinicaltrials.gov/ct2/results?cond=mesenchymal+stem+cells&term=covid&cntry=&state=&city=&dist=
- [89].Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Liang B, Chen J, Li T, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. chinaXiv. DOI: 10.12074/202002.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Abraham A, Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rohde E, Pachler K, Gimona M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy. 2019;21:581–592. [DOI] [PubMed] [Google Scholar]
- [93].Börger V, Weiss DJ, Anderson JD, et al. ISEV and ISCT statement on EVs from MSCs and other cells: considerations for potential therapeutic agents to suppress COVID-19. Cytotherapy. 2020. Epub ahead of print DOI: 10.1016/j.jcyt.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Antounians L, Tzanetakis A, Pellerito O, et al. The regenerative potential of amniotic fluid stem cell extracellular vesicles: lessons learned by comparing different isolation techniques. Sci Rep. 2019;9:1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kalluri R, LeBleu VS. The biology, function,and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Reiner AT, Witwer KW, van Balkom BWM, et al. Concise review: developing best‐practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl Med. 2017;6:1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Pachler K, Lener T, Streif D, et al. A good manufacturing practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy. 2017;19:458–472. [DOI] [PubMed] [Google Scholar]
- [98].Gimona M, Pachler K, Laner-Plamberger S, et al. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int J Mol Sci. 2017;18:E1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Witwer KW, Van Balkom BWM, Bruno S, et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8:1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gardiner C, Harrison P, Belting M, et al. Extracellular vesicles, tissue factor, cancer and thrombosis - discussion themes of the ISEV 2014 educational day. J Extracell Vesicles. 2015;4:26901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Nielsen T, Kristensen AF, Pedersen S, et al. Investigation of procoagulant activity in extracellular vesicles isolated by differential ultracentrifugation. J Extracell Vesicles. 2018;7:1454777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wang T, Chen R, Liu C, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020. Epub ahead of print DOI: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kanada M, Bachmann MH, Hardy JW, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci. 2015;112:E1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Denzer K, van Eijk M, Kleijmeer MJ, et al. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165:1259–1265. [DOI] [PubMed] [Google Scholar]
- [107].Meyer C, Losacco J, Stickney Z, et al. Pseudotyping exosomes for enhanced protein delivery in mammalian cells. Int J Nanomedicine. 2017;12:3153–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yim N, Ryu S-W, Choi K, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ragni E, Banfi F, Barilani M, et al. Extracellular vesicle-shuttled mRNA in mesenchymal stem cell communication. Stem Cells. 2017;35:1093–1105. [DOI] [PubMed] [Google Scholar]
- [110].Lai CP, Kim EY, Badr CE, et al. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun. 2015;6:7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Grange C, Tapparo M, Bruno S, et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med. 2014;33:1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020. Epub ahead of print DOI: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Khan M, Nickoloff E, Abramova T, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Umscheid CA, Margolis DJ, Grossman CE. Key concepts of clinical trials: a narrative review. Postgrad Med. 2011;123:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Miller JE, Ross JS, Moch KI, et al. Characterizing expanded access and compassionate use programs for experimental drugs. BMC Res Notes. 2017;10:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Whitfield K, Huemer K-H, Winter D, et al. Compassionate use of interventions: results of a European Clinical Research Infrastructures Network (ECRIN) survey of ten European countries. Trials. 2010;11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].fda.gov/. U.S. Food & Drug Administration Press Release 2020. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-continues-accelerate-development-novel-therapies-covid-19
- [118].Xing D, Kwong J, Yang Z, et al. Intra-articular injection of mesenchymal stem cells in treating knee osteoarthritis: a systematic review of animal studies. Osteoarthr Cartil. 2018;26:445–461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


