Abstract
Objective
The objective of this study was to assess access to healthcare and to estimate the prevalence of depression and anxiety among persons with epilepsy (PWE) during the ongoing coronavirus disease 2019 (COVID-19) pandemic.
Methods
We conducted a multicountry online survey among PWE. Persons with epilepsy were invited to participate through various social media channels. The Hospital Anxiety and Depression Scale (HADS) and 9-item Patient Health Questionnaire (PHQ-9) scale were used to score anxiety and depression. Logistic regression modeling was used to investigate factors associated with anxiety and depression.
Results
Three hundred ninety-nine PWE were included (mean age: 38.22 ± 12.09 years), the majority were female (80.2%) and living in high-income countries (83.2%). Two hundred three PWE reported symptoms of a cold since January 2020. Nine (25%) of the 36 PWE tested for COVID were positive. A total of 72 PWE (19.6%) reported problems to obtain antiseizure medication (ASM), which in 25% of cases was directly COVID-related. Of the 399 PWE, 201 (50.4%) screened positive for anxiety according to the HADS; 159 (39.8%) and 187 (46.9%) PWE screened positive for depression based on the HADS and PHQ-9 scale, respectively. Female gender and financial problems were associated with both depression and anxiety. A planned follow-up consultation with the treating physician was associated with a lower risk of depression, whereas difficulties to access ASM treatment increased the odds of depression. In 65/137 (47.4%) PWE with a planned follow-up visit with the treating physician, this consultation was canceled.
Conclusions
Innovative approaches are needed to ensure continuity in access to ASM treatment. Healthcare workers should ensure continued follow-up, either through inperson or telehealth appointments, to timely identify symptoms of anxiety and depression and act accordingly.
Keywords: Epilepsy, Mental health, COVID-19, HADS, PHQ-9
Highlights
-
•
We performed a multicountry online survey among 399 persons with epilepsy (PWE).
-
•
During COVID-19, 19.6% of the PWE had difficulties to obtain antiseizure drugs.
-
•
Overall, 39.8% of the PWE screened positive for depression, 50.4% for anxiety.
-
•
Follow-up consultations with the treating physician were canceled in 47.4% of the PWE.
1. Introduction
Epilepsy is a chronic neurological disease, affecting more than 50 million people worldwide [1]. Besides the physical impact of epileptic seizures, epilepsy also has important neurobiological, cognitive, and psychosocial consequences [2]. Psychiatric comorbidities are up to three times more prevalent in persons with epilepsy (PWE) than in the general population. Depression and anxiety are the most frequently encountered, with reported lifetime prevalence rates as high as 30–35% and 22.8%, respectively [[3], [4], [5]]. Psychiatric comorbidities are associated with poor quality of life (QoL) [6], seizure severity [7], more severe adverse effects of antiseizure medication (ASM) [8], therapy resistance [9], and poor outcomes after epilepsy surgery [10]. Despite their tremendous impact, psychiatric comorbidities in PWE are often overlooked. Persons with epilepsy represent a vulnerable population because of increased rates of unemployment, in turn, resulting in financial problems and social stigma [11].
The ongoing coronavirus disease 2019 (COVID-19) pandemic puts the global population under important psychosocial distress. Reports from China, Turkey, and Italy suggest that during the COVID-19 pandemic, up to 25% and 45% of the general population show symptoms of depression and anxiety, respectively [[12], [13], [14]]. The coronavirus crisis leads to feelings of loneliness, boredom, anger, and anxiety and poses a threat to the public mental health [15]. Previous studies have shown higher incidence rates of mental disorders including depression and anxiety during outbreaks of acute respiratory infections, such as the influenza virus [16,17]. Furthermore, in survivors of severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1), an increased prevalence of neuropsychiatric disorders was noted including posttraumatic stress disorder, depression, and panic disorder [18]. Next to posing a mental burden, COVID-19 may also delay access to and provision of care for non-COVID-related health problems, causing deaths that could have been prevented [19].
The prevalence of depression and anxiety among PWE as well as access to healthcare by PWE during the COVID-19 pandemic has not been well studied so far. A study from southwest China by Hao et al. reported that PWE experience more psychosocial distress compared with healthy subjects during the COVID-19 pandemic [20]. In the latter study, the diagnosis of refractory epilepsy and time spent to follow the evolution of the COVID-19 pandemic were predictive of severe psychological distress [20]. In a bid to expand the knowledge regarding the health and medical follow-up of PWE during the COVID-19 pandemic, we conducted a multicountry online survey to evaluate their access to healthcare and the prevalence of anxiety and depression in this population.
2. Methods
2.1. Study design
An online questionnaire survey among PWE was organized between April 10th and May 18th, 2020. At the beginning of the study period, Europe was still in full lockdown with healthcare services focused on COVID-19 and nonpharmaceutical interventions such as physical distancing highly recommended by local governments. By the first half of May, Europe had started to gradually lift lockdown measures. In Brazil and North America, there were large differences between regions in terms of lockdown and healthcare situations.
Persons with epilepsy were invited to participate by clicking an URL link that was shared through various websites or social media channels of associations that support persons with epilepsy and epilepsy research such as the Epilepsy Liga Flanders, the Brazilian Federation of Epilepsy (Epibrasil), the International League Against Epilepsy (ILAE), the Brazilian Association of Epilepsy (ABE), and Citizens United for Research in Epilepsy (CURE). The link was accessible to anyone.
The questionnaire consisted of five parts, inquiring on demographic data, epilepsy-related data, COVID-related data, and anxiety- and depression-related data. The survey was available in English, Dutch, French, and Portuguese. Respondents were included if they provided e-consent, reported to be a PWE or a caretaker/parent of a PWE, and were older than 18 years. Caretakers/parents of a PWE were asked to complete the questionnaire from the perspective of the PWE. A duplicate of the survey template can be found in the supplementary data of this paper. Respondents were categorized as living in low- to middle-income countries or high-income countries according to the World Bank Classification [21].
This study was approved by the ethics committee of the Antwerp University Hospital. All participants provided e-consent.
2.2. Assessment of anxiety and depression
The prevalence of anxiety and depression among the participants was estimated using the Hospital Anxiety and Depression Scale (HADS) and the 9-item Patient Health Questionnaire (PHQ-9) [[22], [23], [24], [25], [26], [27], [28], [29]]. Both screening tools have been validated as a self-reporting tool for use in PWE [[22], [23], [24], [25], [26], [27], [28], [29]]. The HADS consists of two main sections of seven questions each, to screen for depression and anxiety. The answer options followed a 0–3 Likert scale format, with 0 corresponding to no sign of anxiety or depression and 3 for the highest level of anxiety or depression for a particular item in the screening tool. The overall scores ranged from 0 to 21 for anxiety and depression screening, respectively. The PHQ-9 screening tool scores each of the 9 items of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for depression between 0 (not at all) and 3 (nearly every day).
Persons with epilepsy with HADS scores of 7 and above in each section of the scale (anxiety and depression) were considered as screened positive for that condition. A cutoff value of 9 was used to define depression according to the PHQ-9 scale, such that any PWE scoring 9 and above was classified as suffering from depression. These cutoffs were shown to result in the best sensitivity and specificity [[22], [23], [24], [25], [26], [27], [28], [29]].
2.3. Statistical analyses
Continuous variables are reported as mean with standard deviation (mean ± SD) or median with interquartile range (IQR), categorical variables as numbers with percentages. Continuous variables were compared between groups using a Student's t- or Mann–Whitney U test as appropriate, categorical variables using a chi-square or Fisher's exact test. Multiple logistic regression modeling was used to investigate factors associated with anxiety and depression (both based on HADS). Factors with a p < 0.100 in bivariate analysis (uncorrected for multiple testing, with a view on being inclusive) were included in multivariate analyses. All statistical tests were two-sided, and p < 0.05 was considered significant. Statistical analyses were performed in Statistical Product and Service Solutions (SPSS) 26.0.
3. Results
3.1. Respondent characteristics
A total of 460 responses were collected. Of these, 37 respondents who reported not to be a PWE nor a parent/caretaker of a PWE and 24 who were younger than 18 years old were excluded from analysis. Three hundred ninety-nine PWE from 18 countries (57.6% from Belgium, 14.5% from Brazil, 9.8% from The Netherlands, 7.9% from the United States, 11.2% from other countries; Supplementary Table 1) were included in the study; the mean age was 38.22 ± 12.09 years, and 80.2% were female (Table 1). A minority of the PWE lived in a low- to middle-income country (67/399; 16.8%). Of the 399 respondents, 35 reported to be a parent or caretaker of a PWE. We compared responses provided by the caretaker or parent with those directly provided by PWE (data not shown) and did not identify any significant difference. Therefore, we pooled the data of both groups for further analysis.
Table 1.
Characteristics of the persons with epilepsy (PWE).
| Total n = 399 | ||
|---|---|---|
| Demographic data | ||
| Age | In years (mean ± standard deviation) | 38.22 ± 12.09 |
| Gender | Male; n (%) | 79 (19.8%) |
| Country of residence | Low- to middle-income country; n (%) | 67 (16.8%) |
| High-income country; n (%) | 332 (83.2%) | |
| Marital status | Single; n (%) | 133 (33.3%) |
| Highest educational level | Primary school; n (%) | 16 (4.0%) |
| Secondary school; n (%) | 158 (39.6%) | |
| University undergraduate degree; n (%) | 159 (39.8%) | |
| University postgraduate degree; n (%) | 53 (13.3%) | |
| None; n (%) | 13 (3.3%) | |
| Who do you live with? (housemates) | With parents; n (%) | 78 (19.5%) |
| With spouse/partner; n (%) | 221 (55.4%) | |
| With child(ren); n (%) | 160 (40.1%) | |
| With siblings or other family relatives; n (%) | 30 (7.5%) | |
| With friend(s); n (%) | 12 (3.0%) | |
| Alone; n (%) | 58 (14.5%) | |
| Epilepsy data | ||
| Seizure-free during the last three months before COVID | Yes; n (%) | 238 (59.6%) |
| If not seizure-free, number of seizures over the last three months; median (IQR) | 3 (1–9) | |
| On antiseizure medication | Yes; n (%) | 368 (92.2%) |
| If yes, number of antiseizure medications | 1; n (%) | 199 (54.1%) |
| 2; n (%) | 100 (27.2%) | |
| 3; n (%) | 47 (12.8%) | |
| 4; n (%) | 18 (4.9%) | |
| 5; n (%) | 4 (1.1%) | |
| On antidepressants or anxiolytics | No; n (%) | 313 (78.4%) |
| Yes, since a long time; n (%) | 77 (19.3%) | |
| Yes, since the start of COVID-19; n (%) | 7 (1.8%) | |
| No answer reported; n (%) | 2 (0.5%) | |
| Belongs to an association that supports PWE | Yes; n (%) | 69 (17.3%) |
| FU consultation with neurologist planned before onset of corona | Yes; n (%) | 137 (34.3%) |
| If yes, what happened to the consultation | Canceled; n (%) | 65 (47.4%) |
| Changed to telephone or online consult; n (%) | 45 (32.8%) | |
| Took place as planned; n (%) | 27 (19.7%) | |
FU: follow-up.
3.2. Epilepsy characteristics
The majority of the PWE were on ASM (368/399; 92.2%). There was no difference between the proportion of PWE who reported being seizure-free during the last three months before the onset of COVID-19 in their respective countries (238/399; 59.7%) and during the last three months preceding completion of the questionnaire (229/399; 57.4%; p = 0.518). Prior to the COVID-19 outbreak and associated restrictions, 137 PWE had a planned follow-up consultation with the treating physician. For 27 (19.7%) of them, this follow-up visit took place as planned; for 65 (47.4%), this visit was canceled because of the COVID-19 restrictions; and for 45 (32.8%), this visit was changed to a telephone or online consult.
3.3. COVID-19 symptoms and testing
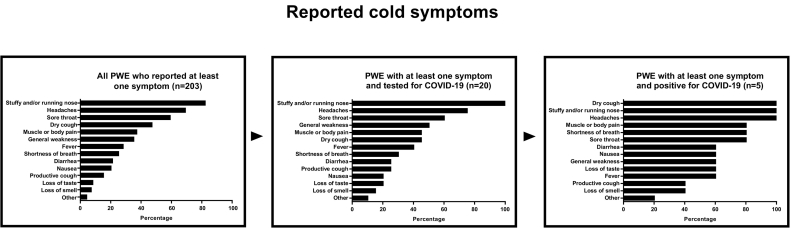
Of the total of 399 responding PWE, 203 reported at least one symptom of a cold since January 2020. The most frequently reported symptoms (Fig. 1) were a stuffy and/or running nose (167/203 PWE; 82.3%), headache (141/203 PWE; 69.5%), and a sore throat (120/203 PWE; 59.1%). Loss of taste and smell were reported by 17/203 (8.4%) and 15/203 (7.4%) PWE, respectively. COVID-19 testing was performed in 36/399 PWE, 16 (44.4%) of whom had not reported a cold (asymptomatic). Nine of 36 PWE tested positive for COVID-19, 4/9 (44.4%) of whom were reportedly asymptomatic. Persons with epilepsy with symptoms who tested positive for COVID-19 all reported a stuffy and/or running nose, dry cough, and headache; three (60%) reported loss of taste and 2 (40%) loss of smell (Fig. 1). Of the nine PWE with a COVID-19 positive test, six reported no change in seizure frequency during the COVID-19 epidemic compared with the period before, but three reported an increase in seizure frequency. Applying the World Health Organization (WHO) clinical diagnostic criteria for COVID-19 [30] on the study population with PWE suggested 56 (14.8%) cases with suspected COVID-19 when ignoring any previous contact with a case with COVID-19. Overall, 39.1% of the PWE (156/399) reported “quite some” to “extreme” difficulties to follow the COVID-19 protective measures.
Fig. 1.
Overview of the reported cold symptoms.
3.4. Access to antiseizure medication and financial difficulties
Of the 368 PWE who reported the use of ASM, 72 (19.6%) had difficulties to obtain ASM during the COVID-19 pandemic. The reasons for this included the following: ASM were not available (n = 50/72; 69.4%), COVID-19-related mobility restrictions made a visit to the hospital/pharmacy impossible (n = 9/72; 12.5%), financial problems as a result of COVID-19-related unemployment (n = 9/72; 12.5%), or other reasons, such as difficulties to obtain new prescriptions (n = 3/72; 4.2%).
Seventy-eight (22.8%) of 342 PWE who answered the question reported financial problems. Of these, 61 (78.2%) reported difficulties to pay for housing/bills, 27 (34.6%) difficulties to eat properly, and 30 (38.5%) difficulties to pay for ASM. Compared with PWE without financial problems, PWE with financial problems were more often living in a low- to middle-income country (35.9% vs 14.8%; p < 0.001).
3.5. Anxiety and depression among PWE
Of the 399 PWE, 201 (50.4%) screened positive for anxiety according to the HADS, and 159 (39.8%) and 187 (46.9%) PWE screened positive for depression based on the HADS and PHQ-9 scale, respectively.
Multiple logistic regression analysis suggested that female gender and financial problems significantly increased the odds of screening positive for both anxiety and depression (Table 2). Indeed, of the 66 female PWE with financial problems, 51 (77.5%) screened positive for anxiety and 44 (66.7%) for depression. In addition, PWE residing in high-income countries had significantly lower odds (0.360, 95% confidence interval [C.I.]: 0.191–0.682) for anxiety. Importantly, PWE who reported difficulties to access ASM had a higher odds (1.885, 95% C.I.: 1.083–3.282) for depression.
Table 2.
Multiple logistic regression analysis to investigate factors associated with depression and anxiety.
| Factors associated with anxiety according to HADS screening tool | ||||||
|---|---|---|---|---|---|---|
| Factor | No anxiety (n = 165) | Anxiety (n = 177) | Crude OR (95% C.I.) | Adjusted OR (95% C.I.) | p | |
| Gender | Female; n (%) | 114 (69.1%) | 156 (88.1%) | 3.489 (1.972–6.175) | 3.691 (2.022–6.740) | < 0.001 |
| Financial problems | Yes; n (%) | 22 (13.3%) | 56 (31.6%) | 3.008 (1.737–5.211) | 2.270 (1.256–4.103) | 0.007 |
| Country of residence | High-income; n (%) | 147 (89.1%) | 128 (72.3%) | 0.320 (0.177–0.577) | 0.360 (0.191–0.682) | 0.002 |
| Problems to obtain ASM | Yes; n (%) | 119 (72.1%) | 58 (64.4%) | 2.206 (1.232–3.331) | 1.605 (0.929–2.771) | 0.090 |
| Factors associated with depression according to HADS screening tool | ||||||
|---|---|---|---|---|---|---|
| Factor | No depression (n = 202) | Depression (n = 140) | Crude OR (95% C.I.) | Adjusted OR (95% C.I.) | p | |
| Financial problems | Yes; n (%) | 28 (13.9%) | 50 (35.7%) | 3.452 (2.036–5.854) | 2.275 (1.271–4.073) | 0.006 |
| Gender | Female; n (%) | 148 (73.3%) | 122 (87.1%) | 0.382 (0.211–0.693) | 2.381 (1.263–4.487) | 0.007 |
| Country of residence | High-income; n (%) | 171 (84.7%) | 104 (74.3%) | 0.524 (0.306–0.897) | 0.670 (0.360–1.245) | 0.205 |
| Living with spouse/partner | Yes; n (%) | 120 (59.4%) | 71 (50.7%) | 0.703 (0.455–1.086) | 0.535 (0.313–0.915) | 0.022 |
| Living with children | Yes; n (%) | 67 (33.2%) | 70 (50.0%) | 2.015 (1.295–3.135) | 2.738 (1.598–4.692) | < 0.001 |
| Problems to obtain ASM | Yes; n (%) | 41 (20.3%) | 49 (35.0%) | 2.114 (1.298–3.445) | 1.885 (1.083–3.282) | 0.025 |
| FU visit planned during COVID-19 lockdown | Yes; n (%) | 52 (25.7%) | 58 (41.4%) | 0.497 (0.313–0.788) | 0.499 (0.300–0.829) | 0.007 |
OR: odds ratio. C.I.: confidence interval. FU: follow-up. ASM: antiseizure medication. Only persons with epilepsy who answered the question on financial difficulties were included (n = 342).
4. Discussion
In this multicountry study, we assessed the access to healthcare and self-reported psychiatric symptoms among PWE during the ongoing COVID-19 pandemic. We detected important problems to access ASM and high prevalence of anxiety and depression. During confinement, follow-up consultations with the treating physician were canceled in almost half of the cases.
Of the 36 PWE (10% of the study population) who were tested for COVID-19, nine reported positive test results, accounting for 2.5% of the total population. However, when we applied the WHO clinical diagnostic criteria on our population, eight times more PWE were classified as cases with suspected COVID-19, suggesting that our participants were being undertested. The COVID-19 infections were asymptomatic in approximately half of the infected PWE, which is in line with previous results [31].
We found that the ongoing COVID-19 pandemic had an impact on the access to ASM. Nearly one-fifth of the PWE on ASM reported problems to obtain ASM; the most frequent constraint to ASM, accounting for approximately 70% of cases, was a shortage of ASM, which is an important problem that is not necessarily related to COVID-19. Our survey does not allow to determine whether COVID-19-related restrictions led to increased problems of ASM availability. Our results, however, do suggest an additional impact of COVID-19 on access to ASM. One-fourth of the PWE with difficulties to obtain ASM indicated COVID-19-related causes such as financial challenges to buy ASM as a consequence of COVID-19-related unemployment (12.5%) and COVID-19-related mobility restrictions making it difficult to go to the hospital/pharmacy for ASM refill (12.5%). Special attention should be paid by governments and healthcare workers to the impact of COVID-19 restrictions on access to ASM. In a longitudinal study conducted in the United States in 2016, ASM shortage caused psychological problems in the PWE related to the fear and anxiety of not being able to get their ASM and the possible consequences thereof [32]. In our study, we observed an increased risk of depression in PWE who reported problems to access ASM. We did not detect an increase in seizure frequency since the start of the COVID-19 pandemic. However, we conducted the survey early during confinement, and a potential impact in case of continuing access problems cannot be excluded.
In our survey, 201/399 (50.4%) and 159/399 (39.8%) PWE scored positive for anxiety and depression, respectively. The prevalence of depression and anxiety in PWE is higher than in the general population [4,20]. In a meta-analysis of 51 cross-sectional studies among PWE performed between 1999 and 2018, the prevalence of depression varied between 5% and 85%, largely depending on the diagnostic criteria that were used [33]. In 8 studies that used the HADS–Depression score, the point prevalence varied between 5% and 43%. Given the differences in study design, it is difficult to know whether COVID-19 increases the rates of depression among PWE [33]. The same is true for anxiety, for which a wide variation of screening tools and ranges of prevalence (9%–45%) have been reported in the literature [[34], [35], [36], [37]]. It is, however, worth noting that the prevalence of depression reported in our study seems to be higher than what has been reported by other authors when investigating the mental health condition of the general population during the COVID-19 pandemic [[12], [13], [14]]. Telehealth appointments may help to timely identify anxiety and depression. Several recent studies have highlighted the feasibility of telehealth appointments in epilepsy care and suggest an added value of interprofessional collaboration [[38], [39], [40], [41]].
Our study has limitations. First, the design was cross-sectional, which largely prohibited the investigation of causal relationships. Secondly, our survey was web-based, which causes an important sampling bias as people who are more active on social media were more prone to respond and low- to middle-income countries may not have equal access to the internet. Also, people with anxiety and depression may be more prone to complete online questionnaires, which may have resulted in an overestimation of the prevalence of depression and anxiety. Studies with a different design are needed to confirm our results. The link to complete the survey was freely accessible to anyone, making it difficult to estimate the reached sample [42]. Nonetheless, our sample size was small, and only 16.8% of PWE were from low-income countries, prohibiting an in-depth study of relationships with demographic features. In addition, the diagnosis of epilepsy was self-reported. As such, some respondents may not be true PWE according the ILAE diagnostic criteria. As we did not collect information on region of origin, we cannot relate individual responses directly to the actual lockdown and healthcare situation at the time of response. An additional limitation was related to the cancelation of follow-up visits: we do not know whether these were canceled by PWE themselves or by the healthcare providers. Four of the 9 (44.4%) PWE with a positive COVID-19 test reported no symptoms. However, we did not ask why the COVID-19 test was done. Those without symptoms may have been tested because of a contact with a person with a confirmed COVID-19 infection and may have developed symptoms since they completed the survey. Lastly, as more than 40% of the responders reported a seizure during the last 3 months, one would suspect that the COVID-19 epidemic increased the frequency of the seizures. Indeed, an increase of frequency of seizures has been observed with anxiety, depression, and healthcare barriers [43]. A prospective study certainly is needed to investigate the effect of the COVID-19 epidemic and lockdown measures on the frequency of seizures of PWE. Notwithstanding, our study provides important information on the impact of COVID-19 on PWE, which should be taken into account to improve their quality of life.
In conclusion, during the COVID-19 pandemic, PWE are confronted with challenges to access ASM. Half of the PWE screened positive for depression and slightly less screened positive for anxiety. Almost half of the respondents had their follow-up consultation with the treating physician canceled. While more research is needed, our results call for innovative approaches to ensure continuity in access to ASM. Physicians should ensure continued follow-up, either through inperson or telehealth appointments, to timely identify symptoms of anxiety and depression and act accordingly.
The following is the supplementary data related to this article.
Number of respondents per country.
Declaration of Competing Interest
All authors disclose no conflict of interest related to this study.
Footnotes
Declaration of competing interest: The authors declare no conflict of interest.
Funding source: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.World Health Organization Epilepsy. https://www.who.int/health-topics/epilepsy#tab=tab_1 Available at:
- 2.Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Cross J.H., Elger C.E. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 3.Josephson C.B., Jetté N. Psychiatric comorbidities in epilepsy. Int Rev Psychiatry. 2017;29:409–424. doi: 10.1080/09540261.2017.1302412. [DOI] [PubMed] [Google Scholar]
- 4.Tellez-Zenteno J.F., Patten S.B., Jetté N., Williams J., Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48:2336–2344. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 5.Kanner A.M., Ribot R. Depression. In: Mula M., editor. Neuropsychiatric symptoms of epilepsy. Springer International Publishing; Cham: 2016. pp. 25–41. [Google Scholar]
- 6.Boylan L.S., Flint L.A., Labovitz D.L., Jackson S.C., Starner K., Devinsky O. Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology. 2004;62:258–261. doi: 10.1212/01.wnl.0000103282.62353.85. [DOI] [PubMed] [Google Scholar]
- 7.Cramer J.A., Blum D., Reed M., Fanning K. The influence of comorbid depression on seizure severity. Epilepsia. 2003;44:1578–1584. doi: 10.1111/j.0013-9580.2003.28403.x. [DOI] [PubMed] [Google Scholar]
- 8.Kanner A.M., Barry J.J., Gilliam F., Hermann B., Meador K.J. Depressive and anxiety disorders in epilepsy: do they differ in their potential to worsen common antiepileptic drug-related adverse events? Epilepsia. 2012;53:1104–1108. doi: 10.1111/j.1528-1167.2012.03488.x. [DOI] [PubMed] [Google Scholar]
- 9.Hitiris N., Mohanraj R., Norrie J., Sills G.J., Brodie M.J. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75:192–196. doi: 10.1016/j.eplepsyres.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Psychiatric outcome after temporal lobectomy: a predictive studyEpilepsia. 2000;41:1608–1615. doi: 10.1111/j.1499-1654.2000.001608.x. [DOI] [PubMed] [Google Scholar]
- 11.de Souza J.L., Faiola A.S., Miziara C., de Manreza M.L.G. The perceived social stigma of people with epilepsy with regard to the question of employability. Neurol Res Int. 2018;2018:4140508. doi: 10.1155/2018/4140508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y., Zhao N. Chinese mental health burden during the COVID-19 pandemic. Asian J Psychiatr. 2020;51:102052. doi: 10.1016/j.ajp.2020.102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazza C., Ricci E., Biondi S., Colasanti M., Ferracuti S., Napoli C. A nationwide survey of psychological distress among Italian people during the COVID-19 pandemic: immediate psychological responses and associated factors. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17093165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Özdin S., Bayrak Özdin Ş. Levels and predictors of anxiety, depression and health anxiety during COVID-19 pandemic in Turkish society: the importance of gender. Int J Soc Psychiatry. 2020;66:504–511. doi: 10.1177/0020764020927051. 20764020927051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang Y.T., Yang Y., Li W., Zhang L., Zhang Q., Cheung T. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7:228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menninger K.A. Influenza and schizophrenia. An analysis of post-influenzal “dementia precox,” as of 1918, and five years later further studies of the psychiatric aspects of influenza. 1926. Am J Psychiatry. 1994;151:182–187. doi: 10.1176/ajp.151.6.182. [DOI] [PubMed] [Google Scholar]
- 17.Honigsbaum M. “An inexpressible dread”: psychoses of influenza at fin-de-siècle. Lancet. 2013;381:988–989. doi: 10.1016/S0140-6736(13)60701-1. [DOI] [PubMed] [Google Scholar]
- 18.Lam M.H., Wing Y.K., Yu M.W., Leung C.M., Ma R.C., Kong A.P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 19.Lazzerini M., Barbi E., Apicella A., Marchetti F., Cardinale F., Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:e10–e11. doi: 10.1016/S2352-4642(20)30108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao X., Zhou D., Li Z., Zeng G., Hao N., Li E. Severe psychological distress among epilepsy patients during the COVID-19 outbreak in southwest China. Epilepsia. 2020;61:1166–1173. doi: 10.1111/epi.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Bank Country and Lending Groups World Bank data help desk. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 22.Lin C.Y., Pakpour A.H. Using Hospital Anxiety and Depression Scale (HADS) on patients with epilepsy: confirmatory factor analysis and Rasch models. Seizure. 2017;45:42–46. doi: 10.1016/j.seizure.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Wiglusz M.S., Landowski J., Michalak L., Cubała W.J. Validation of the Hospital Anxiety and Depression Scale in patients with epilepsy. Epilepsy Behav. 2016;58:97–101. doi: 10.1016/j.yebeh.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 24.de Oliveira G.N., Lessa J.M., Gonçalves A.P., Portela E.J., Sander J.W., Teixeira A.L. Screening for depression in people with epilepsy: comparative study among Neurological Disorders Depression Inventory for Epilepsy (NDDI-E), Hospital Anxiety and Depression Scale Depression subscale (HADS-D), and Beck Depression Inventory (BDI) Epilepsy Behav. 2014;34:50–54. doi: 10.1016/j.yebeh.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Fiest K.M., Patten S.B., Wiebe S., Bulloch A.G., Maxwell C.J., Jetté N. Validating screening tools for depression in epilepsy. Epilepsia. 2014;55:1642–1650. doi: 10.1111/epi.12754. [DOI] [PubMed] [Google Scholar]
- 26.Rampling J., Mitchell A.J., Von Oertzen T., Docker J., Jackson J., Cock H. Screening for depression in epilepsy clinics. A comparison of conventional and visual-analog methods. Epilepsia. 2012;53:1713–1721. doi: 10.1111/j.1528-1167.2012.03571.x. [DOI] [PubMed] [Google Scholar]
- 27.Rathore J.S., Jehi L.E., Fan Y., Patel S.I., Foldvary-Schaefer N., Ramirez M.J. Validation of the Patient Health Questionnaire-9 (PHQ-9) for depression screening in adults with epilepsy. Epilepsy Behav. 2014;37:215–220. doi: 10.1016/j.yebeh.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siarava E., Hyphantis T., Katsanos A.H., Pelidou S.H., Kyritsis A.P., Markoula S. Depression and quality of life in patients with epilepsy in Northwest Greece. Seizure. 2019;66:93–98. doi: 10.1016/j.seizure.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Jamal-Omidi S., Collins C., Fulchiero E., Liu H., Colon-Zimmermann K., Fuentes-Casiano E. Assessing depression severity with a self-rated vs. rater-administered instrument in patients with epilepsy. Epilepsy Behav. 2018;85:52–57. doi: 10.1016/j.yebeh.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance. Published March 2020. https://apps.who.int/iris/rest/bitstreams/1272502/retrieve
- 31.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles' heel of current strategies to control Covid-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukmanji S., Sauro K.M., Josephson C.B., Altura K.C., Wiebe S., Jetté N. A longitudinal cohort study on the impact of the clobazam shortage on patients with epilepsy. Epilepsia. 2018;59:468–478. doi: 10.1111/epi.13974. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y., Yang M., Shi Q., Wang T., Jiang M. Risk factors for depression in patients with epilepsy: a meta-analysis. Epilepsy Behav. 2020;106:107030. doi: 10.1016/j.yebeh.2020.107030. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z., Luo Z., Li S., Luo Z., Wang Z. Anxiety screening tools in people with epilepsy: a systematic review of validated tools. Epilepsy Behav. 2019;99 doi: 10.1016/j.yebeh.2019.06.035. [DOI] [PubMed] [Google Scholar]
- 35.Al-Asmi A., Dorvlo A.S., Burke D.T., Al-Adawi S., Al-Zaabi A., Al-Zadjali H.A. The detection of mood and anxiety in people with epilepsy using two-phase designs: experiences from a tertiary care centre in Oman. Epilepsy Res. 2012;98:174–181. doi: 10.1016/j.eplepsyres.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Lee S.A., Jeon J.Y., No S.K., Park H., Kim O.J., Kwon J.H. Factors contributing to anxiety and depressive symptoms in adults with new-onset epilepsy. Epilepsy Behav. 2018;88:325–331. doi: 10.1016/j.yebeh.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Scott A.J., Sharpe L., Hunt C., Gandy M. Anxiety and depressive disorders in people with epilepsy: a meta-analysis. Epilepsia. 2017;58:973–982. doi: 10.1111/epi.13769. [DOI] [PubMed] [Google Scholar]
- 38.Punia V., Nasr G., Zagorski V., Lawrence G., Fesler J., Nair D. Evidence of a rapid shift in outpatient practice during the COVID-19 pandemic using telemedicine. Telemed J E Health. 2020 doi: 10.1089/tmj.2020.0150. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Shawahna R. Development of key performance indicators to capture in measuring the impact of pharmacists in caring for patients with epilepsy in primary healthcare: a Delphi consensual study. Epilepsy Behav. 2019;98:129–138. doi: 10.1016/j.yebeh.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 40.Axon D.R., Taylor A.M., Vo D., Bingham J. Initial assessment of an interprofessional team-delivered telehealth program for patients with epilepsy. Epilepsy Res. 2019;158:106235. doi: 10.1016/j.eplepsyres.2019.106235. [DOI] [PubMed] [Google Scholar]
- 41.Varley J., Kiersey R., Power R., Byrne J.P., Doherty C., Saris J. Igniting intersectoral collaboration in chronic disease management: a participatory action research study on epilepsy care in Ireland. J Interprof Care. 2019:1–9. doi: 10.1080/13561820.2019.1697655. [DOI] [PubMed] [Google Scholar]
- 42.Lagae L., Brambilla I., Mingorance A., Gibson E., Battersby A. Quality of life and comorbidities associated with Dravet syndrome severity: a multinational cohort survey. Dev Med Child Neurol. 2018;60:63–72. doi: 10.1111/dmcn.13591. [DOI] [PubMed] [Google Scholar]
- 43.Aledo-Serrano Á., Mingorance A., Jiménez-Huete A., Toledano R., García-Morales I., Anciones C. Genetic epilepsies and COVID-19 pandemic: lessons from the caregiver perspective. Epilepsia. 2020;61:1312–1314. doi: 10.1111/epi.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of respondents per country.