Introduction
An increase in acute flaccid paralysis with a longitudinal gray matter lesion of the spinal cord was noted in California in 2012.1 Additional cases in California, Colorado, and across the United States followed in 2014,2 resulting in Centers for Disease Control and Prevention (CDC) surveillance, with acute flaccid myelitis (AFM) defined as a patient aged less than 18 years with the acute onset of flaccid paralysis in the setting of a longitudinal, gray matter predominant lesion of the spinal cord. There were subsequent biennial increases in cases of AFM to a maximum of 238 US cases spread over 42 states in 2018.3 This biennial occurrence presents unique challenges. Just as public and health care provider vigilance begin to wane, we approach another season. With focus and energy diverted to the coronavirus disease 2019 pandemic, 2020 may be even more testing. We aim to review AFM basics, provide guidance on testing and reporting, and discuss the current state of research and therapeutics. We encourage child neurologists to maintain a high level of suspicion for AFM, to review the diagnosis with local front line providers, and to have a plan for rapid evaluation and management of these patients.
Clinical presentation
Children with AFM typically present with proximal greater than distal, asymmetric paralysis of one or more extremities with or without bulbar or cranial nerve findings in the setting of a recent or current febrile illness. Weakness may be preceded by pain in the affected extremity, leading to misdiagnoses of joint conditions or trauma. Weakness may progress over hours to several days to multiple extremity weakness and respiratory decline, with 33% of patients requiring respiratory support.4 Findings on neurological examination include low muscle tone with decreased or absent deep tendon reflexes and typically intact sensation. Weakness is flaccid in the primarily involved extremity, but in cases with severe cord edema there may be secondary upper motor neuron signs, particularly in the lower extremities. There may be bulbar or cranial nerve findings. Given the sudden loss of muscle tone and decreased movement in addition to the spinal cord lesion, patients are also at risk for constipation and urinary retention.
Notably, AFM is a diagnosis that is easy to miss early in the presentation because subtle weakness associated with pain may be attributed to trauma or viral illness. Close examination of all patients for proximal muscle weakness (high five, shoulder shrug, stoop and recover, and stand from floor without using hands) and hyporeflexia is essential to avoid missing an early presentation of AFM.
Testing recommendation
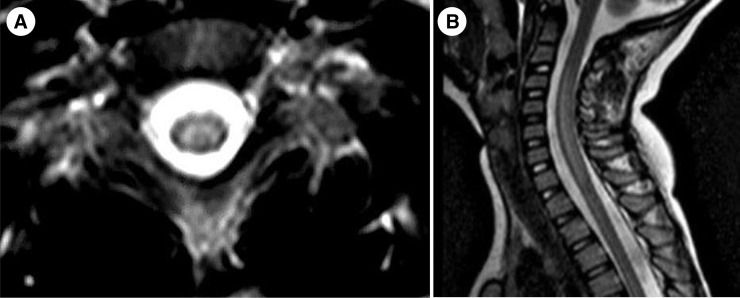
Earlier viral testing increases yield, so specimens should be obtained as quickly as possible. Viral testing has minimal risk, and samples, respiratory in particular, should be obtained as soon as the diagnosis of AFM is suspected, without waiting for magnetic resonance imaging (MRI) confirmation. The Table lists basic testing recommendations. Suspicion for AFM should lead to MRI of the spinal cord to look for central gray matter lesions (Fig ). MRI brain should also be obtained to rule out alternative possibilities and in case of cranial nerve involvement, which may accompany. Repeat imaging may be necessary depending on clinical course. Spinal MRI weeks and months later may show myelomalacia but there is no consensus on the utility of routine follow-up studies. Lumbar puncture is recommended to look for evidence of inflammation, and typically demonstrates a lymphocytic pleocytosis with variable increase in protein. The diagnosis does not require electrodiagnostic confirmation, but electromyography may help when alternative etiologies, such as Guillain-Barré syndrome, are in the differential and may be helpful in prognostication.5
TABLE.
Recommendations for Initial Testing for Suspected AFM
| Specimen | Tests |
|---|---|
| Respiratory (nasopharyngeal swab) | Respiratory virus PCR panel, enteroviral PCR |
| Serum | Enteroviral PCR, NMO (aquaporin 4), and MOG antibody testing, consider Lyme and West Nile antibody testing when clinically appropriate |
| CSF | Basic studies, enteroviral PCR testing |
| Stool | Enteroviral PCR |
| Imaging | MRI of the spinal cord with and without contrast, strongly consider MRI brain with and without contrast |
Abbreviations:
AFM = Acute flaccid myelitis
CSF = Cerebrospinal fluid
MOG = Myelin oligodendrocyte glycoprotein
MRI = Magnetic resonance imaging
NMO = Neuromyelitis optica
PCR = Polymerase chain reaction
FIGURE.
Axial (A) and sagittal (B) T2-weighted images demonstrating gray matter predominant longitudinally extensive lesions characteristic of AFM. AFM, acute flaccid myelitis.
Treatment
We recommend that all patients with possible AFM be admitted to the hospital for close monitoring and testing. All patients need close monitoring of respiratory function, particularly negative inspiratory force. Patients with bulbar findings and/or cervical spine lesions and those with progressive weakness will be at higher risk for respiratory compromise and autonomic dysfunction, and we suggest admission to an intensive care unit.
Unfortunately, there remain no clear recommendations for optimal medical treatment of AFM. Intravenous immunoglobulin is often used to boost humoral immunity and showed efficacy when given early in a mouse model.6 Steroids and plasma exchange have been used and theoretically may have benefit in individuals with significant spinal cord edema and long tract signs; however, the timing and potential risk of exacerbating an underlying infection should be carefully considered on a case-by-case basis. Several centers around the country tried fluoxetine in 2015 to 2016 but a retrospective analysis of data revealed no benefit.
Recent data from colleagues at Vanderbilt demonstrate that, in a mouse model, anti-enterovirus D68 antibodies may limit the course of AFM and are associated with marked recovery in the mouse model.7 Whether these antibodies will be developed as a safe and effective therapy for humans with AFM remains to be seen, but the data are encouraging.
Early involvement of pediatric rehabilitation is a key to maximize functional outcomes. Bracing must balance safety and use of the muscles. In addition, nerve and muscle transfer surgeries may be considered to maximize function in select patients.8 Patients with AFM are at risk for secondary complications such as joint subluxation, limb length discrepancies, scoliosis, and decreased bone density.9 Close monitoring is required for long term. Where feasible, consider referral to centers with AFM experience and multidisciplinary teams.
Reporting
There have been changes to the AFM case definition over the years. Current US CDC guidelines request that clinicians report any patient with sudden onset of flaccid limb weakness and MRI with spinal cord lesions involving at least some gray matter with the exclusion of malignancies, vascular disease, or anatomic abnormalities. The CDC provides clear instructions for reporting cases at https://www.cdc.gov/acute-flaccid-myelitis/hcp/clinicians-health-departments.html. Cases should be reported by a team caring for the patient when the MRI has been obtained and the diagnosis is suspected. As with initial sample acquisition, time is important—earlier reporting improves accuracy of surveillance numbers and makes it easier to procure the appropriate samples.
In addition to surveillance, the National Institutes of Health and the National Institute for Allergy and Infectious Diseases AFM Natural History Study begins this year. Clinicians interested in enrolling patients may find additional information at https://www.cdc.gov/acute-flaccid-myelitis/parents/get-involved-afm-research.html.
Conclusions
AFM is associated with significant morbidity. Early consideration of the diagnosis and testing is a key to identify the etiology, keeping patients safe, and contributing key data to surveillance. We hope that recent research will lead to additional treatments for AFM, in which case early diagnosis will be a key to effective treatment. We ask child neurologists to remind front line colleagues in their region about AFM and urge institutions to have clear protocols in place for testing and management.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report. Dr. Hopkins receives salary support from the US Centers for Disease Control for activities related to acute flaccid myelitis (AFM) surveillance and is the site principal investigator (PI) for the National Institutes of Health AFM Natural History Study. Dr. Desai has received funds from EFGLA, Ovid, Novartis, Aquestive, Neurelis, and UCB. Dr. Benson is a site PI for the AFM Natural History Study and is a paid consultant to the National Vaccine Injury Compensation Program and to the Massachusetts Department of Public Health. She receives funds for Biogen- and Alexion-sponsored clinical trials, and from ROHHAD Fight, Inc and HMS Shore Foundation.
References
- 1.Van Haren K., Ayscue P., Waubant E. Acute flaccid myelitis of unknown etiology in California, 2012-2015. JAMA. 2015;314:2663–2671. doi: 10.1001/jama.2015.17275. [DOI] [PubMed] [Google Scholar]
- 2.Messacar K., Schreiner T.L., VanHaren K. Acute flaccid myelitis: a clinical review of US cases 2012–2015. Ann Neurol. 2016;80:326–338. doi: 10.1002/ana.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC AFM cases and outbreaks. 2020. https://www.cdc.gov/acute-flaccid-myelitis/cases-in-us.html Available at:
- 4.Ayers T., Lopez A., Lee A. Acute flaccid myelitis in the United States: 2015-2017. Pediatrics. 2019;144:e20191619. doi: 10.1542/peds.2019-1619. [DOI] [PubMed] [Google Scholar]
- 5.Martin J.A., Messacar K., Yang M.L. Outcomes of Colorado children with acute flaccid myelitis at 1 year. Neurology. 2017;89:129–137. doi: 10.1212/WNL.0000000000004081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hixon A.M., Clarke P., Tyler K.L. Evaluating treatment efficacy in amouse model of enterovirus D68-associated paralytic myelitis. J Infect Dis. 2017;216:1245–1253. doi: 10.1093/infdis/jix468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogt M., Fu J., Kose N. Human antibodies neutralize enterovirus D68 and protect against infection and paralytic disease. Sci Immunol. 2020;5:eaba4902. doi: 10.1126/sciimmunol.aba4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pino P.A., Intravia J., Kozin S.H., Zlotolow D.A. Early results of nerve transfers for restoring function in severe cases of acute flaccid myelitis. Ann Neurol. 2019;86:607–615. doi: 10.1002/ana.25558. [DOI] [PubMed] [Google Scholar]
- 9.Melicosta M.E., Dean J., Hagen K. Acute flaccid myelitis: rehabilitation challenges and outcomes in a pediatric cohort. J Pediatr Rehabil Med. 2019;12:245–253. doi: 10.3233/PRM-180549. [DOI] [PubMed] [Google Scholar]