Summary:
NTproBNP ≤1000 has favorable prognostic implications in HF, but the prognosis is worse for Black patients at any level of achieved NTproBNP
Racial differences exist in the circulating natriuretic peptides (NPs)1 and they manifest starting in early adulthood.2 N-terminal pro-B-type NP (NT-proBNP) levels have a weaker predictive ability for heart failure (HF) in Black individuals with obesity, and kidney dysfunction.3 Use of serial NT-proBNP levels was tested in the multicenter, randomized Guiding Evidence-Based Therapy Using Biomarker-Intensified Treatment in HF (GUIDE-IT) trial, the results of which were neutral.4 An analysis from GUIDE-IT indicates that treatment to a therapeutic NT-proBNP target of ≤1000 pg/mL is an important component of optimal HF management.5 However, the prognostic value of the achieved threshold of NT-proBNP ≤1,000 pg/ml has not been examined in Black individuals. We sought to evaluate the racial differences in the prognostic implications of guiding HF management to a target NT-proBNP level of ≤1000 pg/mL among the GUIDE-IT trial participants.
Anonymized study data are publically available at the National Heart, Lung, and Blood Institute BioLINCC data repository. All patients provided written informed consent, and the institutional review boards at each site approved the study. Self-identified Black and White individuals who did not experience adverse cardiovascular outcomes (HF hospitalization or death due to cardiovascular causes) in the first six weeks were included in the study. The achievement of target NT-proBNP cutoff was defined using the NT-proBNP levels at the six-week visit. Baseline characteristics were compared using descriptive statistics with the continuous data compared using Wilcoxon rank-sum test and the categorical variables compared using Chi-square test. Survival analyses were conducted for the risk of adverse cardiovascular outcomes using multivariable-adjusted Cox proportional hazard models. The rate of adverse cardiovascular outcomes was estimated using Poisson regression. Multivariable-adjusted incidence rate ratio was computed for the racial groups using six-week NT-proBNP levels. The models were adjusted for age, sex, body-mass index, baseline NT-proBNP, baseline left ventricular ejection fraction (LVEF), blood pressure, atrial fibrillation, heart rate, sodium and potassium levels, creatinine, ischemic heart disease, sleep apnea, depression, use of implantable cardioverter-defibrillator/pacemaker, and treatment arm.5 Interaction testing was done using multiplicative interaction terms (race*achievement of target NT-proBNP levels). All analyses were conducted using SAS 9.4 (Cary, NC).
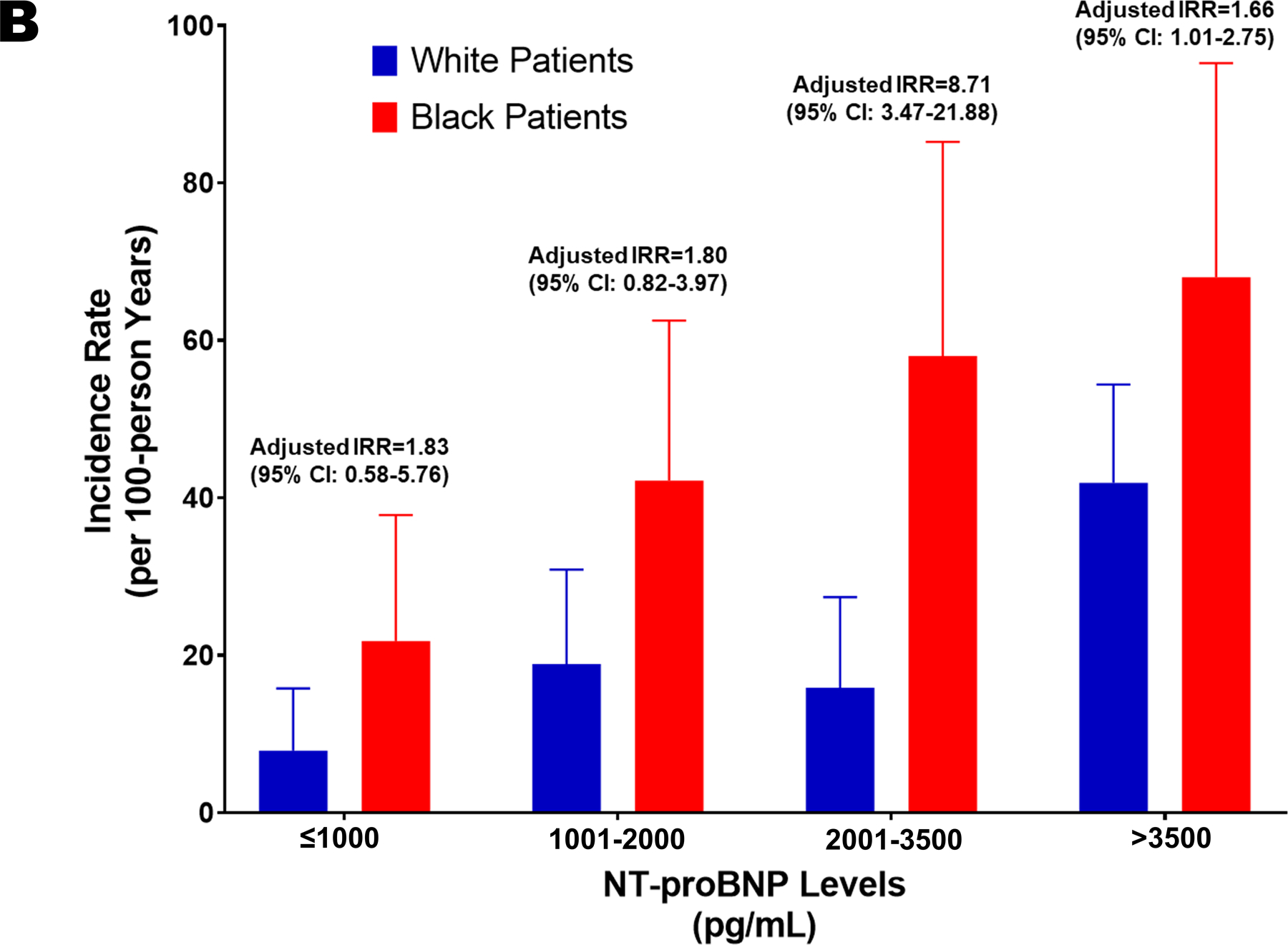
There were 608 patients with available data. Of them, 37.5% were Black patients. Similar demographic profiles and clinical characteristics were noted by race. Significantly lower NT-proBNP levels (2025.5 pg/mL [25th percentile, 75th percentile: 1155.5, 3868.5 pg/mL] vs. 2447.5 pg/mL [1153.0, 4998.5 pg/mL]; adjusted p=0.002) and LVEF (20.0% [15.0, 30.0%] vs. 25.0% [20.0, 30.0%]; adjusted p<0.001) was present among Black patients at baseline. The event rate for adverse cardiovascular outcomes was 9.5% (8/84) and 29.1% (86/296) in White HF patients with NT-proBNP levels ≤1000 pg/mL and >1000 pg/mL, respectively. The event rate among Black patients was 24.1% (13/54) for those who achieved the target NT-proBNP levels and 48.9% (89/174) for those who did not. Among White HF patients, those who did not achieve the target NT-proBNP levels had a greater risk of adverse cardiovascular outcomes compared to those with NT-proBNP levels ≤1000 pg/mL (HR: 3.42 [95% CI: 1.55–7.54]; p=0.002). Similarly, among Blacks, patients who did not achieve the target NT-proBNP levels had a higher risk of developing adverse cardiovascular outcomes compared to those with NT-proBNP levels ≤1000 pg/mL (HR: 2.58 [95% CI: 1.38–4.83]; p=0.003) (Figure 1A). There was no significant interaction between race and achievement of target NT-proBNP levels on the outcome (p>0.10). The rates of adverse cardiovascular outcomes across different strata of NT-proBNP levels is depicted in Figure 1B.
Figure 1. Risk of Adverse Cardiovascular Outcomes in Heart Failure-Stratified by Race and NT-proBNP Levels.
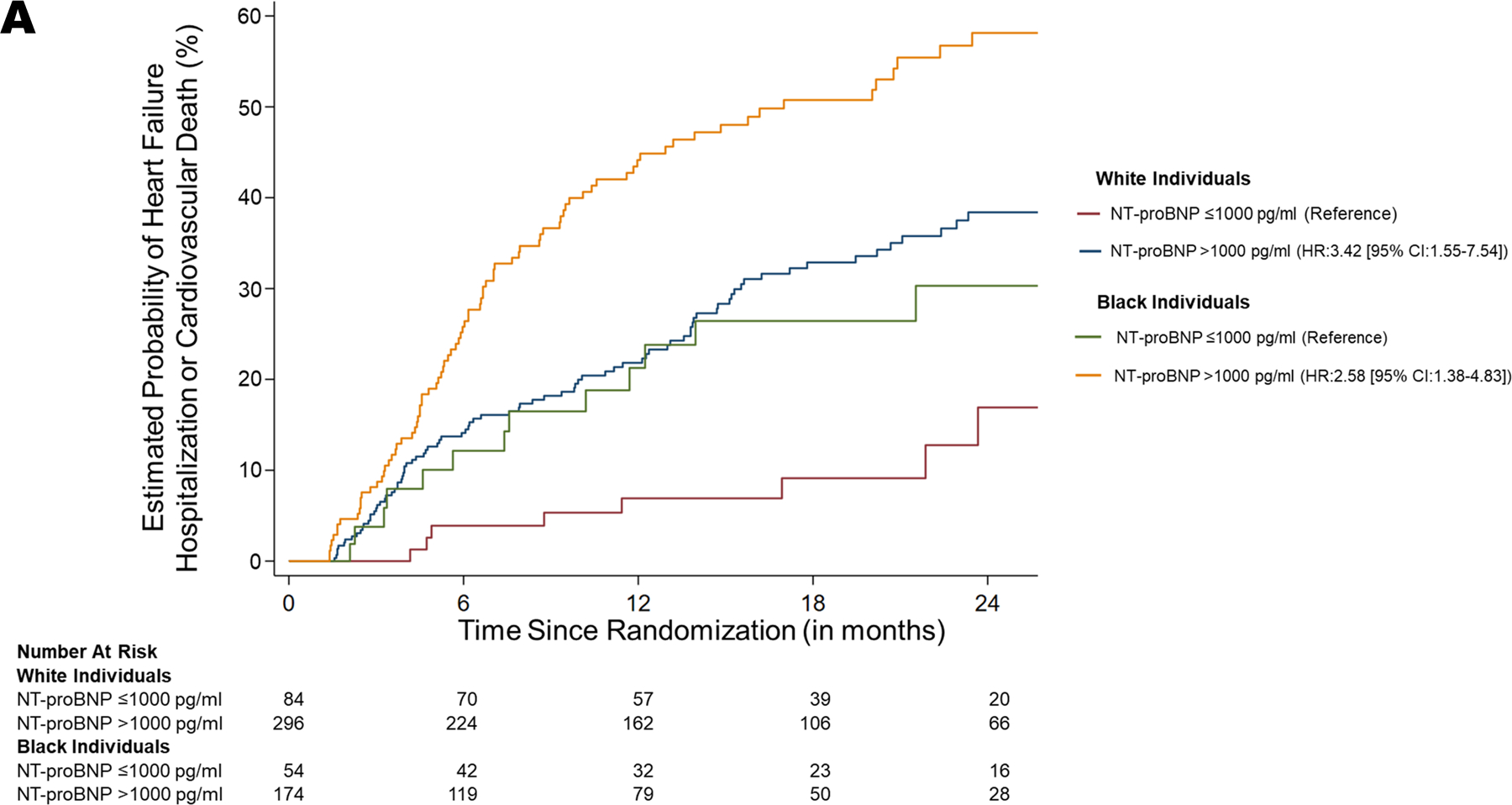
A. Risk of Heart Failure Hospitalization or Cardiovascular Death
Kaplan Meier curves depicting the probability of heart failure hospitalization or death due to cardiovascular causes, stratified by race, and NT-proBNP levels achieved. The curves in red (NT-proBNP ≤1000 pg/mL) and blue (NT-proBNP >1000 pg/mL) represents White patients. The curves in green (NT-proBNP ≤1000 pg/mL) and yellow (NT-proBNP >1000 pg/mL) represents Black patients.
B. Rates of Heart Failure Hospitalization or Cardiovascular Death Across NT-proBNP Level Categories at 6-weeks
The bars in blue represent White patients, and the bars in red represent the Black patients. The error bars represent 95% CI. IRR: Incidence Rate Ratio.
In this study, we observed that Black patients with HF had ~21% lower NT-proBNP levels as compared to White patients. Despite this, NT-proBNP concentrations of ≤1,000 pg/ml had prognostic significance in both Black and White patients, as evidenced by a better prognosis in those who achieve target NT-proBNP levels irrespective of the race. Black HF patients had a higher risk for adverse cardiovascular outcomes compared to their White counterparts, as evidenced by an upward shift of the survival curves and higher clinical event rates across various strata of NT-proBNP. We have previously shown that Black individuals have relative NP deficiency.1, 2 This deficiency may be attributed to impaired NP processing and enhanced clearance of the circulating NPs.2 Racial differences in NP system response to perturbations indicate that similar NT-proBNP levels may signify different states of congestion and treatment response between racial groups. With many HF trials considering NT-proBNP levels as a surrogate therapeutic endpoint, our findings indicate that the achievement of NTproBNP target ≤1,000 pg/ml is of comparable importance in both races. Our study may be underpowered due to the small population size in each stratum and fewer events, to evaluate the interaction of race with achieved target NT-proBNP levels. In summary, achieving a target NT-proBNP level of ≤1000 pg/mL has favorable prognostic implications in both Black and White HF patients, but the prognosis is worse for Black patients at either level of achieved NT-proBNP.
Acknowledgments:
We would like to thank the GUIDE-IT trial investigators for making the study data available for public use through the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository.
Source of Funding: This work is supported by the Minority Health & Health Disparities Research Center, National Institute of Minority Health and Health Disparities [U54MD000502], and the National Institutes of Health Mentored-Patient Oriented Research Award [5K23 HL146887-02] to Dr. Pankaj Arora.
Disclosures: Dr. Januzzi is a Trustee of the American College of Cardiology, has received grant support from Novartis Pharmaceuticals and Abbott Diagnostics, consulting income from Abbott Diagnostics, Janssen, MyoKardia, Novartis, and Roche Diagnostics, and participates in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, Bayer, CVRx, Janssen, and Takeda. Dr. Felker has received research grants from NHLBI, American Heart Association, Amgen, Merck, Cytokinetics, and Roche Diagnostics. He has acted as a consultant to Novartis, Amgen, BMS, Cytokinetics, Medtronic, Cardionomic, Relypsa, V-Wave, Innolife, EBR Systems, Arena, Abbott, Sphingotec, Roche Diagnostics, Alnylam, LivaNova, Windtree Therapeutics, Rocket Pharma, and SC Pharma. Dr. Wang has taken personal fees from Novartis outside of submitted work. None of the other authors had any conflicts of interest or financial disclosures to declare.
Footnotes
Data Sharing Statement: Anonymized study data are publically available at the National Heart, Lung, and Blood Institute BioLINCC data repository and can be accessed at https://biolincc.nhlbi.nih.gov/home/.
References
- 1.Bajaj NS, Gutierrez OM, Arora G, Judd SE, Patel N, Bennett A, Prabhu SD, Howard G, Howard VJ, Cushman M and Arora P. Racial Differences in Plasma Levels of N-Terminal Pro-B-Type Natriuretic Peptide and Outcomes: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. JAMA Cardiol. 2018;3:11–17. doi: 10.1001/jamacardio.2017.4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel N, Russell GK, Musunuru K, Gutierrez OM, Halade G, Kain V, Lv W, Prabhu SD, Margulies KB, Cappola TP, Arora G, Wang TJ and Arora P. Race, Natriuretic Peptides, and High-Carbohydrate Challenge: A Clinical Trial. Circ Res. 2019;125:957–968. doi: 10.1161/CIRCRESAHA.119.315026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel N, Cushman M, Gutierrez OM, Howard G, Safford MM, Muntner P, Durant RW, Prabhu SD, Arora G, Levitan EB and Arora P. Racial differences in the association of NT-proBNP with risk of incident heart failure in REGARDS. JCI Insight. 2019; 4(13): e129979. doi: 10.1172/jci.insight.129979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, Januzzi JL Jr., Mark DB, Pina IL, Passmore G, Whellan DJ, Yang H, Cooper LS, Leifer ES, Desvigne-Nickens P and O’Connor CM. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2017;318:713–720. doi: 10.1001/jama.2017.10565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Januzzi JL Jr., Ahmad T, Mulder H, Coles A, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, Mark DB, Pina IL, Passmore G, Whellan DJ, Cooper LS, Leifer ES, Desvigne-Nickens P, Felker GM and O’Connor CM. Natriuretic Peptide Response and Outcomes in Chronic Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2019;74:1205–1217. doi: 10.1016/j.jacc.2019.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]


