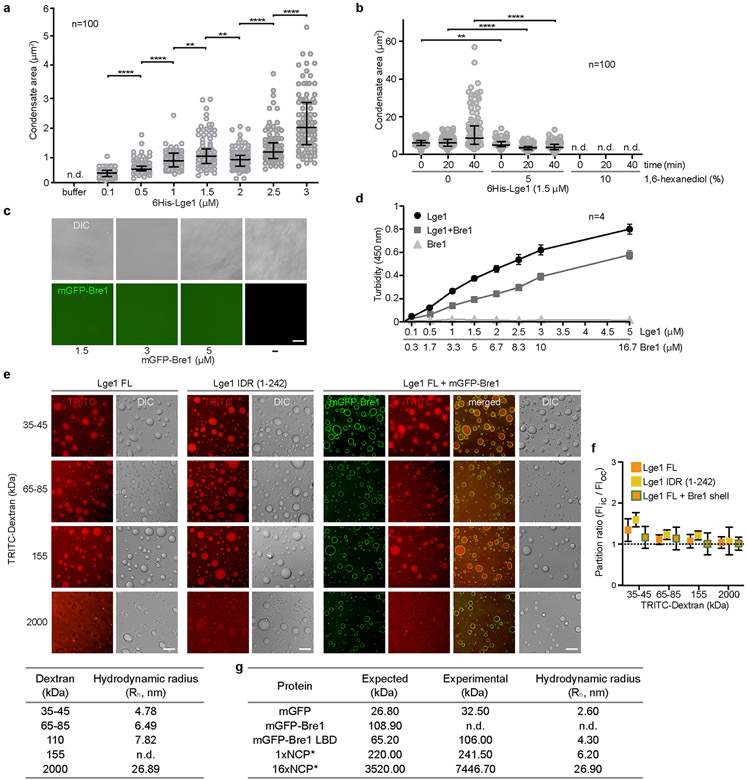
Extended Data Figure 4. Material properties of Lge1 condensates.
a. Quantification of 6His-Lge1 condensate sizes at different protein concentrations after 5 min of incubation at 20 °C. Quantification was done with ImageJ. n = number of condensates. Dot plots show median and interquartile range. **p-value 1 vs. 1.5 = 0.0046, **p-value 1.5 vs. 2 = 0.0029, ****p-value < 0.0001 determined by two-sided Mann-Whitney test, n.d., not determinable, b. Quantification of 6His-Lge1 condensate size in the presence of 1,6-hexanediol indicates an inhibition of LLPS. Concentrated Lge1 protein was diluted to 1.5 μM in buffer with 1,6-hexanediol (%, w/v) and incubated for 15 min before imaging, n = number of condensates. Dot plots show median and interquartile range. **p-value = 0.0032, ****p-value < 0.0001 determined by two-sided Mann-Whitney test, n.d., not determinable, c. Strep-mGFP-Bre1 does not phase-separate under the conditions tested. Concentrated proteins were diluted and incubated at 20 °C for 5 min prior to DIC microscopy. Scale bar, 10 μm. d. Turbidity measurements of 6His-Lge1 at 450 nm with or without Strep-Bre1. Proteins were mixed at the indicated molar ratios. LLPS of Lge1 occurred already at 0.1 μM, Strep-Bre1 shows no LLPS under the conditions tested. Mean and standard deviation are indicated, e. Condensates of 6His-Lge1, 6His-Lge1 IDR or 6His-Lge1 with an mGFP-Bre1 shell were incubated with TRITC-labelled dextran of different sizes (final dextran concentration 0.05 mg/ml) for 15 min at 20 °C. Samples were imaged by DIC and fluorescence microscopy. Scale bar, 10 μm. The table below shows the hydrodynamic radius (Rh) for dextrans in aqueous buffer; adapted from published work34, f. Lge1-Bre1 condensates are permeable to dextrans of different sizes. Dextran is never excluded (partition ratios ≥ 1). Mean and standard deviation are indicated; n = 60 condensates, g. Average Rh of recombinant proteins used in this work as measured by dynamic light scattering (DLS) at 20 °C. The expected molecular mass was calculated according to the protein’s amino-acid composition and compared to the experimental molecular mass obtained by DLS. Final data correspond to the average of at least two independent measures, n.d., not determinable.