Abstract
Background
Chronic urticaria (CU) is a common skin disease; however, its etiology is rarely recognized. Infection due to Helicobacter pylori (H. pylori) has been shown in some studies to play a significant role in the pathogenesis of CU.
Objective
This study was conducted to determine the association between CU and H. pylori infection among patients attending the Regional Dermatology Training Center, Northern Tanzania, from October 2018 to April 2019. Methodology. A matched case-control study that included 55 cases and 55 controls matched by age and sex was conducted. Data were collected through direct interviews, and the results of laboratory investigations were recorded in the extraction sheet. An enzyme-linked immunosorbent assay test was used to detect H. pylori antigen in the stool samples. Conditional logistic regression was used to measure the association between CU and H. pylori.
Results
The total number of participants in this study was 110 patients (55 cases and 55 controls), whereby the median age was 31 (IQR 27–45) among controls versus 34 (IQR: 22–46) years among the cases. Both cases and controls had the same number of females and males. There was no significant association between CU and baseline characteristics of the participants. There was an association between CU and H. pylori infection, such that subjects with CU had a higher number of positive H. pylori test (15/55 = 27%) versus controls (6/55 = 10.1%) (p = 0.0225). The adjusted odds of CU among patients who were positive for H. pylori were sixfolds higher (OR = 6.9; CI: 1.3–36.2; p = 0.021) than those of patients who were negative for H. pylori.
Conclusion
There was a strong and significant association between CU and H. pylori infection. We recommend investigating for H. pylori in all cases of CU and conducting further trials on H. pylori eradication.
1. Introduction
Urticaria, commonly known as hives, is defined by the appearance of short-lived swellings, which are called wheals. This condition presents with either wheals, angioedema, or both [1]. Patients with urticaria present with severe itching that can have effect on the quality of their life [2]. Urticaria can be either acute or chronic. Acute urticaria occurs days to weeks, producing wheals that rarely may last more than 12 hours, and complete resolution of the lesions may occur within six weeks of onset. In comparison, daily episodes lasting more than six weeks are designated as CU [3]. Both children and adults can develop urticaria, although it is more common in adults, and females are more affected than males [4]. Urticaria is a debilitating condition affecting 0.3% to 5% of the general population worldwide [5]. In sub-Saharan Africa, the prevalence of CU is estimated at 1.9% [6]. In Tanzania, there is a paucity of information on CU; however, urticaria accounts for 1.2% of skin diseases among pediatric patients [7]. CU may result from several causes; some are known as bacterial, viral, fungal, and protozoan agents; however, the etiology, for most cases, remains unknown [8]. In recent years, there has been emerging literature associating CU and H. pylori infection [4]. Several theories have been put forward for this association. For example, it is thought that infection with H. pylori increases the permeability of the stomach lining and thus increases the exposure to allergens in the gastrointestinal tract [3]. Also, the immune response to H. pylori produces antibodies that may encourage the release of histamine in the skin [9]. However, other authors suggest that this association is still controversial [4]. H. pylori is a Gram-negative bacterium that is found on the luminal surface of the gastric epithelium. This bacterium is transmitted through the fecal-oral route and has been associated with low-social economic status, poor hygiene, and consumption of untreated water [10]. This bacterium can cause gastric inflammation of the underlying mucosa leading to peptic ulcer, gastritis, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Most of the infected people are asymptomatic, and they develop manifestations of peptic ulcer. Extra-gastrointestinal manifestations such as CU, vascular, and autoimmune disorders occur later in life [9]. CU has continued to be a frustrating condition to both the patient and the care provider and has a significant impact on the patient's well-being especially on sleep, mood, and work. CU hence impacts economically, leads to social isolation, degrades the quality of life, and is enormously challenging to the managing clinician [11]. Management of CU focuses mainly on nonpharmacological and pharmacological measures [12]. In Tanzania, there is a paucity of information on the association between CU and H. pylori infection, and there is no specific management of patients with CU. Patients are managed according to their signs and symptoms [13]. There is a need to determine the extent of association between CU and H. pylori infection in our setting for effective management of CU.
2. Materials and Methods
2.1. Study Area and Design
This study was a case-control,which was conducted at Kilimanjaro Christian Medical Centre (KCMC) in Moshi-Tanzania from October 2018 to April 2019. KCMC is a referral, consultant, research, and teaching hospital located in north-eastern Tanzania and lies at the foot of Mount Kilimanjaro. RDTC is one of the departments at KCMC which offer advanced diploma and specialist degree training in Dermatovenereology in East and Central Africa.
2.2. Study Population
2.2.1. Case Definition
Individuals aged five years and above who attended RDTC and were clinically diagnosed with CU were selected during the study period. Cases were recruited from the outpatient department at RDTC. CU was diagnosed as a patient presenting with the following features: recurrent, transitory, and itchy wheals that occur daily or almost every day and persist for longer than six weeks.
2.2.2. Control Definition
Individuals aged five years and above who attended RDCT and were clinically diagnosed with other dermatological conditions and free from any feature of urticaria were selected. Controls were recruited from the outpatient department at RDTC.
2.2.3. Matching
Cases and controls were matched by sex (female or male) and age (years). For each case selected, control who is in the same sex and within the same 10-year age group was selected (for example, if the case is aged 27 years, then control was chosen who aged between 20 and 29 years).
2.3. Inclusion Criteria
All patients attended the RDTC in the outpatient clinic, inpatient ward, referred to the centre from other departments and consented to the study.
2.4. Exclusion Criteria
We excluded patients who have been taking proton pump inhibitors or any antibiotics within four weeks.
2.5. Sample Size and Power
The Epi Info computer program version 6 was used for sample size determination. We used 95% confidence interval (CI), a power of 90%, and a proportion of 72% and 40% for H. pylori among cases and control group respectively, as found in Tanzania and Iran [3, 10]. The parameters provided a total of 55 cases and 55 matched control patients.
2.6. Case Selection
For every patient, a detailed history and a thorough physical examination were conducted by the principal investigator (PI) and supervised by the consultant dermatologist. A consecutive sampling method was used in this study. Demographic data such as age, sex, gender, area of residence, and medical history were recorded using the questionnaire after the patient's consent. For those less than 18 years, an assent and a written informed consent were given. Those who were diagnosed to have CU were given sterile stool containers and asked to collect stool of about one gram and hand the sample to the PI.
2.7. Control Selection
Patients who had other dermatological conditions during the study period were selected consecutively. Those who consented including patients aged less than 18 years were also asked to collect stool samples. The control group was matched with the cases by age and sex.
2.8. Data Collection Method
Face-to-face interview was done using a questionnaire. Stool samples were analyzed immediately in the microbiology lab and then stored at –20°C. H. pylori antigen test (Tellmefast, Biocan, Canada) was used to test stool samples. The test is a rapid diagnostic method for the detection of H. pylori antigen in the human stool with a sensitivity of 95% and a specificity of 98% [3]. The findings were recorded in the data extraction sheet.
2.9. Statistical Analysis
The findings were summarized into medians with their interquartile range (IQR) for age, and for categorical variables, into frequencies with their respective percentages.
Age was tested for normality and was found to be skewed to the right; thus, the Wilcoxon matched-pair signed-rank test was used to test the association between CU and age. Furthermore, for the categorical variables, the test statistic was based on a Mc Nemar's test or Mantel–Haenszel test stratified by matched pair was used to test the association between CU and age, sex, residency area, occupation, personal or family history of atopy, and H. pylori. Conditional logistic regression was used to determine the strength of association between CU and H. pylori. A p value of less than 0.05 was considered statistically significant, and all p values were two-tailed. Statistical analysis was performed using Stata version 14.1 (Stata Corp LP®, College Station, Texas, USA).
3. Results
The total number of participants in this study was 110 patients (55 cases and 55 controls), whereby the median age was 31 (IQR 27–45) among controls versus 34 (IQR: 22–46) years among the cases. Furthermore, over one-third (n = 19; 34.6%) of controls were aged from 20 to 29 years, and among cases, the majority (n = 18; 32.7%) were in the same age group. Both cases and controls had the same number of each sex, with the majority (n = 78; 70.9%) being females. More than half (n = 37; 67.3%) of the controls and (n = 31; 56.4%) cases reside in urban area (Table 1).
Table 1.
Baseline characteristics of case and control subjects after matching.
| Characteristics | Control subjects (n = 55) | Case subjects (n = 55) | p value |
|---|---|---|---|
| Median age, years (IQR) | 31 (27–45) | 34 (22–46) | 0.1231∗ |
|
| |||
| Age groups (years) | |||
| <20 | 9 (16.4) | 10 (18.2) | 0.3173 |
| 20–29 | 19 (34.6) | 18 (32.7) | — |
| 30–39 | 12 (21.8) | 12 (21.8) | — |
| 40–49 | 8 (14.5) | 8 (14.5) | — |
| >50 | 7 (12.7) | 7 (12.7) | — |
|
| |||
| Sex | — | — | 1.0 |
| Male | 16 (29.1) | 16 (29.1) | — |
| Female | 39 (70.9) | 39 (70.9) | — |
|
| |||
| Residence area | — | — | 0.2008 |
| Urban | 31 (56.4) | 37 (67.3) | — |
| Rural | 24 (43.6) | 18 (32.7) | — |
|
| |||
| Occupational status | — | — | 0.4652 |
| Self-employed | 9 (16.4) | 13 (23.6) | — |
| Formal employed | 21 (38.2) | 17 (30.9) | — |
| Nonemployed/retired/NA | 25 (45.4) | 25 (45.5) | — |
|
| |||
| Specific attack time | — | — | — |
| Cold/hot temp | — | 1 (1.8) | — |
| Not specific | — | 54 (98.2) | — |
|
| |||
| Associated with angioedema | — | — | — |
| Yes | — | 10 (18.2) | — |
| No | — | 45 (81.8) | — |
|
| |||
| Personal or family history of atopy | — | — | 0.8273 |
| Yes | 14 (25.5) | 15 (27.3) | — |
| No | 41 (74.5) | 40 (72.7) | — |
∗Wilcoxon signed-rank test; NA: not applicable (children).
There was no significant association between CU and baseline characteristics of the participants (Table 1).
3.1. Distribution of H. pylori among CU patients
Table 2 shows the matched analysis of the presence of H. pylori in the case-control pairs in the study. Each member of the pair is either exposed (H. pylori present) or unexposed (H. pylori absent) and is a case or a control subject, which yields four possible outcomes. Pairs with the same exposure status for both case and control are concordant pairs, and pairs with different exposures are discordant pairs, such that a total number of concordant pairs = 42 and the total number of discordant pairs = 13. Percentage of subjects who were diagnosed with CU and tested positive for the H. pylori was significantly different from subjects free from urticaria and tested positive for H. pylori (15/55 = 27% versus 6/55 = 10.1%; OR: 5.5, 95% CI: 1.2 to 24.8, p = 0.0225) (Table 2 and Figure 1).
Table 2.
Presence of H.pylori in paired cases and controls (55 matched pairs).
| Positive H. pylori in CU subjects | Positive of H. pylori in non-CU subjects | |
|---|---|---|
| Yes | No | |
| Yes | 4 | 11 |
| No | 2 | 38 |
Figure 1.
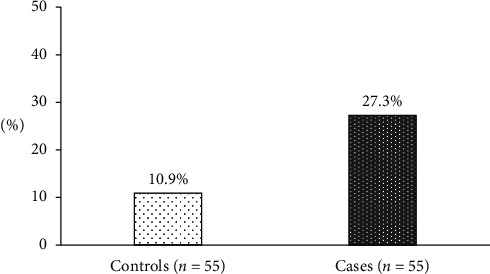
The percentage of the positive test for H. pylori in matched case and control subjects. The portion was significantly different among cases and controls (p=0.0225).
3.2. Crude and Adjusted Conditional Logistic Regression Odds of CU
In the crude analysis, the only variable associated with CU was H. pylori. For multivariable regression analysis, all the variables which had p value ≤0.2 except for age and sex were included in the final model. Backward and forward conditional logistic regression was deployed for multiple regression to determine the predictors of CU. The exposures were adjusted for age and sex of the subjects, area of residence, and the presence of H. pylori accordingly. The odds of CU among patients who tested positive for H. pylori were sixfolds higher (OR = 6.2; CI: 1.3–32.3; p value 0.029) than those patients who tested negative for H. pylori (Table 3).
Table 3.
Odds of association between CU and H. pylori (n = 110).
| Variables | COR (95% CI) | p values | AOR (95% CI) | p value |
|---|---|---|---|---|
| Positive H. pylori antigen test∗ | ||||
| Yes | 5.5 (1.2–24.8) | 0.025 | 6.2 (1.2–32.3) | 0.029 |
| No | Ref | — | Ref | — |
|
| ||||
| Sex | ||||
| Male | Ref | — | Ref | — |
| Female | 0.5 (0.4–2.5) | 1.0 | 1.3 (0.4–4) | 0.607 |
|
| ||||
| Age cantered at the mean (years) | 0.9 (0.8–1.1) | 0.239 | 0.9 (0.7–1.1) | 0.233 |
|
| ||||
| Area of residence | ||||
| Urban | 1.8 (0.7–4.2) | 0.207 | 1.7 (0.6–4.4) | 0.287 |
| Rural | Ref | — | Ref | — |
|
| ||||
| History of treatments β | ||||
| Yes | 4.5 (0.9–20.8) | 0.054 | 4.8 (0.9–25.1) | 0.061 |
| No | Ref | — | Ref | — |
∗Adjusted for age, sex, area of residence, and history of been treated of peptic ulcer/duodenal ulcer/gastritis; βbeen treated of peptic ulcer/duodenal ulcer/gastritis.
4. Discussion
CU is a distressing condition in our everyday practice and is difficult to deal with because of its complex triggering factors [14]. H. pylori has been implicated as a pathogenic agent in a variety of disorders other than gastrointestinal diseases including CU [15]. This organism can be detected easily by a stool antigen test, which meets the requirements of dermatologists treating most patients with CU because it is rapid, noninvasive, cheap, and convenient for pretreatment diagnosis with a high sensitivity of 95% and a specificity of 98% [9]. The current study aimed to look at the association between CU and H. pylori infection among patients attending a tertiary hospital in Tanzania.
In this study, a total number of 110 patients were studied (55 cases and 55 controls) with age and gender matching. The majority were aged from 20 to 29 years in both cases and controls, and there was no significant association between CU and baseline characteristics of the participants. Our findings were similar to Muhemmed and Khalis Mohammed, Alfahaad, Moesbehi et al., Majeed, and Jwan Saleh Khoshnaw [3, 4, 9] which explain that CU occurs mostly in adults. Our findings concur with the literature that CU is related to frequent, long duration, and prolonged exposure to allergens or causative agents for immunological reactions to occur [15–17]. These findings were different from the study done by Shin and Lee because their study population was children who aged less than 18 years [18].
We found that 70.9% of the cases and controls were females, while 29.1% of the cases and controls were males. Our findings were similar to Moesbehi et al., and other authors who explained that CU affects more females than males [3, 4, 9, 19]. The reason could be explained by low levels of dehydroepiandrosterone (DHEA)-S in females, suggesting a possible role for hormone imbalance with CU [3, 4]. However, there is limited information from the literature to support this relationship. Muhemmed and Khalis Mohammed suggest that CU is more among females than males because females are more exposed to household activities and household dust. In such activities, they handle raw foods while preparing, hence prone to acquire H. pylori infection, which will lead to CU more than males [3]. Alfahaad) also explains that females tend to seek medical consultations more compared to males [4].
In this study, 56.4% of the cases and 29.1% of the controls reside in urban areas. Residence area difference may be due to the reason that majority of the patients attending KCMC-RDTC are from inside the town (Moshi). In contrast, those in rural areas usually attend to their health service facilities in their locality.
We found that 27% of CU patients had H. pylori infection while in controls it was 10.1%. The findings of this study were similar to the studies done in Egypt and Iraq [3, 9, 15], which explain that H. pylori infection is more in CU patients than in the controls. These similarities could be explained by the fact that Egypt, Iraq, and Tanzania are developing countries, with a higher prevalence of H. pylori [4, 9, 10]. It was also pointed out by Gu et al., Mogaddam et al., Jaka et al., and Majeed et al. that in these countries, the prevalence of H. pylori is as high as 90% because there is lack of proper sanitation, basic hygiene, poor diet, and overcrowding that favors H. pylori transmission, while in developed countries the prevalence is below 40% [8, 10, 15, 17].
In the current study, the only variable which was associated with CU was H. pylori. We found that the risk of developing CU among patients who were positive for H. pylori was sixfolds higher compared to those who tested negative, and these findings were statistically significant. These findings were similar to a number of other studies. [3, 9, 15]. It was, however, inconsistent with the findings of Frederman et al. [16]. The reason for this difference could be explained by the different geographical locations, which explains the high prevalence of H. pylori infection with the risks that favors H. pylori transmission [3, 4, 17, 20]. In addition, various H. pylori identification methods are used because most of these studies use the H. pylori antibody test instead of the antigen test which detects the active infection as used in our study [3, 9, 16].
Several theories have been put forward for this association [3]. It is thought that infection with H. pylori increases the permeability of the stomach lining and thus increases the exposure to allergens in the gastrointestinal tract [19]. Also, the immune response to H. pylori produces antibodies that may encourage the release of histamine in the skin [9]. However, in some other studies, this association is controversial as to whether H. pylori is an etiological agent for CU or not [21, 22].
This association has enormous potential as eradication of the bacteria could signify potential cure [4, 20]. Studies done in the USA, Iran, Pakistan, and Egypt have shown that among patients who had CU, there was complete resolution of the symptoms after eradication of the bacteria [3, 4, 16, 20, 21].
5. Conclusion
There is a significant association between CU and H. pylori infection, indicating to include the H. pylori tests in the diagnostic workup for CU cases. This organism can be detected with confidence by using rapid, noninvasive, sensitive, specific, and cheaper techniques like serology for the H. pylori stool antigen test.
6. Limitation
This study involved participants who presented at the hospital as dermatology patients; therefore, the results of this study cannot be generalized to the general population.
7. Recommendations
We recommend conducting further randomized controlled studies including using H. pylori eradication drugs.
Acknowledgments
The authors wish to thank Prof. Gail Todd and Ben Naafs, for their assistance and valuable comments on the earlier version of the manuscript. Special appreciation goes to Dr. Wilhellmuss Mauka for his statistical assistance and data analysis of this work, Dr. Hileni Taleni Nangolo, and all the staff at RDTC for their support. We also thank all the patients for voluntarily participating in this study.
Abbreviations
- CU:
Chronic urticaria
- H. pylori:
Helicobacter pylori
- KCMC:
Kilimanjaro Christian Medical Centre
- MALT:
Mucosa-associated lymphoid tissue
- RDTC:
Regional Dermatology Training Center.
Data Availability
Data are available and will be submitted upon request.
Ethical Approval
This study was approved by the KCMUCo Research and Ethics Review Committee with certificate no. 2348.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
All authors contributed equally to this work.
References
- 1.Kaplan A. P. Chronic urticaria and angioedema. New England Journal of Medicine. 2002;346(3):175–179. doi: 10.1056/nejmcp011186. [DOI] [PubMed] [Google Scholar]
- 2.Zuberbier T., Aberer W., Asero R., et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69(7):868–887. doi: 10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 3.Muhemmed F., Khalis Mohammed A. Association of chronic urticaria with Helicobacter Pylori infection in Erbil: A case-control study. Zanco Journal of Medical Sciences. 2016;20(2):1376–1384. doi: 10.15218/zjms.2016.0034. [DOI] [Google Scholar]
- 4.Alfahaad H. A. Chronic urticaria and dyspepsia: association and treatment, an experimental study. J Pakistan Assoc Dermatologists. 2018;28(4):443–448. [Google Scholar]
- 5.Loh J. A., Kanani A. S., Stark D. F. Prevalence of Helicobacter pylori infection and chronic urticaria. The Journal of Allergy and Clinical Immunology. 2013;131(2):p. AB28. doi: 10.1016/j.jaci.2012.12.782. [DOI] [Google Scholar]
- 6.Nnoruka E. N. Current epidemiology of atopic dermatitis in south-eastern Nigeria. International Journal of Dermatology. 2004;43(10):739–744. doi: 10.1111/j.1365-4632.2004.02360.x. [DOI] [PubMed] [Google Scholar]
- 7.Kiprono S. K., Muchunu J. W., Masenga J. E. Skin diseases in pediatric patients attending a tertiary dermatology hospital in Northern Tanzania: A cross-sectional study. BMC Dermatology. 2015;15(1):p. 16. doi: 10.1186/s12895-015-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu H., Li L., Gu M., Zhang G. Association between Helicobacter pylori infection and chronic urticaria : A meta-analysis. Gastroenterology Research and Practice. 2015;2015:1–9. doi: 10.1155/2015/486974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosbeh A. S., Alad A., Tealeb A. S., Ali M. S. Role of Helicobacter pylori in chronic urticaria among Egyptian patients with dyspepsia: A case–control study. International Journal of Dermatology and Clinical Research. 2017;3(1):26–31. doi: 10.17352/2455-8605.000023. [DOI] [Google Scholar]
- 10.Jaka H., Mushi M. F., Mirambo M. M., et al. Sero-prevalence and associated factors of Helicobacter pylori infection among adult patients with dyspepsia attending the gastroenterology unit in a tertiary hospital in Mwanza, Tanzania. African Health Sciences. 2016;16(3):684–689. doi: 10.4314/ahs.v16i3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balp M.-M., Lopes da Silva N., Vietri J., Tian H., Ensina L. F. The burden of chronic urticaria from Brazilian patients’ perspective. Dermatology and Therapy. 2017;7(4):535–545. doi: 10.1007/s13555-017-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yosipovitch G., Ansari N., Goon A., Chan Y. H., Goh C. L. Clinical characteristics of pruritus in chronic idiopathic urticaria. British Journal of Dermatology. 2002;147(1):32–36. doi: 10.1046/j.1365-2133.2002.04758.x. [DOI] [PubMed] [Google Scholar]
- 13.Ministy of Health Tanzania. Standard Treatment Guidelines & National Essential Medicines List Tanzania Mainland. 4th. Ministy of Health Tanzania; 2017. [Google Scholar]
- 14.Hasan M. S., Khan M. S. I., Nayeem J. Study on association between chronic idiopathic urticaria and Helicobacter pylori infection in armed forces personnel. Journal of Armed Forces Medical College, Bangladesh. 2016;12(2):122–126. doi: 10.3329/jafmc.v12i2.41108. [DOI] [Google Scholar]
- 15.AL-Hamdi K. I., Khashan L. S. Role of Helicobacter pylori in chronic ordinary urticaria: A case-control and therapeutic study. The Medical Journal of Basrah University. 2017;35(1):39–47. doi: 10.33762/mjbu.2017.126395. [DOI] [Google Scholar]
- 16.Federman D. G., Kirsner R. S., Moriarty J. P., Concato J. The effect of antibiotic therapy for patients infected with Helicobacter pylori who have chronic urticaria. Journal of the American Academy of Dermatology. 2002;49(5):861–864. doi: 10.1016/s0190-9622(03)00846-6. [DOI] [PubMed] [Google Scholar]
- 17.Majeed P. D., Jwan Saleh Khoshnaw K. Seroprevalence of Helicobacter pylori infection among patients with gastroduodenal disorders in Erbil city. Diyala Journal of Medicine. 2020;18(2):91–101. doi: 10.26505/djm.18014880818. [DOI] [Google Scholar]
- 18.Shin M., Lee S. Prevalence and causes of childhood urticaria. Allergy Asthma Immunology Research. 2017;9(3):p. 189. doi: 10.4168/aair.2017.9.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mogaddam M. R., Yazdanbod A., Ardabili N. S., Maleki N., Isazadeh S. Relationship between Helicobacter pylori and idiopathic chronic urticaria: effectiveness of Helicobacter pylori eradication. Advances in Dermatology and Allergology. 2015;1:15–20. doi: 10.5114/pdia.2015.48729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Essrani R., Sullivan M., Shah H. Chronic urticaria associated with Helicobacter pylori infection. Consultant. 2018;8(2):10–15. doi: 10.7759/cureus.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khashaba S., Diab N., Abdallah E., Said N. Prevalence of Helicobacter pylori and impact of its eradication in acne vulgaris: A prospective cohort study. Journal of the Egyptian Women’s Dermatologic Society. 2020;17(1):45–49. doi: 10.4103/jewd.jewd_50_19. [DOI] [Google Scholar]
- 22.Miftahussurur M., Yamaoka Y. Diagnostic methods of Helicobacter pylori infection for epidemiological studies: Critical importance of indirect test validation. BioMed Research International. 2016;2016:1–4. doi: 10.1155/2016/4819423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available and will be submitted upon request.


