Abstract
Background
Treatment paradigms for borderline resectable pancreatic cancer are evolving with increasing use of neoadjuvant chemotherapy and neoadjuvant chemoradiation. Variations in the definition of borderline resectable pancreatic cancer and neoadjuvant approaches have made standardizing care for borderline resectable pancreatic cancer difficult. We report an effort to standardize management of borderline resectable pancreatic cancer throughout Sanford Health, a large community oncology network.
Methods
Starting in October 2013, cases of pancreatic adenocarcinoma without known metastatic disease were categorized as borderline resectable pancreatic cancer if they met ≥ 1 of the following criteria: (1) abutment of superior mesenteric, common hepatic, or celiac arteries with < 180° involvement, (2) venous involvement deemed potentially suitable for reconstruction, and/or (3) biopsy-proven lymph node involvement. Patients with borderline resectable pancreatic cancer were treated with neoadjuvant chemotherapy followed by reimaging and surgery if venous involvement had improved; if disease remained borderline resectable, patients underwent neoadjuvant chemoradiation and surgical exploration as long as reimaging did not reveal evidence of progressive disease.
Results
Forty-three patients from October 2013 to April 2017 were diagnosed with borderline resectable pancreatic cancer. Twelve of 42 (29%) patients proceeded to surgical exploration directly after neoadjuvant chemotherapy; 23 (55%) received neoadjuvant chemoradiation. Overall, 28/43 (65%) underwent exploration with 19 (44%) able to undergo resection. Of those, 14/19 (74%) attained R0 resection and 11/19 (58%) were pathologic N0. No pretreatment or treatment variables were associated with resection rates; resection was the only variable associated with survival.
Conclusion
This report demonstrates the feasibility of implementing a standardized approach to borderline resectable pancreatic cancer across multiple sites over a wide geographic area. Adherence to protocol therapies was good and surgical outcomes are similar to many reported series.
INTRODUCTION
Pancreatic adenocarcinoma (PC) is historically associated with a dismal prognosis. It is currently the fourth leading cause of cancer death in the United States, claiming more than 45,000 lives annually with an estimated 5-year overall survival of < 10% [1]. Given the rising incidence of PC and poor outcomes despite treatment, PC is expected to rise to the second leading cause of cancer death by 2020 [2]. Surgical resection has long been the mainstay of therapy for PC for patients whose tumor is deemed resectable. Unfortunately, only a small number of patients (15%–20%) present with resectable disease, historically rendering a majority of patients ineligible for curative intent therapy [3,4].
Recent efforts to improve outcomes in PC have focused on the use of systemic therapy, with new combination chemotherapy regimens such as 5-fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) [5] and gemcitabine/nab-paclitaxel [6] leading to significant improvement in survival in patients with metastatic disease. Demonstration of the potential benefits of such combination therapy in this setting has led to efforts to integrate such chemotherapy into the care of patients with less advanced disease. Adjuvant chemotherapy has been a standard of care following surgical resection for more than a decade [7], with further improvement in survival through utilization of combination chemotherapy in this setting [8].
Given the necessity of surgery for curative intent therapy, there has been a recent focus on what has been termed borderline resectable pancreatic cancer (BRPC). Broadly defined, this is a group of patients with PC with localized disease but in intimate relationship with vascular structures, making surgical resection with adequate margins difficult or impossible. The precise criteria characterizing a malignancy as borderline resectable have varied over time and by expert opinion [[9], [10], [11], [12]], which has been a significant issue in developing and executing clinical research investigating standardized approaches to the management of these patients. Given the response rates noted in trials investigating combination chemotherapy in patients with metastatic PC, these same regimens have been integrated into the neoadjuvant (NA) setting in BRPC.
Numerous small series have demonstrated the feasibility of NA therapy in patients with BRPC, using some combination of multiagent chemotherapy, chemoradiation, or both prior to surgical resection, with suggestions of improved outcomes compared to historical controls [[13], [14], [15], [16]]. More recently, the first large-scale randomized trial to assess the role of neoadjuvant therapy in BRPC was reported. The PREOPANC trial [17] compared initial surgery followed by adjuvant gemcitabine to a short course of gemcitabine-based chemoradiation followed by surgery followed by 4 months of adjuvant gemcitabine. Preliminary reports demonstrated an increase in R0 resection rate and improved overall survival associated with neoadjuvant therapy compared to initial surgery.
As these data on optimal management of BRPC evolved, Sanford Health, a multisite community oncology practice treating patients in 3 states in the upper Midwest, implemented a standardized approach to the evaluation and management of patients with BRPC. This was undertaken primarily in an effort to demonstrate the feasibility of adherence to such a standardized approach across multiple rural sites and numerous different specialty providers.
METHODS
Study Design and Data Collection
Given the growing data supporting the use of neoadjuvant therapy and BRPC, a health system-wide gastrointestinal oncology Disease Associated Working Group (DAWG) comprised of surgical oncology, medical oncology, radiation oncology, radiology, and pathology representatives from all 4 hubs of the Sanford Health Cancer Center (Fargo, ND; Sioux Falls, SD; Bismarck, ND; and Bemidji, MN) began meeting regularly in early 2013 to discuss standardization of BRPC care. These meetings were initially held monthly and have since been held quarterly via video meeting software. These meetings led to the development of a standardized BRPC management protocol. Identification of appropriate patients and implementation of the protocol were designated to each individual site coordinated through their site-specific gastrointestinal oncology tumor board, which typically meets either weekly or every other week depending on the practice site. Oncology nurse navigators and providers both identified patients to be considered for this protocol for discussion at the appropriate tumor board.
Starting in October 2013, all patients with PC whose cases met criteria for BRPC (outlined below) following review by a multidisciplinary tumor board were managed in a standardized fashion and followed using a prospectively maintained database across all participating sites. Patients treated on this standardized protocol from October 2013 through April 2017 were included in this retrospective review following institutional review board approval.
Patients included in this cohort had diagnostic evaluation including at least a computed tomography (CT) scan of the chest, abdomen, and pelvis per institutional protocol and CA 19-9 determination following a pathologic diagnosis of pancreatic adenocarcinoma via endoscopic ultrasound. Each case was reviewed in a multidisciplinary tumor board including surgical oncology, medical oncology, radiation oncology, radiology, and pathology. The standardized definition of BRPC used in this trial was adopted following review of multiple previously existing definitions of borderline resectability [[9], [10], [11], [12],18] and is as follows:
-
1.
No evidence of distant/metastatic disease
-
2.
Abutment of superior mesenteric, common hepatic, or celiac arteries with < 180° involvement and/or
-
3.
Venous involvement deemed potentially suitable to reconstruction (adequate neck of superior mesenteric vein (SMV) and portal vein without thrombus and/or tumor which allows for reconstruction) and/or
-
4.
Biopsy-proven regional lymph node involvement
Clinicopathologic variables collected included age, sex, site of primary malignancy within the pancreas, pretreatment CA 19-9, reason for borderline resectable status, nature and duration of neoadjuvant chemotherapy (NAC), receipt of neoadjuvant radiation therapy, and posttreatment/presurgical CA 19-9 levels. For patients who were able to proceed to surgery, variables collected included resection rates, margin status, lymph node status, receipt of adjuvant chemotherapy, and receipt of adjuvant chemoradiation.
This study was approved by the Institutional Review Board for Human Research and was compliant with all Health Insurance Portability and Accountability Act regulations.
Interventions
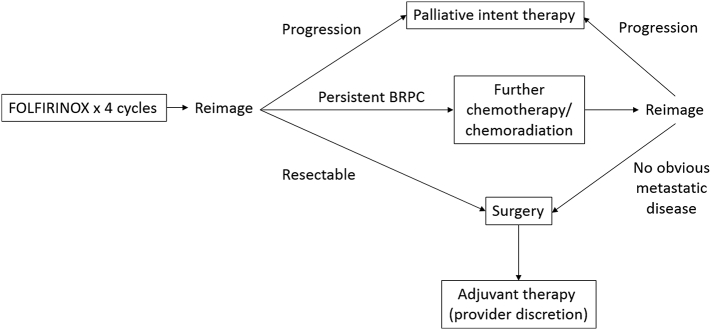
Patients with BRPC per this standardized definition were treated in the neoadjuvant setting per provider preference. Providers were informed of a preferred treatment strategy, including utilization of NA chemotherapy followed by reimaging and reassessment as to resectability. Patient suitability for treatment and the chemotherapy used in this setting were per discretion of the treating provider, although FOLFIRINOX given in every 2-week cycles was the preferred treatment regimen. Gemcitabine/nab-paclitaxel given 3 weeks out of 4 in a monthly cycle was allowed in patients felt unlikely to tolerate FOLFIRINOX. After 2 months of NA chemotherapy, patients underwent CT reevaluation. Patients whose venous involvement had improved with adequate expected surgical planes upon multidisciplinary review proceeded to surgery; a change in lymph node status if noted on imaging was not required to proceed to surgical exploration. If this imaging revealed persistent borderline resectability by vascular criteria, patients could either continue chemotherapy for up to 2 more months or proceed to neoadjuvant chemoradiation (NACR). Chemoradiation, if administered, was administered concurrently with either capecitabine or infusional 5-fluorouracil. Radiation was administered via standard fractionation in all cases, with patients receiving between 50 and 50.4 Gy in 25–28 fractions. Figure 1 demonstrates the basic schema of the recommended treatment protocol.
Fig 1.
Sanford recommended BRPC treatment algorithm.
Following every 2 months of NA chemotherapy or upon completion of chemoradiation (if administered), reassessment of potential resectability was undertaken, and patients with improvement in venous involvement as outlined above proceeded to surgery. Patients maintaining a good performance status following completion of NAC and NACR proceeded to surgical exploration regardless of radiologic response in the absence of evidence of metastatic disease given the well-described discrepancy reported between radiologic findings and surgical resectability following NA therapy [19]. Patients were taken to surgery between 4 and 8 weeks following completion of NAC or NACR. Following surgery, adjuvant therapies including chemotherapy and chemoradiation were allowed per provider discretion, if not received preoperatively. Patients with metastatic disease or performance status concerns precluding surgical intervention were treated as appropriate with systemic chemotherapy and/or best supportive care.
Patients from 5 different community oncology sites were enrolled in the database and treated prospectively in this fashion. NAC was administered in all 5 sites; NACR, surgery, and pathology review were performed at 2 centers. These centers each have 2 dedicated pancreatic surgeons, and these 4 surgeons performed all surgical procedures. During the years encompassing this series, the average number of pancreatic surgeries for malignancy performed at these 2 sites combined was 29 (26–33). Follow-up visits, laboratory studies, and imaging were scheduled per provider discretion.
Outcomes
The primary outcome of this retrospective review was demonstration of adherence to a standardized protocol across multiple primary treatment sites. Secondary outcomes included surgical resection rates, margin and nodal status at the time of surgical resection, recurrence-free survival in patients undergoing surgical resection, and overall survival, defined as the time between date of diagnosis and death. The date of the last medical record review was used to center time for patients that were still alive at the time of the review.
Statistical Analysis
Continuous variables were reported by mean and standard deviation or median and range. Categorical variables are summarized as frequencies and percentages. Comparison of surgery and nonsurgery patients was compared by t test or χ2 test depending on continuous or categorical data type. Logistic regression was used to generate odds ratios and further explore variables and their relationship with outcome of surgery. Unadjusted odds ratios are reported using a surgery indicator as the dependent variable and each variable as a separate covariate in the logistic regression model. Adjusted odds ratios are reported by including all variables in the regression model together. A regression model is fit, considering all available demographic and clinical measures, using rpart18 in R to construct a decision tree. Patient demographic and clinical measures are chosen and optimally split to maximize the sum of squares between surgery and nonsurgery groups. Splitting criteria are based on the information index and complexity parameter of 0.05. Kaplan-Meier curves are created to show survival probability comparison by surgery or nonsurgery patients; the log-rank test is used to compare survival distributions between 2 groups. Potential predictors of survival are explored using Cox proportional hazards model, reporting hazard ratios (HRs).
All statistical analyses were completed in R [20].
RESULTS
Patient/Treatment Characteristics
Forty-three patients were enrolled prospectively from October 2013 through April 2017. Pretreatment patient characteristics are noted in Table 1. Notably, most patients (39/43) were deemed borderline resectable because of vascular involvement, whereas 4 patients were categorized as BRPC because of pathologically proven nodal involvement. Of the 39 patients deemed BRPC due to vascular involvement, 26 had venous-only involvement, 3 had arterial-only involvement, and 8 had both arterial and venous involvement. Of patients with venous only involvement, 9 had isolated portal vein involvement, eight had isolated superior mesenteric vein involvement, and 9 had involvement of both the portal and superior mesenteric veins. Of the 3 patients with arterial only involvement, 2 had SMA involvement and 1 had celiac artery involvement. Of the 43 patients, 42 (98%) received initial treatment per recommendations with chemotherapy; 1 patient received only NACR. Adherence to the recommended initial regimen (FOLFIRINOX) was high, with 88% of patients (38/43) receiving this regimen as initial neoadjuvant therapy. Tolerance of neoadjuvant therapy was good, as 34/38 (89%) patients receiving FOLFIRINOX were able to complete at least the initially recommended 4 cycles of therapy. FOLFIRI (1) and gemcitabine/nab-paclitaxel (3) were also used in a smaller number of patients.
Table 1.
Baseline characteristics of patients with BRPC (N = 43)
| Variable | Overall |
|---|---|
| Age (y), median (range) | 64 (39–83) |
| Sex, n (%) | |
| Male | 24 (55.8) |
| Female | 19 (44.2) |
| Race, n (%) | |
| White | 43 (100) |
| Tumor location, n (%) | |
| Head | 39 (90.6) |
| Body | 3 (7.0) |
| Tail | 1 (2.4) |
| Unresectability factor, n (%) | |
| Vascular involvement | 39 (90.7) |
| Nodal involvement | 4 (9.3) |
| CA19-9, median (range) | 231 (2–11,866) |
| < 35 (normal), n/total (%) | 10/25 (40) |
| ≥ 35 (elevated), n/total (%) | 15/25 (60) |
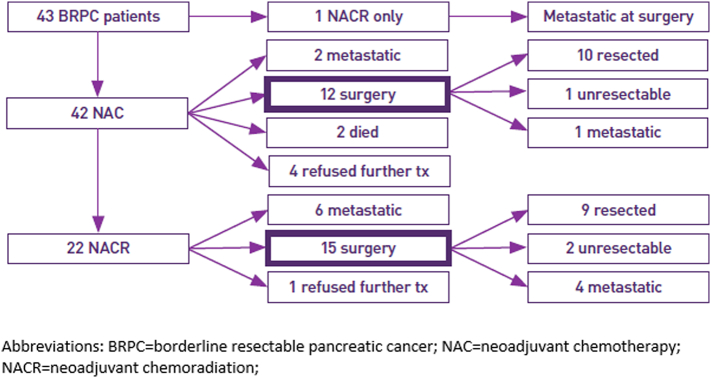
Initial treatment and patient outcomes are outlined in Fig 2 and Table 2. Twelve patients were able to proceed to surgery immediately following NAC; 1 proceeded to surgery directly following NACR. Twenty-two patients proceeded to NACR, with the majority of these patients receiving concurrent infusional 5-FU as a radiosensitizing agent. Fifteen of these 22 patients were able to proceed to surgery, meaning a total of 28/43 patients (65%) were able to proceed to surgery. Of the 28 patients who underwent surgery, 19 (68%) were able to undergo resection, whereas the other 9 did not undergo resection of primary tumor because of discovery of either occult metastatic (6/9) or unresectable local (3/9) disease at the time of surgery. Overall, median time from diagnosis to surgery was 164 days (range 99–295). The large majority of surgical procedures were pancreaticoduodenectomies given the predominance of pancreatic head lesions. Of the 19 patients undergoing resection, 14 were able to obtain an R0 resection (74%) and 11 (58%) were pathologic N0. Postresection irreversible electroporation (IRE) was performed in 4 patients. In these patients, IRE was performed following resection given concerns regarding the adequacy of surgical margins by the performing surgeon; 3 of these 4 patients were found to have R1 resection on pathology review, whereas the fourth obtained an R0 resection. Pathology review revealed significant treatment response in a large majority of patients undergoing resection, including 2 patients with a pathologic complete response.
Fig 2.
BRPC patient treatments/outcomes.
Table 2.
Clinicopathologic/treatment characteristics (N = 43)
| Variable | Overall |
|---|---|
| Initial NA treatment regimen, n (%) | 43 (100) |
| FOLFIRINOX | 38 (88.4) |
| Gemcitabine/nab-paclitaxel | 7 (16.3) |
| FOLFIRI | 1 (0.9) |
| Chemoradiation | 1 (0.9) |
| NA chemoradiation, n (%) | 23 (53.5) |
| Capecitabine | 8 (34.7) |
| 5-Fluorouracil | 15 (65.2) |
| Surgery attempted, n (%) | 28 (65.1) |
| Surgery completed, n (%) | 19 (44.1) |
| Pancreaticoduodenectomy | 17 (89.4) |
| Distal pancreatectomy | 2 (10.6) |
| Tumor size (cm), median (range) | 2.5 (0–5.2) |
| Treatment response, n (%) | |
| Complete | 2 (10.5) |
| Extensive | 2 (10.5) |
| Moderate | 11 (57.8) |
| Absent | 3 (16.7) |
| Nodal disease (present), n (%) | 8 (42.1) |
| Margin status (negative), n (%) | 14 (73.6) |
| Lymphovascular invasion (present), n (%) | 4 (21.1) |
| Perineural invasion (present), n (%) | 8 (42.1) |
| Irreversible electroporation, n (%) | 7 (16.3) |
| Adjuvant/postresection | 4 (57.1) |
| Primary/unresectable | 3 (42.9) |
| Adjuvant chemotherapy, n (%) | 15 (78.9) |
| Adjuvant chemoradiation, n (%) | 5 (26.3) |
Factors Associated With Surgical Resection
Analysis of pretreatment variables and association with surgery is shown in Table 3. On univariable analysis, there is a statistically significant difference between surgery and nonsurgery patients for postchemotherapy CA 19-9 (mean 728.36 U/mL in nonsurgery patients vs 161.04 U/mL in surgery patients; P = .034). Further analysis revealed no statistically significant difference in surgery rates when comparing patients deemed borderline resectable based on nodal versus vascular involvement. There was also no evidence of a statistically significant difference based on the specific vessels involved. Multivariable analysis of these variables via logistic regression modeling revealed no variable significantly associated with surgical resection.
Table 3.
Comparison of surgery versus no surgery patients
| Variable | Surgery | No Surgery | P value⁎ | |
|---|---|---|---|---|
| Total | 28 | 15 | ||
| Age (mean [SD]) | 62.36 (8.69) | 66.47 (12.88) | .220 | |
| Sex (n [%]) | Female | 13 (46.4) | 6 (40.0) | .934 |
| Male | 15 (53.6) | 9 (60.0) | ||
| Location (n [%]) | Body/tail | 3 (10.7) | 1 (6.7) | 1.000 |
| Head | 25 (89.3) | 14 (93.3) | ||
| BRPC factor (n [%]) | Lymph node | 4 (14.3) | 0 (0.0) | .324 |
| Vascular | 24 (85.7) | 15 (100.0) | ||
| Pre CA19-9 (mean [SD]) | 657.79 (1620.34) | 2318.33 (3980.92) | .059 | |
| Post CA19-9 (post CT) (mean [SD]) | 161.04 (222.56) | 728.36 (1231.88) | .034 | |
| Post CA19-9 (post CR) (mean [SD]) | 43.93 (46.10) | 1056.14 (2270.93) | .091 | |
| CA19-9 change (mean [SD]) | − 594.58 (1723.64) | − 1457.55 (2685.42) | .259 | |
| % CA19-9 change (mean [SD]) | − 0.30 (0.90) | − 0.43 (0.83) | .695 | |
| Cycles (mean [SD]) | 4.59 (1.34) | 3.71 (2.67) | .166 | |
| NACR (n [%]) | 0 | 11 (40.7) | 4 (36.4) | 1.000 |
| 1 | 16 (59.3) | 7 (63.6) | ||
| NA chemo (n [%]) | FOLFIRINOX | 26 (96.3) | 11 (73.3) | .066 |
| FOLFIRINOX/GA | 0 (0.0) | 2 (13.3) | ||
| GA | 1 (3.7) | 2 (13.3) | ||
| NACR chemo (n [%]) | C | 6 (37.5) | 2 (28.6) | 1.000 |
| FU | 10 (62.5) | 5 (71.4) |
GA, gemcitabine/nab-paclitaxel; C, capecitabine; FU, 5-fluorouracil.
t test or χ2 test.
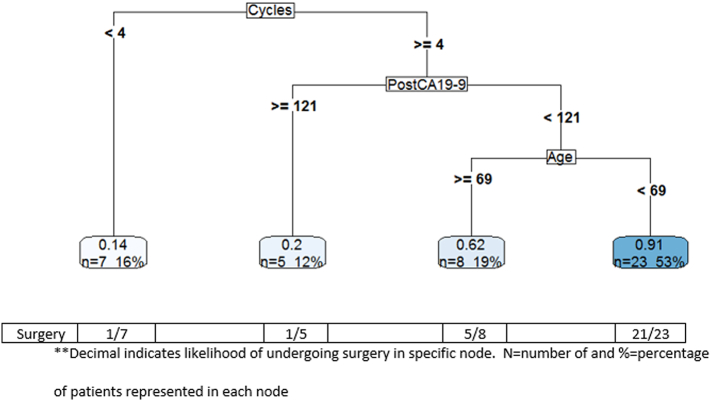
In an effort to better define characteristics of patients likely to proceed to surgery, a decision tree integrating these variables was created. As demonstrated in Fig 3, factors associated with proceeding to surgery include receipt of greater than or equal to 4 cycles of FOLFIRINOX chemotherapy, a posttreatment CA 19-9 of less than 121 U/mL, and age less than 69. Twenty-three patients (53%) met all 3 of these criteria, and 91% of these patients were able to proceed to surgery, as only 2 of these patients did not proceed to surgery. Of the 20 patients not meeting these criteria, only 7 proceeded to surgery (35%).
Fig 3.
Decision tree with surgery as the outcome.
**Decimal indicates likelihood of undergoing surgery in specific node. N = number of and % = percentage of patients represented in each node.
Treatment Outcomes
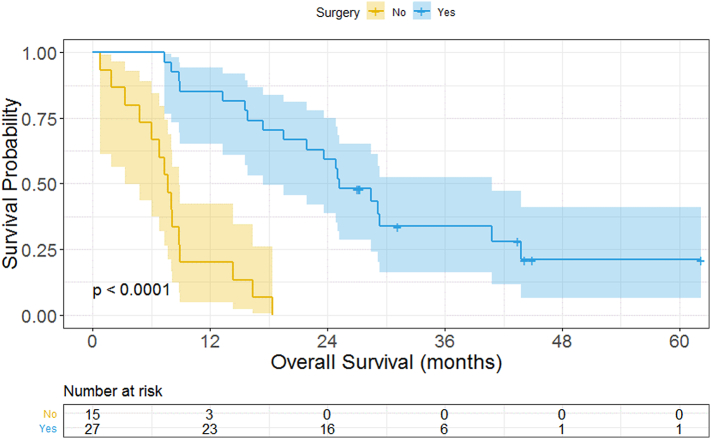
The median follow-up of all patients in the cohort was 44.2 months. Receipt of NACR was not associated with improved resection rates or improved rates of R0 resection, as 8/10 patients who underwent NACR had an R0 resection compared to 6/9 patients who did not undergo NACR. Median overall survival for all patients in the cohort was 16.9 months. The only pretreatment or treatment variable significantly associated with improved overall survival was surgical resection (HR = 0.1043; P = .0006), as demonstrated in Table 4. There was no evidence of a significant difference in survival based on the reason for borderline resectability (nodal versus vascular), specific vascular involvement, or performance of IRE postresection. Overall survival for patients stratified by surgery versus no surgery is displayed in Fig 4. Median survival for patients undergoing surgery was 25.3 months as compared to 7.7 months for nonsurgical patients. Outcomes for patients unable to undergo surgical resection were expectedly dismal. With median follow-up of 16.9 months, 6.7% of patients not undergoing surgery are still alive compared with 74.1% in the surgical group. The 1- and 2-year survival rates in patients with resected disease were 94.7% and 68.4% compared to 34.8% and 13% in the unresected cohort.
Table 4.
Unadjusted and adjusted Cox proportional hazards model on overall survival
|
Unadjusted |
Adjusted |
|||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI for HR | P value | HR | 95% CI for HR | P value |
| Surgery (ref = yes) | 0.1043 | (0.042–0.258) | <.0001 | 0.1043 | (0.029–0.381) | .0006 |
| Age | 1.0353 | (0.99413–1.0782) | .0938 | 1.0170 | (0.967–1.07) | .5084 |
| Sex (ref = male) | 0.9968 | (0.50826–1.9547) | .9925 | 0.8326 | (0.347–1.996) | .6812 |
| Cycles | 0.6566 | (0.44745–0.96346) | .0315 | 0.7673 | (0.34–1.73) | .5230 |
| Pre CA19-9 | 1.0001 | (1–1.0003) | .0523 | 1.0000 | (0.9997–1.0003) | .9966 |
| Post CA19-9 (post CT) | 1.0008 | (1.0002–1.0014) | .0105 | 1.0000 | (0.999–1.002) | .4678 |
| Post CA19-9 (post CR) | 1.0005 | (1.0001–1.001) | .0257 | – | – | – |
| CA19-9 change | 0.9999 | (0.99974–1.0001) | .3470 | – | – | – |
| % CA19-9 change | 1.0714 | (0.70323–1.6323) | .7482 | – | – | – |
| Location (ref = H) | 1.1752 | (0.35481–3.8925) | .7916 | 0.8584 | (0.154–4.771) | .8615 |
| NACR | 0.8805 | (0.41958–1.8476) | .7364 | 0.5981 | (0.23–1.555) | .2916 |
H, head of pancreas.
Fig 4.
Overall survival and log-rank results for all patients; surgery versus no surgery.
In patients who underwent surgical resection, median recurrence-free survival was 15.8 months, with 10/19 (52.6%) of patients having experienced recurrence at a median of 11.8 months following surgery. Of the 10 recurrences, 9 (90%) had a component of distant recurrence, with only 1 local recurrence. The only variable associated with improved survival with statistical significance in the surgical population was a lower postchemotherapy CA 19-9 (HR = 0.9975; P = .046).
DISCUSSION
This case series adds to the growing body of evidence as to the feasibility and potential benefit of administering NA therapy to patients with BRPC prior to surgical resection. One of the challenges in studying patients with BRPC has been establishing a standardized definition of BRPC as well as adhering to a standardized treatment approach. All patients enrolled in this retrospective series were reviewed in a multidisciplinary fashion and did meet the internal criteria outlined above as BRPC without deviation. Our definition of BRPC was informed by numerous expert guidelines [3,[9], [10], [11], [12]], with the addition of biopsy-proven node positivity as a criterion for BRPC status. This addition was based on evidence that node positivity is an indicator of biologically unfavorable disease and that node positivity at surgical resection has been associated with increased rates of distant but not local recurrence and inferior survival [21,22]. Integration of neoadjuvant therapies in this setting would aim to decrease the rate of distant recurrence and improve mortality; similar rationale has led to increasing interest in neoadjuvant therapy in all pancreatic cancer patients, even in tumors considered “resectable” by classic criteria, given the high rate of occult lymph node positivity in this population [23,24].
Treatment strategies were recommended rather than mandated in this retrospective series, allowing for provider preference and assessment of individual patient status for aggressive FOLFIRINOX chemotherapy to be integrated into decision making. Although perhaps a limitation, this series does still demonstrate that adherence to a guideline-based treatment strategy was good and a large majority of patients were able to complete the planned course of FOLFIRINOX therapy without need for dose or schedule modification. Although no particular treatment modality was associated with improved surgical rates or survival outcomes, this series does identify a wide variety of potentially appropriate neoadjuvant treatment strategies that can be used safely and can lead to improved patient outcomes compared to historical controls.
Our observed rates of resection, R0 resection, and overall survival are similar to other case series using similar approaches in BRPC [15,16]. As has been demonstrated in other similar trials in this setting, many patients do not undergo surgery because of development of metastatic disease, poor patient fitness, or patient desire to forego surgery. Although initially discouraging, this illustrates an important role of NA therapy as a potential “screening” modality, identifying patients with biologically unfavorable disease unlikely to benefit from aggressive surgical intervention. Local and distal failure rates are relatively similar to other presented series, with most relapses being distant in nature, suggesting better systemic therapy as a primary goal for further development of BRPC treatment protocols. Discovery of occult disease in 6 of the 28 patients undergoing surgical exploration is higher than described in many studies. Our center does not use preneoadjuvant exploratory laparoscopy in patients with BRPC, as has become a recommendation in some other gastrointestinal malignancies prior to neoadjuvant therapy. The remainder of the patients who developed metastatic disease while on neoadjuvant therapy had this disease detected via imaging prior to surgery. Postchemotherapy CA 19-9 emerged from these data as a potential predictor of ability to proceed with surgery and of improved survival in the surgical population. CA 19-9 is emerging as an important biomarker associated with improved response rates and prognosis following neoadjuvant therapy for BRPC [[25], [26], [27]], and these data further support the potential clinical utility of this biomarker in selecting patients for surgery.
This review has a number of limitations including the small sample size included in the series, potentially contributing into type II error. Further limitations include the retrospective nature of the review and nonstandardized treatment approaches. Despite our best efforts to standardized definition of BRPC, we acknowledge that a lack of central review of all cases as to vascular involvement may contribute to some lack of consistency in terms of patients included in the series.
Despite these limitations, we believe this series effectively demonstrates the ability to deliver standardized care in a difficult disease such as BRPC across multiple sites over a wide geographic region with good adherence to recommended treatment guidelines leading to surgical outcomes similar to other reported series. Given the ability to safely and effectively administer neoadjuvant therapy for BRPC in our rural setting, our centers have adopted the protocol outlined here as a basic framework to build on multidisciplinary management of BRPC and have been involved in numerous cooperative group trials to further investigate advancing neoadjuvant therapy in this setting. Factors enhancing our ability to standardize care across multiple sites and a wide geographic area include a centralized core of pancreatic surgeons and oncologists dedicated to developing this protocol and willing to review all cases preoperatively. Secondly, the development of the DAWG meeting on a monthly and then quarterly basis allowed for ongoing conversation and troubleshooting as to implementation of the protocol, and a shared electronic medical record allowed for easy sharing of patient information and coordination of care across the geographic scope of our footprint. This communication infrastructure remains in place with quarterly meetings across the enterprise and frequent site-specific tumor boards to implement recommendations from the DAWGs. We have also begun using a similar paradigm in the management of resectable pancreatic cancer on a case-by-case basis; further large-scale research will be essential in clarifying the optimal approach to patient with BRPC, with a focus on cooperative group trials incorporating community cancer centers.
Conflict of interest
Jonathan S. Bleeker, MD—none.
Christopher J. Sumey, MD—None.
Steven F. Powell, MD—.
COI/disclosure: consulting (BMS)
COI/disclosure: institutional research support (BMS).
COI/disclosure: (Merck).
COI/disclosure: (Vyriad).
COI/disclosure: (Pfizer).
COI/disclosure: (Genentech).
COI/disclosure: (Actuate).
COI/disclosure: (Incyte).
COI/disclosure: (Astra Zeneca).
Preston D. Steen, MD—none.
Michael D. Keppen, MD—none.
Michele Lohr, MD—none.
Thavam Thambi-Pillai, MD—none.
Peter Kurniali, MD—none.
Miroslaw Mazurczak, MD—none.
Mark M. Gitau, MD—none.
Miran J. Blanchard, MD—none.
Ryan K. Nowak, MD—none.
Steven McGraw, MD—none.
Robert Sticca, MD—none.
Daniel Tuvin, MD—none.
Gary Timmerman, MD—none.
Funding Sources
This project had no external funding sources.
Author Contribution
Jonathan S. Bleeker: Conceptualization, Methodology, Funding acquisition, Formalanalysis, Investigation, Data curation, Writing - original draft, Writing - review &editing, Software, Supervision, Project administration. Christopher J. Sumey: Investigation, Writing - originaldraft, Writing - review & editing. Steven F. Powell: Investigation, Writing - original draft, Writing - review &editing. Preston D. Steen: Conceptualization, Investigation, Writing - original draft, Writing - review &editing. Michael D. Keppen: Investigation, Writing - original draft, Writing - review & editing. Michele Lohr:Investigation, Writing - original draft, Writing - review & editing. Thavam Thambi-Pillai:Conceptualization, Investigation, Writing - original draft, Writing - review & editing. Peter Kurniali:Investigation, Writing - original draft, Writing - review & editing. Miroslaw Mazurczak: Investigation, Writing- original draft, Writing - review & editing. Mark M. Gitau: Investigation, Writing - original draft, Writing -review & editing. Miran J. Blanchard: Investigation, Writing - original draft, Writing - review & editing. RyanK. Nowak: Investigation, Writing - original draft, Writing - review & editing. Steven McGraw:Investigation, Writing - original draft, Writing - review & editing. Robert Sticca:Conceptualization, Investigation, Writing - original draft, Writing - review & editing. Daniel Tuvin:Conceptualization, Investigation, Writing - original draft, Writing - review & editing. Gary Timmerman:Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Project administration.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rozenweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network Pancreatic cancer. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
- 4.Li D., Xie K., Wolff R., Abbruzzse J.L. Pancreatic cancer. The Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T., Desseigne F., Ychou M., Bouche O, Guimbaud R, Becouarn Y. FOLFIRINOX versus gemcitabine for metastatic pancreatic Cancer. New England Journal of Medicine. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 6.Romano A., Lu B., Von Hoff D.D., Penenberg D.N., Goldstein D., Tortora G. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. JNCI: J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju413. [DOI] [PubMed] [Google Scholar]
- 7.Oettle H., Neuhaus P., Hochhaus A., Hartmann J.T., Gellert K., Ridwelski K. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 8.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 9.Callery M.P., Chang K.J., Fishman E.K., Talamonti M.S., William Traverso L., Linehan D.C. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 10.Katz M.H., Marsh R., Herman J.M., Shi Q., Collison E., Venook A.P. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787–2795. doi: 10.1245/s10434-013-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varadhachary G.R., Tamm E.P., Abbruzzese J.L., Xiong H.Q., Crane C.H., Wang H. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Tsai S., Erickson B.A., Dua K., Ritch P.S., Tolat P., Evans D.B. Evolution of the management of resectable pancreatic cancer. J Oncol Pract. 2016;12:772–778. doi: 10.1200/JOP.2016.015818. [DOI] [PubMed] [Google Scholar]
- 13.Faris J.E., Blaszkowsky L.S., McDermott S., Guimaraes A.R., Szymonifka J., Huynh M.A. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer center experience. Oncologist. 2013;18:543–548. doi: 10.1634/theoncologist.2012-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz M.H.G., Shi Q., Ahmad S.A., Herman J.M., Marsh R.D., Collisson E. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA. 2016;151:e161137. doi: 10.1001/jamasurg.2016.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Versteijne E., Vogel J.A., Besselink M.G., Busch O.R.C., Wilmink J.W., Daams J.G. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. BJS. 2018;105:946–958. doi: 10.1002/bjs.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose J.B., Rocha F.G., Alseidi A., Biehl T.R., Lin B.S., Picozzi V.J. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann Surg Oncol. 2014;21:1530–1537. doi: 10.1245/s10434-014-3486-z. [DOI] [PubMed] [Google Scholar]
- 17.Van Tienhoven G., Versteijne E., Suker M., Groothuis K.B.C, Busch O.R., Bonsing B.A. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): a randomized, controlled, multicenter phase III trial. J Clin Oncol. 2018;36:LBA4002. [Google Scholar]
- 18.Tempero M.A., Malafa M.P., Al-Hawary M., Absbun A., Bain A., Behrman S.W. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1028–1061. doi: 10.6004/jnccn.2017.0131. [DOI] [PubMed] [Google Scholar]
- 19.Katz M.H.G., Fleming J.B., Bhosale P., Varadhachary G., Lee J.E., Wolff R. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–5756. doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 20.Team RC . Austria; R Foundation for Statistical Computing. Vienna: 2019. R: a language and environment for statistical computing. [Google Scholar]
- 21.Cameron J.L., Riall T.S., Coleman J., Belcher K.A. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang M.J., Jang J.-Y., Chang Y.R., Kwon W., Jung W., Kim S.W. Revisiting the concept of lymph node metastases of pancreatic head cancer: number of metastatic lymph nodes and lymph node ratio according to N stage. Ann Surg Oncol. 2014;21:1545–1551. doi: 10.1245/s10434-013-3473-9. [DOI] [PubMed] [Google Scholar]
- 23.Sohal D., McDonough S., Ahmad S.A., Gandhi N., Beg M.S., Wang-Gilliam A. SWOG S1505: initial findings on eligibility and neoadjuvant chemotherapy experience with mfolfirinox versus gemcitabine/nab-paclitaxel for resectable pancreatic adenocarcinoma. J Clin Oncol. 2019;37:414. [Google Scholar]
- 24.Motoi F., Kosuge T., Ueno H., Yamaue H., Satoi S., Sho M. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05) Jpn J Clin Oncol. 2019;49:190–194. doi: 10.1093/jjco/hyy190. [DOI] [PubMed] [Google Scholar]
- 25.Aoki S., Motoi F., Murakami Y., Sho M., Satoi S., Honda G. Decreased serum carbohydrate antigen 19-9 levels after neoadjuvant therapy predict a better prognosis for patients with pancreatic adenocarcinoma: a multicenter case-control study of 240 patients. BMC Cancer. 2019;19:252. doi: 10.1186/s12885-019-5460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai S., Mahmoud A., George B., Kelly T.R., Ritch P.S., Erickson B. Association of decline in serum Ca19-9 after neoadjuvant therapy with improved survival among borderline resectable pancreatic cancer patients. J Clin Oncol. 2013;31:e15082. [Google Scholar]
- 27.Rose J.B., Edwards A.M., Alseidi A., Biehl T.R., Lin B.S., Picozzi V.J. Pattern of CA19-9 response to neoadjuvant chemotherapy in locally advanced, borderline resectable pancreatic cancer to predict progression. J Clin Oncol. 2016;34:321. [Google Scholar]