Abstract
Central nervous system involvement in severe acute respiratory syndrome caused by coronavirus disease 2019 (COVID-19) has increasingly been recognised in the literature, and possible mechanisms of neuroinvasion, neurotropism and neurovirulence have been described. Neurological signs have been described in 84% of COVID-19 intensive care unit patients, and haemostatic abnormalities in such patients may play an important role, with a broad spectrum of neuroimaging findings. This report describes the magnetic resonance imaging neurovascular findings in an acutely ill patient with COVID-19, including perfusion abnormalities depicted in the arterial spin labelling technique.
Keywords: COVID-19, arterial spin labelling (ASL), neurointensivism, neuroradiology, neurovascular, cerebral blood flow (CBF)
Introduction
Central nervous system (CNS) involvement in severe acute respiratory syndrome (SARS) caused by SARS coronavirus 2 (SARS-CoV-2) has increasingly been recognised in the literature, and possible mechanisms of neuroinvasion, neurotropism and neurovirulence have been described.1,2 The dissemination of SARS-CoV-2 starts in the bloodstream and cribriform plate of ethmoid cells. After it enters host cells through the angiotensin-converting enzyme 2 receptor (ACE2),3 causing a severe clinical syndrome, it is mainly manifested in the respiratory tract.4 ACE2 is widely found in the lungs, heart, kidneys, intestines, testicles and the brain, mostly in glial cells but also in the motor cortex, brainstem and reticular activating system, therefore playing a role in modulating respiratory function.4,5
SARS-CoV-2 propagation to the CNS is suggested to occur via both haematogenous dissemination of infected leucocytes through damaged cells of the blood–brain barrier and retrograde propagation along the olfactory tract, supposedly determining anosmia.4,6,7
In a recent review, neurological signs were present in 84% of coronavirus disease 2019 (COVID-19) intensive care unit (ICU) patients, the most prevalent being agitation (69%), positive findings on Confusion Assessment Method in an Intensive Care Unit (CAM-ICU; 65%) and corticospinal tract signs (67%), with the least prevalent being dysexecutive syndrome (36%) and a temperature >38.5°C (16%).8
Acute necrotising encephalitis, brain leucoencephalopathy, leptomeningeal enhancement and neurovascular manifestations have all been reported in patients with COVID-19, despite an unclear causative link.6,8–10
In this report, the authors describe the magnetic resonance imaging (MRI) neurovascular findings in an acutely ill patient with COVID-19. To the best of our knowledge, this is the first report that encompasses in a single case a wide range of vascular manifestations, along with perfusion abnormalities, as depicted through arterial spin labelling (ASL) and dynamic susceptibility contrast (DSC) techniques. We hypothesise that COVID-19 could manifest as perfusion alterations in brain MRI scans.
Case presentation
A 67-year-old man, with a history of hypertension, diabetes and dyslipidaemia, was admitted to a tertiary hospital with fever and cough for 6 days, being hospitalised on 26 March. He was diagnosed with COVID-19 with a positive reverse transcriptase polymerase chain reaction assay of a nasopharyngeal sample and pulmonary involvement >50% on chest computed tomography (CT).
The patient was transferred to the ICU with acute respiratory distress syndrome, requiring sedation and mechanical ventilation. Orotracheal intubation was performed without complications. He received antibiotic therapy and pharmacological prophylaxis for venous thromboembolism with 40 g of enoxaparin from 28 March. No other antiplatelet or anticoagulant medication was given.
He remained on prolonged sedation and mechanical ventilation due to COVID-19-related lung impairment. During the sedation-weaning process, there was a reduction in psychomotor agitation and uncoupling from mechanical ventilation, with the necessary adjustment for analgo-sedation and the introduction of antipsychotics. On the 16th day of mechanical ventilation, he underwent a tracheostomy and subsequent sedation weaning. After sedation was removed, he had little interaction with the environment, with only spontaneous eye opening. He did not attend to commands, and was unresponsive to a verbal, tactile and painful stimulus. Under neurological assessment, he presented reduced patellar reflexes bilaterally, tonic deviation from the vertical look down and with a CAM-ICU negative for delirium. There were no clinical signs of intracranial hypertension or focal deficits. An electroencephalogram was performed on 10 April which showed diffuse attenuation of brain electrical activity and no epileptiform activity. Non-invasive monitoring of intracranial pressure showed P2>P1, demonstrating alterations in brain complacency.
Due to the unchanging clinical picture, a MRI scan was performed on 20 April, which showed bleeding. The MRI was performed on a 1.5 T General Electric (GE) scanner using an eight-channel head coil, without sedation, with an average blood pressure of 86 mmHg, oxygen saturation of 94% and heart rate of 97 beats per minute. The ASL perfusion was acquired in a pulsed labelling process with the following parameters: repetition time (TR) = 4546 ms, echo time (TE) = 10.5 ms, field of view (FOV) = 240 mm, flip angle = 180°, slice thickness = 4.0 mm, number of excitations (NEX) = 3. The DSC MRI perfusion was acquired with the following parameters: TR = 2300 ms, TE = 80 ms, FOV = 240 mm, flip angle = 180°, slice thickness = 5.0 mm, NEX = 1. The MRIs were assessed by two experienced neuroradiologists (L.L.F.A. and V.H.F.M.) with more than 30 and 15 years of experience, respectively.
Conventional MRI sequences are demonstrated in Figure 1. Susceptibility weighted imaging (SWI) showed diffuse small hypo-intense dots related to microbleeds located at the cortico-subcortical junction and corpus callosum. There was also intraparenchymal haematoma in the left thalamus and posterior internal capsule draining to the lateral ventricle. Axial FLAIR images showed subarachnoid haemorrhage in the right fronto-parietal convexity.
Figure 1.
(a), (b) and (c) Susceptibility weighted imaging (SWI) showing diffuse small hypo-intense dots related to microbleeds located at the cortico-subcortical junction and corpus callosum (yellow arrows). There is also intraparenchymal haematoma in the left thalamus and posterior internal capsule draining to the lateral ventricle (red arrow). (d), (e) and (f) Axial FLAIR images showing subarachnoid haemorrhage in the right fronto-parietal convexity (yellow arrows in (e) and (f)) and haematoma in (d).
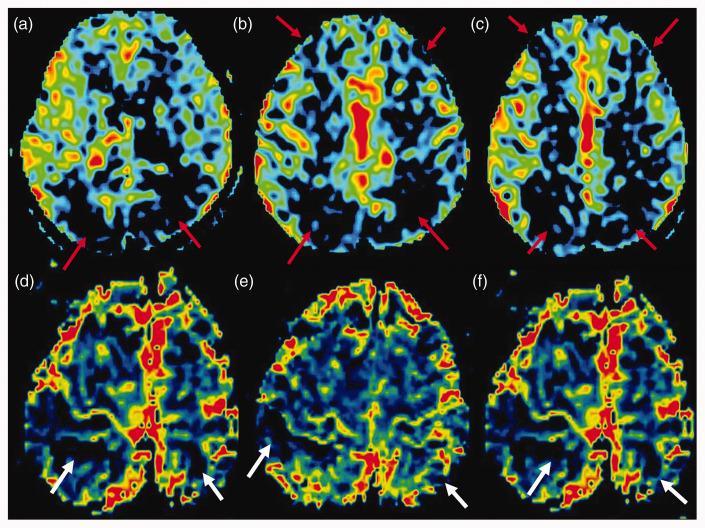
ASL demonstrated an asymmetric marked reduction of cerebral blood flow in the bilateral fronto-parietal regions, presumably related to vascular frontier zones (Figure 2). A few areas of decreased cerebral blood volume were also shown in the right fronto-parietal region, and the left parietal lobe was also shown in the DSC perfusion maps (Figure 2).
Figure 2.
(a), (b) and (c) Perfusion arterial spin labelling (ASL) imaging showing reduction of cerebral blood flow in the bilateral fronto-parietal regions (red arrows). (d), (e) and (f) Some areas also demonstrate decreased cerebral blood volume in the dynamic susceptibility contrast perfusion (white arrows).
During the ICU stay, the patient did not show any hypertensive symptoms. Moreover, during the 24 hours of the MRI scan, he had a mean arterial pressure between 79 and 116 mmHg, not contributing to hypoperfusion. Pharmacological prophylaxis for venous thromboembolism was suspended, and secondary injury prevention measures were maintained, such as blood pressure, glycaemic, sodium and temperature controls, maintaining pCO2 between 35 and 40 mmHg. A CT scan was performed in 24 hours, in which the haematoma was practically unchanged compared with the previous imaging exam.
At the present moment, the patient is on the 60th day of hospital stay, under rehabilitation, alert, contact, with no focal deficits, and is undergoing dehospitalisation.
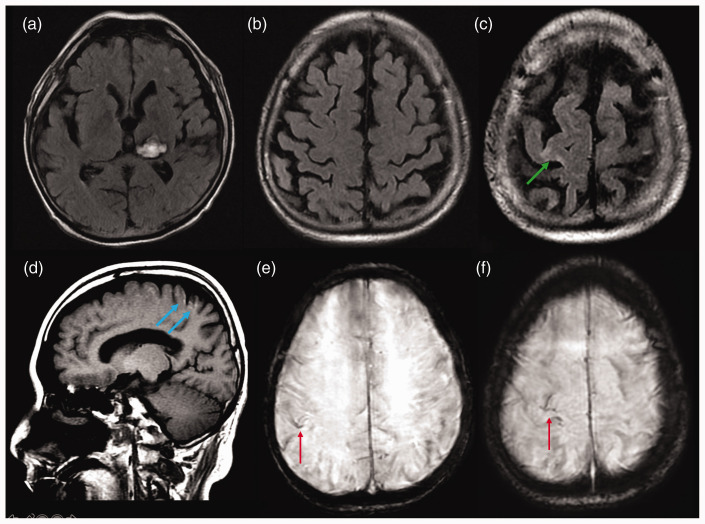
A follow-up brain MRI with conventional imaging (Figure 3) depicted partial reabsorption of the subarachnoid haemorrhage, foci of cortical laminar necrosis, gliosis in the right prefrontal cortex, superficial siderosis, resolution of both cortical and subarachnoid contrast-enhancement, with no additional signs of acute ischaemia. Unfortunately, we were not able to acquire perfusion imaging.
Figure 3.
Follow-up brain magnetic resonance imaging with conventional imaging using axial FLAIR ((a), (b) and (c)), sagittal T1 (d) and axial SWI ((e )and (f)), showing partial reabsorption of the subarachnoid haemorrhage, foci of cortical laminar necrosis (blue arrows), gliosis in the right prefrontal cortex (green arrow), superficial siderosis (red arrows) and resolution of both cortical and subarachnoid contrast-enhancement, with no additional signs of acute ischaemia.
Discussion
To date, what we know about CNS changes in patients with COVID-19 leads us to postulate a multifactorial mechanism of action. Most likely, there is an association of coagulation changes, endothelial dysfunction, cytokine storm and hypoxaemia, in addition to alterations secondary to multiple-organ dysfunction.11–14
SARS-CoV-2 may induce a cytokine storm syndrome in a subgroup of patients, and it is usually correlated with manifestations such as encephalitis, acute necrotising encephalopathy, leucoencephalopathy and Guillain–Barré syndrome.9,10,15,16 In addition, haemostatic abnormalities have been increasingly described in acutely ill patients with COVID-19, including thrombocytopaenia, increased D-dimer levels, arterial and venous thrombotic events and multifocal haemorrhages.17 Studies have also demonstrated positivity for anticardiolipin immunoglobulin (Ig)A antibodies as well as anti-β2-glycoprotein I IgA and IgG antibodies in patients with multiple cerebral infarctions.11
A recent case series of COVID-19 patients from Wuhan, PR China, reported that of 36% of patients with neurological manifestations, 6% had acute cerebrovascular events.8 Although the exact mechanism is unclear, a transient hypercoagulable state and endothelial dysfunction have been suggested.18 Cerebral vascular events are more common in critically ill patients with co-morbidities, corroborating a multifactorial mechanism for their occurrence. However, it should be noted that a recent study demonstrated ischaemic events in large vessels as the initial finding of COVID-19 in younger patients, without previous significant systemic symptoms.19
In COVID-19 patients, hypoxaemia related to perfusion and vascular abnormalities has recently also been proposed to occur due to pulmonary vascular shunting.20 Another postulation for hypoxia is related to the virus attack on the haem group within haemoglobin, separating haem from globin.21
Largely because of the logistical difficulties in performing perfusion brain MRI in COVID-19 patients, only a few studies have been conducted. In studies with CT and angio-CT, both ischaemic strokes and haemorrhages have been described. To the best of our knowledge, there is only one study with a larger group of patients who underwent MRI (13 patients), which demonstrated ischaemic events in 23%, meningeal impregnation in 62% and perfusion abnormalities in 100% of cases,6 and no possible mechanisms for perfusion abnormalities were made.
Our patient presented with cortico-subcortical and callosal microbleeds, subarachnoid haemorrhage, intraparenchymal haematoma, as well as decreased cerebral blood flow (CBF) in the fronto-parietal regions juxtaposed to the vascular border zones, depicted in both ASL and DSC perfusion techniques.
Brain haemorrhages are frequent in COVID-19, and their detection is clinically important, as they are associated with a worse respiratory, neurological and biological status and are more often found in ICU patients.22 A study demonstrated juxta-cortical and callosal micro-haemorrhages in more than half of COVID-19 patients, which were thought to be related to hypoxaemia and disruption of the blood–brain barrier.23 A report of two patients with catastrophic haemorrhages also infers such putative mechanisms, despite therapeutic anticoagulation.24 In addition, post-mortem brain MRI studies have shown subcortical micro- and macrobleeds,25 and along with hypoxemic-induced mechanisms, small-vessel disease was considered in their aetiopathogenesis.26
In our case, the association of multifocal microbleeds (including the corpus callosum), normal blood pressure and evidence of reduced brain perfusion support the fact that prophylactic anticoagulation or hypertension were unlikely to be solely responsible for the haemorrhages.22,24 Moreover, no other anticoagulant or antiplatelet was given.
ASL is a non-invasive imaging technique that permits the evaluation of blood flow and perfusion without the need for contrast injection and is already widely used in many clinical settings in neuroradiology.27,28 Perfusion abnormalities in brain viral infections have been described in the literature and oscillate from high to low CBF according to different studies and viral agents, without a clear causative mechanism. In general, an increase in CBF using the ASL technique has been demonstrated in most cases, especially in herpes simplex virus (HSV) and tick-borne encephalitis, while a reduction in CBF occurred in human immunodeficiency virus (HIV) patients.29–31 In addition, studies have reported a positive correlation between a decreased brain CBF in acute viral infections and a poorer prognostic outcome, highlighting the potential additional value of using the ASL technique.31–33 Regarding SARS-CoV-2, it is important to note that most studies so far have demonstrated neurological and neuroradiological changes in patients with moderate to severe conditions, which is detrimental to state that the findings are due only to the direct action of the virus, as in the cases of HSV and HIV described above.
Based on current knowledge of the pathophysiological mechanisms of action of SARS-CoV-2, we postulate that the perfusion change found in our patient in the projection of vascular boundary zones occurred due to a haemodynamic mechanism of cerebral vascular auto-regulation dysfunction promoted by hypoxia and endothelial injury.
In conclusion, neuroradiologists should be aware of the wide range of neurological manifestations, including cerebrovascular disease, haemorrhages and perfusion abnormalities, in patients with COVID-19. Although yet unclear, we believe the association of hypoxaemia and blood–brain barrier disruption are the most probable underlying mechanisms. The ASL technique may add further contributions in both identifying patients with perfusion dysfunction and helping to elucidate the biological mechanisms of SARS-CoV-2. Further studies with a large number of patients and in a prospective design would help to clarify the potential causative link between brain perfusion abnormalities and COVID-19 infection.
Declaration of conflicting interests
The authors declared no potential conflicts of interest concerning the research, authorship and/or publication of this article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD
Matheus Dorigatti Soldatelli https://orcid.org/0000-0002-1544-4398
References
- 1.Desforges M, Le Coupanec A, Stodola JK, et al. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res 2014; 194: 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020; 11: 995–998. [DOI] [PubMed] [Google Scholar]
- 4.Mankad K, Perry MD, Mirsky DM, et al. COVID-19: a primer for neuroradiologists. Neuroradiology. 2020; 62: 647–648.
- 5.Netland J, Meyerholz DK, Moore S, et al. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008; 82: 7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. Epub ahead of print 15 April 2020. DOI: 10.1056/nejmc2008597. [DOI] [PMC free article] [PubMed]
- 7.Dubé M, Le Coupanec A, Wong AHM, et al. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol 2018; 92: e00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachs JR, Gibbs KW, Swor DE, et al. COVID-19-associated leukoencephalopathy. Radiology. Epub ahead of print 14 May 2020. DOI: 10.1148/radiol.2020201753. [DOI] [PMC free article] [PubMed]
- 10.Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. Epub ahead of print 21 March 2020. DOI: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed]
- 11.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020; 382: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci 2020; 413: 116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zubair AS, McAlpine LS, Gardin T, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019. JAMA Neurol. Epub ahead of print 29 May 2020. DOI: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed]
- 14.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. Epub ahead of print 6 April 2020. DOI: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed]
- 15.Zhao H, Shen D, Zhou H, et al. Guillain–Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 2020; 19: 383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma J, Xia P, Zhou Y, et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol 2020; 214: 108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020; 382: e60. [DOI] [PMC free article] [PubMed]
- 20.Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. Epub ahead of print April 2020. DOI: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed]
- 21.Liu W, Li H. COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv. Epub ahead of print 27 April 2020. DOI: 10.26434/CHEMRXIV.11938173.V8.
- 22.Kremer S, Lersy F, De Sèze J, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Neuroradiology. Epub ahead of print 16 June 2020. DOI: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed]
- 23.Radmanesh A, Derman A, Lui YW, et al. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. Epub ahead of print 21 May 2020. DOI: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed]
- 24.Carroll E, Lewis A. Catastrophic intracranial hemorrhage in two critically ill patients with COVID-19. Neurocrit Care. Epub ahead of print 26 May 2020. DOI: 10.1007/s12028-020-00993-5. [DOI] [PMC free article] [PubMed]
- 25.Coolen T, Lolli V, Sadeghi N, et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. Epub ahead of print 16 June 2020. DOI: 10.1212/WNL.0000000000010116. [DOI] [PubMed]
- 26.Reichard RR, Kashani KB, Boire NA, et al. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol 2020; 140: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soldozy S, Galindo J, Snyder H, et al. Clinical utility of arterial spin labeling imaging in disorders of the nervous system. Neurosurg Focus 2019; 47: 1–10. [DOI] [PubMed] [Google Scholar]
- 28.Van Osch MJP.Teeuwisse WM, Chen Z, et al. Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow. J Cereb Blood Flow Metab 2018; 38: 1461–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyrakowska-Dadełło Z, Tarasów E, Janusek D, et al. Brain perfusion alterations in tick-borne encephalitis – preliminary report. Int J Infect Dis 2018; 68: 26–30. [DOI] [PubMed] [Google Scholar]
- 30.Koeller KK, Shih RY. Viral and prion infections of the central nervous system: radiologic-pathologic correlation. Radiographics 2017; 37: 199–233. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi T, Yakushiji Y, Nishihara M, et al. Arterial spin-labeling in central nervous system infection. Magn Reson Med Sci 2016; 15: 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao CH, Wang SJ, Mak SC, et al. Viral encephalitis in children: detection with technetium-99m HMPAO brain single-photon emission CT and its value in prediction of outcome. Am J Neuroradiol 1994; 15: 1369–1373. [PMC free article] [PubMed] [Google Scholar]
- 33.Lee BY, Newberg AB, Liebeskind DS, et al. FDG-PET findings in patients with suspected encephalitis. Clin Nucl Med 2004; 29: 620–625. [DOI] [PubMed] [Google Scholar]