Abstract
Eye drops producing long-acting ocular anesthesia would be desirable for corneal pain management. Here we present liposome-based formulations to achieve very long ocular anesthetic effect after a single eye drop instillation. The liposomes were functionalized with succinyl-Concanavalin A (sConA-Lip), which can bind corneal glycan moieties, to significantly prolong the dwell time of liposomes on the cornea. sConA-Lip were loaded with tetrodotoxin and dexmedetomidine (sConA-Lip/TD), and provided sustained release for both. A single topical instillation of sConA-Lip/TD on the cornea could achieve 105 min of dense analgesia and 540 min of partial analgesia, which was significantly longer than analgesia with proparacaine, tetrodotoxin/dexmedetomidine solution or unmodified liposomes containing tetrodotoxin and dexmedetomidine. sConA-Lip/TD were not cytotoxic in vitro to human corneal limbal epithelial cells or corneal keratocytes. Topical administration of sConA-Lip/TD provided prolonged corneal anesthesia without delaying corneal wound healing. Such a formulation may be useful for the management of acute surgical and nonsurgical corneal pain, or for treatment of other ocular surface diseases.
Keywords: Liposome, Ocular anesthesia, Tetrodotoxin, Dexmedetomidine, Concanavalin A
1. Introduction
Conventional amino-ester and amino-amide local anesthetics are commonly used to reduce ocular pain related to corneal injury [1, 2] and ophthalmic surgery [3–5]. However, these agents only produce short periods of analgesia (15 to 20 minutes) after a single topical instillation [6]. Thus, continuous analgesia would require frequently repeated administration, which could cause anterior segment inflammation, corneal ulceration, and delay epithelial healing [7, 8]. Long-acting ocular anesthetics with minimal toxicity are clinically desirable, particularly during longer surgical procedures and for outpatient management of minor corneal injury during the period when ocular pain is most intense.
Tetrodotoxin (TTX) is a naturally occurring toxin found in several organisms, whose mechanism of action is unimolecular blockade of site 1 on the extracellular surface of sodium channels on nerves[9, 10]. TTX is an extremely potent local anesthetic [11, 12]. Unlike commercially available amino-amide and amino-ester local anesthetics, tissue toxicity from TTX after injection at peripheral nerves can be minimal [13], even when delivered for prolonged periods [14]. Co-administration of TTX with adjuvant agents can enhance anesthetic effect. For example, the combination of TTX and the α2-agonist dexmedetomidine can significantly prolong the duration of corneal local anesthesia over that from TTX alone [8].
Liposomes are a class of versatile carriers for encapsulating both hydrophilic and hydrophobic drugs [15–17]. Liposomes are biocompatible and biodegradable, and have been widely investigated for delivery of a variety of therapeutic agents for ocular diseases [18–20]. Liposome-based eye drops can improve the bioavailability of encapsulated drugs [21]. TTX and DMED can be co-encapsulated in liposomes, exhibiting sustained release profiles and generating prolonged local anesthetic effects on periphery nerves [22–24].
Concanavalin A (ConA) is a lectin (carbohydrate-binding protein) that binds specifically to glycoproteins and glycopeptides [25]. ConA has been previously reported to bind corneal epithelial cells at low concentrations in vitro, without apparent toxicity [26, 27]. sConA is the succinylated ConA [28], which exhibits comparable binding affinity to glycan but less effects on the migration of corneal epithelial cells [29]. We hypothesize that sConA modified liposomes (sConA-LipB) would remain on the cornea for longer periods than unmodified ones, enabling sustained release of TTX/DMED on the cornea and long-acting ocular anesthesia.
Here, we report liposome-based formulations encapsulating TTX and DMED for long-acting topical ocular anesthesia, and compare them with TTX/DMED solution and the widely used amino-ester ocular anesthetic, 0.5% (wt/vol) proparacaine. We also characterize the cytotoxicity of those formulations to corneal cells in vitro and their effect on corneal healing in vivo.
2. Materials and methods
2.1. Materials
Tetrodotoxin (TTX) was obtained from Abcam PLC (Cambridge, MA); dexmedetomidine hydrochloride (DMED) was from R&D systems, Inc. (Minneapolis, MN), and dexmedetomidine free base was prepared by alkaline precipitation. Lipids (DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine and DOPG, 1,2-dioleoyl-sn-glycero-3-phospho-(1’-rac- glycerol)) were acquired from CordenPharma International (Plankstadt, Germany). 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (mal-PEG-DSPE) was purchased from Laysan Bio, Inc. (Arab, AL). Succinyl-Concanavalin A (sConA), Traut’s reagent and cholesterol were obtained from Sigma (St, Louis, MO). Tetrodotoxin ELISA kits were from Reagen LLC (Moorestown, NJ).
2.2. Animal care
Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) 6–8 weeks in age were cared for in accordance with protocols approved by the Animal Care and Use Committee at Children’s Hospital, and the Guide for the Care and Use of Laboratory Animals of the U.S. National Research Council. They were housed in groups, in a 7 am to 7 pm light-dark cycle.
2.3. Preparation of liposomal formulations
Liposomes were synthesized by hydrating a thin lipid film, using DOPC, DOPG and cholesterol [22, 30]. The molar ratios between DOPC and DOPG varied from 16:0 to 12:4 (Table 1). The content of cholesterol was fixed at a 6:16 molar ratio to the total lipids (DOPC + DOPG) in all liposomal formulations. 160 mg lipids (DOPC + DOPG), 30 mg cholesterol and 200 μg dexmedetomidine free base were dissolved in a chloroform : methanol (v/v, 9:1) mixture and the thin lipid film formed under reduced pressure, followed by hydration at 60 °C with 2 mL TTX solution (1 mg/mL) or rhodamine 6G (R6G) in phosphate buffered saline (PBS). The formed liposomes were further homogenized at 10,000×g with a 3/8” MiniMicro workhead on a L4RT-A Silverson Laboratory Mixer (East Longmeadow, MA) for 10 min to narrow the size distribution of liposomes and drug loading was increased after 10 freeze-thaw cycles [14]. For the determination of drug loading, unencapsulated drugs were removed by dialysis bag with MWCO 50 KDa (Spectrum Laboratories, Inc., Rancho Dominguez, CA) at 4oC. Size and zeta-potential of liposomes were analyzed by a particle analyzer (Delsa Nano C, Beckman Counter). To assess the release kinetics from TTX and DMED, liposomes (0.4 mL) were placed in dialysis membranes (molecular weight cutoff 10 kDa) and dialyzed against PBS. At each predetermined time point, the buffer was changed with fresh PBS buffer. TTX and DMED contents were determined using a TTX ELISA kit (Reagen LLC, Moorestown, NJ) and analytic HPLC (WondaCract ODS-2 column, 5 um, 4.6×150 mm, mobile phase: Water/Acetonitrile = 4/6, UV absorbance at 254 nm), respectively. Lipid concentration in the final formulations were measured using the Bartlett assay [14, 31].
Table 1.
Characterization of liposomal formulations.
| PC/PG | Size/nm | PDI | Zeta potential/mV | EEa/% | LCb/% | |||
|---|---|---|---|---|---|---|---|---|
| DMED | TTX | DMED | TTX | |||||
| LipA | 16:0 | 2010 | 0.62 | − 1 ± 1 | 58 ± 7 | 26 ± 5 | 0.07 ± 0.01 | 0.3 ± 0.1 |
| LipB | 15:1 | 446 | 0.33 | − 12 ± 1 | 65 ± 5 | 44 ± 4*** | 0.08 ± 0.01 | 0.5 ± 0.1 |
| LipC | 14:2 | 502 | 0.33 | − 25 ± 1 | 72 ± 3††† | 42 ± 3*** | 0.08 ± 0 | 0.5 ± 0.1 |
| LipD | 12:4 | 449 | 0.32 | − 29 ± 1 | 73 ± 6†† | 52 ± 3*** | 0.09 ± 0 | 0.6 ± 0.1 |
| sConA-LipB | 15:1 | 508 | 0.32 | − 14 ± 1 | 62 ± 3 | 43 ± 5 | 0.07 ± 0.01 | 0.5 ± 0.1 |
EE = Encapsulation efficiency,
LC = Loading capacity,
Data are means ± SD (n = 3). Groups were compared using 2-way analysis of variance with Bonferroni post hoc test.
p < 0.001 compared with TTX encapsulation efficiency in LipA
p < 0.01 and
p < 0.001 compared with DMED encapsulation efficiency in LipA. Encapsulation efficiency of both drugs in LipB was not statistically significantly different from that in sConA-LipB.
sConA was thiolated (sConA-SH) with Traut’s reagent according to the manufacturer’s specifications, and the final concentration of sConA-SH in PBS was determined by UV absorbance at 280 nm [32]. Liposomes for this application were prepared with mal-PEG-DSPE (1 mol% of the total lipid), to provide a maleimide for the sConA-SH to react with, mixed with lipids (DOPC, DOPG and cholesterol at a molar ration of 15:1:6) to form a thin film. Hydration and drug loading were carried out as above. To prepare sConA decorated liposomes, sConA-SH (with a final concentration of 25 μg/mL) was incubated with maleimide-bearing overnight at 4 oC. The conjugation efficiency of sConA was measured using the UV spectroscopic assay (at 280 nm using a molar extinction coefficient of 33920) and the BCA protein assay (ThermoFisher, Waltham, MA) according to the manufacturer’s instruction.
2.4. Cell viability assay
Cell viability assay was determined as reported [33]. Immortalized human corneal limbal epithelial cells (HCLE) were cultured in keratinocyte serum-free (KSF) medium (Invitrogen, Carlsbad, CA) supplemented with epidermal growth factor (EGF) and bovine pituitary extract, until cells reached 50% confluence. Culturing medium was switched to a 1:1 mixture of KSF medium and a combination of 1:1 unsupplemented low-calcium Dulbecco’s minimum essential medium (DMEM) and F12 Ham’s nutrient mixture (Invitrogen). For differentiation and stratification, HCLE cells were exposed to 1:1 DMEM/F12 medium (Mediatech, Manassas, VA) supplemented with newborn calf serum and EGF. Human corneal keratocytes (HCK) were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. All cells were incubated at 37°C in a 5% CO2 environment.
Corneal keratocytes and differentiated HCLE cells were exposed to media containing liposomes (50 mM lipids), and cellular viability was assessed at 4, 8, 16, and 24 hours, using the MTS colorimetric assay (CellTiter96 Proliferation Assay; Promega, Fitchburg, WI). Results are presented as percent viability from 4 separate experiments, normalized to cultured cells that were not exposed to test compounds.
2.5. Ocular analgesia testing
Towel-restrained rats received formulations to the left eye, while the right eye served as an untreated control. All test formulations were administered in 30 μL. Corneal tactile sensitivity was tested using a Cochet–Bonnet esthesiometer (Luneau Ophtalmologie, Chartres, France) [33], which consists of a retractable nylon monofilament that exerts pressure inversely proportional to its length. At full extension, the monofilament is 6 cm. Testing began by gently placing the tip of the fully extended filament on the cornea, followed by application of sufficient force to slightly buckle the filament. A reflexive blink was considered a positive response. In the absence of blink response, the filament length was reduced by 0.5 cm. The process of filament length reduction and retesting was repeated until a blink was elicited. Animals were tested every 15 minutes after the administration of a test substance until animals had a positive response to filament length of 6.0 cm. The duration of nonresponsiveness to filament length of 0.5 cm (block0.5) was defined as the duration of complete corneal block. Similarly, nonresponsiveness to 2.0 cm (block2) and 2.0 to 6.0 cm (block6) were considered the duration of dense and partial corneal block, respectively.
2.6. Ocular persistence study
Towel-restrained rats received 30 μL fluorescently labeled formulations or R6G solution (2 mg/mL) to the left eye, with care taken to avoid spillage of dye from the eye. Imaging of the left eye with an IVIS in vivo imaging system (PerkinElmer, Waltham, MA) was conducted at 5 minutes before administration, and at different time points up to 24 hours after administration. Imaging was done under isoflurane in oxygen anesthesia. Fluorescence on the eye was quantitated with the IVIS image software. The fluorescence at 5 minutes was set as 100%, and that at following time points were normalized accordingly.
2.7. Assessment for sedation
Rats were assessed for sedation from systemically absorbed DMED immediately after application of liposomes and every 15 minutes thereafter until ocular sensation returned to baseline. The sedation rating scale [34] is a 6-point scale ranging from 0 (asleep, eye fully closed, loss of right reflex) to 5 (fully awake, eyes wide open, grooming, feeding and ambulating).
2.8. Corneal Debridement Studies
To assess the effect of formulations on the rate of cornea epithelialization, rats were anesthetized with isoflurane in oxygen. A corneal trephine (3-mm diameter) was placed on the left cornea and filled with 50% ethanol for 90 seconds. This resulted in loosening and detachment of the ethanol-soaked cornea within the trephine. Care was taken to ensure no ethanol leaked outside the trephine. The eye was flushed with warm saline, and the detached cornea was gently scraped away using a sterile surgical blade. Immediately after the lesion was made, a single dose of 30 mL of the formulation was placed in the wounded eye. An external light source with a cobalt blue filter was used to better visualize the fluorescein filled corneal defect. Photographs were taken after fluorescein instillation (FUL-GLO strips; Akorn, Inc, Lake forest, IL), using a Nikon D60 camera, every 4 hours until complete reepithelialization. All images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD) for corneal wound area estimation. The rate of corneal epithelialization was calculated as previously reported [33].
2.9. Statistical analysis
Data are presented as means ± SDs for in vitro data, and medians with 25th and 75th quartiles for in vivo data unless noted otherwise. Statistical differences in mean corneal block duration and average rate of corneal wound healing between the experimental groups were compared using 1-way analysis of variance with Bonferroni post hoc test. Data from in vitro analysis at various time points were compared using 2-way analysis of variance with Bonferroni post hoc test; P < 0.05 was considered statistically significant. All analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA).
3. Results
3.1. Characterization of liposomal formulations
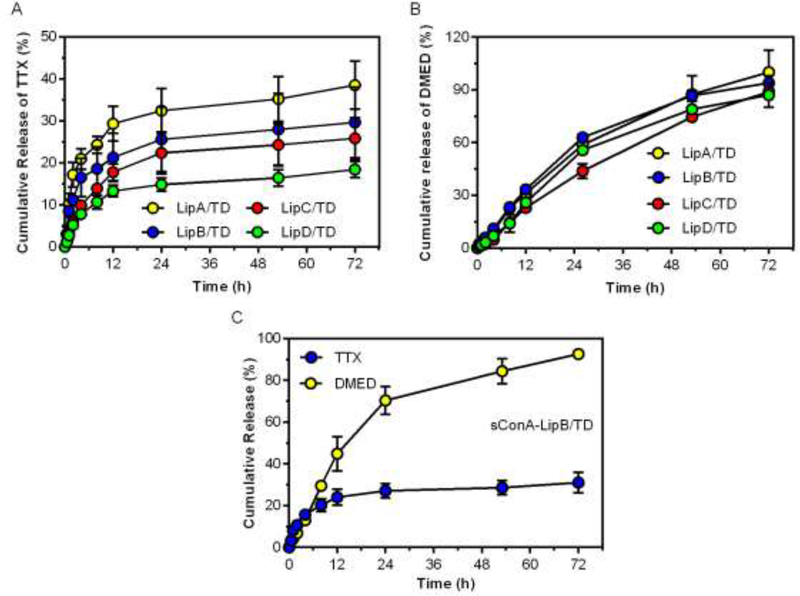
Liposomal formulations were synthesized by hydrating thin lipid films (containing DMED free base) with TTX solution (in PBS, 1 mg/mL). Since these liposomes were intended to stay on the cornea for a relatively short period (days) the low-transition temperature lipids DOPC and DOPG were used, as they would provide relatively rapid drug release [35]. The cholesterol content was fixed at a 6:16 molar ratio to total lipids (DOPC + DOPG) in all liposomal formulations. Since the negatively charged lipid DOPG could enhance the loading capacity and slow the release of TTX (a positively charged small molecule) [14], we prepared four populations of liposomes consisting of different DOPC/DOPG molar ratios, and studied their effect on drug loading and release (Table 1, Fig 1A and 1B). LipA was formulated with DOPC (without DOPG) and cholesterol, had a neutral zeta potential and relatively low encapsulation efficiency for TTX (26.3%). LipA was 2010 nm with a high polydispersity (0.62). Incorporation of DOPG decreased the size of liposomes to around 500 nm. Increasing the DOPG content did not significantly affect the size and polydispersity of liposomes, but did decrease the zeta potential significantly. DOPG also greatly increased the efficiency of encapsulation of TTX. DOPG had a modest but statistically significant effect on the encapsulation of the hydrophobic DMED free base.
Fig 1.
Cumulative release from liposomes. Release of TTX (A) and DMED (B) from LipA, B, C, D. (C) Release of TTX and DMED from sConA-LipB/TD. Data are means ± SD (n = 3).
3.2. Drug release in vitro
The release profile of TTX/DMED could be crucial for achieving long-acting corneal anesthesia. Liposomes (0.4 mL, 80 mg/mL of lipids) were placed in a dialysis bag (MWCO 50 KDa) against PBS at 37 oC. The release of TTX and DMED were determined by ELISA and HPLC, respectively. Release of DMED was substantially similar between all the formulations (no statistically significant differences, Fig. 1B). TTX release from LipB/TD was slightly higher than from LipC/TD and LipD/TD over the desired time frame of effect in vivo, indicating that negative charge (imparted by DOPG) could extend TTX release to some extent. Given that the duration of local anesthesia in vivo depends on the release of local anesthetics above a threshold (unknown a priori) level [36] - i.e. higher release is more likely to be effective – LipB/TD was used in all subsequent in vitro and in vivo experiments (LipA/TD was not used due to its low encapsulation efficiency of TTX, see Table 1)..
sConA was covalently conjugated on the surface of LipB (sConA-LipB). The unconjugated sConA was separated in the supernatant by centrifugation and quantified spectroscopically by UV measurements at 280 nm using a molar extinction coefficient of 33920, calculated according to a published algorithm [37], showing that 73.5% of sConA was conjugated on the surface of liposomes. In another independent test, we detected 64.8% of sConA conjugating on liposome surface by using the BCA protein assay kit (ThermoFisher, Waltham, MA), which was close to the result of the aforementioned assay. As shown in Table 1 and Fig 1C, sConA conjugation did not significantly alter the size, zeta potential or release profiles of LipB.
3.3. Cytotoxicity in vitro
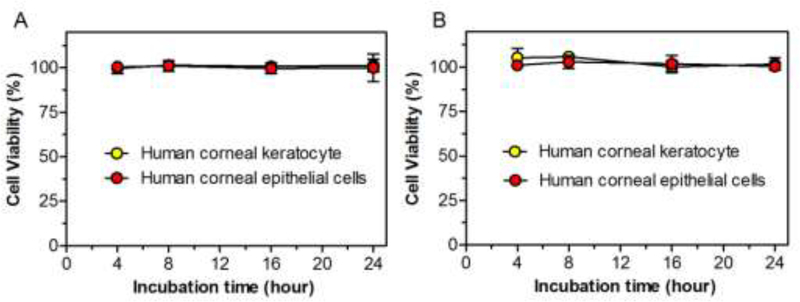
To determine the cytotoxicity of LipB/TD and sConA-LipB/TD, human corneal keratocytes and human corneal limbal epithelial (HCLE) cells were incubated with media containing liposomes (40 mg/mL lipids) (Fig 2). The viability of human corneal keratocytes and HCLE cells, as measured by the MTS assay was not reduced over 24 h incubation with LipB/TD or sConA-LipB/TD.
Fig 2.
Effect of (A) LipB/TD and (B) sConA-LipB/TD on viability of human corneal keratocytes and HCLE cells, as determined by the MTS assay. Data are means ± SD, n = 4.
3.4. Ocular anesthesia after treatment with drug solutions and liposomal formulations
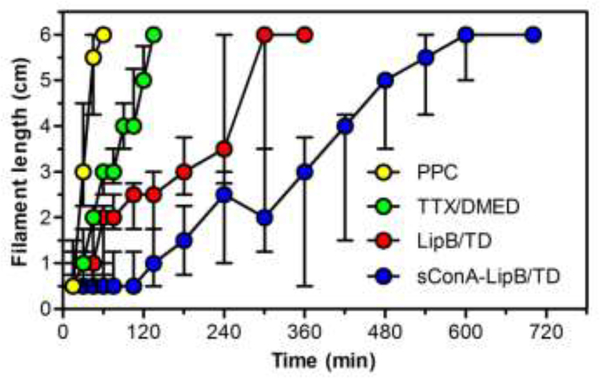
Animals received a single 30-μL eye drop of PPC (5 mg/mL; a local anesthetic commonly used in ophthalmic practice [38]), TTX/DMED solution (1 mg/mL TTX and 100 μg/mL DMED), LipB/TD and sConA-LipB/TD (1 mg/mL TTX and 100 μg/mL DMED; ~60% DMED and ~40% TTX were encapsulated in liposomes, see Table 1). The liposomal formulations included encapsulated and free drugs; the latter were not removed after preparation of drug loaded liposomes so as to achieve immediate deep anesthetic effect. We used a validated rat model to measure corneal sensitivity to touch after anesthetic solutions were applied (Fig 3, Table 2).
Fig 3.
Effect of PPC solution, TTX/DMED solution, LipB/TD and sConA-LipB/TD on corneal nerve block. Longer filament length denotes less anesthesia. Data are medians with 25th and 75th quartiles, n = 5.
Table 2.
Duration of corneal anesthesia achieved by various agents.
| Block0.5 (min) | Block2 (min) | Block6 (min) | |
|---|---|---|---|
| PPC | 9 ± 8 | 18 ± 7 | 57 ± 7 |
| TTX/DMED Solution | 18 ± 13 | 45 ± 0 | 129 ± 13 |
| LipB/TD | 27 ± 25 | 105 ± 75§,ɸ | 300 ± 60§§§,ɸɸɸ |
| sConA-LipB/TD | 105 ± 50*,†,‡ | 282 ± 152***,†††,‡ | 608 ± 72***,†††,‡‡‡ |
Data are means ± SD (n = 5), and are compared by a one-way ANOVA with Bonferonni post hoc test.
P < 0.05
P < 0.01
P < 0.001 when sConA-LipB/TD was compared with PPC.
P < 0.05 and
P < 0.001 when sConA-LipB/TD was compared with TTX/DMED solution.
P < 0.05 and
P < 0.001 when sConA-LipB/TD was compared with LipB/TD.
P < 0.05
P < 0.001 when LipB/TD was compared with PPC
P < 0.05
P < 0.001 when LipB/TD was compared with TTX/DMED solution.
Animals treated with PPC achieved complete corneal block (block0.5) for 9 minutes, deep block (block2) for 18 minutes, and partial block (block6) for 57 minutes. TTX/DMED in solution yielded 18 minutes complete corneal block (block0.5), extended block2 to 45 minutes, and block6 to 129 minutes. LipB/TD also did not significantly extend the duration of block0.5, but prolonged block2 to 2.3-fold of that of the TTX/DMED solution group (p<0.05), and block6 to 2.3-fold of that of TTX/DMED solution group (p<0.001). Topical application of 30 μL sConA-LipB/TD greatly prolonged the duration of corneal anesthesia in comparison to the unmodified liposomal formulation, yielding 3.9-fold prolongation of complete block0.5 to 105 minutes (p<0.05 in comparison to LipB/TD group), and 2.7-fold prolongation of block2 (p<0.001 in comparison to LipB/TD group), and 2-fold prolongation of block6 (p<0.001 in comparison to LipB/TD group). LipB or sConA-LipB (formulations without drug loading) did not achieve any effective corneal nerve block.
The Sedation Rating Scale (see Methods) was used to measure potential sedation from dexmedetomidine. All groups, regardless of treatment, received scores of 5±0 (i.e., no sedation was observed).
3.5. Liposome persistence on the cornea
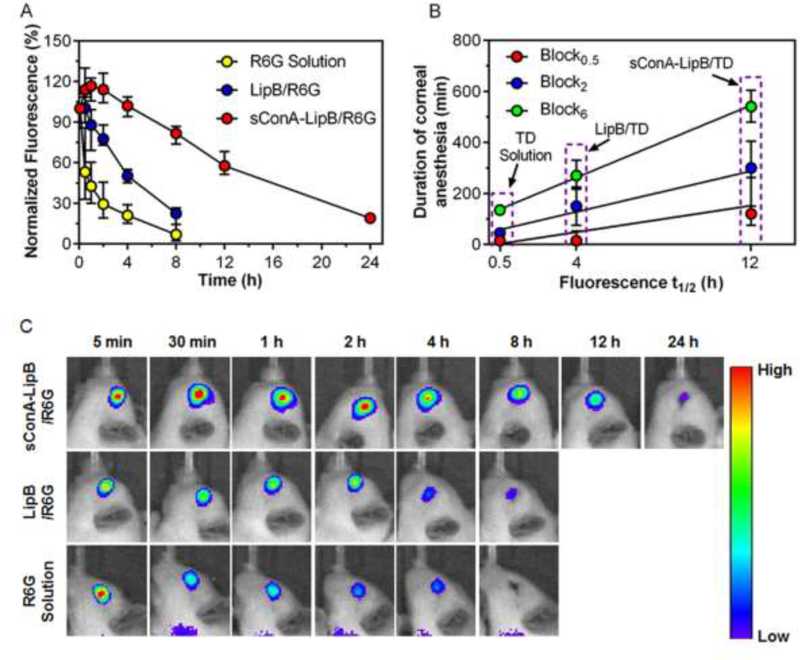
Liposomes were loaded with the fluorescent dye rhodamine 6G (R6G; loaded particles referred to as LipB/R6G and sConA-LipB/R6G; see Methods) to track their persistence on the cornea. R6G in saline, LipB/R6G and sConA-Lip-R6G were topically administered on the cornea, at a concentration of 2 mg/mL R6G (n = 5). Eyes were imaged by an in vivo imaging system (IVIS) five minutes after administration and the fluorescence was quantitated (Fig 4A and 4C). Fluorescence at subsequent time points were normalized to the fluorescence at that first time point.
Fig 4.
Persistence on the cornea of various formulations. (A) Timecourse of fluorescence on the cornea. (B) Relationship between duration of corneal anesthesia and the time to for fluorescence to reach 50% of initial value (fluorescence t1/2), determined by interpolation. Data are medians with 25th and 75th percentiles (n = 5). (C) Representative fluorescence images of rat eyes at different time points after topical administration of R6G solution, LipB/R6G or sConA-LipB/R6G.
With free R6G solution, 45% of fluorescence was eliminated from the eye after 30 min. With LipB/R6G, 50% of dye was still detectable on the eye after 4 hours, and fluorescence returned to baseline after more than 8 hours. With sConA-modified liposomes, 57% of fluorescence was still retained on the eye after 12 h, and returned to baseline after more than 24 hours. Those results were consistent with the durations of corneal nerve block observed with the various formulations (Fig 4B). A large fraction of eye drops are lost from the ocular surface by tearing and nasolacrimal drainage, although some can persist in conjunctiva [39, 40]. Irrespective of exact location on the ocular surface, it is clear that cConA greatly prolonged liposomal presence (Fig. 4). It is unlikely that liposomes of this size would be able to cross the cornea/conjunctiva [41], but the encapsulated drugs probably could [42], depending on their loading and physicochemical properties.
3.6. Effect on the rate of corneal healing
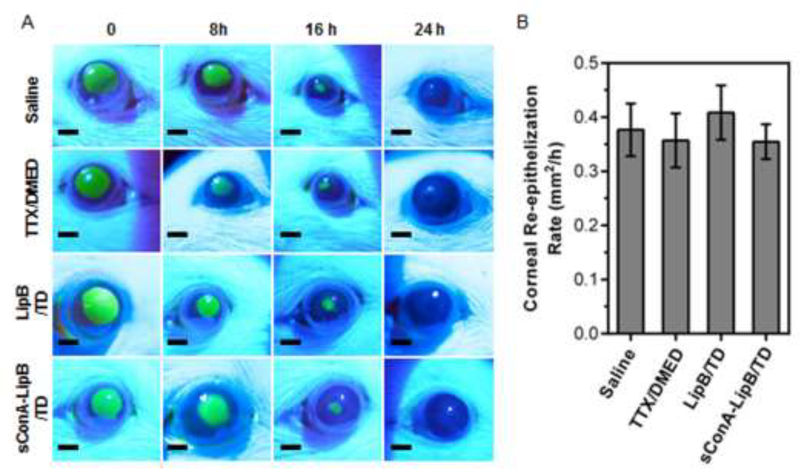
The combination of TTX and DMED has been has been reported to have no effect on corneal re-epithelialization [8]. However, ConA has been reported to delay the migration of corneal epithelial cells at high concentration in vitro [27], which could cause delayed corneal healing. To assess whether sConA-LipB/TD altered corneal healing, a 2 mm-diameter area of the corneal epithelium was debrided, then exposed to a single instillation of test solutions. Fluorescein was instilled, eyes were illuminated with an external light source using a cobalt-blue filter, and photographs were taken for digital analysis to measure the size of the defect. The rate of re-epithelialization was not decreased in any treatment group compared to the saline control (Fig 5; P > 0.05 for all comparisons, n = 5). All defects were healed by 36 hours.
Fig 5.
Effect of test formulations on corneal healing. (A) Representative fluorescence images of rat eyes after debridement and treatment of formulations. (B) Average rate of corneal re-epithelialization after debridement of a 2 mm2 area of corneal epithelium. Data are means ± SD, n = 5.
4. Conclusion
Unmodified liposomes in the present study persisted on the cornea longer than R6G solution, which is consistent with previous studies [43]. Modification with ConA greatly prolonged liposomal persistence on the corneal surface and the resulting duration of corneal anesthesia. The longer period of intense analgesia (block0.5 and block2) afforded by the ConA-modified liposomes, a median of 5 hours, could be of practical value in prolonging the time frame for performing surgery under topical anesthesia. The longer timeframe for lesser degrees of analgesia (block6), a median of 9 hours, could be of benefit in postoperative care or in the management of some painful non-surgical conditions. Moreover, the modified liposomes loaded with local anesthetics did not have any effect on corneal wound healing, suggesting adequate biocompatibility. This approach to topical corneal drug delivery could be applied to other situations where drugs have to be delivered frequently to the eye [44, 45].
5. Acknowledgement
This work was supported by NIH Grant GM116920 (to D.S.K).
Reference
- [1].Becker DE, Reed KL. Essentials of local anesthetic pharmacology. Anesthesia progress. 2006;53:98–108; quiz 9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Grant RL, Acosta D. Comparative toxicity of tetracaine, proparacaine and cocaine evaluated with primary cultures of rabbit corneal epithelial cells. Experimental eye research. 1994;58:469–78. [DOI] [PubMed] [Google Scholar]
- [3].Ting JY, Barns KJ, Holmes JL. Management of Ocular Trauma in Emergency (MOTE) Trial: A pilot randomized double-blinded trial comparing topical amethocaine with saline in the outpatient management of corneal trauma. Journal of emergencies, trauma, and shock. 2009;2:10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schwartz DM, Duncan KG, Duncan JL. Experimental use of tetrodotoxin for corneal pain after excimer laser keratectomy. Cornea. 1998;17:196–9. [DOI] [PubMed] [Google Scholar]
- [5].Nam SM, Lee HK, Kim EK, Seo KY. Comparison of corneal thickness after the instillation of topical anesthetics: proparacaine versus oxybuprocaine. Cornea. 2006;25:51–4. [DOI] [PubMed] [Google Scholar]
- [6].Nomura K, Singer DE, Aquavella JV. Corneal sensation after topical anesthesia. Cornea. 2001;20:191–3. [DOI] [PubMed] [Google Scholar]
- [7].Schwartz DM, Fields HL, Duncan KG, Duncan JL, Jones MR. Experimental study of tetrodotoxin, a long-acting topical anesthetic. American journal of ophthalmology. 1998;125:481–7. [DOI] [PubMed] [Google Scholar]
- [8].McAlvin JB, Zhan C, Dohlman JC, Kolovou PE, Salvador-Culla B, Kohane DS. Corneal Anesthesia With Site 1 Sodium Channel Blockers and Dexmedetomidine. Investigative ophthalmology & visual science. 2015;56:3820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Catterall WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. [DOI] [PubMed] [Google Scholar]
- [10].Terlau H, Heinemann SH, Stuhmer W, Pusch M, Conti F, Imoto K, et al. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett. 1991;293:93–6. [DOI] [PubMed] [Google Scholar]
- [11].Schwarz JR, Ulbricht W, Wagner HH. The rate of action of tetrodotoxin on myelinated nerve fibres of Xenopus laevis and Rana esculenta. The Journal of physiology. 1973;233:167–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang GK. Binding affinity and stereoselectivity of local anesthetics in single batrachotoxin-activated Na+ channels. The Journal of general physiology. 1990;96:1105–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Padera RF, Tse JY, Bellas E, Kohane DS. Tetrodotoxin for prolonged local anesthesia with minimal myotoxicity. Muscle Nerve. 2006;34:747–53. [DOI] [PubMed] [Google Scholar]
- [14].Epstein-Barash H, Shichor I, Kwon AH, Hall S, Lawlor MW, Langer R, et al. Prolonged duration local anesthesia with minimal toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lu W, Zhan C, Hou H. Targeted Liposomes and Micelles as Carriers for Cancer Therapy In: Alonso MJ, Garcia-Fuentes M, editors. Nano-Oncologicals: Springer International Publishing; 2014. p. 95–122. [Google Scholar]
- [16].Mallick S, Choi JS. Liposomes: versatile and biocompatible nanovesicles for efficient biomolecules delivery. Journal of nanoscience and nanotechnology. 2014;14:755–65. [DOI] [PubMed] [Google Scholar]
- [17].Feng L, Cheng L, Dong Z, Tao D, Barnhart TE, Cai W, et al. Theranostic Liposomes with Hypoxia-Activated Prodrug to Effectively Destruct Hypoxic Tumors Post-Photodynamic Therapy. ACS nano. 2017;11:927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Morand K, Bartoletti AC, Bochot A, Barratt G, Brandely ML, Chast F. Liposomal amphotericin B eye drops to treat fungal keratitis: physico-chemical and formulation stability. International journal of pharmaceutics. 2007;344:150–3. [DOI] [PubMed] [Google Scholar]
- [19].Natarajan JV, Chattopadhyay S, Ang M, Darwitan A, Foo S, Zhen M, et al. Sustained release of an anti-glaucoma drug: demonstration of efficacy of a liposomal formulation in the rabbit eye. PloS one. 2011;6:e24513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bourlais CL, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems--recent advances. Progress in retinal and eye research. 1998;17:33–58. [DOI] [PubMed] [Google Scholar]
- [21].Monem AS, Ali FM, Ismail MW. Prolonged effect of liposomes encapsulating pilocarpine HCl in normal and glaucomatous rabbits. International journal of pharmaceutics. 2000;198:29–38. [DOI] [PubMed] [Google Scholar]
- [22].Zhan C, Wang W, Santamaria C, Wang B, Rwei A, Timko BP, et al. Ultrasensitive Phototriggered Local Anesthesia. Nano letters. 2017;17:660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rwei AY, Zhan C, Wang B, Kohane DS. Multiply repeatable and adjustable on-demand phototriggered local anesthesia. Journal of controlled release : official journal of the Controlled Release Society. 2017;251:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhan C, Wang W, McAlvin JB, Guo S, Timko BP, Santamaria C, et al. Phototriggered Local Anesthesia. Nano letters. 2016;16:177–81. [DOI] [PubMed] [Google Scholar]
- [25].Panjwani N, Moulton P, Alroy J, Baum J. Localization of lectin binding sites in human, cat, and rabbit corneas. Investigative ophthalmology & visual science. 1986;27:1280–4. [PubMed] [Google Scholar]
- [26].Gipson IK, Anderson RA. Effect of lectins on migration of the corneal epithelium. Investigative ophthalmology & visual science. 1980;19:341–9. [PubMed] [Google Scholar]
- [27].Gipson IK, Riddle CV, Kiorpes TC, Spurr SJ. Lectin Binding to Cell-Surfaces - Comparisons between Normal and Migrating Corneal Epithelium. Dev Biol. 1983;96:337–45. [DOI] [PubMed] [Google Scholar]
- [28].Gunther GR, Wang JL, Yahara I, Cunningham BA, Edelman GM. Concanavalin A derivatives with altered biological activities. Proceedings of the National Academy of Sciences of the United States of America. 1973;70:1012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jaison PL, Cao Z, Panjwani N. Binding of Acanthamoeba to 23 mannose-glycoproteins of corneal epithelium: effect of injury. Current Eye Research. 1998;17:770–6. [PubMed] [Google Scholar]
- [30].Lu W, Zhan C, Hou H. Targeted Liposomes and Micelles as Carriers for Cancer Therapy In: Alonso MJ, Garcia-Fuentes M, editors. Nano-Oncologicals: New Targeting and Delivery Approaches. Cham: Springer International Publishing; 2014. p. 95–122. [Google Scholar]
- [31].Bartlett GR. Phosphorus assay in column chromatography. The Journal of biological chemistry. 1959;234:466–8. [PubMed] [Google Scholar]
- [32].Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- [33].Wang L, Shankarappa SA, Tong R, Ciolino JB, Tsui JH, Chiang HH, et al. Topical drug formulations for prolonged corneal anesthesia. Cornea. 2013;32:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Salamone JD, Cousins MS, Maio C, Champion M, Turski T, Kovach J. Different behavioral effects of haloperidol, clozapine and thioridazine in a concurrent lever pressing and feeding procedure. Psychopharmacology. 1996;125:105–12. [DOI] [PubMed] [Google Scholar]
- [35].Shimanouchi T, Ishii H, Yoshimoto N, Umakoshi H, Kuboi R. Calcein permeation across phosphatidylcholine bilayer membrane: effects of membrane fluidity, liposome size, and immobilization. Colloids and surfaces B, Biointerfaces. 2009;73:156–60. [DOI] [PubMed] [Google Scholar]
- [36].Kohane DS, Lipp M, Kinney RC, Lotan N, Langer R. Sciatic nerve blockade with lipid-protein-sugar particles containing bupivacaine. Pharmaceutical research. 2000;17:1243–9. [DOI] [PubMed] [Google Scholar]
- [37].Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Science. 1995;4:2411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu JC, Steinemann TL, McDonald MB, Thompson HW, Beuerman RW. Topical bupivacaine and proparacaine: a comparison of toxicity, onset of action, and duration of action. Cornea. 1993;12:228–32. [DOI] [PubMed] [Google Scholar]
- [39].Fitzgerald P, Hadgraft J, Kreuter J, Wilson CG. A γ-scintigraphic evaluation of microparticulate ophthalmic delivery systems: liposomes and nanoparticles. International journal of pharmaceutics. 1987;40:81–4. [Google Scholar]
- [40].Pleyer U, Lutz S, Jusko WJ, Nguyen KD, Narawane M, Ruckert D, et al. Ocular absorption of topically applied FK506 from liposomal and oil formulations in the rabbit eye. Investigative ophthalmology & visual science. 1993;34:2737–42. [PubMed] [Google Scholar]
- [41].Milani JK, Pleyer U, Dukes A, Chou HJ, Lutz S, Ruckert D, et al. Prolongation of corneal allograft survival with liposome-encapsulated cyclosporine in the rat eye. Ophthalmology. 1993;100:890–6. [DOI] [PubMed] [Google Scholar]
- [42].McCalden TA, Levy M. Retention of topical liposomal formulations on the cornea. Experientia. 1990;46:713–5. [DOI] [PubMed] [Google Scholar]
- [43].Andonova VY. A New Direction in Ophthalmic Development: Nanoparticle Drug Delivery Systems. Current pharmaceutical design. 2016;22:6313–29. [DOI] [PubMed] [Google Scholar]
- [44].Matsuo T, Tsuchida Y, Morimoto N. Trehalose eye drops in the treatment of dry eye syndrome. Ophthalmology. 2002;109:2024–9. [DOI] [PubMed] [Google Scholar]
- [45].Cochereau I, Meddeb-Ouertani A, Khairallah M, Amraoui A, Zaghloul K, Pop M, et al. 3-day treatment with azithromycin 1.5% eye drops versus 7-day treatment with tobramycin 0.3% for purulent bacterial conjunctivitis: multicentre, randomised and controlled trial in adults and children. The British journal of ophthalmology. 2007;91:465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]