Abstract
We and others propose vimentin as a possible cellular target for the treatment of COVID-19. This innovative idea is so recent that it requires further attention and debate. The significant role played by vimentin in virus-induced infection however is well established: (1) vimentin has been reported as a co-receptor and/or attachment site for SARS-CoV; (2) vimentin is involved in viral replication in cells; (3) vimentin plays a fundamental role in both the viral infection and the consequent explosive immune-inflammatory response and (4) a lower vimentin expression is associated with the inhibition of epithelial to mesenchymal transition and fibrosis. Moreover, the absence of vimentin in mice makes them resistant to lung injury. Since vimentin has a twofold role in the disease, not only being involved in the viral infection but also in the associated life-threatening lung inflammation, the use of vimentin-targeted drugs may offer a synergistic advantage as compared with other treatments not targeting vimentin. Consequently, we speculate here that drugs which decrease the expression of vimentin can be used for the treatment of patients with COVID-19 and advise that several Food and Drug Administration-approved drugs be immediately tested in clinical trials against SARS-CoV-2, thus broadening therapeutic options for this type of viral infection.
Keywords: pneumonia, viral infection, respiratory infection, ARDS
Introduction
The COVID-19 has triggered a global public health crisis with more than 10 million infected people up to date (30 June 2020) worldwide and a global incidence rate which is still growing.1 SARS-CoV-2 infection causes an explosive immune response, characterised by a cytokine storm, a subsequent progression to lung injury and an acute respiratory distress syndrome leading to death.2 3 Despite many promising therapeutic options for the treatment of COVID-19, anti-SARS-CoV-2 drugs or vaccines are still under investigation.4
Vimentin, known as a cytoskeletal protein belonging to the intermediate filament (IF) family, plays an important role in stabilising intracellular architecture through its mechanical role in cell plasticity and organelle anchoring, in the metabolism of lipids and in cell remodelling through its interaction with signalling molecules and components of gene regulatory networks.5–10 At cellular level, vimentin has been shown to be implicated in cell proliferation, stiffness, adhesion, migration, differentiation, senescence and apoptosis.7–10 Vimentin also plays a role in inflammation and immune responses, as well as in the epithelial to mesenchymal transition (EMT); the latter, in turn, being involved in the opening of epithelial barriers and cell migration.11 12
Strikingly, intracellular vimentin has been shown to be implicated in the processes of virus fusion, replication and assembly (for a review, see Ramos et al8). Vimentin was additionally found to be located outside of the cell, where it serves as an attachment site for viral proteins, in the majority of cases favouring viral binding/entry.13–16 One possible exception was reported for the human papillomavirus type 16 (HPV-16) infection, in which vimentin actually had the opposite effect.17 In this case, the authors reported that cell surface vimentin interfered with viral binding to its receptor, thus having a protective effect against viral infection. However, the role of vimentin for this specific virus is debated and contradictory reports have been published. As evidence to the contrary, Jacobs et al18 experimentally reduced the level of vimentin on the surface of cervical cancer cells by exposing the cells to the hookworm Nippostrongylus brasiliensis, with the result of decreasing the internalisation of the HPV-16 in these cells.18 One of the proposed mechanisms underlying this observation was the production of T helper-derived cytokines (eg, interleukin (IL)-4) associated with the helminth infection, which could, in turn, have modified the expression of HPV receptors in cancer cells.
During the submission period of the present manuscript in which we propose vimentin as a potential therapeutic target against viral infection, another two papers were written advocating the same hypothesis.8 9 Vimentin was also been previously proposed as a potential therapeutic target in cancer treatment and other disease conditions such as fibrosis. The upregulation of vimentin typically observed in oncogenic transformation and its involvement in cancer cell migration highlighted the possibility that vimentin is not only a marker but also a player in the development of cancer (reviewed in Refs 10 and 19). Therefore, molecules such as withaferin A, FiVe1 and simvastatin were used to perturb the expression and/or the assembly of vimentin filament and led to the downregulation of soluble vimentin, accompanied by decreased fibrosis, the disruption of mitotic cells, filament disorganisation and cell death.20–22
This paper summarises the scientific evidence indicating that reducing the expression of vimentin could be a powerful therapeutic option for COVID-19, since it could hamper SARS-CoV-2 infection and, at the same time, decrease the immunological response that is ultimately responsible for the often lethal acute respiratory distress syndrome. This hypothesis can be readily tested through clinical trials since Food and Drug Administration (FDA)-approved pharmacological treatments targeting vimentin for other pathologies are already available.
Vimentin is a co-receptor for SARS-CoV and possibly for SARS-CoV-2
ACE 2 (ACE2) is a cellular receptor for the SARS-CoV spike protein.23 However, it has been shown that ACE2 is not sufficient to make host cells susceptible to infection24–26 and vimentin has been proposed as a co-receptor for the entry of SARS-CoV into cells.13 Indeed, the authors reported that the SARS-CoV virus enhances cell surface vimentin expression and, importantly, showed the existence of a direct interaction between vimentin and the SARS-CoV spike protein during viral entry.13 Moreover, SARS-CoV and SARS-CoV-2 share similar spike protein sequences and the same cell entry route.27 Interestingly, it has been recently reported that two proteins that are well known to be involved in the entry of SARS-CoV-2 into the cell—ACE2 and TMPRSS2—are both expressed by two cell types, that is, lung type II pneumocytes and nasal goblet secretory cells28; it is worth noting these two cell types also express high levels of vimentin.28–30 In addition, a potential interaction between vimentin and the SARS-CoV-2 spike protein is revealed in a SARS-CoV-2 protein–protein interaction map (see supplemental data).31
Vimentin starts being expressed in the early stages of embryonic development by highly plastic precursor cells, whereas in postnatal life it is found in fibroblasts, endothelial cells, smooth muscle cells and in the lining epithelial cells of the lung, gut and other mucosae.29 30 32 33 Here vimentin expression is quickly increased in response to viral infection and inflammatory stimuli34–36 since vimentin belongs to the ‘immediate early genes’ family, a group of genes which are quickly activated in the presence of infection and inflammation. In this condition, due to its translocation to the cell surface, vimentin works as a co-receptor or attachment site for several viruses, including the Japanese encephalitis virus,37 the porcine reproductive and respiratory syndrome virus,38 the enterovirus 71,39 cowpea mosaic virus,14 dengue virus16 as well as the coronavirus.13 Interestingly, an upsurge of vimentin expression accompanies, and favours, viral entry of the majority of viruses including SARS-CoV13 with the exception of HPV-16 in which viral entry is actually hampered by an increase in vimentin expression.17 Consistently, interfering with vimentin expression or treating cells with the neutralising ‘anti-vimentin’ antibodies attenuates some types of viral infection.8 9 18 37 38 However, it is unknown if vimentin is involved in the infection of the Middle East respiratory syndrome coronavirus (MERS-CoV) which uses dipeptidyl peptidase 4 as the host cell receptor.40
Vimentin is involved in different steps of viral replication or assembly since it colocalises with viral capsid proteins in the endoplasmic reticulum of the infected cells,41 cooperating to produce virus particles.42 It is reported that vimentin is implicated—through the control of lysosomal trafficking and reduced translocation of viral ribonucleoproteins—in the progression of the influenza A virus, acting as a restriction factor for viral replication.43 44 In addition, vimentin influences the hepatitis C virus (HCV) and Theiler’s virus replication through its interaction with virus core proteins.45 46 A strong reduction of HIV-1 replication was reported in vimentin knockdown cells, showing that vimentin is necessary for viral replication.47 Vimentin contributes to EMT, a process by which epithelial cells, including those lining the lung mucosa, lose their polarity and adhesion. EMT thus confers migratory and invasive properties to the cells in a variety of pathological conditions such as viral infections, angiogenesis, chronic autoimmune inflammatory diseases, inflammatory bowel diseases, chronic obstructive pulmonary disease, cystic fibrosis and cancer. Overall, EMT promotes the creation of a suitable environment for viral infection and inflammation.
Vimentin expression is regulated by a plethora of transcription factors such as NF-κB (nuclear factor-kappa B), Stat3 (signal transducer and activator of transcription 3), ZBP-89 (Kruppel-type zinc-finger family transcription factor), Smads (small mothers against decapentaplegic transcription factors), AP-1 (activator protein 1), Sp1 (proximal specificity protein 1) and PEA3 (polyoma enhancer activator 3)—see figure 1. In response to viral infection or other insults, vimentin expression quickly increases. There are other transcription factors, such as Snail, Twist, ZEB2 and Slug,12 48–52 that regulate vimentin expression during EMT so that the overexpression of vimentin and N-cadherin in epithelial cells leads to the loss of the epithelial phenotype.
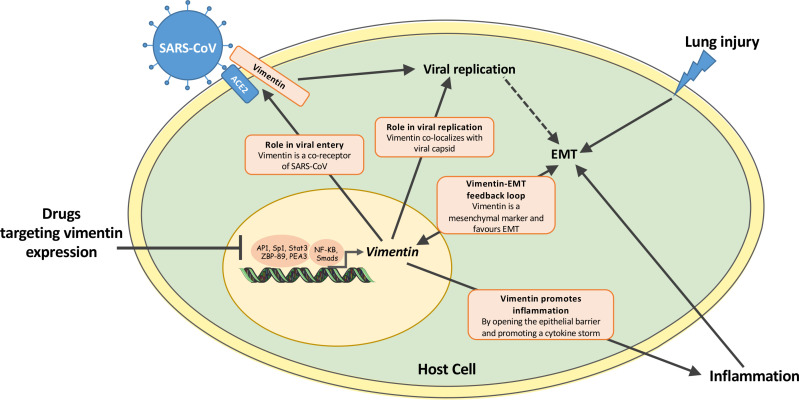
Figure 1.
Schematic representation of the hypothetical role of vimentin in the crucial steps of viral infection and in the inflammation leading to lung injury. Drugs inhibiting vimentin expression in the nucleus of infected cells offer the advantage of a synergic effect as compared with other treatments not targeting vimentin. This is due to the fact that the vimentin protein is involved in: (1) viral entry13–16; (2) viral replication8; (3) endothelial to mesenchymal transition (EMT)12 and (4) inflammation.11 56–60 Therefore, decreasing the expression of vimentin would contribute to hampering viral infection and, in parallel, reducing the inflammatory response ultimately leading to COVID-19 lung injury, acute respiratory distress syndrome and fatalities. Worth noting, several transcription factors (including NF-kB, AP1 and Sp1) are implicated in the regulation of vimentin expression.34 48–52 The figure includes material from SMART Servier Medical Art (https://smart.servier.com/) under a Creative Commons (CC) license 3.0.AP1, activator protein 1; NF-κB, nuclear factor-kappa B; PEA3, polyoma enhancer activator 3; Smads, small mothers against decapentaplegic transcription factors; Sp1, proximal specificity protein 1; Stat3, signal transducer and activator of transcription 3; ZBP-89, Kruppel-type zinc-finger family transcription factor
In addition, vimentin expression is also regulated epigenetically through the chromatin modifications of its promoter.53 These modifications have been studied in cancer cells since they promote EMT—a crucial event in the formation of tumour metastases. In particular, the methylation level of the vimentin promoter inversely correlates with the expression of vimentin in gastric cancer.54 Finally, vimentin expression is also regulated by post-transcriptional mechanisms including that of non-coding microRNAs.12
Vimentin actively participates in inflammation and immune response
Targeting vimentin could have additional effects against the progression of COVID-19 by directly modulating the inflammatory response of patients with COVID-19. Indeed, a subgroup of these patients suffer from a cytokine storm syndrome, that is, an excessive cytokine production sustained by a positive feedback loop producing an inflammatory response, often leading to respiratory failure from acute respiratory distress syndrome.2 3
The NLRP3 inflammasome (nucleotide-binding and oligomerisation domain-like receptor protein 3 inflammasome) is an intracellular multiprotein complex that triggers the immune response of the host against pathogens including viruses and is implicated in acute lung injury.55 It has been demonstrated that vimentin is a key regulator of the NLRP3 inflammasome by direct interaction.11 Important events in lung inflammation and the onset of injury are the increase in IL-1β levels, the permeability of the endothelial–alveolar epithelium barrier and irreversible fibrosis. The relevance of vimentin in these responses was demonstrated in animal models in which the inflammatory response of vimentin-knockout mice to lipopolysaccharide (LPS), bleomycin or asbestos treatment was considerably lower, entailing a less significant lung injury.11 Leucocyte adhesion to vascular endothelium and platelets is an early step in acute inflammatory response and attenuation of this adhesion may be beneficial in acute lung injury. By increasing circulating IL-1β—the major proinflammatory cytokine— vimentin is responsible for activating and recruiting inflammatory cells. In this context, it was shown that the vimentin IFs of both endothelial cells and lymphocytes form an anchoring structure between these two cell types.56 Further evidence of the importance of vimentin for leucocyte extravasation comes from the report that the transendothelial migration of blood T and B lymphocytes is markedly reduced in vimentin-deficient lymphocytes and endothelial cells.56 On the other hand, the presence of vimentin on the platelet and endothelial cell surface also serves as an adhesive receptor for the von Willebrand factor (VWF) and causes the binding of platelets to the subendothelial collagen and the subsequent intravascular generation of thrombin.57 58 The latter being responsible for microthrombus formation and contributing to a non-haemostatic effect, that is, the inflammatory response.
The inhibition of the vimentin–VWF interaction, by anti-vimentin antibodies, is effective in interfering with the VWF-mediated platelet adhesion to different matrices.57 58 The administration of exogenous recombinant human vimentin, binding specifically to P-selectin, stops leucocyte adhesion to platelets and endothelium by blocking P-selectin interaction with P-selectin glycoprotein ligand, ultimately decreasing endotoxin-induced acute lung injury.59 The oxidised form of membrane-bound vimentin is a marker of senescent cells,60 which, as such, produce a plethora of potentially harmful proinflammatory cytokines, chemokines and growth factors. Virus incubation times in senescent cells is longer, as observed in the case of HPV-1661 and studying coronavirus infection in aged cells has been proposed, since this important issue for this virus is still unexplored.9
As shown above, vimentin is expressed by many cell types, it has multiple cellular localisations, it plays multiple roles in cell behaviour by interacting with a plethora of molecular partners. The complexity of the functions exercised by vimentin, especially as concerns viral infection, is exemplified by contradictory reports in the literature showing opposite roles for vimentin, depending on the type of virus and the specific partners involved in its direct or indirect interactions. In the following paragraph and in table 1, we report and comment on a list of vimentin-targeting drugs, which are globally known to downregulate vimentin expression, since we think that such a downregulation would be beneficial. However, we decided to provide only a few significant case by case comments. Each single drug, depending on the type of virus and host cell type concerned, should be further investigated.
Table 1.
Compounds downregulating vimentin expression
| Name of molecule | Type | Effects | Modes of action | Models | Reference |
| 17-DMCHAG | Geldanamycin derivative | Antimigratory; antiproliferative |
Inhibits HSP90; induces the proteasome-dependent degradation of androgen receptor | Human prostate cancer cells; tumour-bearing mice | 79 |
| Alpha-lipoic acid* | Acid; antioxidant |
Inhibitor of EMT; antiproliferative |
Inhibits TGF-ß; activates AMPK and downregulates mTOR-S6 signalling | Human thyroid cancer cells; tumour-bearing mice | 80 |
| Apigenin*† | Flavonoid derivative | Antiapoptosis; pro-proliferative; antifibrotic |
Decreases miR-34a | Mouse mesothelial peritoneal cells | 81 |
| Berberine* | Alkaloid derivative | Inhibitor of EMT; antiproliferative |
Decreases PI3/AKT, Ras-Raf-ERK and TGF-ß1 signalling | Mouse neuroblastoma cells | 82 |
| Bergamottin | Furanocoumarin derivative | Inhibitor of EMT; antimigratory |
Decreases PI3K/AKT/mTOR, TGF-ß signalling | Human lung adenocarcinoma cells | 83 |
| Beta-asarone | Phenylpropanoid derivative | Inhibitor of EMT; antimigratory |
Decreases hnRNP A2/B1, MMP-9, p-STAT3 expression | Human glioma cells | 84 |
| Beta-lapachone | Quinone derivative | Inhibitor of EMT; antimigratory; proapoptosis |
Induces apoptosis through caspase-3, -8 and -9 activation and poly(ADP-ribose) cleavage; decreases MMP-2 and MMP-9 | Mouse colon cancer cells; tumour-bearing mice | 85 |
| BHX | Pyrazoline derivative | Inhibitor of EMT; antimigratory; proapoptosis |
Inhibits Wnt/β-catenin signalling | Human breast cancer cells; tumour-bearing mice | 86 |
| BMS345541 | Selective I kappa B kinase inhibitor | Inhibitor of EMT; anti-inflammatory |
Inhibits NF–κB/RelA pathway | Human epithelial cells; polyinosinic–polycytidylic acid-treated mice | 87 |
| Chrysin* | Flavonoid derivative | Inhibitor of EMT; antimigratory |
Reduces MMP-2 activity; induces zona occludens protein-1 and occludin expression | Human renal epithelial cells; db/db mice | 88 |
| Chrysotobibenzyl | Inhibitor of EMT; antimigratory |
Decreases integrins (β1, β3, αν), p-FAK, p-AKT, caveolin-1 expression | Human lung cancer cells | 89 | |
| Cisplatin | Anticancer | Inhibitor of EMT; antimigratory; antiproliferative |
Reduces YAP activity | Human colon cancer cells; tumour-bearing mice | 90 |
| Compound 11 | Selective class I HDAC inhibitor | Inhibitor of EMT; antiproliferative |
Decreases p-AKT, p-ERK activity | Human colorectal cancer cells; tumour-bearing mice | 91 |
| Curcumin* | Polyphenolic compound | Inhibitor of EMT; antimigratory; antiproliferative |
Decreases TGF-ß/Smad2/3 signalling | Human thyroid cancer cells | 92 |
| Cypripedin | Phenanthrenequinone derivative | Inhibitor of EMT; antimigratory |
Decreases AKT/GSK-3β signalling | Human lung cancer cells | 93 |
| D-4F | Apolipoprotein A-I mimetic | Inhibitor of EMT; antifibrotic |
Inhibits TGF-ß1 | Human alveolar epithelial cells | 94 |
| Dehydroepiandrosterone | Steroid | Inhibitor of EMT; antimigratory |
Decreases N-cadherin and Snail expression | Human breast cancer cells; tumour-bearing mice | 95 |
| Dictamnine | Alkaloid | Inhibitor of EMT; antiproliferative; proapoptosis |
Decreases mTOR/p70S6K/eIF4E and MAPK signalling | Human lung cancer cells | 96 |
| Ginsenoside Rg3*† | Steroidal saponin | Inhibitor of EMT; antimigratory; proapoptosis |
Inhibits LncRNA colon cancer-associated transcript 1 expression and PI3K/AKT signalling; inhibits MAPK and NF–κB signalling | Human colorectal cancer cells; lung cancer cells; tumour-bearing mice | 97 98 |
| Hydrogen sulfide*† | – | Inhibitor of EndMT; decrease ER stress; vasodilator; cardioprotective | Decreases Smad2 and Src signalling | Human umbilical vein endothelial cells | 99 |
| Hydroxygenkwanin | Flavonoid derivative | Antimigratory; antiproliferative |
Activates p21 signalling | Human oral squamous cell carcinoma | 44 |
| Icariin*† | Flavonoid derivative | Antimigratory; antiproliferative; proapoptosis |
Inhibits PI3K, AKT and MEK/ERK signalling; decreases miR-625-3p and MMP-9 expression | Human thyroid cancer cells | 100 |
| Icariside II | Flavonol glycoside | Inhibitor of EMT; antimigratory |
Inhibits NF-κB and AKT/GSK-3ß signalling | Human lung cancer cells; tumour-bearing mice | 101 |
| Inositol*† | Lipid | Inhibitor of EMT; antimigratory |
Inhibits PI3K/AKT and NF-κB signalling | Human breast cancer cells | 102 |
| Melatonin*† | Hormone | Inhibitor of EMT; antimigratory; antiproliferative |
Inhibits PI3K/AKT signalling; decreases MMP-2, MMP-9, NF-κB p65 expression |
Human ovarian cancer cells; gastric cancer cells; tumour-bearing mice |
103 104 |
| Metapristone* | RU486 derivative | Antimigratory; antiproliferative; proapoptosis |
Decreases AKT and ERK phosphorylation and Bcl-2; upregulates total p53 and Bax expression | Mouse skin melanoma cells | 105 |
| Metformin*† | Biguanide derivative; antidiabetic | Inhibitor of EMT; antimigratory |
Downregulates HIF-1alpha, CAIX, miR-34a, SNAIL1 and ZEB1; upregulates miR-200a, miR-200c and miR-429 | Human cervical squamous cancer cells; human colorectal cancer cell | 106 107 |
| Moscatilin | Bibenzyl derivative | Inhibitor of EMT; proapoptosis |
Decreases ERK and AKT activity; downregulates caveolin-1 level | Human lung cancer cells | 108 |
| N-acetylcysteine* | Antioxidant | Reduce fibrosis; anti-inflammatory |
Enhances antioxidant enzyme activities; downregulates oxidising enzymes’ expression | Crystalline silica-induced pulmonary fibrosis mice model | 109 |
| Niclosamide*† | Antihelminthic | Inhibitor of EMT; antimigratory |
Inhibits Wnt/β-catenin signalling | Human oral squamous cell carcinoma | 110 |
| Norcantharidin | Cantharidin derivative | Inhibitor of EMT; antimigratory |
Inhibits αvβ6-ERK-Ets1 signalling | Human colon cancer cells | 111 |
| Osthole* | Coumarin derivative | Inhibitor of EMT; antimigratory; antiproliferative; proapoptosis |
Decreases MMP-2, MMP-9, Smad-3, Snail-1, Twist-1 expression | Human renal cell carcinoma | 112 |
| PAC* | Curcumin analogue | Inhibitor of EMT; antimigratory; antiproliferative |
Inhibits JAK2/STAT3, AKT/mTOR and MEK/ERK signalling; inhibits STAT3/cyclin D1 pathway | Human colorectal cancer cells; tumour-bearing mice | 113 |
| Palbociclib | Selective CDK4/6 inhibitor | Inhibitor of EMT; antimigratory |
Inhibits c-Jun/COX-2 signalling | Human breast cancer cells; tumour-bearing mice | 114 |
| Pargyline | Lysine-specific demethylase 1 inhibitor | Inhibitor of EMT; antimigratory |
Reduces prostate-specific antigen | Human prostate cancer cells; tumour-bearing mice | 115 |
| Physcion | Anthraquinone derivative | Inhibitor of EMT; antimigratory |
Activates ROS/AMPK/GSK3β signalling; inhibits SOX2 | Human colorectal cancer cells | 116 |
| Pterostilbene*† | Antioxidant | Inhibitor of EMT; antimigratory |
Reduces Src/Fak signalling; upregulates miR-205 expression | Human breast cancer cells; tumour-bearing mice | 117 |
| Salidroside* | Glucopyranoside derivative | Inhibitor of EMT; antimigratory; antiproliferative |
Downregulates miR-891b expression; inhibits PI3K/AKT/mTOR and NF-κB signalling | Wilms' tumour cells; mouse prostate cancer | 118 |
| Selenium*† | Metal | Inhibitor of EMT | Downregulates genes involved in cellular migration, inflammation and mesenchymal markers; upregulates genes involved in epithelial markers | Human prostate | 119 |
| Simvastatin*† | Statin | Inhibitor of EMT; antifibrotic |
Decreases Toll-like receptor 4 and NF-κB signalling | Human biliary epithelial cells | 120 |
| Sinomenine*† | Alkaloid | Antimigratory; antiproliferative |
Downregulates miR-23a expression; inhibits PI3K/AKT and JAK/STAT signalling | Human prostate cancer cells | 121 |
| Swainsonine | Indolizidine alkaloid | Antimigratory; antiproliferative; proapoptosis |
Downregulates miR-92a, inhibits PI3K/AKT/mTOR signalling | Human glioblastoma cells | 122 |
| Tanshinone IIA*† | Lipophilic compound | Inhibitor of EMT; antimigratory; antiproliferative |
Inhibits STAT3-CCL2 signalling | Human bladder cancer cells | 123 |
| Tetramethylpyrazine nitrone | Tetramethylpyrazine derivative | Reduces brain infarction; preserves neurological function | Decrease expression of neuroinflammatory markers | Cynomolgus macaques brain ischaemic stroke model | 124 |
| Tetrandrine | Alkaloid | Inhibitor of EMT; antimigratory |
Reduces glioma-associated oncogene family zinc finger 1 (Gli-1) expression | Human bladder cancer cells | 125 |
| Toosendanin | Alkaloid | Inhibitor of EMT; antimigratory; antiproliferative |
Inhibits AKT/mTOR signalling | Human pancreatic cancer cells; tumour-bearing mice | 126 |
| Triptolide*† | Diterpenoid derivative | Antifibrotic | Inhibits TLR4-induced NF-κB/IL-1β immune pathway; inhibits NF-κB/TNF-α/VCAM-1 inflammatory pathway; downregulates TGF-β1/α-SMA/vimentin fibrosis pathway | Diabetic rats | 127 |
| Valproic acid*† | Acid | Inhibitor of EMT; antiproliferative |
Downregulates Smad4 expression; upregulates transcriptional intermediary factor-1γ (TIF1γ) expression | Human prostate carcinoma cells; tumour-bearing mice | 128 129 |
| Wogonin* | Flavonoid derivative | Inhibitor of EMT; antimigratory |
Inhibits IL-6/STAT3 signalling pathway | Human alveolar adenocarcinoma cells | 130 |
A selection of compounds downregulating vimentin expression and their major cellular and molecular effects. Some of these molecules already have FDA approval, thus can be immediately used to set up clinical trials against COVID-19.
*Indicates the compounds have been referenced by the FDA; the latter group of drugs is briefly discussed in the text.
†Indicates the compounds that have been reported to play a role in acute lung injury.
AMPK, AMP-activated protein kinase; ANG, Angiotensin; Bax, Bcl-2–associated X; Bcl-2, B-cell lymphoma 2; CCL2, Chemokine ligand 2; 17-DMCHAG, 17-(6-(3,4-dimethoxycinnamamido)hexylamino)-17-demethoxy-geldanamycin; eIF4E, Eukaryotic translation initiation factor 4E; EMT, epithelial to mesenchymal transition; EndMT, endothelial to mesenchymal transition; ER, endoplasmic reticulum; ERK, Extracellular signal-regulated kinase; FAK, Focal adhesion kinase; FDA, Food and Drug Administration; GSK-3, Glycogen synthase kinase-3; HDAC, Histone deacetylase; HIF, Hypoxia Inducible Factor; hRNP, Heterogeneous Nuclear Ribonucleoprotein; HSP, Heat Shock Protien; IL, Interleukin; JAK2, Janus kinase 2; LncRNA, Long noncoding RNAs; MAPK, Mitogen-activated protein kinase; MMP, Matrix metallopeptidase; mTOR, Mechanistic target of rapamycin; NF-κB, nuclear factor-kappa B; PEA3, polyoma enhancer activator 3; PI3K, Phosphatidylinositol 3-kinase; ROS, Reactive oxygen species; SMA, Smooth muscle actin; Smad, small mothers against decapentaplegic transcription factor; STAT3, Signal transducer and activator of transcription 3; TGF, Transforming growth factor; TLR4, Toll-like receptor 4; TNF, Tumor necrosis factor; VCAM, Vascular cell adhesion molecule; YAP, Yes-associated protein.
Drugs targeting vimentin could combat coronavirus-related effects
Based on the fact that vimentin is important for viral infection and the associated inflammatory response, we have searched the literature for compounds targeting vimentin so as to select drugs that may treat COVID-19. Some of these drugs are as common and as easily available as melatonin, which makes our hypothesis immediately verifiable through clinical trials on COVID-19 specifically.
Some of the 49 compounds that we have identified (table 1) are completely new molecules that have recently started to be studied, others have never been employed in clinical practice although they are referenced by the FDA, while others are already being tested in clinical trials. However, all these molecules in table 1 affect vimentin levels, as demonstrated by western blot and/or real-time PCR analysis. Several of these compounds have been identified and characterised in in vitro experiments only, while others have been validated in vivo against cell growth and dispersion in cancer, and for their capacity to inhibit EMT which, as mentioned, amounts to fighting inflammation.
Interestingly, a significant fraction (16 out of 49) of the drugs listed in table 1 have been reported to play a role in acute lung injury and are briefly discussed below for this reason. Melatonin markedly reduces pulmonary injury and decreases the infiltration of macrophages and neutrophils into the lungs of LPS-treated mice, by inhibiting the NLRP3 inflammasome, in a model of acute lung injury.62 Worth noting, it has already been speculated that melatonin would be beneficial in patients with COVID-19.63 Niclosamide, a cheap antihelminthic drug, is effective against several viruses such as SARS-CoV, MERS-CoV, Zika virus, HCV and human adenovirus.64 In particular, niclosamide has been shown to be an inhibitor of SARS-CoV 3CL protease, involved in viral replication.64 Endogenous hydrogen sulfide participates in the regulation of important biological processes in the respiratory tract, such as airway tone, pulmonary circulation, cell proliferation and apoptosis, fibrosis, oxidative stress and inflammation.65 Since hydrogen sulfide exerts a broad-spectrum antiviral activity, as well as having an anti-inflammatory action, the controlled release of hydrogen sulfide from chemical donors has been proposed as a treatment in lung diseases.65 Simvastatin has protective vascular effects in the lungs since it improves the function of the endothelial barrier. The efficacy of simvastatin against LPS-induced lung injury involves the stabilisation of the cell cytoskeleton and adherent junctions.66 The ability of triptolide to inhibit the proinflammatory NF–κB signalling pathway makes it another promising therapeutic agent for acute lung injury.67 Sinomenine attenuates septic shock-dependent acute lung injury, probably thanks to the inhibition of inflammation and oxidative stress.68 Tanshinone IIA inhibits the production of proinflammatory factors in LPS-induced acute lung injury through the regulation of calcium in pulmonary interstitial macrophages.69 Ginsenoside Rg3 attenuates LPS-induced acute lung injury by decreasing the production of proinflammatory factors and increasing the synthesis of anti-inflammatory cytokines, through the activation of the PI3K/AKT/mTOR pathway downstream of the Mer receptor.70 Icariin reduces acute lung injury by enhancing the expression of the glucocorticoid receptor alpha in lung tissues and by inhibiting the expression of p65, c-Jun, Stat3, IL-6 and tumour necrosis factor-alpha.71 The therapeutic window of Valproic acid is narrower but interesting, since it is effective in a mouse model of Gram-negative bacteria-induced pneumonia.72 Apigenin C-glycosides inhibit acute inflammation and apoptosis by suppressing the activation of the TLR4/TRPC6 signalling pathway in a murine model of acute lung injury.73 Inositol derivatives present in surfactant preparations diminish the activation of key inflammatory pathways in lung diseases.74 Pterostilbene 4′-β-glucoside, the glycosylated form of the antioxidant pterostilbene, diminishes intracellular and mitochondrial reactive oxygen species production, thus reducing the inflammatory response to LPS.75 Osthole exerts beneficial effects on bleomycin-induced pulmonary fibrosis in rats by modulating the ACE2/ANG-(1–7) axis and by inhibiting lung inflammation.76 Selenium restores the antioxidant capacity of the lungs and reduces inflammatory responses, thus improving lung mechanics.77 All the previous examples concerned infection-induced lung injury, while lung injury may also be caused by the mechanical stress caused by forced ventilation. To specifically address this type of damage, Tsaknis et al78 set up an elegant experimental model, exercising high-pressure ventilation on ex vivo lung preparations thereby increasing their microvascular permeability, oedema and microhaemorrhages; in this model, pretreatment with metformin decreased the severity of the damage preserving alveolar capillary permeability. Additional FDA-approved drugs that have already been used in clinical trials, but have not been discussed here, are metapristone, N-acetylcysteine, chrysin, berberine, salidroside, dehydroepiandrosterone and cisplatin. It is worth noting that many of the compounds above have been tested against cancer and reported to inhibit cell growth and EMT, but will not be discussed in this article. High-throughput screening in cell culture systems using a reporter system driven by the vimentin promoter will identify additional novel drugs for their capacity to specifically decrease vimentin transcription. In addition, alternative therapeutic interventions based on the downregulation of vimentin can be designed, such as the administration of antisense oligonucleotides, anti-vimentin RNA, humanised neutralising antibodies or dominant-negative peptides capable of blocking the interaction between vimentin and viral proteins.
Conclusions
Vimentin functions as a co-receptor for SARS-CoV—and likely for SARS-CoV-2—thus contributing to viral infection. Evidence of the significant role played by vimentin in virus-induced infection comes from the following observations: vimentin expression increases during viral infection in several clinical settings, while the absence of vimentin in knockout mice makes them more resistant to inflammation and acute lung injury than wild-type mice. Therefore, vimentin can be a target for the treatment of COVID-19-related pneumonia. It is a matter of fact that drugs that have proven efficient against viral infection downregulate vimentin expression. While the correlative nature of this evidence does not yet prove a causative role for vimentin downregulation in diminishing viral infection, it indicates that establishing whether drugs which decrease vimentin expression can be used for the treatment of patients with COVID-19 is the point at issue. Since vimentin has a twofold role in the disease, not only being involved in the viral infection but also in the associated life-threatening lung inflammation, the use of vimentin-targeted drugs may offer a synergistic advantage as compared with other treatments not targeting vimentin. In particular: (1) the decreased amount of vimentin on the cell surface would decrease its interaction with SARS-CoV-2 spike protein, thus interfering with viral entry; (2) the decreased amount of vimentin within the cell would affect virus replication; (3) the lesser amount of vimentin within inflammatory cells would exert negative effects on the inflammasome. This in turn would avoid the cytokine storm and curb the arrival of infiltrating inflammatory cells; (4) lower vimentin expression in epithelial cells and fibroblasts would decrease EMT and fibrosis. Taken together, all these considerations strongly suggest that vimentin represents a valuable therapeutic target at the early stages of the SARS-CoV infection, may help avoid the progression to serious complications and indicate that several FDA-approved drugs (such as melatonin, niclosamide, selenium, hydrogen sulfide, inositol) should be tested in clinical trials against SARS-CoV-2.
Acknowledgments
The authors are grateful to Anna Luisa Mazzotti and Lydia Colin Mazzotti for the editing of the manuscript.
Footnotes
Contributors: ZL and OA developed the idea, wrote the first draft and are the guarantors of the article jointly. DC and OA expanded and fine-tuned the original version of the paper. DP and PL provided their expertise to give further depth to the review of the literature the manuscript is based on.
Funding: This research was partly supported by Sorbonne Université, INSERM, CNRS. ZL and OA are supported by the AFM-Téléthon (contract numbers: 21833 and 22142) and the Fédération Française de Cardiologie. DC is supported by Sapienza University Ateneo 2019 and the AFM-Téléthon (contract number: 20603).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article.
References
- 1.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265–9. 10.1038/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular Endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120–8. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangalmurti N, Hunter CA. Cytokine Storms: understanding COVID-19. Immunity 2020;53:19–25. 10.1016/j.immuni.2020.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020. 10.1001/jama.2020.12839. [Epub ahead of print: 10 Jul 2020]. [DOI] [PubMed] [Google Scholar]
- 5.Pekny M, Lane EB. Intermediate filaments and stress. Exp Cell Res 2007;313:2244–54. 10.1016/j.yexcr.2007.04.023 [DOI] [PubMed] [Google Scholar]
- 6.Herrmann H, Aebi U. Intermediate filaments: structure and assembly. Cold Spring Harb Perspect Biol 2016;8. 10.1101/cshperspect.a018242. [Epub ahead of print: 01 Nov 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klymkowsky MW. Filaments and phenotypes: cellular roles and orphan effects associated with mutations in cytoplasmic intermediate filament proteins. F1000Res 2019;8. 10.12688/f1000research.19950.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos I, Stamatakis K, Oeste CL, et al. Vimentin as a multifaceted player and potential therapeutic target in viral infections. Int J Mol Sci 2020;21. 10.3390/ijms21134675. [Epub ahead of print: 30 Jun 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapagain P. Potential role of cellular senescence on coronavirus infections 2020.
- 10.Danielsson F, Peterson MK, Caldeira Araújo H, et al. Vimentin diversity in health and disease. Cells 2018;7. 10.3390/cells7100147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.dos Santos G, Rogel MR, Baker MA, et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun 2015;6:6574. 10.1038/ncomms7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rout-Pitt N, Farrow N, Parsons D, et al. Epithelial mesenchymal transition (EMT): a universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir Res 2018;19:136. 10.1186/s12931-018-0834-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu YT-C, Chien S-C, Chen I-Y, et al. Surface vimentin is critical for the cell entry of SARS-CoV. J Biomed Sci 2016;23:14. 10.1186/s12929-016-0234-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koudelka KJ, Destito G, Plummer EM, et al. Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin. PLoS Pathog 2009;5:e1000417. 10.1371/journal.ppat.1000417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das S, Ravi V, Desai A. Japanese encephalitis virus interacts with vimentin to facilitate its entry into porcine kidney cell line. Virus Res 2011;160:404–8. 10.1016/j.virusres.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Zou L, Yang Y, et al. Superficial vimentin mediates DENV-2 infection of vascular endothelial cells. Sci Rep 2016;6:38372. 10.1038/srep38372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schäfer G, Graham LM, Lang DM, et al. Vimentin modulates infectious internalization of human papillomavirus 16 pseudovirions. J Virol 2017;91. 10.1128/JVI.00307-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs B-A, Chetty A, Horsnell WGC, et al. Hookworm exposure decreases human papillomavirus uptake and cervical cancer cell migration through systemic regulation of epithelial-mesenchymal transition marker expression. Sci Rep 2018;8:11547. 10.1038/s41598-018-30058-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strouhalova K, Přechová M, Gandalovičová A, et al. Vimentin intermediate filaments as potential target for cancer treatment. Cancers 2020;12. 10.3390/cancers12010184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bargagna-Mohan P, Deokule SP, Thompson K, et al. Withaferin a effectively targets soluble vimentin in the glaucoma filtration surgical model of fibrosis. PLoS One 2013;8:e63881. 10.1371/journal.pone.0063881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bollong MJ, Pietilä M, Pearson AD, et al. A vimentin binding small molecule leads to mitotic disruption in mesenchymal cancers. Proc Natl Acad Sci U S A 2017;114:E9903–12. 10.1073/pnas.1716009114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trogden KP, Battaglia RA, Kabiraj P, et al. An image-based small-molecule screen identifies vimentin as a pharmacologically relevant target of simvastatin in cancer cells. Faseb J 2018;32:2841–54. 10.1096/fj.201700663R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-Converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450–4. 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.To KF, Lo AWI. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J Pathol 2004;203:740–3. 10.1002/path.1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan PKS, To K-F, Lo AWI, et al. Persistent infection of SARS coronavirus in colonic cells in vitro. J Med Virol 2004;74:1–7. 10.1002/jmv.20138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol 2003;200:282–9. 10.1002/path.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–80. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020;181:1016–35. 10.1016/j.cell.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milara J, Peiró T, Serrano A, et al. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax 2013;68:410–20. 10.1136/thoraxjnl-2012-201761 [DOI] [PubMed] [Google Scholar]
- 30.Nishioka M, Venkatesan N, Dessalle K, et al. Fibroblast-epithelial cell interactions drive epithelial-mesenchymal transition differently in cells from normal and COPD patients. Respir Res 2015;16:72. 10.1186/s12931-015-0232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020;583:459–68. 10.1038/s41586-020-2286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cochard P, Paulin D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J Neurosci 1984;4:2080–94. 10.1523/JNEUROSCI.04-08-02080.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langlois B, Belozertseva E, Parlakian A, et al. Vimentin knockout results in increased expression of sub-endothelial basement membrane components and carotid stiffness in mice. Sci Rep 2017;7:11628. 10.1038/s41598-017-12024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lilienbaum A, Paulin D. Activation of the human vimentin gene by the Tax human T-cell leukemia virus. I. mechanisms of regulation by the NF-kappa B transcription factor. J Biol Chem 1993;268:2180–8. [PubMed] [Google Scholar]
- 35.Lilienbaum A, Duc Dodon M, Alexandre C, et al. Effect of human T-cell leukemia virus type I Tax protein on activation of the human vimentin gene. J Virol 1990;64:256–63. 10.1128/JVI.64.1.256-263.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozden S, Mouly V, Prevost M-C, et al. Muscle wasting induced by HTLV-1 TAX-1 protein: an in vitro and in vivo study. Am J Pathol 2005;167:1609–19. 10.1016/S0002-9440(10)61245-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang J-J, Yu C-Y, Liao C-L, et al. Vimentin binding is critical for infection by the virulent strain of Japanese encephalitis virus. Cell Microbiol 2011;13:1358–70. 10.1111/j.1462-5822.2011.01624.x [DOI] [PubMed] [Google Scholar]
- 38.Kim J-K, Fahad A-M, Shanmukhappa K, et al. Defining the cellular target(s) of porcine reproductive and respiratory syndrome virus blocking monoclonal antibody 7G10. J Virol 2006;80:689–96. 10.1128/JVI.80.2.689-696.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du N, Cong H, Tian H, et al. Cell surface vimentin is an attachment receptor for enterovirus 71. J Virol 2014;88:5816–33. 10.1128/JVI.03826-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu G, Hu Y, Wang Q, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 2013;500:227–31. 10.1038/nature12328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maruri-Avidal L, Weisberg AS, Moss B. Vaccinia virus L2 protein associates with the endoplasmic reticulum near the growing edge of crescent precursors of immature virions and stabilizes a subset of viral membrane proteins. J Virol 2011;85:12431–41. 10.1128/JVI.05573-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Risco C, Rodríguez JR, López-Iglesias C, et al. Endoplasmic reticulum-Golgi intermediate compartment membranes and vimentin filaments participate in vaccinia virus assembly. J Virol 2002;76:1839–55. 10.1128/JVI.76.4.1839-1855.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu W, Panté N. Vimentin plays a role in the release of the influenza a viral genome from endosomes. Virology 2016;497:41–52. 10.1016/j.virol.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 44.Huang Y-C, Lee P-C, Wang JJ, et al. Anticancer effect and mechanism of Hydroxygenkwanin in oral squamous cell carcinoma. Front Oncol 2019;9:911. 10.3389/fonc.2019.00911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nitahara-Kasahara Y, Fukasawa M, Shinkai-Ouchi F, et al. Cellular vimentin content regulates the protein level of hepatitis C virus core protein and the hepatitis C virus production in cultured cells. Virology 2009;383:319–27. 10.1016/j.virol.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 46.Nédellec P, Vicart P, Laurent-Winter C, et al. Interaction of Theiler's virus with intermediate filaments of infected cells. J Virol 1998;72:9553–60. 10.1128/JVI.72.12.9553-9560.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernández-Ortega C, Ramírez A, Casillas D, et al. Identification of vimentin as a potential therapeutic target against HIV infection. Viruses 2016;8. 10.3390/v8060098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen JH, Vercamer C, Li Z, et al. PEA3 transactivates vimentin promoter in mammary epithelial and tumor cells. Oncogene 1996;13:1667–75. [PubMed] [Google Scholar]
- 49.Paulin D, Lilienbaum A, Duprey P, et al. Regulatory elements of the human vimentin gene: activation during proliferation. Reprod Nutr Dev 1990;30:423–9. 10.1051/rnd:19900316 [DOI] [PubMed] [Google Scholar]
- 50.Salvetti A, Lilienbaum A, Li Z, et al. Identification of a negative element in the human vimentin promoter: modulation by the human T-cell leukemia virus type I Tax protein. Mol Cell Biol 1993;13:89–97. 10.1128/MCB.13.1.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Y, Diab I, Zhang X, et al. Stat3 enhances vimentin gene expression by binding to the antisilencer element and interacting with the repressor protein, ZBP-89. Oncogene 2004;23:168–78. 10.1038/sj.onc.1207003 [DOI] [PubMed] [Google Scholar]
- 52.Wu Y, Zhang X, Salmon M, et al. TGFbeta1 regulation of vimentin gene expression during differentiation of the C2C12 skeletal myogenic cell line requires Smads, AP-1 and Sp1 family members. Biochim Biophys Acta 2007;1773:427–39. 10.1016/j.bbamcr.2006.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer 2016;15:18. 10.1186/s12943-016-0502-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cong H, Yao R-Y, Sun Z-Q, et al. DNA hypermethylation of the vimentin gene inversely correlates with vimentin expression in intestinal- and diffuse-type gastric cancer. Oncol Lett 2016;11:842–8. 10.3892/ol.2015.3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas PG, Dash P, Aldridge JR, et al. NLRP3 (NALP3/CIAS1/Cryopyrin) mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 2009;30:566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nieminen M, Henttinen T, Merinen M, et al. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol 2006;8:156–62. 10.1038/ncb1355 [DOI] [PubMed] [Google Scholar]
- 57.Cruz MA, Vijayan KV. Vimentin exposed on platelets serves as an adhesive receptor for Von Willebrand factor. Blood 2012;120:259–60. 10.1182/blood.V120.21.259.25922596257 [DOI] [Google Scholar]
- 58.Da Q, Behymer M, Correa JI, et al. Platelet adhesion involves a novel interaction between vimentin and von Willebrand factor under high shear stress. Blood 2014;123:2715–21. 10.1182/blood-2013-10-530428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lam FW, Da Q, Guillory B, et al. Recombinant human vimentin binds to P-selectin and blocks neutrophil capture and rolling on platelets and endothelium. J Immunol 2018;200:ji1700784–26. 10.4049/jimmunol.1700784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frescas D, Roux CM, Aygun-Sunar S, et al. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc Natl Acad Sci U S A 2017;114:E1668–77. 10.1073/pnas.1614661114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broniarczyk J, Ring N, Massimi P, et al. HPV-16 virions can remain infectious for 2 weeks on senescent cells but require cell cycle re-activation to allow virus entry. Sci Rep 2018;8:811. 10.1038/s41598-017-18809-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Li X, Grailer JJ, et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res 2016;60:405–14. 10.1111/jpi.12322 [DOI] [PubMed] [Google Scholar]
- 63.Zhang R, Wang X, Ni L, et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci 2020;250:117583. 10.1016/j.lfs.2020.117583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu J, Shi P-Y, Li H, et al. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect Dis 2020;6:909–15. 10.1021/acsinfecdis.0c00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bazhanov N, Ansar M, Ivanciuc T, et al. Hydrogen sulfide: a novel player in airway development, pathophysiology of respiratory diseases, and antiviral defenses. Am J Respir Cell Mol Biol 2017;57:403–10. 10.1165/rcmb.2017-0114TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu Y, Jing L, Zhang X, et al. Simvastatin attenuates acute lung injury via regulating CDC42-PAK4 and endothelial microparticles. Shock 2017;47:378–84. 10.1097/SHK.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Zhang L, Duan W, et al. Anti-Inflammatory effects of triptolide by inhibiting the NF-κB signalling pathway in LPS-induced acute lung injury in a murine model. Mol Med Rep 2014;10:447–52. 10.3892/mmr.2014.2191 [DOI] [PubMed] [Google Scholar]
- 68.Wang W, Yang X, Chen Q, et al. Sinomenine attenuates septic-associated lung injury through the Nrf2-Keap1 and autophagy. J Pharm Pharmacol 2020;72:259–70. 10.1111/jphp.13202 [DOI] [PubMed] [Google Scholar]
- 69.Li J, Zheng Y, Li M-X, et al. Tanshinone IIA alleviates lipopolysaccharide-induced acute lung injury by downregulating TRPM7 and pro-inflammatory factors. J Cell Mol Med 2018;22:646–54. 10.1111/jcmm.13350 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Yang J, Li S, Wang L, et al. Ginsenoside Rg3 attenuates lipopolysaccharide-induced acute lung injury via MerTK-dependent activation of the PI3K/Akt/mTOR pathway. Front Pharmacol 2018;9:850. 10.3389/fphar.2018.00850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun X, Cheng H, Liu B, et al. Icariin reduces LPS-induced acute lung injury in mice undergoing bilateral adrenalectomy by regulating GRα. Eur J Pharmacol 2020;876:173032. 10.1016/j.ejphar.2020.173032 [DOI] [PubMed] [Google Scholar]
- 72.Kasotakis G, Galvan MD, Osathanugrah P, et al. Timing of valproic acid in acute lung injury: prevention is the best therapy? J Surg Res 2017;220:206–12. 10.1016/j.jss.2017.06.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li K, He Z, Wang X, et al. Apigenin C-glycosides of Microcos paniculata protects lipopolysaccharide induced apoptosis and inflammation in acute lung injury through TLR4 signaling pathway. Free Radic Biol Med 2018;124:163–75. 10.1016/j.freeradbiomed.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 74.Spengler D, Winoto-Morbach S, Kupsch S, et al. Novel therapeutic roles for surfactant-inositols and -phosphatidylglycerols in a neonatal piglet ARDS model: a translational study. Am J Physiol Lung Cell Mol Physiol 2018;314:L32–53. 10.1152/ajplung.00128.2017 [DOI] [PubMed] [Google Scholar]
- 75.Park J, Chen Y, Zheng M, et al. Pterostilbene 4'-β-Glucoside Attenuates LPS-Induced Acute Lung Injury via Induction of Heme Oxygenase-1. Oxid Med Cell Longev 2018;2018:2747018. 10.1155/2018/2747018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hao Y, Liu Y. Osthole Alleviates Bleomycin-Induced Pulmonary Fibrosis via Modulating Angiotensin-Converting Enzyme 2/Angiotensin-(1-7) Axis and Decreasing Inflammation Responses in Rats. Biol Pharm Bull 2016;39:457–65. 10.1248/bpb.b15-00358 [DOI] [PubMed] [Google Scholar]
- 77.Mahmoodpoor A, Hamishehkar H, Shadvar K, et al. The effect of intravenous selenium on oxidative stress in critically ill patients with acute respiratory distress syndrome. Immunol Invest 2019;48:147–59. 10.1080/08820139.2018.1496098 [DOI] [PubMed] [Google Scholar]
- 78.Tsaknis G, Siempos II, Kopterides P, et al. Metformin attenuates ventilator-induced lung injury. Crit Care 2012;16:R134. 10.1186/cc11439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang J, Li Z, Lin Z, et al. 17-DMCHAG, a new geldanamycin derivative, inhibits prostate cancer cells through Hsp90 inhibition and survivin downregulation. Cancer Lett 2015;362:83–96. 10.1016/j.canlet.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 80.Jeon MJ, Kim WG, Lim S, et al. Alpha lipoic acid inhibits proliferation and epithelial mesenchymal transition of thyroid cancer cells. Mol Cell Endocrinol 2016;419:113–23. 10.1016/j.mce.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y, Sun Q, Li X, et al. Apigenin suppresses mouse peritoneal fibrosis by down-regulating miR34a expression. Biomed Pharmacother 2018;106:373–80. 10.1016/j.biopha.2018.06.138 [DOI] [PubMed] [Google Scholar]
- 82.Naveen CR, Gaikwad S, Agrawal-Rajput R. Berberine induces neuronal differentiation through inhibition of cancer stemness and epithelial-mesenchymal transition in neuroblastoma cells. Phytomedicine 2016;23:736–44. 10.1016/j.phymed.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 83.Ko J-H, Nam D, Um J-Y, et al. Bergamottin suppresses metastasis of lung cancer cells through abrogation of diverse oncogenic signaling cascades and epithelial-to-mesenchymal transition. Molecules 2018;23. 10.3390/molecules23071601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. LiL, WuM, WangC, et al. β-Asarone inhibits invasion and EMT in human glioma U251 cells by suppressing splicing factor hnRNP A2/B1. Molecules 2018;23:671 10.3390/molecules23030671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kee J-Y, Han Y-H, Park J, et al. β-Lapachone inhibits lung metastasis of colorectal cancer by inducing apoptosis of CT26 cells. Integr Cancer Ther 2017;16:585–96. 10.1177/1534735416681638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bao H, Zhang Q, Zhu Z, et al. BHX, a novel pyrazoline derivative, inhibits breast cancer cell invasion by reversing the epithelial-mesenchymal transition and down-regulating Wnt/β-catenin signalling. Sci Rep 2017;7:9153. 10.1038/s41598-017-09655-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian B, Patrikeev I, Ochoa L, et al. NF-κB Mediates Mesenchymal Transition, Remodeling, and Pulmonary Fibrosis in Response to Chronic Inflammation by Viral RNA Patterns. Am J Respir Cell Mol Biol 2017;56:506–20. 10.1165/rcmb.2016-0259OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang M-K, Park S-H, Choi Y-J, et al. Chrysin inhibits diabetic renal tubulointerstitial fibrosis through blocking epithelial to mesenchymal transition. J Mol Med 2015;93:759–72. 10.1007/s00109-015-1301-3 [DOI] [PubMed] [Google Scholar]
- 89.Petpiroon N, Bhummaphan N, Tungsukruthai S, et al. Chrysotobibenzyl inhibition of lung cancer cell migration through caveolin-1-dependent mediation of the integrin switch and the sensitization of lung cancer cells to cisplatin-mediated apoptosis. Phytomedicine 2019;58:152888. 10.1016/j.phymed.2019.152888 [DOI] [PubMed] [Google Scholar]
- 90.Li K, Guo J, Wu Y, et al. Suppression of YAP by DDP disrupts colon tumor progression. Oncol Rep 2018;39:2114–26. 10.3892/or.2018.6297 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Chen C-H, Lee C-H, Liou J-P, et al. Molecular mechanisms underlying the antitumor activity of (E)-N-hydroxy-3-(1-(4-methoxyphenylsulfonyl)-1,2,3,4-tetrahydroquinolin-6-yl)acrylamide in human colorectal cancer cells in vitro and in vivo. Oncotarget 2015;6:35991–6002. 10.18632/oncotarget.5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang L, Cheng X, Gao Y, et al. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-β/Smad2/3 signaling pathway. Exp Cell Res 2016;341:157–65. 10.1016/j.yexcr.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 93.Treesuwan S, Sritularak B, Chanvorachote P, et al. Cypripedin diminishes an epithelial-to-mesenchymal transition in non-small cell lung cancer cells through suppression of Akt/GSK-3β signalling. Sci Rep 2018;8:8009. 10.1038/s41598-018-25657-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.You J, Wang J, Xie L, et al. D-4F, an apolipoprotein A-I mimetic, inhibits TGF-β1 induced epithelial-mesenchymal transition in human alveolar epithelial cell. Exp Toxicol Pathol 2016;68:533–41. 10.1016/j.etp.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 95.Colín-Val Z, González-Puertos VY, Mendoza-Milla C, et al. DHEA increases epithelial markers and decreases mesenchymal proteins in breast cancer cells and reduces xenograft growth. Toxicol Appl Pharmacol 2017;333:26–34. 10.1016/j.taap.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 96.Wang JY, Wang Z, Li MY, et al. Dictamnine promotes apoptosis and inhibits epithelial-mesenchymal transition, migration, invasion and proliferation by downregulating the HIF-1α and Slug signaling pathways. Chem Biol Interact 2018;296:134–44. 10.1016/j.cbi.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 97.Tian L, Shen D, Li X, et al. Ginsenoside Rg3 inhibits epithelial-mesenchymal transition (EMT) and invasion of lung cancer by down-regulating FUT4. Oncotarget 2016;7:1619–32. 10.18632/oncotarget.6451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J, Qi Y. Ginsenoside Rg3 inhibits cell growth, migration and invasion in Caco-2 cells by downregulation of lncRNA CCAT1. Exp Mol Pathol 2019;106:131–8. 10.1016/j.yexmp.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 99.Ying R, Wang X-Q, Yang Y, et al. Hydrogen sulfide suppresses endoplasmic reticulum stress-induced endothelial-to-mesenchymal transition through Src pathway. Life Sci 2016;144:208–17. 10.1016/j.lfs.2015.11.025 [DOI] [PubMed] [Google Scholar]
- 100.Fang L, Xu W, Kong D. Icariin inhibits cell proliferation, migration and invasion by down-regulation of microRNA-625-3p in thyroid cancer cells. Biomed Pharmacother 2019;109:2456–63. 10.1016/j.biopha.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 101.Song J, Feng L, Zhong R, et al. Icariside II inhibits the EMT of NSCLC cells in inflammatory microenvironment via down-regulation of Akt/NF-κB signaling pathway. Mol Carcinog 2017;56:36–48. 10.1002/mc.22471 [DOI] [PubMed] [Google Scholar]
- 102.Dinicola S, Fabrizi G, Masiello MG, et al. Inositol induces mesenchymal-epithelial reversion in breast cancer cells through cytoskeleton rearrangement. Exp Cell Res 2016;345:37–50. 10.1016/j.yexcr.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 103.Akbarzadeh M, Movassaghpour AA, Ghanbari H, et al. The potential therapeutic effect of melatonin on human ovarian cancer by inhibition of invasion and migration of cancer stem cells. Sci Rep 2017;7:17062. 10.1038/s41598-017-16940-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X, Wang B, Zhan W, et al. Melatonin inhibits lung metastasis of gastric cancer in vivo. Biomed Pharmacother 2019;117:109018. 10.1016/j.biopha.2019.109018 [DOI] [PubMed] [Google Scholar]
- 105.Zheng N, Chen J, Liu W, et al. Metapristone (RU486 derivative) inhibits cell proliferation and migration as melanoma metastatic chemopreventive agent. Biomed Pharmacother 2017;90:339–49. 10.1016/j.biopha.2017.03.076 [DOI] [PubMed] [Google Scholar]
- 106.Tyszka-Czochara M, Lasota M, Majka M. Caffeic acid and metformin inhibit invasive phenotype induced by TGF-β1 in C-4I and HTB-35/SiHa human cervical squamous carcinoma cells by acting on different molecular targets. Int J Mol Sci 2018;19. 10.3390/ijms19010266. [Epub ahead of print: 16 Jan 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y, Wu Z, Hu L. The regulatory effects of metformin on the [SNAIL/miR-34]:[ZEB/miR-200] system in the epithelial-mesenchymal transition(EMT) for colorectal cancer(CRC). Eur J Pharmacol 2018;834:45–53. 10.1016/j.ejphar.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 108.Busaranon K, Plaimee P, Sritularak B, et al. Moscatilin inhibits epithelial-to-mesenchymal transition and sensitizes anoikis in human lung cancer H460 cells. J Nat Med 2016;70:18–27. 10.1007/s11418-015-0931-7 [DOI] [PubMed] [Google Scholar]
- 109.Huang H, Chen M, Liu F, et al. N-Acetylcysteine tiherapeutically protects against pulmonary fibrosis in a mouse model of silicosis. Biosci Rep 2019;39 10.1042/BSR20190681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang L-H, Xu M, Fu L-Q, et al. The antihelminthic niclosamide inhibits cancer stemness, extracellular matrix remodeling, and metastasis through dysregulation of the nuclear β-catenin/c-Myc axis in OSCC. Sci Rep 2018;8:12776. 10.1038/s41598-018-30692-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peng C, Li Z, Niu Z, et al. Norcantharidin suppresses colon cancer cell epithelial-mesenchymal transition by inhibiting the αvβ6-ERK-Ets1 signaling pathway. Sci Rep 2016;6:20500. 10.1038/srep20500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu L, Mao J, Wang Q, et al. In vitro anticancer activities of osthole against renal cell carcinoma cells. Biomed Pharmacother 2017;94:1020–7. 10.1016/j.biopha.2017.07.155 [DOI] [PubMed] [Google Scholar]
- 113.Al-Qasem A, Al-Howail HA, Al-Swailem M, et al. PAC exhibits potent anti-colon cancer properties through targeting cyclin D1 and suppressing epithelial-to-mesenchymal transition. Mol Carcinog 2016;55:233–44. 10.1002/mc.22271 [DOI] [PubMed] [Google Scholar]
- 114.Qin G, Xu F, Qin T, et al. Palbociclib inhibits epithelial-mesenchymal transition and metastasis in breast cancer via c-Jun/COX-2 signaling pathway. Oncotarget 2015;6:41794–808. 10.18632/oncotarget.5993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang M, Liu X, Guo J, et al. Inhibition of LSD1 by Pargyline inhibited process of EMT and delayed progression of prostate cancer in vivo. Biochem Biophys Res Commun 2015;467:310–5. 10.1016/j.bbrc.2015.09.164 [DOI] [PubMed] [Google Scholar]
- 116.Han Y-tao, Chen X-hong, Gao H, et al. Physcion inhibits the metastatic potential of human colorectal cancer SW620 cells in vitro by suppressing the transcription factor Sox2. Acta Pharmacol Sin 2016;37:264–75. 10.1038/aps.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Su C-M, Lee W-H, Wu ATH, et al. Pterostilbene inhibits triple-negative breast cancer metastasis via inducing microRNA-205 expression and negatively modulates epithelial-to-mesenchymal transition. J Nutr Biochem 2015;26:675–85. 10.1016/j.jnutbio.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 118.Li H, Huang D, Hang S. Salidroside inhibits the growth, migration and invasion of Wilms' tumor cells through down-regulation of miR-891b. Life Sci 2019;222:60–8. 10.1016/j.lfs.2019.02.052 [DOI] [PubMed] [Google Scholar]
- 119.Kok DEG, Kiemeney LALM, Verhaegh GW, et al. A short-term intervention with selenium affects expression of genes implicated in the epithelial-to-mesenchymal transition in the prostate. Oncotarget 2017;8:10565–79. 10.18632/oncotarget.14551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim Y, Lee EJ, Jang HK, et al. Statin pretreatment inhibits the lipopolysaccharide-induced epithelial-mesenchymal transition via the downregulation of Toll-like receptor 4 and nuclear factor-κB in human biliary epithelial cells. J Gastroenterol Hepatol 2016;31:1220–8. 10.1111/jgh.13230 [DOI] [PubMed] [Google Scholar]
- 121.Xu F, Li Q, Wang Z, et al. Sinomenine inhibits proliferation, migration, invasion and promotes apoptosis of prostate cancer cells by regulation of miR-23a. Biomed Pharmacother 2019;112:108592. 10.1016/j.biopha.2019.01.053 [DOI] [PubMed] [Google Scholar]
- 122.Sun L, Jin X, Xie L, et al. Swainsonine represses glioma cell proliferation, migration and invasion by reduction of miR-92a expression. BMC Cancer 2019;19:247. 10.1186/s12885-019-5425-7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Huang S-Y, Chang S-F, Liao K-F, et al. Tanshinone IIA inhibits epithelial-mesenchymal transition in bladder cancer cells via modulation of STAT3-CCL2 signaling. Int J Mol Sci 2017;18. 10.3390/ijms18081616. [Epub ahead of print: 25 Jul 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Z, Zhang G, Sun Y, et al. Tetramethylpyrazine nitrone, a multifunctional neuroprotective agent for ischemic stroke therapy. Sci Rep 2016;6:37148. 10.1038/srep37148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Y, Liu W, He W, et al. Tetrandrine reverses epithelial-mesenchymal transition in bladder cancer by downregulating gli-1. Int J Oncol 2016;48:2035–42. 10.3892/ijo.2016.3415 [DOI] [PubMed] [Google Scholar]
- 126.Pei Z, Fu W, Wang G. A natural product toosendanin inhibits epithelial-mesenchymal transition and tumor growth in pancreatic cancer via deactivating Akt/mTOR signaling. Biochem Biophys Res Commun 2017;493:455–60. 10.1016/j.bbrc.2017.08.170 [DOI] [PubMed] [Google Scholar]
- 127.Guo X, Xue M, Li C-J, et al. Protective effects of triptolide on TLR4 mediated autoimmune and inflammatory response induced myocardial fibrosis in diabetic cardiomyopathy. J Ethnopharmacol 2016;193:333–44. 10.1016/j.jep.2016.08.029 [DOI] [PubMed] [Google Scholar]
- 128.Lan X, Lu G, Yuan C, et al. Valproic acid (VPA) inhibits the epithelial-mesenchymal transition in prostate carcinoma via the dual suppression of Smad4. J Cancer Res Clin Oncol 2016;142:177–85. 10.1007/s00432-015-2020-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qi G, Lu G, Yu J, et al. Up-Regulation of TIF1γ by valproic acid inhibits the epithelial mesenchymal transition in prostate carcinoma through TGF-β/Smad signaling pathway. Eur J Pharmacol 2019;860:172551. 10.1016/j.ejphar.2019.172551 [DOI] [PubMed] [Google Scholar]
- 130.Zhao Y, Yao J, Wu X-P, et al. Wogonin suppresses human alveolar adenocarcinoma cell A549 migration in inflammatory microenvironment by modulating the IL-6/STAT3 signaling pathway. Mol Carcinog 2015;54 Suppl 1:E81–93. 10.1002/mc.22182 [DOI] [PubMed] [Google Scholar]