Abstract
Among transgender women (TGW), the effects of feminizing hormone therapy use on rectal mucosal (RM) HIV transmission are largely unknown. In this small pilot study, we compared the RM transcriptome in TGW utilizing feminizing hormone therapy with a group of cisgender men who have sex with men (MSM) engaging in condomless receptive anal intercourse (AI) and to a group of cisgender men who had never engaged in AI. There were 498 differentially expressed genes (DEGs) in TGW compared with men who had never engaged in AI, and 154 DEGs compared with the MSM. Among TGW, a unique RM transcriptome was identified that implicated pathways critical for mucosal immune responses, including upregulation of genes that mediate immune cell activation and the production of cytokines and other immune signaling molecules. Furthermore, gene set enrichment analyses identified immune signatures that implicated enrichment of proinflammatory immunological pathways in TGW, specifically involving interferon-α, interleukin-6, and tumor necrosis factor-α signaling, whereas metabolic pathways were shown to be enriched among the cisgender male groups. These findings suggest that TGW have a distinct RM immune environment influenced by the use of feminizing hormones, and consequently, there is an urgent need for further investigation into the immunological effects of gender-affirming hormone therapy and its potential impact on HIV mucosal transmission risk for transgender individuals.
Keywords: transgender women, feminizing hormone therapy, HIV transmission, rectal mucosal transcriptome
The transgender population, particularly transgender women (TGW) or people who are recorded male at birth but who have a female identity, are disproportionately at risk for HIV acquisition with an estimated prevalence of 18.8% among TGW in the United States.1 Numerous contextual factors affecting transgender individuals, including sexual behavior, adverse social determinants of health, and violence, contribute to higher rates of HIV transmission.1 In HIV prevention research, TGW are traditionally grouped with men who have sex with men (MSM) due to presumed similar risk of HIV exposure and acquisition, primarily through receptive anal intercourse (AI). Use of feminizing hormone therapy by TGW could influence HIV transmission risk among this population, yet, remains underinvestigated. Considering the use of exogenous hormone therapy has been associated with augmentation of immune responses to HIV and other sexually transmitted pathogens in the female genital tract (FGT),2 the potential effects of feminizing hormone therapy on rectal mucosal (RM) HIV transmission should be further examined given the alarmingly high rates of HIV infection affecting this population.
To explore biological differences in gene expression related to feminizing hormone use, we conducted this exploratory pilot study to compare the RM transcriptome of HIV-negative TGW using feminizing hormone therapy and two groups of HIV-negative cisgender men, MSM engaging in condomless receptive anal intercourse (CRAI) and control males who had never engaged in AI. In this cross-sectional study, RM tissue biopsies were obtained through rigid sigmoidoscopy from nine TGW on stable feminizing hormone therapy for a minimum of 6 months before enrollment. Total RNA was extracted from two pinch biopsies for RNA-Seq analysis using methods previously described.3 The RM transcriptome of TGW was compared with stored specimens from a prior study that included 10 MSM engaging in CRAI and 10 men who reported no history of AI. The previously stored specimens were resequenced from extracted RNA to control for sequencing batch effect. The RNA-Seq data were deposited to the GEO database (GSE141785). Clustering was visualized by covariance principal components analysis. Differential gene expression was compared between the three groups using negative binomial generalized linear models. Significant differential gene expression was determined by a 0.67 > fold-change >1.5 with adjusted p-value of <.01. In addition, gene set enrichment analysis (GSEA)4 was performed utilizing the Hallmark geneset5 in the MSigDB database.6 Informed consent was obtained from all participants and the Institutional Review Board at Emory University approved this study.
Among the cohort of TGW, median age was 48 years [interquartile range (IQR) 33–49] and six out of nine participants reported Caucasian race. The median age was 32 (IQR 29–37) and 24 years (IQR 24–26) in the MSM and control groups, respectively, and 9 out of 10 MSM and 8 out of 10 controls reported Caucasian race. All nine TGW were using estrogen, whereas five were also taking spironolactone, two were taking progesterone, and one was taking growth hormone. Five of the nine TGW reported engaging in RAI during their lifetimes.
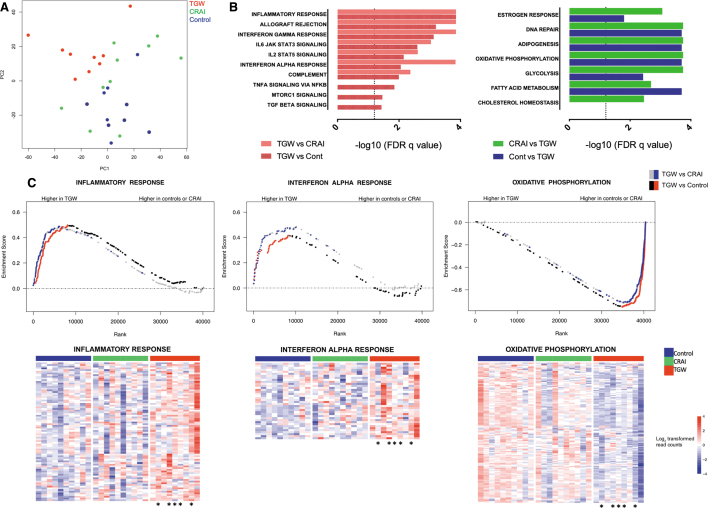
Two-dimensional plots of the principal components demonstrated a discrete separation between the TGW on feminizing hormone therapy and the cisgender men (Fig. 1A). There were 498 differentially expressed genes (DEGs) ascribed to TGW compared with men who had never engaged in AI, and 154 DEGs compared with MSM engaging in CRAI. Many of the DEGs upregulated in TGW were discovered to be mediators of immune responses, including those contributing to immune cell activation and exhaustion (e.g., NFATC3, CD80, CD83, CD28, PDCD1LG2). Other genes identified are known to promote the production of multiple cytokines and other signaling molecules important for mucosal immune responses (e.g., TNFRSF9, CD27, NFKBIZ).
FIG. 1.
Comparison of the RM RNA transcriptome results between TGW on feminizing hormone therapy, MSM engaging in CRAI, and men with no history of engaging in AI. (A) Principal components analysis of the RM transcriptome for TGW on feminizing hormone therapy (red), MSM engaging in CRAI (green) and men who do not engage in AI (blue) demonstrating a distinct separation between TGW and the cisgender male cohorts. (B) Results of GSEA using the Hallmark geneset. The left panel shows immune gene pathways enriched among TGW as compared with MSM engaging in CRAI (red) and men who do not engage in AI (controls, shaded red; dotted line: adjusted p-value = .05). The right panel demonstrates estrogen response and metabolic gene pathways enriched among MSM engaged in CRAI compared with TGW (green) and men who do not engage in AI compared with TGW (controls, blue; dotted line: adjusted p-value = .05). (C) Upper panel: graphic depiction of leading edge genes for three representative pathways of interest identified by GSEA using the Molecular Signatures Database program. Red dots represent leading edge genes significantly enriched among TGW versus men who never engaged in AI (control). Blue dots represent the distribution of those same genes in comparison of TGW versus MSM engaging in CRAI to demonstrate significant overlap between the two comparison groups. Lower panel: heat maps of the leading edge genes from select pathways determined to be enriched by GSEA (as shown in the upper panel). The color scales were determined by gene-centric median normalization; blue denotes downregulation, whereas red denotes upregulation. Asterisks indicate transgender female subjects who have previously engaged in receptive AI. AI, anal intercourse; CRAI, condomless receptive anal intercourse; GSEA, gene set enrichment analysis; MSM, men who have sex with men; RM, rectal mucosal; TGW, transgender women.
In addition, GSEA identified immune signatures in the MSigDB database that were significantly enriched among TGW. Consistent with the individual DEG identified, the Hallmark geneset implicated enrichment of immunological pathways in TGW that are critical in the development of inflammatory responses, including interferon-α, interleukin-6, and tumor necrosis factor-α pathways signaling. A decrease in estrogen receptor signaling was also observed among TGW compared with the cisgender men, a finding that was difficult to interpret although it may be related to postsignaling receptor downregulation. In contrast, metabolic pathways involving DNA repair, adipogenesis, oxidative phosphorylation, glycolysis, and fatty acid/cholesterol metabolism were enriched in the two cisgender male groups in comparison with TGW (Fig. 1B, C).
These novel findings provide evidence for a distinct RM transcriptome for TGW using feminizing hormone therapy, most notably involving pathways implicated in immune cell activation and mucosal inflammation. In a prior study from our group comparing the RM transcriptome between MSM engaging in CRAI and males not engaging in AI, 54 genes were differentially expressed between the two groups, many of which were involved in mucosal injury/repair, cell proliferation, and immune activation, likely attributed to engagement in CRAI.3 In this study, we have shown distinct differences between the RM transcriptome of TGW using feminizing hormone therapy when compared with both cisgender MSM and men not engaging in AI underscoring the need to consider TGW uniquely from cisgender MSM in HIV prevention research. In addition, many of the genes and pathways identified in our study are immune related and further exploration of the RM immune environment among TGW utilizing feminizing hormone therapy is essential to understand how exogenous sex hormone use may facilitate or hinder HIV transmission.
There is virtually no known research evaluating the effects of feminizing hormone therapy on the rectal mucosa. In the FGT, the influence of sex hormones on immune processes and the potential implications for HIV transmission remain controversial and poorly understood issues, despite accumulating investigation into this area of research. Generally, the immunomodulatory effects of estrogen in the FGT promote the production of inflammatory cytokines, induce propagation of B cells and antibody production, increase the recruitment of certain innate immune cell subsets, and directly repress HIV transcriptional activity.7,8 In contrast, the findings from ex vivo studies suggest that the use of exogenous progesterone has the potential to impair the immune responses against HIV through the inhibition of immune cell activity and reduction in tissue infiltration of NK cells, lymphocytes, and macrophages.9–12 However, the results from a randomized clinical trial determined no substantial difference in HIV incidence between female cisgender cohorts utilizing progesterone-only and hormone-free contraceptive methods.13 Nonetheless, findings from the FGT cannot be extrapolated to the rectum in TGW. The human intestine is structurally and immunologically distinct from the FGT, has the capacity to produce glucocorticoids, and contains both glucocorticoid and estrogen receptors.14,15 As there can be permissive hormonal effects between sex hormones and glucocorticoids,16 the potential impact of feminizing hormone use within the steroid-responsive environment of the gut could further influence rectal HIV susceptibility.
An important limitation of this study is that age and engagement in various sexual practices, including RAI, were not controlled for in the analysis given the small sample size. There is a critical need for larger studies that provide sufficient power to determine whether perturbations to the RM immune environment derive from exogenous sex hormone use or possibly from other demographic or behavioral characteristics.
In conclusion, the findings from this pilot study suggest that TGW utilizing feminizing hormone therapy have a distinct RM immune environment that could influence susceptibility to HIV infection. Based on these hypothesis-generating results, our group is currently initiating a larger follow-up study among U.S. and Thai TGW to further unravel the effects of feminizing hormone therapy on the rectal mucosa. These findings could inform the development and optimization of biomedical HIV prevention interventions to the direct benefit of TGW at increased risk for HIV acquisition.
Acknowledgments
We thank the study volunteers for their participation in this research.
Author Disclosure Statement
C.F.K. has received research grants from Gilead Sciences. The other authors declare no conflicts of interest.
Funding Information
This study was supported in parts by the following grant resources: K23 AI108335 (C.F.K.), T32 DK108735 (C.G.A.), UL1 TR002378 (C.G.A.), and the Emory Center for AIDS Research P30 AI050409.
References
- 1. Becasen JS, Denard CL, Mullins MM, Higa DH, Sipe TA: Estimating the prevalence of HIV and sexual behaviors among the US transgender population: A systematic review and meta-analysis, 2006–2017. Am J Public Health 2019;109:e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hall OJ, Klein SL: Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol 2017;10:1097–1107 [DOI] [PubMed] [Google Scholar]
- 3. Kelley CF, Kraft CS, de Man TJ, et al. : The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: Implications for HIV transmission and prevention. Mucosal Immunol 2017;10:996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Subramanian A, Tamayo P, Mootha VK, et al. : Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P: The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 2015;1:417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Godec J, Tan Y, Liberzon A, et al. : Compendium of immune signatures identifies conserved and species-specific biology in response to inflammation. Immunity 2016;44:194–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Straub RH: The complex role of estrogens in inflammation. Endocr Rev 2007;28:521–574 [DOI] [PubMed] [Google Scholar]
- 8. Scully EP: Sex differences in HIV infection. Curr HIV/AIDS Rep 2018;15:136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hel Z, Stringer E, Mestecky J: Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev 2010;31:79–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polis CB, Curtis KM: Use of hormonal contraceptives and HIV acquisition in women: A systematic review of the epidemiological evidence. Lancet Infect Dis 2013;13:797–808 [DOI] [PubMed] [Google Scholar]
- 11. Hickey M, Marino JL, Tachedjian G: Mechanisms of HIV transmission in Depo-Provera users: The likely role of hypoestrogenism. J Acquir Immune Defic Syndr 2016;71:1–7 [DOI] [PubMed] [Google Scholar]
- 12. Ralph LJ, McCoy SI, Shiu K, Padian NS: Hormonal contraceptive use and women's risk of HIV acquisition: A meta-analysis of observational studies. Lancet Infect Dis 2015;15:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahmed K, Baeten JM, Beksinska M, et al. : HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: A randomised, multicentre, open-label trial. Lancet 2019;394:303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kostadinova F, Schwaderer J, Sebeo V, Brunner T: Why does the gut synthesize glucocorticoids? Ann Med 2014;46:490–497 [DOI] [PubMed] [Google Scholar]
- 15. Williams C, DiLeo A, Niv Y, Gustafsson JA: Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett 2016;372:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Da Silva JAP: Sex hormones, glucocorticoids and autoimmunity—Facts and hypotheses. Ann Rheum Dis 1995;54:6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]