Abstract
Background: Despite the excellent survival of most patients with differentiated thyroid cancer (DTC), recurrent and persistent disease remain major concerns for physicians and patients. However, studies on patient report of recurrent and persistent disease are lacking.
Methods: Between February 1, 2017, and October 31, 2018, we surveyed eligible patients who were diagnosed with DTC between 2014 and 2015 from the Georgia and Los Angeles Surveillance, Epidemiology, and End Results cancer registries (N = 2632; response rate, 63%). Patients who reported current disease status were included in this study (n = 2454). Patient-reported data were linked to registry data. A multivariable, multinomial logistic regression analysis was conducted to determine patient and tumor characteristics associated with recurrent and persistent thyroid cancer. Quality of life was evaluated using the Patient-Reported Outcomes Measurement Information System-Global Health v1.2 questionnaire. Meaningful change in global health was defined as a minimal difference of a half standard deviation or 5 points compared with the mean (T score = 50) of a sample population matching the United States 2000 General Census.
Results: Of the 2454 patients completing the survey, 95 (4.1%) reported recurrent disease and 137 (5.8%) reported persistent disease. In multinomial analyses, T3/T4 classification and cervical lymph node involvement (N1) were associated with both report of recurrent (adjusted relative risk ratio [RRR] 1.99, 95% confidence interval [CI 1.16–3.42]; adjusted RRR 2.03 [CI 1.29–3.21], respectively) and persistent disease (adjusted RRR 3.48 [CI 1.96–6.20]; adjusted RRR 3.56 [CI 2.41–5.24], respectively). Additionally, Hispanic ethnicity was associated with report of recurrent disease (adjusted RRR 1.99 [CI 1.23–3.24]). Regarding quality of life, the median scores in patients with persistent disease met criteria for meaningful change in global physical health (T-score = 44.9) and global mental health (T-score = 43.5) when compared with the general population norms. Median scores in patients with cured or recurrent disease did not meet criteria for meaningful change.
Conclusions: Patient report is a reasonable method of assessing recurrent and persistent disease. Impact on quality of life is more marked for patients with reported persistent disease. Our findings will help personalize treatment and long-term follow-up in these patients.
Keywords: thyroid cancer, recurrence, persistent, quality of life, population-based study
Introduction
Differentiated thyroid cancer (DTC) affects more than 50,000 Americans every year and is generally associated with an excellent prognosis, with a five-year overall survival rate reaching 98% (1). It has been shown that adequate initial management, including completeness of surgical resection and in some cases adjunctive radioactive iodine (RAI) and thyrotropin suppression therapy, leads to a high likelihood of cure in these patients (2–5).
Despite its excellent survival, recurrent and persistent DTC remain major concerns for physicians and patients (6–10). Recurrent and persistent thyroid cancer are thought to represent elements of the same pathological process (5,11). Persistent disease typically occurs in patients with high disease burden when cancer could not adequately be eradicated or in those with inadequate initial treatment resulting in residual treatable disease (5,12,13). Patients with recurrent disease either had microscopic disease following initial treatment that grew to a detectable level or they had inadequate initial treatment and at a later time, residual disease was noted (5,12,14–17). Blurring the line between recurrent and persistent disease, in a smaller study of patients with papillary thyroid cancer who underwent a total thyroidectomy (n = 69), an alarming 40% of reoperations occurred within a year from the initial surgery, raising suspicion that some “recurrences” are actually persistent disease, that is disease that should have been detected at time of initial treatment (12).
However, although physiologically physicians understand that recurrent and persistent disease are parts of a continuum, patients would typically define their disease as “gone,” “came back,” or “never gone.” In view of information on recurrent and persistent thyroid cancer not routinely captured in national cancer registries, and the fact that patient report may be what physicians initially rely on when first seeing a patient, it is important to understand patient-reported recurrent and persistent disease in a population-based sample of thyroid cancer patients.
The main objective of this study was to determine characteristics correlating with patient report of recurrent and persistent disease in a diverse population-based sample of patients with DTC. We hypothesized that patient report of recurrent and persistent thyroid cancer correlates with factors expected to be associated with these entities and thus may constitute a reasonable measure. We also hypothesized that although patients with report of recurrent and persistent disease would be similar in many ways, impact of reported persistent disease on quality of life would be greater.
Methods
Study population and data collection
We conducted a large population-based survey of patients aged 18–79 years with incident DTC, as reported to the Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and Los Angeles County from January 1, 2014, to December 31, 2015. Patients completed surveys from February 1, 2017, to October 31, 2018. The modified Dillman method of survey administration was employed to encourage a greater response rate (18), which included multiple mailings, follow-up of nonrespondents by phone, and a $20 cash incentive. Patients with Hispanic surnames were mailed materials in both English and Spanish, and bilingual interviewers conducted follow-up calls. A double data entry method was used to ensure <1% error.
Survey responses, which included information on current disease status and patient-reported quality of life, were subsequently linked to clinical data reported to the respective SEER registries and a de-identified data set was generated. Of the 4317 patients identified, 4185 were response-eligible and 2632 responded to the mailed survey. The response rate was 63% (2632/4185) and the cooperation rate was 77% (2632/3435) (19). Patients who did not report current disease status (n = 178) were excluded, resulting in a final analytic sample of 2454 patients.
The study was approved by the University of Michigan, the University of Southern California, the Committee for the Protection of Human Subjects (California State Institutional Review Board), the Georgia Department of Public Health, and the Emory University Institutional Review Board and received California Cancer Registry approval.
Questionnaire design and content
The survey questionnaire was developed based on systematic review, a conceptual framework, and prior research experience in the thyroid cancer population. To assess content validity, the instrument was reviewed by a multidisciplinary team of clinicians and methodologists and piloted in a select cohort of thyroid cancer patients at the University of Michigan before survey administration. Relevant key survey items are included in Appendix A1.
Measures
Patient-reported current disease status
Respondents were asked to state which of the following best described their thyroid cancer at the time of completing the survey: “My cancer was gone after initial treatment,” “My cancer was gone but then came back (recurrence),” or “My cancer was never gone after initial treatment; I have persistent disease.” For patients who reported that their cancer was gone and came back (recurrence), we also asked them to report mode of discovery (imaging, laboratory work, biopsy, surgery) and location of their recurrence (neck, lungs, bone, brain, liver, other).
Patient-reported health-related quality of life
Quality of life was measured using the Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaire (v1.2), a 10-item instrument representing global mental health (GMH) and global physical health (GPH) domains. The PROMIS scales are validated measures of health-related quality of life and have been used extensively to efficiently evaluate physical and mental quality of life in cancer patients (20–22). Raw scores are converted to T-score values, and T-score distributions are standardized such that a 50 represents the mean for the United States general population, and the standard deviation around that mean is 10 points. For example, a person who has a T-score of 60 is one standard deviation better (healthier) than the general population.
Meaningful change in global health was defined as a minimally important difference of a half standard deviation, or 5 points, compared with the mean (T score = 50) of a sample population matching the United States 2000 General Census (22). This method has been employed in other studies using the PROMIS health measures (23,24). Minimally important difference refers to the smallest meaningful difference that carries implications for patient care. T-scores reported are unadjusted.
Covariates
Covariates for our analyses included patient demographic and clinical factors. Demographic covariates obtained from the survey included sex (male or female), race (white, black, Asian, multiracial, other), and ethnicity (Hispanic or non-Hispanic). For the purpose of the analyses, the race categories of black, Asian, multiracial, and other were coded as non-white. Clinical characteristics obtained from the SEER registries included patient age at diagnosis (years), tumor size and extension (T1a, T1b, T2, or T3/T4), and regional lymph node involvement (N0 or N1) at diagnosis. Registries capture these last four variables from combined clinical/pathological sources and then derive stage categories (T, N, M) and a stage group (I, II, III, IV) using definitions from the AJCC 7th edition. The stage (I, II/III, VI) was considered separately; stage II and III were combined due to low counts. The stage distribution of our cohort is representative of patients diagnosed with DTC in Los Angeles and Georgia between 2014 and 2015.
Statistical analyses
Descriptive statistics were generated for the entire sample and then separately for patients cured, patients with persistent, and patients with recurrent thyroid cancer. Non-weighted frequencies and weighted percentages were reported. A multivariable, multinomial logistic regression analysis was used to determine correlates with report of persistent and recurrent disease, adjusting for the covariates mentioned above. All statistical analyses incorporated weights to account for differential probabilities of sample selection and nonresponse.
Subsequently, we separately compared unadjusted PROMIS T-scores for patient-reported GPH and patient-reported GMH between the three patient samples: cured, persistent thyroid cancer, recurrent thyroid cancer, and determined which patients had a minimally important difference in quality of life, as described above. Because the scoring table is prepared for a fixed set of items, for these analyses, we excluded patients who did not respond to all items used in each of the PROMIS scales (N = 49 [2.0%] for PROMIS-GPH, N = 47 [1.9%] for PROMIS-GMH).
Adjusted relative risk ratios (RRRs) with 95% confidence intervals [CIs] are reported for all multinomial logistic regression models, with p-values <0.05 considered statistically significant. All analyses were performed using Stata version 15.1 (StataCorp LLC, College Station, TX).
Results
Table 1 shows the demographic and clinical characteristics of the entire analytic sample and by disease status, categorized as cured, persistent thyroid cancer, and recurrent thyroid cancer. A total of 77.7% of patients were female and 21.5% reported Hispanic ethnicity. Overall, 69.1% of patients were white, 14.0% black, 12.5% Asian, and 0.4% other (including American Indian/Alaskan Native, Native Hawaiian, or other Pacific Islander, other). A total of 2293 (93.5%) patients had papillary thyroid cancer, 112 (4.6%) had follicular thyroid cancer, and 49 (1.9%) had Hürthle cell thyroid cancer.
Table 1.
Patient Demographics and Tumor Characteristics
| Patient characteristic | Overall Na(%)b(N = 2454) | Cured Na(%)b(n = 2222) | Recurrent disease Na(%)b(n = 95) | Persistent disease Na(%)b(n = 137) |
|---|---|---|---|---|
| Age at diagnosis (years) | ||||
| ≤44 | 863 (38.3) | 755 (37.1) | 50 (56.3) | 58 (44.5) |
| 45–54 | 596 (23.5) | 542 (23.6) | 21 (21.0) | 33 (23.3) |
| 55–64 | 564 (21.6) | 529 (22.4) | 11 (11.0) | 24 (16.9) |
| ≥65 | 431 (16.6) | 396 (16.9) | 13 (11.7) | 22 (15.3) |
| Sex | ||||
| Female | 1899 (77.7) | 1730 (78.1) | 73 (79.3) | 96 (70.3) |
| Male | 555 (22.3) | 492 (21.9) | 22 (20.7) | 41 (29.7) |
| Race | ||||
| White | 1857 (72.3) | 1678 (72.2) | 78 (79.0) | 101 (69.8) |
| Non-white/multiracial | 595 (27.7) | 542 (27.8) | 17 (21.0) | 36 (30.2) |
| Ethnicity | ||||
| Non-Hispanic | 1971 (78.5) | 1805 (79.5) | 60 (59.2) | 106 (75.9) |
| Hispanic | 483 (21.5) | 417 (20.5) | 35 (40.8) | 31 (24.1) |
| T classificationc | ||||
| T1a | 852 (35.4) | 812 (37.1) | 23 (25.9) | 17 (13.9) |
| T1b | 562 (22.3) | 519 (22.7) | 14 (14.6) | 29 (20.8) |
| T2 | 424 (16.8) | 392 (17.2) | 12 (12.5) | 20 (14.1) |
| T3/T4 | 579 (25.5) | 472 (23.0) | 41 (47.0) | 66 (51.2) |
| N classificationc | ||||
| N0 | 1859 (76.5) | 1750 (79.6) | 50 (54.1) | 59 (44.7) |
| N1 | 574 (23.5) | 452 (20.4) | 44 (45.9) | 78 (55.3) |
Unweighted number.
Weighted percentage (to compensate for differential probability of selection and survey nonresponse).
Derived from combined clinical and pathological tumor characteristics reported to SEER registries.
SEER, Surveillance, Epidemiology, and End Results.
Of the 2454 patients completing the survey, 95 (4.1%) reported recurrent thyroid cancer and 137 (5.8%) reported persistent thyroid cancer. Patients with recurrent and persistent disease were more likely to be younger than the cured group. Of those reporting recurrent thyroid cancer, 40.8% were of Hispanic ethnicity compared with 24.1% of those reporting persistent thyroid cancer and 20.5% of those reporting being cured. Overall, of those with recurrence, 61 (64.2%) patients reported that their recurrence was detected by an imaging test (such as ultrasound, computed tomography, or positron emission tomography), 46 (48.4%) by laboratory work (such as elevated thyroglobulin), 36 (37.9%) by biopsy, and 18 (18.9%) by surgical pathology. Of note, several patients checked multiple answers. A total of 88 (92.6%) patients with recurrence reported recurrence in the neck and the rest (7.4%) reported recurrence in other sites, such as the lungs, bone, brain, and other (data not shown).
Using SEER data linked to the patient surveys, we found that 167 (34.6%) Hispanic patients had lymph node involvement at initial surgery (N1 classification) compared with 389 (20.7%) non-Hispanics (p < 0.001). Additionally, there was a significant difference between Hispanics and non-Hispanics in regard to extent of surgery (p < 0.001) with 282 (59.1%) Hispanic patients undergoing total thyroidectomy with lymph node dissection compared with 890 (46.5%) non-Hispanics.
Table 2 displays the results of the multinomial logistic regression, with “cured” being the reference disease status. Report of recurrent thyroid cancer was associated with T3/T4 classification (adjusted RRR 1.99 [CI 1.16–3.42], compared with T1a) and cervical lymph node involvement, denoted as N1 classification (adjusted RRR 2.03 [CI 1.29–3.21], compared with N0). Additionally, Hispanic ethnicity was associated with report of recurrent disease (adjusted RRR 1.99 [CI 1.23–3.24], compared with non-Hispanic ethnicity). We also found that the use of RAI but not extent of surgery was significantly associated with Hispanic ethnicity (p = 0.020 and p = 0.950, respectively). Furthermore, it is shown that T3/T4 classification (adjusted RRR 3.48 [CI 1.96–6.20], compared with T1a) and cervical lymph node involvement, denoted as N1 classification (adjusted RRR 3.56 [CI 2.41–5.24], compared with N0), were associated with report of persistent thyroid cancer.
Table 2.
Patient Characteristics Associated with Recurrent and Persistent Thyroid Cancer
| Patient characteristic | Patients with recurrent disease |
Patients with persistent disease |
||
|---|---|---|---|---|
| n (%) | Adjusted RRR [CI] | n (%) | Adjusted RRR [CI] | |
| Age at diagnosis (years) | ||||
| ≤44 | 58 (7.1) | Ref | 50 (6.5) | Ref |
| 45–54 | 33 (6.0) | 1.00 [0.62–1.60] | 21 (3.9) | 0.69 [0.40–1.19] |
| 55–64 | 24 (4.6) | 0.83 [0.49–1.40] | 11 (2.2) | 0.34 [0.17–0.68] |
| ≥65 | 22 (5.5) | 0.88 [0.51–1.52] | 13 (3.1) | 0.56 [0.29–1.09] |
| Sex | ||||
| Male | 41 (8.0) | Ref | 22 (4.1) | Ref |
| Female | 96 (5.5) | 0.80 [0.55–1.18] | 73 (4.4) | 1.15 [0.69–1.93] |
| Race | ||||
| White | 101 (5.9) | Ref | 78 (4.8) | Ref |
| Non-white/multiracial | 36 (6.5) | 1.04 [0.68–1.60] | 17 (3.3) | 0.75 [0.41–1.34] |
| Ethnicity | ||||
| Non-Hispanic | 106 (5.8) | Ref | 60 (3.3) | Ref |
| Hispanic | 31 (7.0) | 0.86 [0.55–1.35] | 35 (8.3) | 1.99 [1.23–3.24] |
| T classification | ||||
| T1a | 17 (2.3) | Ref | 23 (2.9) | Ref |
| T1b | 29 (5.4) | 1.94 [1.01–3.73] | 14 (2.7) | 0.74 [0.37–1.48] |
| T2 | 20 (4.9) | 1.83 [0.93–3.62] | 12 (3.0) | 0.84 [0.40–1.75] |
| T3/T4 | 66 (12.2) | 3.48 [1.96–6.20] | 41 (8.1) | 1.99 [1.16–3.42] |
| N classification | ||||
| N0 | 59 (3.5) | Ref | 50 (3.0) | Ref |
| N1 | 78 (15.0) | 3.56 [2.41–5.24] | 44 (9.3) | 2.03 [1.29–3.21] |
RRR, relative risk ratio; CI, 95% confidence interval.
As a secondary analysis, disease severity was considered two additional ways: based on stage and T/N groupings (data not shown). Stage IV was associated with both report of persistent and recurrent disease (p < 0.001 and p = 0.025, respectively) when compared with stage I in univariate analyses. Only T3/T4 N1 was associated with report of recurrent disease compared with T1aN0 (p < 0.001). All T/N groups (except T2N0) were associated with report of persistent disease compared with T1aN0 (T1aN1, p = 0.039; T1bN0, p = 0.009; T1bN1, p = 0.001; T2N1, p < 0.001; T3/T4 N0, p = 0.005; T3/T4 N1, p < 0.001).
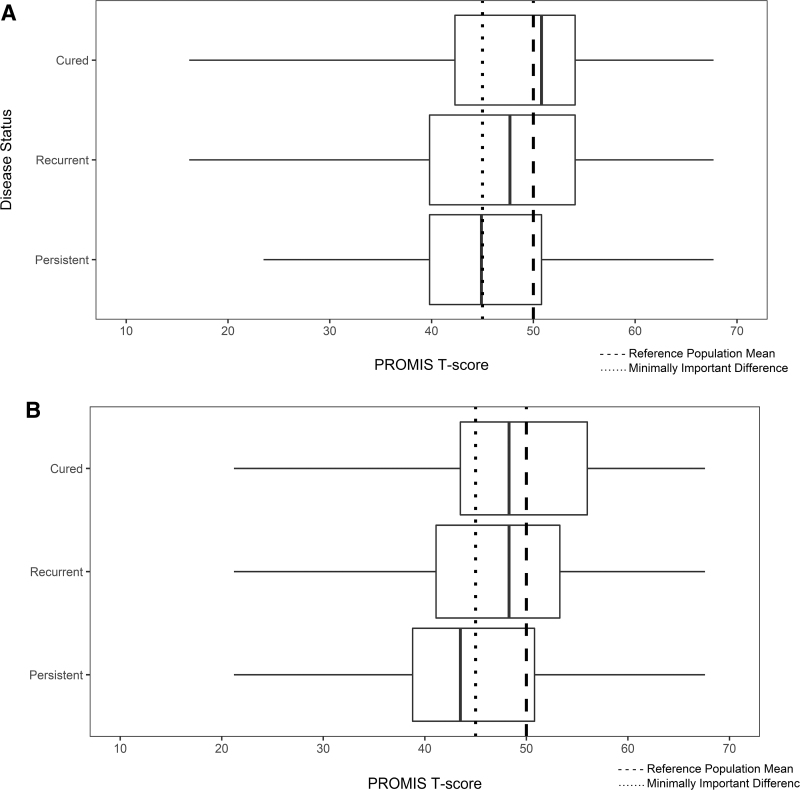
Figure 1A and B demonstrates that the median scores in patients with persistent thyroid cancer were lower and met criteria for meaningful change in GPH (T-score = 44.9) and GMH (T-score = 43.5) when compared with the general population norms. More explicitly, 81 (59.7%) of values from patients with persistent thyroid cancer were ≤45 for GPH and 70 (50.7%) of values were ≤45 for GMH. Although patients with recurrent thyroid cancer had a lower median global physical and mental health score compared with the reference healthy adult population, the difference did not meet criteria for meaningful change. More specifically, 44 (49.3%) of values from patients with recurrent thyroid cancer were ≤45 for GPH and 40 (43.7%) of values were ≤45 for GMH. Finally, 836 (38.5%) of values from patients with cured disease were ≤45 for GPH and 761 (35.7%) were ≤45 for GMH.
FIG. 1.
(A) Patient-reported GPH. The median scores in patients with persistent thyroid cancer were lower and met criteria for meaningful change in GPH when compared with the general population norms. (B) Patient-reported GMH. The median scores in patients with persistent thyroid cancer were lower and met criteria for meaningful change in GMH when compared with the general population norms. GMH, global mental health; GPH, global physical health.
Discussion
In this diverse population-based sample of patients with DTC, 4.1% reported recurrent disease and 5.8% reported persistent disease. We found that T3/T4 classification and cervical lymph node involvement were more likely to be associated with report of both recurrent and persistent thyroid cancer when compared with thyroid cancer patients cured of their disease. Less expected was the finding that Hispanic ethnicity was also associated with report of recurrent disease. Additionally, the impact on quality of life was greater in patients with reported persistent disease.
Despite recent updates on risk-adapted approaches to thyroid cancer management with “dynamic” risk stratification (5), little attention has been given to studying persistent and recurrent disease with only a few prior studies addressing these entities (11,12,25). Part of the difficulty lies on the fact that national cancer registries lack information on recurrent and persistent thyroid cancer. In our study, clinical factors that one would expect to be associated with persistent and recurrent disease did correlate, thus suggesting that patient report is reliable and reasonably accurate. Self-reported information is an important tool for physicians for collecting data in clinical settings where electronic medical records are not employed or shared. Additionally, high-quality clinical care requires patients to provide information that directly reflects the impact of disease and its treatment from their perspective, such as impact on quality of life (7,26–28).
Our finding that Hispanic ethnicity was associated with report of recurrent disease could either suggest differences in severity of disease at the time of presentation and/or ethnic disparities in DTC care. Interestingly, prior population-based and single institution studies have shown that considerable variability exists in the patient characteristics and clinical presentation of thyroid cancer across ethnic groups, with Hispanics less likely to be insured and having more lymph node involvement compared with non-Hispanics at presentation (29,30). Our study similarly found ethnic differences in severity of disease. More lymph node involvement in Hispanics may be indicative of greater extent of disease at the time of presentation, placing these patients at higher risk for true recurrence (17,31). This could explain our study's finding of higher rates of more extensive surgery in these patients.
A large study by Sosa et al. (32) (N = 16,878) found that total thyroidectomy was performed more frequently among whites (43%) compared with other race groups (40% Hispanics, 37% blacks), whereas subtotal thyroidectomy was more common among blacks and Hispanics (both 57% compared with 53% whites). Additionally, the authors of this study showed that the majority of Hispanics (55%) had surgery by the lowest volume surgeons (1–9 cases per year) compared with only 44% of whites. Racial disparities in outcomes, including mean total cost and length of hospital stay, persisted even after adjustment for surgeon volume group (32).
In our study, even when we controlled for lymph node involvement (N1) in our multivariable analyses, Hispanic patients still had more report of recurrence compared with non-Hispanics. Thus, although Hispanic patients likely present with advanced disease, other reasons may also account for more recurrence in these patients, which could be related to molecular characteristics of their cancer, more surveillance for recurrence, or disparities in use of suppressive doses of thyroid hormone. These findings indicate a potential need for future studies to better understand the intersection between ethnicity, disease severity, treatment, and surveillance.
Our study reveals worse patient-reported quality of life as it relates to both physical and mental health in patients with reported persistent thyroid cancer compared with the general population. Patients with persistent disease tend to have higher tumor burden and more advanced-stage disease (25). In addition to impaired quality of life resulting from increased morbidity due to more advanced underlying disease, treatment-related complications may play a role as these patients are more likely to receive more aggressive treatments (33–35). Thyroid cancer-specific worry about progression of disease and the impact on social functioning may also be contributing factors. Recent studies focusing on the evaluation of quality of life for thyroid cancer survivors have demonstrated that overall self-reported quality of life in thyroid cancer patients is similar, or worse, to that of other cancers with poorer prognoses (36,37). Our study is unique in that it provides further details regarding quality of life according to patient-reported disease status.
Strengths of this study include the inclusion of a large, diverse population-based cohort of patients with DTC providing unique patient-reported data on disease status and quality of life (n = 2454), a high participation rate, and linkage of survey data to SEER data, which include details on tumor characteristics. In addition, since recurrence data are not readily available in large cancer registries and single institution studies are prone to selection bias, this study provides an important method for studying persistent and recurrent disease (38).
This study also has some limitations. As patients were surveyed two to four years following diagnosis, recall bias is a possibility as report of recurrent or persistent disease is based on patient report and recurrence may have occurred anytime in the prior two to four years. Despite this potential limitation, prior studies have shown that cancer patient self-reporting is reliable and feasible as the emotionally charged nature of a cancer diagnosis increases the validity of recall about the experience (39–44). Another study limitation is that even though the PROMIS questionnaire provides an efficient, reliable, and valid assessment of adult overall health and allows for comparison to the general population and other disease states, it may not capture all effects of thyroid cancer and its treatments on quality of life, as many of these can be very specific (e.g., voice impairment, fatigue) (45).
Additionally, there is a potential for subjective differences in how providers explain and patients interpret the difference between persistence and recurrence. Also, even though we recognize that specific histological subtypes may play a role in recurrent and persistent disease, we were not able to conduct analyses with histology as a covariate due to relatively low event rates and the small sample size of certain histology subgroups. Finally, patients included in this study lived in two geographic regions and may not be representative of the general population. However, these two SEER regions were carefully selected regions to provide population-level assessments of cancer care.
Our findings place us a step closer to understanding the implications of patient-reported persistent and recurrent thyroid cancer. As shown by our findings, recurrent and persistent disease are similar in the fact that advanced diseases, as characterized by tumor size and lymph node status, are associated with both reported persistent and recurrent disease. However, Hispanic ethnicity is a risk factor for reporting recurrence, which may suggest potential disparities. Additionally, quality of life is impacted more in patients with persistent disease, likely related to greater disease burden.
As the inherent biology of the tumors that may underline tumor burden is not modifiable, perioperative management is critical to achieve favorable outcomes in these patients. Since risk for persistent and recurrent thyroid cancer is expected to be higher in patients with more aggressive and advanced tumors, physicians should consider referral to high-volume surgeons in patients with more advanced disease, ensure adequate and high-quality operative procedures at initial resection, and safeguard efforts to standardize care among those with Hispanic ethnicity to improve health-related quality of life.
In conclusion, our study suggests that patient report of persistent and recurrent thyroid cancer is a reasonable outcome measure, and patients with reported persistent disease exhibit worse quality of life compared with the general population. Efforts should be made to ensure that patients receive optimal initial therapy, avoid unnecessary complications and side effects, undergo appropriate long-term follow-up, and are offered support services when needed. Understanding the impact of patient-reported persistent and recurrent thyroid cancer on thyroid cancer survivors' lives will help personalize treatment and follow-up in these patients.
Appendix A1
Appendix A1
E1. Which of the following best describes your thyroid cancer?
□ My cancer was gone after initial treatment.
□ My cancer was never gone after initial treatment. I have persistent disease.
□ My cancer was gone but then it came back (recurrence).
[E4 was answered by those who indicated in question E1 that their cancer had come back (recurrence)].
E4. How did you find out that your cancer came back or that you had cancer recurrence?
Please mark ALL that apply.
□ My doctor said that an imaging test (such as ultrasound, CT, or PET) showed that my cancer came back
□ My doctor said that laboratory work, such as an elevated thyroglobulin, showed that my cancer came back
□ I had a biopsy and it showed that my cancer came back
□ I had surgery and the surgical pathology showed that my cancer came back
E5. Where was the recurrence found?
Please mark ALL that apply.
□ Neck □ Lungs
□ Bone □ Brain
□ Liver □ Other (please specify)_________________
Quality of Life
| In general… | Excellent | Very good | Good | Fair | Poor |
|---|---|---|---|---|---|
| H1. Would you say your health is……………… | |||||
| H2. Would you say your quality of life is……… | |||||
| H3. How would you rate your physical health? | |||||
| H4. How would you rate your mental health, including your mood and your ability to think?.….….….….….….….….….….….….….…. | |||||
| In general… | Excellent | Very good | Good | Fair | Poor |
| H5. How would you rate your satisfaction with your social activities and relationships? |
| Excellent | Very good | Good | Fair | Poor | |
|---|---|---|---|---|---|
| H6. In general, please rate how well you carry out your usual social activities and roles. (This includes activities at home, at work and in your community, and responsibilities as a parent, child, spouse, employee, friend, etc.) |
| Completely | Mostly | Moderately | A little | Not at all | |
|---|---|---|---|---|---|
| H7. To what extent are you able to carry out your everyday physical activities such as walking, climbing stairs, carrying groceries, or moving a chair? |
| Never | Rarely | Sometimes | Often | Always | |
|---|---|---|---|---|---|
| H8. In the past 7 days, how often have you been bothered by emotional problems such as feeling anxious, depressed, or irritable? |
| None | Mild | Moderate | Severe | Very severe | |
|---|---|---|---|---|---|
| H9. How would you rate your fatigue on average? |
| No pain | Worst imaginable pain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H10. How would you rate your pain on average? | ||||||||||
Disclaimer
The ideas and opinions expressed herein are those of the authors and endorsement by the State of California and State of Georgia Departments of Public Health, the National Cancer Institute and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work is supported by R01 CA201198 from the National Cancer Institute to Dr. M.R.H. Dr. M.R.H. is also supported by R01 HS024512 from Agency for Healthcare Research and Quality and Dr. M.P. by K08 AG049684 from the National Institute on Aging. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention's National Program of Cancer Registries, under cooperative agreement 5NU58DP006344 and the National Cancer Institute's Surveillance, Epidemiology, and End Results Program under contract HHSN261201800015I awarded to the University of Southern California. The collection of cancer incidence data in Georgia was supported by contract HHSN261201800003I, Task Order HHSN26100001 from the National Cancer Institute, and cooperative agreement 5NU58DP003875-04 from the Centers for Disease Control and Prevention.
References
- 1. NIH National Cancer Institute. Surveillance, epidemiology, and end results program 2019. Cancer stat facts: thyroid cancer. Available at https://seer.cancer.gov/statfacts/html/thyro.html (accessed December14, 2019)
- 2. Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. 1993. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057; discussion 1057–1058. [PubMed] [Google Scholar]
- 3. Shah MD, Hall FT, Eski SJ, Witterick IJ, Walfish PG, Freeman JL. 2003. Clinical course of thyroid carcinoma after neck dissection. Laryngoscope 113:2102–2107 [DOI] [PubMed] [Google Scholar]
- 4. Wang TS, Dubner S, Sznyter LA, Heller KS. 2004. Incidence of metastatic well-differentiated thyroid cancer in cervical lymph nodes. Arch Otolaryngol Head Neck Surg 130:110–113 [DOI] [PubMed] [Google Scholar]
- 5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hedman C, Strang P, Djarv T, Widberg I, Lundgren CI. 2017. Anxiety and fear of recurrence despite a good prognosis: an interview study with differentiated thyroid cancer patients. Thyroid 27:1417–1423 [DOI] [PubMed] [Google Scholar]
- 7. Hedman C, Djarv T, Strang P, Lundgren CI. 2018. Fear of recurrence and view of life affect health-related quality of life in patients with differentiated thyroid carcinoma: a prospective Swedish population-based study. Thyroid 28:1609–1617 [DOI] [PubMed] [Google Scholar]
- 8. Papaleontiou M, Reyes-Gastelum D, Gay BL, Ward KC, Hamilton AS, Hawley ST, Haymart MR. 2019. Worry in thyroid cancer survivors with a favorable prognosis. Thyroid 29:1080–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bresner L, Banach R, Rodin G, Thabane L, Ezzat S, Sawka AM. 2015. Cancer-related worry in Canadian thyroid cancer survivors. J Clin Endocrinol Metab 100:977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazzaferri EL, Jhiang SM. 1994. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97:418–428 [DOI] [PubMed] [Google Scholar]
- 11. Urken ML, Milas M, Randolph GW, Tufano R, Bergman D, Bernet V, Brett EM, Brierley JD, Cobin R, Doherty G, Klopper J, Lee S, Machac J, Mechanick JI, Orloff LA, Ross D, Smallridge RC, Terris DJ, Clain JB, Tuttle M. 2015. Management of recurrent and persistent metastatic lymph nodes in well-differentiated thyroid cancer: a multifactorial decision-making guide for the Thyroid Cancer Care Collaborative. Head Neck 37:605–614 [DOI] [PubMed] [Google Scholar]
- 12. Bates MF, Lamas MR, Randle RW, Long KL, Pitt SC, Schneider DF, Sippel RS. 2018. Back so soon? Is early recurrence of papillary thyroid cancer really just persistent disease? Surgery 163:118–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oltmann SC, Schneider DF, Chen H, Sippel RS. 2015. All thyroid ultrasound evaluations are not equal: sonographers specialized in thyroid cancer correctly label clinical N0 disease in well differentiated thyroid cancer. Ann Surg Oncol 22:422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang LY, Migliacci JC, Tuttle RM, Shaha AR, Shah JP, Patel SG, Ganly I. 2017. Management and outcome of clinically evident neck recurrence in patients with papillary thyroid cancer. Clin Endocrinol (Oxf) 87:566–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Durante C, Montesano T, Torlontano M, Attard M, Monzani F, Tumino S, Costante G, Meringolo D, Bruno R, Trulli F, Massa M, Maniglia A, D'Apollo R, Giacomelli L, Ronga G, Filetti S, Group PTCS. 2013. Papillary thyroid cancer: time course of recurrences during postsurgery surveillance. J Clin Endocrinol Metab 98:636–642 [DOI] [PubMed] [Google Scholar]
- 16. Liu FH, Kuo SF, Hsueh C, Chao TC, Lin JD. 2015. Postoperative recurrence of papillary thyroid carcinoma with lymph node metastasis. J Surg Oncol 112:149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schneider DF, Mazeh H, Chen H, Sippel RS. 2013. Lymph node ratio predicts recurrence in papillary thyroid cancer. Oncologist 18:157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dillman DA. 2007. Mail and Internet Surveys: The Tailored Design Method. Second ed. Wiley, New York [Google Scholar]
- 19. American Association for Public Opinion Research Response rates 2016. Available at https://aapor.org/Education-Resources/For-Researchers/Poll-Survey-FAQ/Response-Rates-An-Overview.aspx (accessed December15, 2019).
- 20. Weaver KE, Forsythe LP, Reeve BB, Alfano CM, Rodriguez JL, Sabatino SA, Hawkins NA, Rowland JH. 2012. Mental and physical health-related quality of life among U.S. cancer survivors: population estimates from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev 21:2108–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. 2009. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res 18:873–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Health Measures. Patient-Reported Outcomes Measurement Information System 2018. Available at http://www.healthmeasures.net/explore-measurement-systems/promis (accessed December14, 2019)
- 23. Shaw BE, Syrjala KL, Onstad LE, Chow EJ, Flowers ME, Jim H, Baker KS, Buckley S, Fairclough DL, Horowitz MM, Lee SJ. 2018. PROMIS measures can be used to assess symptoms and function in long-term hematopoietic cell transplantation survivors. Cancer 124:841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Homco J, Rodriguez K, Bardach DR, Hahn EA, Morton S, Anderson D, Kendrick D, Scholle SH. 2019. Variation and change over time in PROMIS-29 survey results among primary care patients with type 2 diabetes. J Patient Cent Res Rev 6:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sapuppo G, Tavarelli M, Belfiore A, Vigneri R, Pellegriti G. 2019. Time to separate persistent from recurrent differentiated thyroid cancer: different conditions with different outcomes. J Clin Endocrinol Metab 104:258–265 [DOI] [PubMed] [Google Scholar]
- 26. Wang T, Jiang M, Ren Y, Liu Q, Zhao G, Cao C, Wang H. 2018. Health-related quality of life of community thyroid cancer survivors in Hangzhou, China. Thyroid 28:1013–1023 [DOI] [PubMed] [Google Scholar]
- 27. Hedman C, Djarv T, Strang P, Lundgren CI. 2017. Effect of thyroid-related symptoms on long-term quality of life in patients with differentiated thyroid carcinoma: a population-based study in Sweden. Thyroid 27:1034–1042 [DOI] [PubMed] [Google Scholar]
- 28. Singer S, Lincke T, Gamper E, Bhaskaran K, Schreiber S, Hinz A, Schulte T. 2012. Quality of life in patients with thyroid cancer compared with the general population. Thyroid 22:117–124 [DOI] [PubMed] [Google Scholar]
- 29. Ahmadi S, Rojas CR, Policarpio-Nicolas ML, Metter D, Avery D, Prihoda TJ, Sabra MM. 2017. Variability in thyroid cancer management and prognosis among Hispanic versus non-Hispanic patients: 17 years data from University of Texas Health Science Center and University Health System at San Antonio. Endocr Pract [Epub ahead of print]; DOI: 10.4158/EP171852.OR [DOI] [PubMed]
- 30. Moo-Young TA, Panergo J, Wang CE, Patel S, Duh HY, Winchester DJ, Prinz RA, Fogelfeld L. 2013. Variations in clinicopathologic characteristics of thyroid cancer among racial ethnic groups: analysis of a large public city hospital and the SEER database. Am J Surg 206:632–640 [DOI] [PubMed] [Google Scholar]
- 31. Robinson TJ, Thomas S, Dinan MA, Roman S, Sosa JA, Hyslop T. 2016. How many lymph nodes are enough? Assessing the adequacy of lymph node yield for papillary thyroid cancer. J Clin Oncol 34:3434–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sosa JA, Mehta PJ, Wang TS, Yeo HL, Roman SA. 2007. Racial disparities in clinical and economic outcomes from thyroidectomy. Ann Surg 246:1083–1091 [DOI] [PubMed] [Google Scholar]
- 33. Papaleontiou M, Hughes DT, Guo C, Banerjee M, Haymart MR. 2017. Population-based assessment of complications following surgery for thyroid cancer. J Clin Endocrinol Metab 102:2543–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goswami S, Peipert BJ, Mongelli MN, Kurumety SK, Helenowski IB, Yount SE, Sturgeon C. 2019. Clinical factors associated with worse quality-of-life scores in United States thyroid cancer survivors. Surgery 166:69–74 [DOI] [PubMed] [Google Scholar]
- 35. Almeida JP, Vartanian JG, Kowalski LP. 2009. Clinical predictors of quality of life in patients with initial differentiated thyroid cancers. Arch Otolaryngol Head Neck Surg 135:342–346 [DOI] [PubMed] [Google Scholar]
- 36. Applewhite MK, James BC, Kaplan SP, Angelos P, Kaplan EL, Grogan RH, Aschebrook-Kilfoy B. 2016. Quality of life in thyroid cancer is similar to that of other cancers with worse survival. World J Surg 40:551–561 [DOI] [PubMed] [Google Scholar]
- 37. van de Wal M, van de Poll-Franse L, Prins J, Gielissen M. 2016. Does fear of cancer recurrence differ between cancer types? A study from the population-based PROFILES registry. Psychooncology 25:772–778 [DOI] [PubMed] [Google Scholar]
- 38. In H, Bilimoria KY, Stewart AK, Wroblewski KE, Posner MC, Talamonti MS, Winchester DP. 2014. Cancer recurrence: an important but missing variable in national cancer registries. Ann Surg Oncol 21:1520–1529 [DOI] [PubMed] [Google Scholar]
- 39. Ye F, Moon DH, Carpenter WR, Reeve BB, Usinger DS, Green RL, Spearman K, Sheets NC, Pearlstein KA, Lucero AR, Waddle MR, Godley PA, Chen RC. 2017. Comparison of patient report and medical records of comorbidities: results from a population-based cohort of patients with prostate cancer. JAMA Oncol 3:1035–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Basch E, Dueck AC, Rogak LJ, Minasian LM, Kelly WK, O'Mara AM, Denicoff AM, Seisler D, Atherton PJ, Paskett E, Carey L, Dickler M, Heist RS, Himelstein A, Rugo HS, Sikov WM, Socinski MA, Venook AP, Weckstein DJ, Lake DE, Biggs DD, Freedman RA, Kuzma C, Kirshner JJ, Schrag D. 2017. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol 3:1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qureshi ZP, Ganz PA, Bennett CL. 2017. Improving the evidence base for delivery of high-quality cancer care. JAMA Oncol 3:1029–1031 [DOI] [PubMed] [Google Scholar]
- 42. Phillips KA, Milne RL, Buys S, Friedlander ML, Ward JH, McCredie MR, Giles GG, Hopper JL. 2005. Agreement between self-reported breast cancer treatment and medical records in a population-based Breast Cancer Family Registry. J Clin Oncol 23:4679–4686 [DOI] [PubMed] [Google Scholar]
- 43. Schootman M, Jeffe DB, West MM, Aft R. 2005. Self-report by elderly breast cancer patients was an acceptable alternative to surveillance, epidemiology, and end results (SEER) abstract data. J Clin Epidemiol 58:1316–1319 [DOI] [PubMed] [Google Scholar]
- 44. Clegg LX, Potosky AL, Harlan LC, Hankey BF, Hoffman RM, Stanford JL, Hamilton AS. 2001. Comparison of self-reported initial treatment with medical records: results from the prostate cancer outcomes study. Am J Epidemiol 154:582–587 [DOI] [PubMed] [Google Scholar]
- 45. Kovatch KJ, Reyes-Gastelum D, Hughes DT, Hamilton AS, Ward KC, Haymart MR. 2019. Assessment of voice outcomes following surgery for thyroid cancer. JAMA Otolaryngol Head Neck Surg 145:823–829 [DOI] [PMC free article] [PubMed] [Google Scholar]