Abstract
Introduction: Multikinase inhibitors have clinical activity in radioactive iodine refractory (RAIR) differentiated thyroid cancers (DTCs) but are not curative; optimal management and salvage therapies remain unclear. This study assessed clinical effects of pazopanib therapy in RAIR-DTC patients with progressive disease, examining in parallel biomarker that might forecast/precede therapeutic response.
Methods: Assessment of responses and toxicities and of any association between thyroglobulin (Tg) changes cycle 1 and RECIST (response evaluation criteria in solid tumors) response to pazopanib therapy were prospectively undertaken in Tg antibody negative RAIR-DTC patients. RECIST progressive metastatic disease <6 months preceding enrollment was required. With a sample size of 68 (assuming 23 attaining partial response [PR]), there would be 90% chance of detecting a difference of >30% when the proportion of patients attaining PR whose Tg values decrease by >50% is >50% cycle 1 (one-sided α = 0.10, two sample test of proportions). Mean corpuscular volume (MCV) change or mutational status or pretreatment were also explored as early correlates of eventual RECIST response.
Results: From 2009 to 2011, 60 individuals were treated and evaluated; (one additional patient withdrew; another was found ineligible before therapy initiation); 91.7% had previous systemic therapy beyond RAI. Adverse events included one death (thromboembolic) deemed possibly pazopanib associated. Twenty-two confirmed RECIST PRs resulted (36.7%, confidence interval; CI [24.6–50.1]); mean administered 4-week cycles was 10. Among 44 fully accessible patients, the Tg nadir was greater among the 20 attaining PR (median: −86.8%; interquartile range [IQR]: −90.7% to −70.9%) compared with the 28 who did not (median: −69.0%; IQR: −78.1% to −27.7%, Wilcoxon rank-sum test: p = 0.002). However, the difference in the proportion of PRs among those whose Tg fell ≥50% after cycle 1 versus those that did not were not significantly correlated (−23.5% [CI: −55.3 to 8.3]; Fisher's exact test p-value = 0.27). RECIST response was also not correlated with/predicted by early MCV change, receipt of prior therapy, or tumor mutational status.
Conclusions: This trial prospectively confirmed pazopanib to have clinical activity and manageable toxicities in patients with progressive RAIR-DTC. Response to pazopanib, however, was not robustly forecast by early associated changes in Tg or MCV, by prior therapy, or by tumor mutational status. ClinicalTrials.gov NCT00625846.
Keywords: pazopanib, radioactive iodine refractory, differentiated thyroid cancer, kinase inhibitor
Introduction
Over the past decade multikinase inhibitors (MKIs) have emerged as therapeutically useful disease-modifying agents in thyroid cancers (1–11), with sorafenib (1,2) and lenvatinib (3) approved by the US Food and Drug Administration for use in progressive metastatic radioactive iodine refractory differentiated thyroid cancer (RAIR-DTC). Importantly, other MKIs [including cabozantinib (4), vandetanib (5), axitinib (6), sunitinib (7–9), motesanib (10), and pazopanib (11)] also have activity in metastatic RAIR-DTC and are finding increasing application especially among patients who progress through initial, or even second-line, MKI therapy.
Our group had previously reported a high level of activity of the MKI pazopanib in a phase 2 study in largely therapy-naive RAIR-DTC patients [response evaluation criteria in solid tumors, RECIST, partial response (PR) 49%, N = 37] (11). However, the results of our prior phase 2 trial have yet to be formally replicated/validated, and they have also not been revisited in a more heavily pretreated patient population. Moreover, the question arose as to whether baseline, or alternatively early changes in, biomarkers might forecast/predict eventual response to pazopanib therapy, so as to prospectively guide the application of pazopanib therapy most intensively among patients with the greatest potential for benefit.
In this article, we, therefore, report results of a second 12 site international phase 2 clinical trial of pazopanib in RAIR-DTC, with goals of assessing the efficacy and safety of pazopanib in an independent patient cohort, wherein patients were more heavily pretreated, and with the primary additional experimental endpoint of assessing any potential correlation between early (cycle 1, four-week) change in thyroglobulin (Tg) level and eventual RECIST response.
Patients and Methods
Study design and statistics
The intentions of this prospective trial were multiple; primary among them was to validate the safety and efficacy of pazopanib monotherapy in a larger and more heavily pretreated RAIR-DTC cohort than in our previously published trial (11). This said, statistical design and power calculations for this Phase II international therapeutic clinical trial required a single primary endpoint; we elected, therefore, a design intended to provide adequate statistical power to in parallel assess whether cycle 1 induced Tg changes might forecast eventual RECIST response to pazopanib monotherapy among Tg antibody negative patients—so as to formally and prospectively define whether early induced changes in Tg might be sufficiently robust to parse patients into responder and nonresponder cohorts early on.
We reasoned that, if we might be able to predict eventual response the pazopanib therapy, we might be able to use pazopanib more selectively and in patients with greatest changes of eventual benefit. Based on the published findings of MC057H cohort 1 (11), we expected twice the number of patients to have RECIST stable or progressive disease compared with PR, and we also expected that the proportion of patients with at least a 50% decrease in Tg values from pretreatment levels would be 50% in patients with stable/progression disease—and 80% in patients with RECIST PR.
Given these assumptions, with a sample size of 68 patients (predicted to yield 23 with PR) and a one-sided α = 0.10, two sample test of proportions would have a 90% chance of detecting a difference of at least 30% when the proportion of patients with stable/progressive disease whose Tg values decreased by at least 50% is at most 50%. In the end, we closed the trial to accrual after enrollment of 60 patients, having attaining the required number of responses (22) as per initial power calculations.
Moreover, we sought also to in parallel explore other candidate early predictors of eventual RECIST response to pazopanib therapy. In a former study, we had in particular anecdotally noted increasing mean corpuscular volume (MCV) in response to pazopanib therapy, and thus also wondered whether MCV increase might alternatively represent a predictive biomarker of response to pazopanib therapy. However, to assess a correlation in the former study would have been biased, so we chose to undertake prospective evaluation of the hypothesis that induced changes in MCV might correlate with pazopanib response in this prospective study.
We further wondered whether response to pazopanib might alternatively correlate with prior therapy or instead with somatic/tumor mutational status. However, our goal was to define whether pazopanib therapeutic response might be predicted by these candidate biomarkers, seeking in particular to forecast response before response occurs, wishing to parse patients early-on into those who stand to gain the greatest therapeutic benefit. In short, the seminal questions posed were not related to whether correlations might be observed, but whether there might be predictive correlations.
RECIST 1.0 was used to assess disease response, and the Kaplan–Meier method was used to generate survival curves in this National Cancer Institute sponsored and Institutional Review Board approved trial. Exploratory assessments of potential correlations of RECIST response outcomes with changes in erythrocyte MCV, prior therapy, or with mutational status when available used the Wilcoxon rank-sum test or Fisher's exact tests as specified.
Patient eligibility and assessments
This trial enrolled individuals ≥18 years of age with histologically or cytologically confirmed advanced or metastasized DTC that was Tg antibody negative and RAIR—so as to assess efficacy and toxicities and any potential correlation between early Tg changes and RECIST therapeutic response. Enrollment of patients with unresectable locoregionally advanced disease was allowed at investigator discretion. Eligibility criteria also included objective evidence of (RECIST) disease progression ≤6 months before registration; Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2; at least one lesion that could be accurately measured in at least one dimension as ≥2.0 cm by conventional imaging or ≥1.0 cm by spiral CT scan (RECIST 1.0); adequate blood chemistries (absolute neutrophil count ≥1500/mm (3); platelet count ≥100,000/mm (3); leukocyte count ≥3000/mm (3); total bilirubin ≤1.5 times the institutional upper limit of normal [ULN]; serum creatinine ≤1.5 × ULN, AST ≤2.5 × ULN, proteinuria ≤1+ and INR ≤1.2 × ULN); and systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg (ongoing antihypertensive therapy allowed).
RAIR-DTC was defined as disease unresponsive to, or progressive despite, administered therapeutic doses or RAI regardless of cumulative lifetime dosage of administered RAI.
Individuals were excluded from participation if they had >2 prior systemic therapeutic regimens in addition to RAI/for RAIR-DTC; had discontinued radiation, surgery, or systemic therapy ≤28 days before registration; had >1+ proteinuria on two consecutive dipstick tests taken ≥1 week apart; had QTc prolongation (>500 mseconds) or other significant ECG abnormalities; were receiving concomitant medications associated with a risk of QTc prolongation or Torsades de Pointes, cytochrome P450 drug interactions, or affecting the activity or pharmacokinetics of pazopanib; were currently taking therapeutic warfarin (low-molecular-weight heparin was allowed); had any condition that impaired ability to swallow and retain pazopanib, active or untreated brain metastases, or brain metastases requiring ongoing therapy; comorbid conditions such as nonhealing wound; ulcer; bone fracture; poorly controlled depression or anxiety disorder; cerebrovascular accident; abdominal fistula, gastrointestinal perforation; active diverticulitis, or gastrointestinal bleeding ≤28 days before registration; venous thrombosis <12 weeks before registration; history of bleeding disorder; NYHA Class III/IV heart failure; cardiac arrhythmia, or myocardial infarction.
Women of child-bearing potential also underwent serum pregnancy test within seven days of registration and agreed to use effective contraception during treatment. Institutional Review Boards of all participating institutions (12 institutions in total) approved this study and informed consent was obtained from each patient before study entry.
Within four weeks of study entry—and before each cycle of treatment—patients underwent a complete medical examination with blood chemistries and adverse event assessment. Blood pressure measurements were taken twice daily beginning day ≤8 until treatment discontinuation. Tumor RECIST measurements were required every eight weeks. Although not required, when tumor materials were available for analysis, samples were procured and subject to an in-house panel assessing somatic mutations, including BRAF, PIKCA3, PTEN, H/K/NRAS, JAK1–3, and TP53; MCV was assessed at each baseline and cycle, as were Tg and Tg antibodies, with changes in these markers scrutinized as a part of this trial.
Treatment and dose modification
Pazopanib was administered orally fasting (two hours after and one hour before food intake) once daily, initially at 800 mg, until disease progression, required dosage reduction, or intolerability/refusal; cycle length was 28 days.
Dosage reductions and toxicities were based upon preset toxicity criteria, graded based upon Common Terminology Criteria for Adverse Events (CTCAE) 3.0. At most, three dosage reductions were allowed [for grade ≥3 adverse events (excluding proteinuria, hypertension, hemorrhage/bleeding, perforation, thrombosis or thrombocytopenia, neutropenia or anemia)] and treatment could not be resumed if toxicity did not resolve to ≤grade 1 within three weeks. Pazopanib dose reductions per preordained toxicity were 600 mg/day, then 400 mg/day, and finally 200 mg/day. Treatment was permanently discontinued in the event of any intestinal perforation; grade 3 or 4 hemorrhage (or a second instance of grade 2 hemorrhage); symptomatic grade 4 thrombosis (or second instance of grade 2 or higher thrombosis); second instance of grade 3 or 4 thrombocytopenia, neutropenia, or anemia; elevated blood pressure not adequately controlled with antihypertensives; or urine proteinuria >1+.
Results
Study cohort
From May 1, 2009 to December 31, 2011, 62 individuals across 12 international sites were enrolled onto this trial of 68 initially postulated required slots. The lower number of patients accrued than expected occurred after discussions between the principal investigator upon attainment of the intended number of patient RECIST PRs per initial power calculations. The data were locked on July 24, 2016, with the long time elapsed since study closure deliberate, so as to allow robust patient follow-up. One of the 62 enrolled patients was found ineligible, having started treatment before registration, and another patient cancelled participation before starting treatment, resulting in 60 treated and evaluable patients.
Patient and disease characteristics of the cohort of 60 patients (males: 55%), including tumor histology, performance status, and number of prior therapies, are presented in Table 1. The most common sites of metastases were lung (90.0%), lymph nodes (75.0%), bone (35.0%), and liver (13.3%). Medications being taken at the time of registration included antihypertensives (56.7%) and narcotics (18.3%). All patients have been followed until treatment discontinuation or a maximum of 65 cycles.
Table 1.
Patient Characteristics
| n = 60 | |
|---|---|
| Median age (25th–75th percentile) | 60 Years (51–69) |
| Male, n (%) | 33 (55) |
| ECOG performance status, n (%) | |
| 0 | 37 (61.7) |
| 1 | 19 (31.7) |
| 2 | 4 (6.7) |
| Histological subtype, n (%) | |
| Follicular | 16 (26.7) |
| Hürthle cell | 8 (13.3) |
| Papillary | 36 (60.0) |
| Prior radiation | 35 (58.3) |
| No. of prior systemic therapies, n (%) | |
| 0 | 4 (6.7) |
| 1 | 40 (66.7) |
| 2–4 | 16 (22.7) |
| Prior systemic therapies, n (%) | |
| Radioiodine | 54 (90.0) |
| Sorafenib ± Everolimus (trial) | 6 (10.0) |
| Lenalidomide | 3 (5.0) |
| Panobinostat | 2 (3.3) |
| Bexarotene | 2 (3.3) |
| DepsiPeptide | 1 (1.7) |
| Doxorubicin ± cisplatin | 1 (1.7) |
| Gemcitabine | 1 (1.7) |
| Lenvatinib | 1 (1.7) |
| Octreotide | 1 (1.7) |
| Sirolimus | 1 (1.7) |
| Strontium 89 | 1 (1.7) |
| Sunitinib | 1 (1.7) |
| Sites of metastatic disease, n (%) | |
| Lung | 54 (90.0) |
| Nodes | 45 (75.0) |
| Bone | 21 (35.0) |
| Liver | 8 (13.3) |
| Subcutaneous/soft tissue | 6 (10.0) |
| Trachea ± larynx | 2 (3.3) |
| Abdomen | 1 (1.7) |
| Brain | 1 (1.7) |
| Symptoms at registration, n (%) | |
| Grade 3 hypertension | 1 (1.7) |
| Grade 2 hypertension | 1 (1.7) |
| Grade 1 hypertension | 15 (25.0) |
| Grade 1 fatigue | 19 (31.7) |
| Grade 1 anorexia | 2 (3.3) |
| Grade 2 anemia | 3 (5.0) |
| Grade 1 anemia | 15 (30.0) |
| Narcotic use | 11 (18.3) |
| Hypertension medication use | 34 (56.7) |
ECOG, Eastern Cooperative Oncology Group.
Dose reductions and adverse events
Thirty-three patients (55.5%) had at least one dosage reduction, with median cohort pazopanib dosage of 600 mg/day. The most common severe (CTCAE v3.0 grades 3–5) toxicities reported included hypertension (21.7%), fatigue (8.3%), and neutropenia (8.3%); these and all-grade toxicities are enumerated in Table 2. Reasons for discontinuation of treatment include disease progression (42 patients, 72%); adverse events (6 patients, 10.3%; grade 4 vaginal hemorrhage: 1 patient; grade 4 thrombosis: 1 patient; grade 3 oral mucositis: 1 patient; grade 3 alanine aminotransferase increase: 1 patient; grade 3 hand and foot syndrome: 1 patient; grade 4 hypertension: 1 patient); patient refusal (5 patients, 8.6%); comorbid conditions (3 patients, 5.2%), and death (2 patients, 3.4%).
Table 2.
Adverse Events
| Type | Severe (grade 3–5) (%) | Any grade (%) |
|---|---|---|
| Abdominal pain | 1.7 | 8.3 |
| Alanine aminotransferase increase | 5.0 | 20.0 |
| Anorexia | 3.3 | 43.3 |
| Aspartate aminotransferase increase | 3.3 | 43.3 |
| Diarrhea | 6.7 | 75.0 |
| Dyspnea | 1.7 | 1.7 |
| Encephalopathy | 1.7 | 1.7 |
| Fatigue | 8.3 | 78.3 |
| Hand-foot syndrome | 6.7 | 16.7 |
| Hypertension | 21.7 | 71.7 |
| Leukocyte count decrease | 3.3 | 53.3 |
| Lipase increase | 1.7 | 1.7 |
| Lymphocyte count decrease | 5.0 | 13.3 |
| Neutrophil count decrease | 8.3 | 46.7 |
| Oral mucositis | 3.3 | 8.3 |
| Serum calcium decrease | 1.7 | 6.7 |
| Serum sodium decrease | 1.7 | 3.3 |
| Sinus tachycardia | 1.7 | 1.7 |
| Thrombosis | 3.3 | 5.0 |
| Vaginal hemorrhage | 1.7 | 1.7 |
| Vomiting | 1.7 | 28.3 |
| Weight loss | 3.3 | 10.0 |
During the course of treatment, 14 of the 26 patients (53.8%) not taking antihypertensive agents at study entry began taking them; 14 of the 49 patients (28.5%) not taking narcotics at study entry began taking them. Two deaths on study were noted: a 53-year-old male whose pazopanib was withheld after nine cycles to receive radiation treatment to his femoral neck who had a seizure and died two days later off pazopanib therapy (deemed therapy unrelated); and a 61-year-old female who, during the first cycle of treatment, was admitted to the hospital with shortness of breath and chest pain dying two days later due to respiratory failure with hypotension, dyspnea, and sinus tachycardia (possible pulmonary embolism, undocumented; considered possibly therapy related and possibly disease related).
Treatment and tumor response
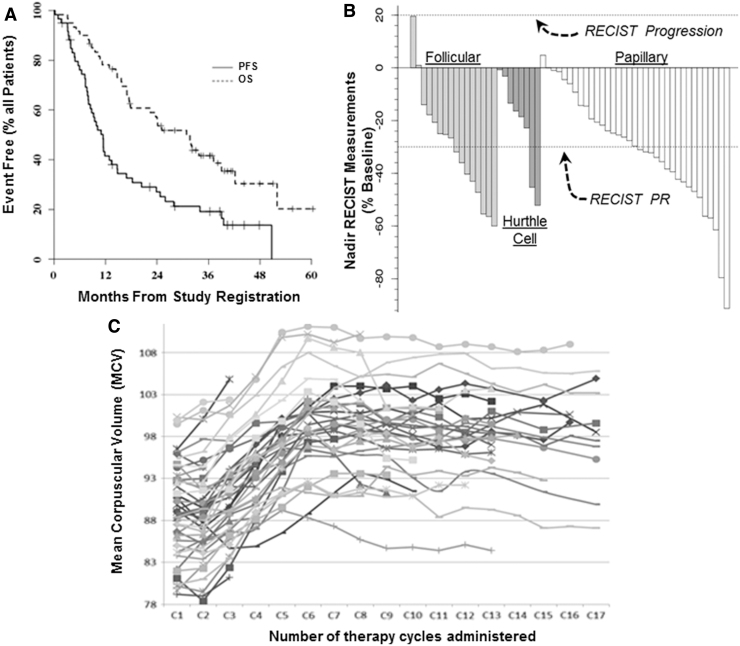
Median progression-free and overall survival (PFS and OS, defined from start of study therapy initiation) were estimated to be 11.4 months and 2.6 years, respectively (Fig. 1A). The median number of cycles administered thus far is 10 (range: 1–67+). Two patients were still on study treatment at the time of article preparation. There have been 22 partial tumor responses (36.7% [CI 24.6–50.1]; see Fig. 1B for waterfall plot of best RECIST response by histology) among 60 enrolled patients; this PR confidence interval (CI) overlaps with our previously reported phase 2 RAIR-DTC PR rate of 49% (11). The median duration of attained RECIST PRs was 10.3 months.
FIG. 1.
Effects of pazopanib study therapy on PFS and OS; (A) best RECIST response by histology (B), and on mean corpuscular volume (C). OS, overall survival; PFS, progression-free survival; RECIST, response evaluation criteria in solid tumors.
Tumor response rates by histological subtype are presented in Table 3 (see Fig. 1B for histology-specific waterfall plots); in contrast to our previous report (11), we did not observe a higher response rate among Hürthle cell thyroid cancer patients. The waterfall plots of best RECIST response (Fig. 1B) demonstrate that the vast majority of patients experienced lessened disease burden in response to pazopanib therapy regardless of DTC histology. At the time of the data lock, 8 patients (13.3%) were alive without disease progression, 11 (18.3%) were alive with disease progression, and 41 (68.3%) have died of disease.
Table 3.
Response Evaluation Criteria In Solid Tumors Partial Response Rates by Histology
| Histology | N | RECIST PR rate [CI] | Median duration of response (months) |
|---|---|---|---|
| Follicular | 16 | 37.5% [15.2–64.6] | 8.5 |
| Hürthle cell | 8 | 25.0% [3.2–65.1] | 5.4 |
| Papillary | 36 | 38.9% [23.1–56.5] | 17.0 |
CI, confidence interval; PR partial response; RECIST, Response Evaluation Criteria In Solid Tumors.
Primary statistical endpoint—changes in Tg level after one cycle of treatment
Tg levels after one cycle of treatment were available for 44 of 60 otherwise evaluable patients (18, 40.9%, patients with PR); reasons for paired cycle 1 Tg unavailability in the remaining 16 patients (27%) included Tg antibody detection/emergence upon on-study reassessment or detection of interfering substances preventing quantitation of Tg levels (at the time of this study, assessment of Tg by mass spectrometry was not yet available at study centers).
Of 44 patients with Tg levels available after one cycle of treatment, 18 attained PR: 2 of the 9 patients (22.2%) whose Tg levels fell ≥50% after one cycle had a PR, whereas 16 of the 35 patients (45.7%) whose Tg levels fell <50% after one cycle of treatment had a PR, not supporting a correlation between the magnitude of change in Tg and attainment of RECIST PR after one cycle of pazopanib therapy.
Formally, the difference in the proportion of PRs among those patients whose Tg level fell ≥50% after one cycle of treatment and those that did not was −23.5% [CI −55.3 to 8.3]; Fisher's exact test p-value = 0.27); thus, there was also no statistical support to indicate that cycle one Tg results might forecast PR. There were 2 patients among the 18 patients (11.1%) who met RECIST criteria for PR and 7 patients among the 26 patients (26.9%) who did not meet the RECIST criteria for PR who had a ≥50% decrease in Tg levels after one cycle of treatment further amplifying the observed noncorrelation between declining Tg occurring cycle one and best anatomical tumor response.
Although not an indication that change in Tg can serve as a valid predictive biomarker, “all cycle”/eventual Tg nadir was greater among the 20 patients attaining PR (median: −86.8%; interquartile range [IQR]: −90.7 to −70.9) compared with the 28 who did not (median: −69.0%; IQR: −78.1 to −27.7, Wilcoxon rank-sum test: p = 0.002). Hence, the study results demonstrated a statistically significant correlation between best RECIST response and best eventual Tg response—but not an analogous correlation after but a single cycle of pazopanib therapy.
Exploratory endpoint—changes in MCV induced with therapy
Noting MCV anecdotally often to increase in our historical pazopanib-treated patients, we also assessed MCV change as another candidate correlate of early RECIST response. MCV as a function of patient therapy cycle is shown in Figure 1C. Sixteen patients had MCV levels measured before the start of treatment and after one cycle of treatment. Ten of these patients had at most a 1 fL change in MCV after one treatment cycle, but correlation between progression-free survival and change in MCV after one cycle of treatment was not found to be significant (Spearman rank correlation = 0.408; p = 0.1168). All 16 of these patients have since had disease progression, 8 after having attained PR. Although not a robust early correlate of RECIST response, study MCV data clearly indicate increasing MCV on pazopanib therapy that plateaus at about cycle 6 (Fig. 1C).
Exploratory endpoint—mutational status and therapeutic response
Although this trial was conducted before uniform mutational somatic assessment of our advanced DTC patients—and before report of The Cancer Genome Atlas data for thyroid cancer—mutational analysis was retrospectively undertaken as feasible using an in-house panel, including BRAF, PIKCA3, PTEN, H/K/NRAS, JAK1–3, and TP53, and were accordingly assessed for these specific somatic mutations. Fourteen of 60 patients (23%) had sufficient tumor available for genotyping. No mutations were found in the tumors of the two patients with follicular thyroid cancer; neither of these two tumors responded to treatment.
Mutations were found in the tumors of the eight patients with papillary thyroid cancer. Two of these eight patients had disease that responded to treatment: one had a HRAS c.182A>G p.Q61R mutated tumor and the other a BRAF c.1799T>A, V600E and PTEN D331G mutated tumor. As expected, in the papillary cohort, five of eight genotyped patients (62.5%) had BRAFV600E mutations. Mutations were found in tumors of three of the six patients with Hürthle cell thyroid cancer; two of these six patients had disease that responded to treatment: one had no mutations detected in their tumors and the other had a PTEN c.955dupA, p.T319NfsX6; TP53 c.626_627delGA, p.Arg209LysfsX6 mutated tumor (Table 4).
Table 4.
Mutational Findings for Subset of 16 Patients by Histological Subtype
| Histological subtype | |
|---|---|
| Follicular | n = 2 |
| None identified, n (%) | 2 (100) |
| Hürthle cell | n = 6 |
| None identified, n (%) | 3 (50.0) |
| JAK3 c.2164G>A, p.V722I, n (%) | 1 (16.7) |
| TP53 S241F, n (%) | 1 (16.7) |
| PTEN c.955dupA, p.T319NfsX6; TP53 c.626_627delGA, p.Arg209LysfsX6, n (%) | 1 (16.7) |
| Papillary | n = 8 |
| BRAF c.1799T>A, V600E, n (%) | 3 (37.5) |
| BRAF c.1799T>A, V600E; PIK3CA c.3140A>T, p.H1047L, n (%) | 1 (12.5) |
| BRAF c.1799T>A, V600E; PTEN D331G, n (%) | 1 (12.5) |
| HRAS c.182A>G p.Q61R, n (%) | 2 (25.0) |
| TP53 c.484A>T, p.I162F, n (%) | 1 (12.5) |
We were unable to detect a difference in either the clinical response (PR) rate (Fisher's exact test p = 0.999) or PFS (Wilcoxon rank-sum test p = 0.821) between mutation detected/undetected cohorts (Table 4). We were also unable to discern a difference in RECIST response rate with respect to whether histological subtype was papillary or not (Fisher's exact test p = 0.999); whether the patient had BRAFV600E mutated DTC or not (Fisher's exact test p = 0.999); whether the patient had BRAFV600E or HRAS mutated DTC or not (Fisher's exact test p = 0.999); or whether the patient had TP53 mutated or not (Fisher's exact test p = 0.529); data shown in Tables 5 and 6.
Table 5.
Response Evaluation Criteria in Solid Tumors Tumor Response Rates by Presence/Absence of Specific Mutations and Histology
| Mutation | Histological subtype |
||
|---|---|---|---|
| Follicular (n = 2), n (%) | Hurthle cell (n = 6), n (%) | Papillary (n = 8), n (%) | |
| No mutation identified | 2 (100) | 3 (50) | |
| BRAF, V600E | 3 (37.5) | ||
| BRAF, V600E, PIKCA3 | 1 (12.5) | ||
| BRAF, V600E, PTEN | 1 (12.5) | ||
| HRAS | 2 (25.0) | ||
| JAK3 | 1 (16.7) | ||
| PTEN, TP53 | 1 (16.7) | ||
| TP53 | 1 (16.7) | 1 (12.5) | |
Table 6.
Response Evaluation Criteria in Solid Tumors Tumor Response Rates by Presence/Absence of Specific Mutations
| Clinical response by subgroups (patients with mutational interrogation only) | |
|---|---|
| Histological subtype | |
| Follicular | 0/2 (0%) |
| Hürthle cell | 2/6 (33.3%) |
| Papillary | 2/8 (25.0%) |
| BRAF mutation | |
| Yes | 1/5 (20.0%) |
| No | 3/11 (27.3%) |
| BRAF or HRAS mutation | |
| Yes | 2/7 (28.6%) |
| No | 2/9 (22.2%) |
| TP53 mutation | |
| Yes | 0/3 (0%) |
| No | 4/13 (30.8%) |
Discussion
The need for additional therapeutics in RAIR-DTC is clear; although approved for use in RAIR-DTC by the US FDA, neither sorafenib nor lenvatinib has curative potential in this disease. Additional therapeutic options are thus inevitably required for patients seeking additional therapy upon progression through, or poorly tolerating, the two FDA-approved agents. In this context, the present report importantly confirms our prior reported high RECIST response rate to pazopanib in RAIR-DTC in a larger and more heavily pretreated 60 patient cohort.
In particular, the response rates in our two phase two studies of pazopanib were 49% in a less heavily pretreated 37 patient cohort (11), and 37% in this larger and more heavily pretreated 60 patient RAIR-DTC cohort. These results are comparable with other outcomes reported for other kinase inhibitors used in this setting, as are the waterfall plots presented in Figure 1B (1–10). Results from now two independent prospective clinical trials involving almost 100 patients, therefore, support clinically meaningful efficacy and tolerability of pazopanib in RAIR-DTC.
This 12 site international therapeutic clinical trial of pazopanib is distinguished as one of the larger therapeutic clinical trials yet conducted in metastatic and progressive RAIR-DTC. Only four larger analogous trials have been published to date—the FDA registration trials for sorafenib (1) and lenvatinib (3), a phase 2 randomized trial of vandetanib (approved in medullary thyroid cancer) (5), and a trial of the investigational agent motesanib (an agent no longer in clinical development) (10). Moreover, most published therapeutic phase 2 trials in RAIR-DTC remain unreplicated/validated in confirmatory trials, also distinguishing the present trial and pazopanib from many other kinase inhibitors previously assessed in this disease.
However, in terms of the primary prospective statistical endpoint of this phase 2 trial, that of assessing whether early Tg changes might serve as a predictive biomarker of eventual response to pazopanib therapy, no correlation was demonstrated. This said, we nevertheless found a correlation between the magnitude of RECIST tumor measurement decline and all cycle Tg nadir, but cycle one Tg decline did not forecast/allow prediction of tumor RECIST responses.
As to why a late, but not an early and predicative correlation between Tg decline and RECIST response was not observed in this study, we can only speculate. Thyrotropin (TSH) often increases in response to pazopanib therapy, as can occur in response to any MKI, potentially obscuring a correlation between Tg changes and RECIST response, as Tg is expected to increase in the event of TSH increase.
In this study, overall, TSH levels increased by ≥15% between baseline and cycle one/4-week reassessment in 64.6%, indicating that early TSH increment is frequent in response to pazopanib therapy. Also of consideration is that Tg data were incomplete in 27%, reflecting the realities of serial Tg assessment wherein Tg antibodies were found fluctuating, and interfering substances were sometimes found confounding, potentially also lessening our ability to detect correlations.
Regardless, when we examined for correlation on an “intention to treat” basis, early Tg changes were not found sufficiently reliable to stratify patient into groups more or less likely to respond to pazopanib therapy. In the final analysis, that we detected a statistically significant correlation between all cycle Tg nadir and best RECIST response indicates that methodological issues seem unlikely to have obscured detection of an analogous correlation, if present, between cycle one/4-week Tg change and eventual RECIST response, suggesting the absence of true correlation.
Of interest is that this study appears to be the first to report that pazopanib is associated with increased MCV (Fig. 1C)—which we also examined as a nonprospectively specified candidate predictive biomarker pf RECIST response. Other multikinase inhibitors have been previously shown to increase MCV, perhaps due to the effects of inhibition of c-Kit on hematopoietic stem cells (12–14), so the noted pazopanib-associated increase in MCV appears to reflect a “class-effect.”
Unlike reports in renal cell carcinoma in the case of other MKIs (12–14), the extent of MCV elevation did not in this study correlate with early RECIST response in RAIR-DTC. These data, combined with Tg and somatic mutational data, add to the body of growing evidence arguing against any yet identified credible early predictive candidate biomarkers of response to MKIs in RAIR DTC (15,16).
The question arises as to what might distinguish pazopanib from other MKIs and why pazopanib might be of particular appeal in treating RAIR-DTC, especially as other MKIs are US FDA and EU approved in this space, yet pazopanib is not. First, we note that the response rates reported in RAIR-DTC to pazopanib therapy are high (36%, 49%). Secondly, comparative analyses of pazopanib relative to other MKIs indicate lessened toxicities with regard to plantar/palmar erythroderma and hematologic toxicities, lesser negative impact on quality of life, and also show that providers prefer pazopanib over other MKIs 2:1 at least in the setting of treating renal cell carcinoma (17–19). Moreover, many published phase 2 trials of other MKIs in thyroid cancer remain unreplicated, and are thus difficult to interpret in the context of data indicating that 40% of published phase 2 trials cannot be reproduced in later confirmatory trials (20).
Limitations of this study, however, are several. First, it is not valid to directly compare outcomes across published studies, as study populations might vary. Hence, although pazopanib RECIST PR rates are numerically high, a randomized blinded multicohort trial will be necessary to judge differences between individual MKI response rates. We can, therefore, declare the results of this study numerically encouraging, but statistical proof of equivalence, superiority, or inferiority to other MKIs in RAIR-DTC remains lacking.
Next, in terms of our primary statistical goal of assessing the predictive value of cycle 1 changes in Tg in forecasting RECIST response, several obstacles hindering a positive outcome should be noted. First, it was not consistently possible to assess Tg levels longitudinally, as 27% of patients had incomplete data principally due to intermittently elevated levels of Tg antibody or the presence of interfering substances. Moreover, as pazopanib induced elevations in TSH in the majority, and as elevations in TSH can in parallel increase Tg, the chances of detection of any potential true predictive value of early changes in Tg in forecasting RECIST response might thereby be attenuated. These issues, however, are unavoidable, adding further credence to the assertion that early changes in Tg cannot be relied upon to stratify pazopanib-treated patients into responding and nonresponding subsets.
Also of note is that somatic mutational data related to this study are available on only 26% of enrolled patients, primarily due to the accrual of patients between 2009 and 2011. Hence, assessment of correlations between somatic mutations and pazopanib response can only be considered exploratory. It should further be noted that, although heavily pretreated with systemic therapies beyond RAI, only eight study patients (10%) had been previously treated with MKIs. Hence, that four of these eight patients attained RECIST PRs is promising, but not definitive in terms of concluding that pazopanib represents an effective salvage approach among RAIR-DTC patients progressing despite prior MKI therapy.
In conclusion, no predictive biomarkers were found to facilitate the robust early identification of patients likely to respond to pazopanib therapy in RAIR DTC. The present trial importantly nevertheless provides substantiating and confirmatory evidence in support of the therapeutic efficacy and tolerability of pazopanib in treating RECIST progressive and metastatic RAIR-DTC. We posit, therefore, that providers should consider the use of pazopanib in RAIR-DTC among other therapeutic systemic therapeutic options.
Acknowledgments
The authors thank the patients who participated in this trial for their seminal contributions to the study, and for their confidence in our care; we also thank the National Cancer Institute for funding this study. The administrative assistance of Candace Kostelec and Bobbi-Ann Jebens is also very much appreciated.
Contributor Information
Collaborators: the Mayo Phase 2 Consortium
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported in part by the National Cancer Institute grants CA15083 and CM62205.
References
- 1. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JWA, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ; DECISION Investigators. 2014. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim M, Kim TH, Shin DY, Lim DJ, Kim EY, Kim WB, Chung JH, Shong YK, Kim BH, Kim WG; Korean Thyroid Cancer Study Group (KTCSG) 2018. Tertiary care experience of sorafenib in the treatment of progressive radioiodine-refractory differentiated thyroid carcinoma: a Korean multicenter study. Thyroid 28:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim S-B, Krzyzanowska MK, Dutcus CE, de las Heras B J Zhu, Sherman SI. 2015. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621–630 [DOI] [PubMed] [Google Scholar]
- 4. Cabanillas ME, de Souza JA, Geyer S, Wirth LJ, Menefee ME, Liu SV, Shah K, Wright J, Shah MH. 2017. Cabozantinib as salvage therapy for patients with tyrosine kinase inhibitor-refractory differentiated thyroid cancer: results of a multicenter phase II International Thyroid Oncology Group Trial. J Clin Oncol 35:3315–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leboulleux S, Bastholt L, Krause T, de la Fouchardiere C, Tennvall J, Awada A, Gómez JM, Bonichon F, Leenhardt L, Soufflet C, Licour M, Schlumberger MJ. 2012. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol 13:897–905 [DOI] [PubMed] [Google Scholar]
- 6. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB. 2008. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 26:4708–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, Bauman JE, Martins RG. 2010. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res 16:5260–5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bikas A, Kundra P, Desale S, Mete M, O'Keefe K, Clark BG, Wray L, Gandhi R, Barett C, Jelinek JS, Wexler JA, Wartofsky L, Burman KD. 2016. Phase 2 clinical trial of sunitinib as adjunctive treatment in patients with advanced differentiated thyroid cancer. Eur J Endocrinol 174:373–380 [DOI] [PubMed] [Google Scholar]
- 9. Ravaud A, de la Fouchardière C, Caron P, Doussau A, Do Cao C, Asselineau J, Rodien P, Pouessel D, Nicolli-Sire P, Klein M, Bournaud-Salinas C, Wemeau JL, Gimbert A, Picat MQ, Pedenon D, Digue L, Daste A, Catargi B, Delord JP. 2017. A multicenter phase II study of sunitinib in patients with locally advanced or metastatic differentiated, anaplastic or medullary thyroid carcinomas: mature data from the THYSU study. Eur J Cancer 76:110–117 [DOI] [PubMed] [Google Scholar]
- 10. Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, Licitra L, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Schlumberger MJ; Motesanib Thyroid Cancer Study Group 2008. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med 359:31–42 [DOI] [PubMed] [Google Scholar]
- 11. Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Sideras K, Morris JC III, McIver B, Burton JK, Webster KP, Bieber C, Traynor AM, Flynn PJ, Goh BC, Tang H, Ivy SP, Erlichman C; Endocrine Malignancies Disease Oriented Group; Mayo Clinic Cancer Center; Mayo Phase 2 Consortium 2010. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol 11:962–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bourlon MT, Gao D, Trigero S, Clemons JE, Breaker K, Lam ET, Flaig TW. 2016. Clinical significance of sunitinib-associated macrocytosis in metastatic renal cell carcinoma. Cancer Med 5:3386–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kucharz J, Giza A, Dumnicka P, Kuzniewski M, Kusnierz-Cabala B, Bryniarski P, Herman R, Zygulska AL, Krzemieniecki K. 2016. Macrocytosis during sunitinib treatment predicts progression-free survival in patients with metastatic renal cell carcinoma. Med Oncol 33:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kloth JSL, Hamberg P, Mendelaar PAJ, Dulfer RR, van der Holt B, Eechoute K, Wiemer EAC, Kruit WHJ, Sleijfer S, Mathijssen RHJ. 2016. Macrocytosis as a potential parameter associated with survival after tyrosine kinase inhibitor treatment. Eur J Cancer 56:101–106 [DOI] [PubMed] [Google Scholar]
- 15. Werner RA, Lückerath K, Schmid JS, Higuchi T, Kreissl MC, Grelle I, Reiners C, Buck AK, Lapa C. 2016. Thyroglobulin fluctuations in patients with iodine-refractory differentiated thyroid carcinoma on lenvatinib treatment—Initial experience. Sci Rep 6:28081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sugino K, Nagahama M, Kitagawa W, Ohkuwa K, Uruno T, Matsuzu K, Suzuki A, Masaki C, Akaishi J, Hames KY, Tomoda C, Ogimi Y, Ito K. 2018. Clinical factors related to the efficacy of tyrosine kinase inhibitor therapy in radioactive iodine refractory recurrent differentiated thyroid cancer patients. Endocr J 65:299–306 [DOI] [PubMed] [Google Scholar]
- 17. Kim JH, Park I, Lee JL. 2016. Pazopanib versus sunitinib for the treatment of metastatic renal cell carcinoma patients with poor-risk features. Cancer Chemother Pharmacol 78:325–332 [DOI] [PubMed] [Google Scholar]
- 18. Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK. 2013. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722–731 [DOI] [PubMed] [Google Scholar]
- 19. Escudier B, Porta C, Bono P, Powles T, Eisen T, Sternberg CN, Gschwend JE, De Giorgi U, Parikh O, Hawkins R, Sevin E, Négrier S, Khan S, Diaz J, Redhu S, Mehmud F, Cella D. 2014. Randomized, controlled, double-blind, crossover trial assessing treatment preference for pazopanib vs. sunitinib in patients with metastatic renal cell carcinoma: PISCES study. J Clin Oncol 32:1412–1418 [DOI] [PubMed] [Google Scholar]
- 20. Sharma MR, Stadler WM, Ratain MJ. 2011. Randomized phase II trials: a long-term investment with promising returns. J Natl Cancer Inst. 103:1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]