Abstract
Novel therapies are needed to address the increasing prevalence of chronic kidney disease. Mesenchymal stem/stromal cells (MSCs) and MSC-derived extracellular vesicles (EVs) augment tissue repair. We tested the hypothesis that EVs are as effective as MSCs in protecting the stenotic kidney, but target different injury pathways. Pigs were studied after 16 weeks of renal injury achieved by diet-induced metabolic syndrome (MetS) and renal artery stenosis (RAS). Pigs were untreated or treated 4 weeks earlier with intrarenal delivery of autologous adipose tissue-derived MSCs (107) or their EVs (1011). Lean pigs and sham RAS served as controls (n = 6 each). Stenotic-kidney function was studied in vivo using computed tomography and magnetic resonance imaging. Histopathology and expression of necroptosis markers [receptor-interacting protein kinase (RIPK)-1 and RIPK-3], inflammatory, and growth factors (angiopoietin-1 and vascular endothelial growth factor) were studied ex vivo. Stenotic-kidney glomerular filtration rate and blood flow in MetS + RAS were both lower than Lean and increased in both MetS + RAS + MSC and MetS + RAS + EV. Both MSCs and EV improved renal function and decreased renal hypoxia, fibrosis, and apoptosis. MSCs were slightly more effective in preserving microvascular (0.02–0.2 mm diameters) density and prominently attenuated renal inflammation. However, EV more significantly upregulated growth factor expression and decreased necroptosis. In conclusion, adipose tissue–derived MSCs and their EV both improve stenotic kidney function and decrease tissue injury in MetS + RAS by slightly different mechanisms. MSCs more effectively preserved the microcirculation, while EV bestowed better preservation of renal cellular integrity. These findings encourage further exploration of this novel approach to attenuate renal injury.
Keywords: mesenchymal stem cells, extracellular vesicles, renal artery stenosis
Introduction
Renal artery stenosis (RAS) is the major cause for renovascular hypertension and may lead to kidney ischemia and eventually fibrosis. RAS frequently coexists with metabolic syndrome (MetS), a constellation of cardiovascular risk factors that promotes renal injury and is associated with unfavorable renal outcomes [1]. Coexistence of RAS and MetS is associated with poorer outcomes after revascularization [2], underscoring the need for targeted interventions capable of improving the stenotic kidney in subjects with MetS + RAS.
Mesenchymal stem/stromal cells (MSCs) are multipotent cells with robust self-renewal, multilineage differentiation, proliferative, and regenerative potential. MSCs constitute strong candidates for cell-based therapies for preservation of kidney diseases [3]. MSCs possess potent immunomodulatory and pro-angiogenic properties, can be harvested from a variety of tissues, and are easily expanded in vitro [4]. In addition, MSCs play a key role in regulation of renal blood flow (RBF), capillary permeability, endothelial survival, and immunologic surveillance [5]. Importantly, MSCs release several growth factors and cytokines that can trigger tissue regeneration. However, concerns regarding safety and transplantation of viable replicating cells, which may cause induction of tumors, malformations, or microinfarctions, might limit their translational capacity [6].
MSC-derived extracellular vesicles (EVs) emerged as a novel noncellular alternative for cell-based treatment. EV from MSCs contains a cargo of mRNA and microRNA capable of regulating transcription of genetic information and modulating inflammation, angiogenesis, and other pathways in recipient cells [7,8]. Therefore, MSC-derived EV may exert trophic and reparative effects, representing an attractive noncellular approach for treating renal disease [9]. Indeed, recent studies have shown that MSC-derived EVs are safe and improve kidney function in small animal models of kidney disease [10]. We have also demonstrated that infusion of MSC-derived EV into the ischemic pig kidney ameliorates structural and functional decline [11]. In mice, systemically delivered bone marrow–derived MSCs and MSC-derived EVs within a week after 5/6 subtotal nephrectomy [12] or glycerol-induced acute kidney injury [13] have comparable efficacy for improving renal function. However, the relative efficacy of adipose tissue–derived MSCs and EVs for repair of chronically ischemic kidneys in a large animal model has not been fully compared.
The stenotic kidney of coexisting experimental RAS and MetS is characterized by functional deterioration secondary to substantial inflammation, hypoxia, fibrosis, and microvascular loss [14]. In addition to inflammation and apoptosis, receptor-interacting protein kinase (RIPK)-1 and RIPK-3 mediate necroptosis cell death that plays a role in renal cell depletion. Prolonged reduction of renal perfusion and vasoconstriction resulting from activation of the renin–angiotensin system leads to permanent changes in microvascular structure associated with inadequate renal angiogenic signaling involving vascular endothelial growth factor (VEGF) and angiopoietin (Ang)-1. These mechanisms contribute to renal dysfunction and, thereby, might offer different therapeutic targets. This study was designed to test the hypothesis that MSCs and EV both achieve effective kidney repair in MetS + RAS pigs, which might be attained by different mechanisms.
Materials and Methods
Animal experiment
All procedures were approved by Mayo Clinic Institutional Animal Care and Use Committee. Thirty domestic female pigs (50–60 kg; Manthei Hog Farm, Elk River, MN) were studied during 16 weeks of observation. Pigs were randomly divided into five groups as follows: Lean, RAS, MetS + RAS, MetS + RAS + MSC, and MetS + RAS + EV (n = 6 each).
At baseline, 18 MetS pigs started a high-cholesterol/high carbohydrate diet [MetS (5B4L; protein 16.1%, ether extract fat 43.0%, and carbohydrates 40.8%; Purina Test Diet, Richmond, IN)], while 12 other pigs were fed regular pig chow (Lean; Purina Animal Nutrition LCC, MN) [15]. Six weeks later (Fig. 1A), pigs were anesthetized with intramuscularly (IM) tiletamine hydrochloride/zolazepam hydrochloride (Telazol®; Zoetis, Kalamazoo, MI; 0.25 g) and xylazine (Xylamed, VetOne; Bimeda-MTC Animal Health, Cambridge, ON, Canada; 0.5 g) and maintained with intravenous ketamine (0.2 mg/kg/min, Ketaset; Zoetis) and xylazine (0.03 mg/kg/min). Then RAS was induced in the MetS and six Lean pigs by placing a local-irritant coil in the main renal artery [1]. Sham renal angiography was performed in the remaining pigs. Fat tissue was collected by abdominal subcutaneous biopsy and was subsequently used to harvest autologous MSCs and isolate their EVs.
FIG. 1.
MSC and EV tracking and the decrease in renal fibrosis. (A) Schematic of the experimental protocol. (B) Images of CM-Dil-labeled (red) MSCs (white arrows) and PKH26-labeled EV in the poststenotic kidney of pigs with RAS + MetS 4 weeks after delivery of MSCs or EV. (C, D) Representative trichrome staining images and quantitation of fibrosis in the renal cortex and medulla. *P < 0.05 versus Lean; &P < 0.05 versus RAS; #P < 0.05 versus MetS + RAS. MetS, metabolic syndrome; RAS, renal artery stenosis; MSC, mesenchymal stem/stromal cell; EV, extracellular vesicle. Color images are available online.
Six weeks later, the degree of stenosis was determined using renal angiography. Then, EV (1011), MSCs (107), or vehicle were injected into the stenotic kidney of MetS + RAS over 5 min through a 5F catheter positioned proximal to the stenosis.
Four weeks later, pigs were anesthetized and a catheter placed under fluoroscopic guidance in the right atrium. Mean arterial pressure (MAP) was monitored during multidetector computed tomography (MDCT) studies using an arterial catheter. Then, glomerular filtration rate (GFR) and RBF were measured using MDCT (Somatom Sensation-128; Siemens Medical Solution, Forchheim, Germany). Images were acquired following a central venous injection of iopamidol (0.5 mL/kg) and calculated from time density curves, as previously described [14]. Urine, systemic, and renal vein blood samples were taken for measurement of biochemical parameters. Single-kidney oxygenation was assessed using Blood-oxygen-level-dependent (BOLD)-magnetic resonance imaging (MRI) 2 days later on a 3 T scanner (GE Medical Systems, Milwaukee, WI) using Fast Gradient Echo sequence with multiple echo times [1].
One week later, the pigs were euthanized and the kidneys collected for histological and molecular assays (Fig. 1).
BOLD imaging
BOLD imaging was performed on a 3 T scanner (GE Medical Systems). Animals were anesthetized with 1%–2% isoflurane and scanned under suspended respiration. T2*-weighed images were acquired using a multiecho gradient echo sequence with the following parameters: repetition time (TR) = 100 ms, echo time (TE) = 2.1–27 ms, Flip angle = 40°, field-of-view (FOV) = 32 cm, Slice thickness = 7 mm, and Matrix = 256 × 256. The renal hypoxia index R2* was quantified by an exponential fitting of the signal intensity and echo times [16,17], after which the renal cortical and medullary R2* values were measured [18].
Microcomputed tomography for microvascular architecture
First, the segmental renal artery perfusing a kidney lobe was cannulated ex-vivo and infused initially with heparin-saline (10 U/mL) and then with a radiopaque silicon polymer (Microfil; FlowTech, Carver, MA; 0.8 mL/min). Finally, the kidney tissue was cut into small cubes and scanned at 20 μm voxels-resolution using an in-house micro-computed tomography (CT). Images were analyzed using Analyze™ (Biomedical Imaging Resource; Mayo Clinic, Rochester, MN), as previously shown [1].
MSCs and EV isolation, characterization, and tracking
Autologous MSCs were isolated from abdominal subcutaneous adipose tissue (5–10 g) using collagenase with standard protocol. Cells were cultured with advanced minimum essential medium (MEM) (Gibco/Invitrogen) supplemented with 5% platelet lysate (PLTMax; Mill Creek Life Sciences, Rochester, MN), in 37°C, 5% CO2, and kept in cell recovery medium at −80°C. MSCs were characterized by the expression of common MSC markers (CD44, CD90, and CD105), and their ability to differentiate into adipocytes, chondrocytes, and osteocytes was assessed as previously described [1].
EV was isolated from supernatants of the same dose of MSCs used for injection (107), cultured for 48 h in advanced MEM medium without supplements. Briefly, after two initial centrifugations at 2,000g and 100,000g (Beckman Coulter Optima L-90K) for 1 h at 4°C, EV was washed in serum-free medium 199 containing HEPES 25 mM and underwent a final ultracentrifugation. Finally, pellets were suspended and protein content quantified (Bradford method; BioRad). Limulus testing was performed to rule out endotoxin contamination (Charles River) and EV stored at −80°C until delivery.
Transmission electron microscopy was performed to confirm the morphology of MSC-derived EV using digital electron microscopy (JEOL 1200 EXII; Mayo Clinic's Electron Microscopy Core). EVs were then characterized for size using nanoparticle tracking analysis, as well as based on the expression of EV (CD9, CD29, and CD63) surface markers using western blotting.
Before injection, autologous MSCs and EVs were labeled with CM-Dil and PKH26, respectively [1]. Labeled MSCs and EVs were tracked and localized in frozen 5 μm sections of the stenotic kidneys by immunofluorescence staining with 4′,6-diamidino-2-phenylindole (D9542; Sigma, St. Louis, MO).
Kidney tissue studies
Tubulointerstitial fibrosis was assessed in slides stained with Masson's trichrome and then examined by light microscopy ( × 40) in a blinded manner. Twenty randomly selected nonoverlapping fields from each kidney were analyzed.
Tubular apoptosis was assessed using a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay with an In Situ Cell Death Detection Kit (Roche, Germany). Briefly, kidney tissues were fixed in 4% paraformaldehyde, incubated with TUNEL reaction mixture, and then followed by anti-Fluorescein-POD conjugate. The degree of apoptosis was estimated using a scale based on the mean number of TUNEL-positive cells per field.
Renal injury pathways
Inflammation, necroptosis, and angiogenesis associated with renal injury were studied. Renal vein levels of the inflammatory factors interleukin (IL)-1α, IL-β, IL-4, IL-6, IL-8, IL-10 and tumor necrosis factor-α (TNF-α) were evaluated by Luminex (Austin, TX). Plasma renin activity (72-REN_CT2; ALPCO Diagnostics) and serum creatinine (Scr, KBO2-H1; Arbor Assays) were measured using kits following vendor instructions.
All real-time polymerase chain reaction (RT-PCR; Thermofisher Scientific) and western blotting studies were performed in whole kidney RNA or homogenates.
Gene expressions of renal fibrosis markers [transforming growth factor-β (TGF-β), Collagen-I, α-smooth muscle actin (α-SMA)] were studied by RT-PCR. Necroptosis markers were tested by standard western blotting protocols [1], using specific antibodies against RIPK-1 (NBP-177077; Novus Biologicals) and RIPK-3 (ARP31513_T100; Aviva Systems Biology). GAPDH was used as loading control. Protein expression was quantified using densitometry and averaged in each group. Renal expression of RIPK1 (NBP-177077; Novus Biologicals) was also evaluated by immunofluorescent staining. Their gene expressions were studied by RT-PCR.
Renal angiogenic signaling involving VEGF-A (ab119; Abcam, United Kingdom) and Ang-1 (sc-8357; Santa-Cruz) was tested by western blotting and RT-PCR. Gene expression of microRNA-532-5p (001518; Thermofisher Scientific), which participates in regulating Ang-1 [19], was studied by RT-PCR. The expression of the peritubular capillary endothelial cell marker PL-VAP (NBP2-19868; Novus Biologicals) was measured by immunofluorescent staining.
Statistical analysis
Data were analyzed using GraphPad Prism 5 (GraphPad Software, San Diego, CA), and results are expressed as mean ± standard deviation for normally distributed variables and median (range) for non-Gaussian distributed data. Comparisons within groups were performed using the paired Student's t-test and among groups using analysis of variance and unpaired t-test with Bonferroni correction. A statistical difference was considered significant for P ≤ 0.05.
Results
MSC and EV tracking
The characterization of MSCs and EV has been reported in our previous studies [1]. EVs showed typical cup-shape morphology under electron microscopy, and their size distribution ranged mainly between 100 and 200 nm (Supplementary Fig. S1). MSCs and clusters of EV labeled with red fluorescent signal were detected in the stenotic kidney 4 weeks after intrarenal administration (5–10 MSCs per 20 × field, 4–10 EV clusters per 40 × field, Fig. 1B). We have previously shown that both MSCs [20] and EV [1] colocalize with proximal and distal tubular, interstitial, and endothelial cells.
Renal function
In RAS pigs, renal volume, RBF, and GFR were lower than normal. Body weight, renal volume, Scr, blood pressure, degree of stenosis, and lipid fraction levels were comparably elevated in MetS + RAS groups compared to Lean (P < 0.05), except that MAP in MetS + RAS + MSC was not different from Lean. The increased Scr was attenuated and RBF and GFR increased in MetS + RAS by both MSCs and EV (P < 0.05, Table 1), whereas plasma renin activity and triglyceride levels were unchanged.
Table 1.
Systemic Characteristics and Single-Kidney Function in Study Groups (n = 6 Each) at Sixteen Weeks
| Lean | RAS | Mets + RAS | Mets + RAS + MSC | Mets + RAS + EV | |
|---|---|---|---|---|---|
| Body weight (kg) | 55.1 ± 5.6 | 50.3 ± 5.9 | 86.2 ± 9.0*,† | 77.3 ± 19.2*,† | 90.5 ± 7.0*,† |
| Renal volume (cc) | 127.4 ± 15.4 | 93.1 ± 25.2* | 186.2 ± 23.3*,† | 140.5 ± 24.9†,‡ | 201.2 ± 13.9*,† |
| Scr (mg/dL) | 1.48 ± 0.25 | 1.66 ± 0.21 | 2.07 ± 0.18*,† | 1.71 ± 0.32*,‡ | 1.67 ± 0.37*,‡ |
| MAP (mmHg) | 96.1 ± 7.9 | 113.2 ± 24.5 | 118.5 ± 128* | 113.5 ± 21.2 | 114.7 ± 4.2* |
| Degree of stenosis (%) | 0 | 84.9 ± 11.0* | 89.2 ± 25.1* | 77.3 ± 23.3* | 75.0 ± 10.5* |
| GFR (mL/min) | 92.9 ± 13.5 | 58.4 ± 18.6* | 83.8 ± 4.8† | 118.6 ± 14.2*,†,‡ | 127.7 ± 19.3*,†,‡ |
| RBF (mL/min) | 610.9 ± 70.6 | 377.5 ± 89.4* | 505.7 ± 36.7*,† | 724.4 ± 97.7*,†,‡ | 889.8 ± 85.3*,†,‡ |
| PRA (ng/mL/h) | 0.13 ± 0.09 | 0.14 ± 0.07 | 0.23 ± 0.10 | 0.15 ± 0.13 | 0.14 ± 0.07 |
| Total cholesterol | 82.4 ± 6.8 | 94.9 ± 18.4 | 477.5 ± 199.9*,† | 455.6 ± 240.3*,† | 599.6 ± 154.0*,† |
| HDL cholesterol | 49.8 ± 17.2 | 50.7 ± 13.4 | 98.2 ± 40.8*,† | 97.8 ± 17.1*,† | 118.5 ± 39.3*,† |
| LDL cholesterol | 37 ± 16.6 | 41.6 ± 5.4 | 436 ± 134.1*,† | 395 ± 222.5*,† | 452 ± 118.0*,† |
| Triglycerides | 9.2 ± 2. 9 | 12.9 ± 2.0 | 13.2 ± 11.5 | 14.8 ± 8.8 | 24.4 ± 12.0 |
P < 0.05 versus Lean; †P < 0.05 versus RAS; ‡P < 0.05 versus Mets + RAS.
Scr, serum creatinine; MAP, mean arterial pressure; PRA, plasma renin activity; HDL, high density lipoprotein; LDL, low density lipoprotein; GFR, glomerular filtration rate; RBF, renal blood flow; MetS, metabolic syndrome; RAS, renal artery stenosis; MSC, mesenchymal stem/stromal cell; EV, extracellular vesicle.
Renal injury
Cortical and medullary fibrosis (trichrome staining) markedly increased in MetS + RAS compared with Lean and RAS, but decreased significantly in MetS + RAS + MSC and MetS + RAS + EV (Fig. 1C, D). Gene expression of collagen-I, TGF-β, and α-SMA (Supplementary Fig. S2) was concordant with trichrome staining.
The number of apoptotic cells in the stenosis kidney tissue was significantly increased in RAS and MetS + RAS kidneys and included glomerular endothelial, epithelial tubular, and interstitial cells. Apoptosis signals decreased by both MSCs and EV (Fig. 2A, B). The number of RIPK-1+ cells, consistent with necroptosis, increased in RAS and MetS + RAS kidneys, composed mainly of tubular cells. The number of RIPK-1+ cells decreased after treatment with EV (Fig. 2C, D), but not MSCs.
FIG. 2.
MSC and EV ameliorate renal injury. (A, B) Representative images and semiquantitative analysis of TUNEL and RIPK1 staining in the kidney. (C, D) RIPK-1 and RIPK-3 (necroptosis markers) renal protein expression in the experimental groups. GAPDH was used as an internal control. *P < 0.05 versus Lean; #P < 0.05 versus MetS + RAS. TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling; RIPK, receptor-interacting protein kinase; white arrows, apoptotic cells. Color images are available online.
Furthermore, western blot (Fig. 2E, F) showed that RIPK-1 protein expression was upregulated in RAS and MetS + RAS and that both necroptosis markers, RIPK-1 and RIPK-3, were decreased in MetS + RAS + EV kidneys compared with MetS + RAS (P < 0.05), but not in MetS + RAS + MSC. Their gene expression (Supplementary Fig. S2) showed a similar pattern.
Renal microcirculation
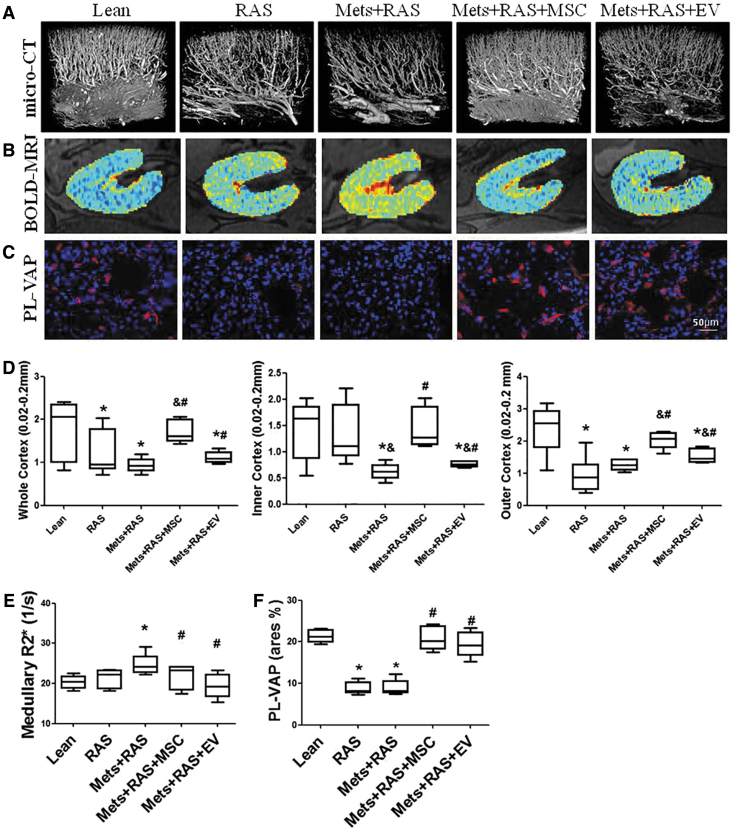
RAS and MetS + RAS pigs showed a decrease in the inner and outer cortical density of microvessels with diameters between 0.02 and 0.2 mm, which was improved but not normalized by EV and restored by MSCs (Fig. 3A, D). Hence, MSCs improved the microvasculature more effectively that EV.
FIG. 3.
MSC and EV improved renal microcirculation and oxygenation. Representative images of pig kidney from Lean, RAS, MetS + RAS, MetS + RAS + MSC, MetS + RAS + EV obtained by micro-computed tomography (A) or blood-oxygen-level-dependent magnetic-resonance imaging (B). (C) Endothelial cell (PL-VAP) staining. (D–F) Quantifications of images. The spatial density of renal microvessels was lower than normal throughout the cortex in MetS + RAS and increased in MetS + RAS + MSC, but not in MetS + RAS + EV. Medullary hypoxic regions (red) were increased in MetS + RAS, but similarly decreased by MSCs and EV. Similarly, PL-VAP expression was decreased in MetS + RAS, but similarly increased by MSCs and EV. *P < 0.05 versus Lean; &P < 0.05 versus RAS; #P < 0.05 versus MetS + RAS. Color images are available online.
BOLD-MRI showed elevated levels of the BOLD hypoxia index R2* in the medulla of MetS + RAS, which decreased to normal levels in MetS + RAS + MSC and MetS + RAS + EV kidneys, suggesting improved oxygenation (Fig. 3B, E).
Renal expression of PL-VAP, a marker of peritubular capillary endothelial cells, was diminished in RAS and MetS + RAS, but restored to normal in both MetS + RAS + MSC and MetS + RAS + EV (Fig. 3C, F).
Moreover, RAS and MetS + RAS also attenuated renal protein expression of the growth factors Ang-1 and VEGF, in which EV improved more effectively than MSCs (Fig. 4A, B). Congruently, renal expression of microRNA-532-5p was markedly increased in MetS + RAS + EV compared with other groups (Fig. 4C). The pattern of Ang-1 gene expression was comparable to its protein levels, whereas VEGF gene expression was similar among the groups (Supplementary Fig. S2). Indeed, we have previously shown that VEGF protein expression in renal ischemia is not adequately upregulated [21].
FIG. 4.
The effect of MSC and EV on growth and inflammatory factors. (A, B) Angiopoietin-1 and VEGF protein expression in the kidney was lower than normal in MetS + RAS and upregulated by EV, but not MSCs. (C) Renal microRNA 532-5p expression increased fourfold only by EV compared to Lean. (D) Renal vein levels of the inflammatory factors IL-1α, IL-1β, TNF-α, IL-1rα, and IL-6 were elevated in MetS + RAS versus Lean. Most were more effectively improved by MSCs than EV. IL-10 was decreased in MetS + RAS and improved by both MSCs and EV. *P < 0.05 versus Lean; #P < 0.05 versus MetS + RAS. VEGF, vascular endothelial growth factor; IL, interleukin; TNF, tumor necrosis factor.
Renal inflammation
In blood samples collected from the renal vein, levels of the pro-inflammatory cytokines IL-1α, IL-1β, TNF-α, IL-1rα, and IL-6 were elevated in RAS and MetS + RAS pigs. Their levels selectively decreased in MetS + RAS + MSC compared to MetS + RAS, but not in MetS + RAS + EV pigs, except for TNF-α. Contrarily, levels of the anti-inflammatory cytokine IL-10 were reduced in RAS and MetS + RAS, but similarly restored in MetS + RAS + MSC and MetS + RAS + EV pigs (Fig. 4), as was its gene expression (Supplementary Fig. S2).
Discussion
This study demonstrated that MSCs and EV delivered into the poststenotic kidney of a preclinical pig model induced comparable improvements in renal function that were achieved by slightly different mechanisms. EV elicited greater improvement in angiogenic signaling and necroptosis, while MSCs increased the microvasculature and suppressed inflammatory cytokines to a larger extent. Nevertheless, both strategies restored peritubular capillary density and increased RBF, GFR, and oxygenation of the stenotic kidney, suggesting overall comparable efficacy in preserving the stenotic kidney.
RAS is the primary etiology underlying renovascular hypertension, may lead to end-stage kidney disease [14], and is often accompanied by metabolic abnormalities. Kidney damage distal to the stenosis is characterized by microvascular loss, inflammation, and interstitial fibrosis [1,14]. However, restoration of renal arterial patency infrequently restores renal function [22].
MSCs are undifferentiated nonembryonic stromal cells with multilineage potential reflecting their stem cell-like properties. Their ability to differentiate into a range of mesenchymal cell lineages and their immunomodulatory properties may permit therapeutic avenues for both tissue repair and regeneration [20]. The beneficial action of MSCs is associated with their ability to repair tissues and ameliorate inflammation. Importantly, MSCs may exert their effects by transient engraftment without longstanding accumulation at the site of inflammation or tissue injury, suggestive of actions through paracrine mechanisms. In line with these observations, we have previously shown in both pigs and mouse ischemic kidney injury models that both MSCs [23,24] and their EV [14] progeny engraft in tubular and endothelial cells, promote tubular regeneration, and reduce inflammation and vascular injury. However, the efficacy of EV compared to MSCs in a large animal model has not been established.
Inflammation plays an important role in microvascular loss in ischemic tissues [25–27]. The current study demonstrates that although both strategies blunted inflammation, MSCs modulated the levels of inflammatory cytokines more effectively than EV, which may indirectly account for the greater improvement in microvascular structure in MetS + RAS. Loss of arterioles and small arteries might be mediated by activation of vascular smooth muscle cells in those vessels in response to myriad local pro-inflammatory stimuli [28]. In contrast, the equivalent decrease in TNF-α levels might have elicited the comparable improvement in peritubular capillary density [29]. The superiority of MSCs over EV in reducing inflammation may relate to a need for cell–cell contact of MSCs with inflammatory cells like macrophages [7]. Suppression of inflammation might also require a longer duration, and given that MSCs proliferate in the kidney [11], they are more likely than acellular EV to achieve this goal. However, concerns that MSCs may promote tumor growth, malformation, or microinfarctions prompted a search for safer and effective alternatives.
Direct comparison of the efficacy of EV and MSCs on tissue disease mechanisms is challenging, partly because the latter may constitute a long-term source of EV on site and because EV injection may not recapitulate the action of EV released by resident MSCs [30]. MSC-derived EVs mediate their paracrine effect by transferring proteins, lipids, and genetic material to target cells [31] and emulate some effects of MSCs in various experimental models, stimulating cell proliferation and repair. Our previous study demonstrated that EVs decrease renal damage in renovascular disease [14], yet the effects of EVs and MSCs on kidney disease mechanisms in a large preclinical model have not been directly compared. MSC-derived EVs appear to be as effective as their parental MSCs in mitigating lung inflammation or attenuating hyperoxic lung injuries [32], but might have shown modest potency for bone regeneration [33]. This study demonstrates that EV reduces inflammation and apoptosis and improves capillary density similar to MSCs, yet increases the expression of growth factors more effectively than MSCs. The mechanism by which EV improves capillary density may include their enrichment with microRNA-532-5p [34,35], which modulates pro-angiogenic activity through transcriptional regulation of Ang-1 [19]. Indeed, both were upregulated in kidneys of EV-treated pigs although we cannot exclude the possibility that microRNA-532-5p expression was locally upregulated secondary to decreased hypoxia or altered signaling, rather than through delivery by EVs.
Necroptosis is a recently-identified form of cell death contributing to tissue damage [36], and increasing evidence has implicated it in the pathogenesis of renal ischemic disease [37]. RIPK-1 mediates necroptosis and contributes to renal injury [38]. Yuan et al. [36] found that human-induced pluripotent stem cell-EV inhibited tubular cell necroptosis in an acute kidney injury rat model. Our findings extend previous observations and show that EVs offer an advantage in reducing necroptosis compared to MSCs and effectively reduce the expression of the necroptosis marker RIPK-1. However, the mechanism by which EVs reduce necroptosis and the implications for renal viability warrant further studies.
Limitations
Limitations in our study include the use of young animals with short duration of the disease. Nevertheless, the strengths of the current investigation include many human-like features of the preclinical pig model, with similar renal injury and dysfunction compared to humans. Moreover, this model enabled focused intrarenal delivery. We have shown before the time course and pattern of EV distribution among different organs [14]. Additional studies are needed to determine the optimal dose and timing of cell delivery and the role of specific pathways (such as necroptosis or microRNA-532-5p) in driving kidney injury and repair. Given their small size, mostly EV clusters are detectable using light microscopy, and some EVs or their membrane fragments might have been internalized by kidney cells or macrophages. The multiple groups and comparisons reduced the ability to detect subtle differences between these therapeutic modalities. Furthermore, given that our follow-up concluded 4 weeks after delivery, longer term benefits of MSC and EV treatment remain to be determined. In particular, the implications of reduced necroptosis may require longer studies to discern. Nevertheless, the short-term improvement of renal function and increased vascular density might sustain kidney function and structure.
Conclusion
Taken together, our data demonstrated that MSCs and EVs induced similar restoration of renal function, fibrosis, oxygenation, and capillary density in MetS + RAS, yet used slightly different mechanisms. EVs may possess greater necroptosis-improving potency, while MSCs more effectively suppress inflammatory cytokines and improve small blood vessels. Both strategies consequently recovered renal structure and function. Therefore, while the underlying pathophysiology may need to be considered during selection of cellular or noncellular therapy for kidney repair, our observations suggest that MSCs and EVs exhibited overall comparable efficacy in preserving stenotic kidney function. Furthermore, our study supports rigorous development of regenerative strategies to improve the damaged kidney.
Supplementary Material
Acknowledgment
The authors thank the China Scholarship Council for support.
Author Disclosure Statement
Dr. L.O.L. is a consultant for AstraZeneca and Weijian Technologies and receives research funding from Novo Nordisk. The other authors declare no conflicts of interest.
Funding Information
This research was partly supported by National Institutes of Health grants DK122734, DK120292, DK104273, and DK102325. The other authors declare no conflicts of interest.
Supplementary Material
References
- 1. Eirin A, Zhu XY, Jonnada S, Lerman A, van Wijnen AJ and Lerman LO (2018). Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transplant 27:1080–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang X, Kim SR, Ferguson CM, Ebrahimi B, Hedayat AF, Lerman A and Lerman LO (2018). The metabolic syndrome does not affect development of collateral circulation in the poststenotic swine kidney. Am J Hypertens 31:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aghajani Nargesi A, Lerman LO and Eirin A (2017). Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther 8:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hickson LJ, Eirin A and Lerman LO (2016). Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int 89:767–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang FY, Zhang XH, Tsang LL, Chan HC and Jiang XH (2019). Dedifferentiation-reprogrammed mesenchymal stem cells for neonates with hypoxic-ischaemic brain injury. Hong Kong Med J 25 (Suppl. 5):12–16 [PubMed] [Google Scholar]
- 6. Kouroupis D, Sanjurjo-Rodriguez C, Jones E and Correa D (2019). Mesenchymal stem cell functionalization for enhanced therapeutic applications. Tissue Eng Part B Rev 25:55–77 [DOI] [PubMed] [Google Scholar]
- 7. Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, Kwong A, Mitsialis SA and Kourembanas S (2018). Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med 197:104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang TT, Lv LL, Wang B, Cao JY, Feng Y, Li ZL, Wu M, Wang FM, Wen Y, et al. (2019). Employing macrophage-derived microvesicle for kidney-targeted delivery of dexamethasone: an efficient therapeutic strategy against renal inflammation and fibrosis. Theranostics 9:4740–4755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathew B, Ravindran S, Liu X, Torres L, Chennakesavalu M, Huang CC, Feng L, Zelka R, Lopez J, Sharma M and Roth S (2019). Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials 197:146–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao L, Hu C, Zhang P, Jiang H and Chen J (2019). Genetic communication by extracellular vesicles is an important mechanism underlying stem cell-based therapy-mediated protection against acute kidney injury. Stem Cell Res Ther 10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC and Lerman LO (2012). Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 30:1030–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He J, Wang Y, Sun S, Yu M, Wang C, Pei X, Zhu B, Wu J and Zhao W (2012). Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton) 17:493–500 [DOI] [PubMed] [Google Scholar]
- 13. Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, et al. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20:1053–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A and Lerman LO (2017). Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int 92:114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eirin A, Ebrahimi B, Kwon SH, Fiala JA, Williams BJ, Woollard JR, He Q, Gupta RC, Sabbah HN, et al. (2016). Restoration of mitochondrial cardiolipin attenuates cardiac damage in swine renovascular hypertension. J Am Heart Assoc 5:e003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang K, Ferguson CM, Ebrahimi B, Tang H, Kline TL, Burningham TA, Mishra PK, Grande JP, Macura SI and Lerman LO (2017). Noninvasive assessment of renal fibrosis with magnetization transfer MR imaging: validation and evaluation in murine renal artery stenosis. Radiology 283:77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang K, Ferguson CM, Woollard JR, Landes VL, Krier JD, Zhu X, Nayak KS and Lerman LO (2019). Magnetization transfer imaging is unaffected by decreases in renal perfusion in swine. Invest Radiol 54:681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang K, Ferguson CM, Woollard JR, Zhu X and Lerman LO (2017). Magnetization transfer magnetic resonance imaging noninvasively detects renal fibrosis in swine atherosclerotic renal artery stenosis at 3.0 T. Invest Radiol 52:686–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Slater SC, Jover E, Martello A, Mitic T, Rodriguez-Arabaolaza I, Vono R, Alvino VV, Satchell SC, Spinetti G, Caporali A and Madeddu P (2018). MicroRNA-532-5p regulates pericyte function by targeting the transcription regulator BACH1 and angiopoietin-1. Mol Ther 26:2823–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eirin A, Zhu XY, Ebrahimi B, Krier JD, Riester SM, van Wijnen AJ, Lerman A and Lerman LO (2015). Intrarenal delivery of mesenchymal stem cells and endothelial progenitor cells attenuates hypertensive cardiomyopathy in experimental renovascular hypertension. Cell Transplant 24:2041–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A and Lerman LO (2004). Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol 24:1854–1859 [DOI] [PubMed] [Google Scholar]
- 22. Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, et al. (2014). Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zou X, Jiang K, Puranik AS, Jordan KL, Tang H, Zhu X and Lerman LO (2018). Targeting murine mesenchymal stem cells to kidney injury molecule-1 improves their therapeutic efficacy in chronic ischemic kidney injury. Stem Cells Transl Med 7:394–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ebrahimi B, Eirin A, Li Z, Zhu XY, Zhang X, Lerman A, Textor SC and Lerman LO (2013). Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS One 8:e67474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paulus WJ and Tschope C (2013). A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62:263–271 [DOI] [PubMed] [Google Scholar]
- 26. Ericson H, Abu Hamdeh S, Freyhult E, Stiger F, Backryd E, Svenningsson A, Gordh T and Kultima K (2019). Cerebrospinal fluid biomarkers of inflammation in trigeminal neuralgia patients operated with microvascular decompression. Pain 160:2603–2611 [DOI] [PubMed] [Google Scholar]
- 27. Duan Y, Li X, Zuo X, Shen T, Yu S, Deng L and Gao C (2019). Migration of endothelial cells and mesenchymal stem cells into hyaluronic acid hydrogels with different moduli under induction of pro-inflammatory macrophages. J Mater Chem B 7:5478–5489 [DOI] [PubMed] [Google Scholar]
- 28. Ackers-Johnson M, Talasila A, Sage AP, Long X, Bot I, Morrell NW, Bennett MR, Miano JM and Sinha S (2015). Myocardin regulates vascular smooth muscle cell inflammatory activation and disease. Arterioscler Thromb Vasc Biol 35:817–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koller GM, Schafer C, Kemp SS, Aguera KN, Lin PK, Forgy JC, Griffin CT and Davis GE (2020). Proinflammatory mediators, IL (interleukin)-1beta, TNF (tumor necrosis factor) alpha, and thrombin directly induce capillary tube regression. Arterioscler Thromb Vasc Biol 40:365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wiklander OPB, Brennan MA, Lotvall J, Breakefield XO and El Andaloussi S (2019). Advances in therapeutic applications of extracellular vesicles. Sci Transl Med 11:eaav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lv LL, Feng Y, Tang TT and Liu BC (2019). New insight into the role of extracellular vesicles in kidney disease. J Cell Mol Med 23:731–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahn SY, Park WS, Kim YE, Sung DK, Sung SI, Ahn JY and Chang YS (2018). Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp Mol Med 50:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xie H, Wang Z, Zhang L, Lei Q, Zhao A, Wang H, Li Q, Cao Y, Jie Zhang W and Chen Z (2017). Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci Rep 7:45622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nargesi AA, Lerman LO and Eirin A (2017). Mesenchymal stem cell-derived extracellular vesicles for renal repair. Curr Gene Ther 17:29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O'Brien D, van Wijnen AJ and Lerman LO (2014). MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 551:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yuan X, Li D, Chen X, Han C, Xu L, Huang T, Dong Z and Zhang M (2017). Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis 8:3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen B, He Y, Zhou S, Zhao H, Mei M and Wu X (2016). TRPC6 may protect renal ischemia-reperfusion injury through inhibiting necroptosis of renal tubular epithelial cells. Med Sci Monit 22:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U and Krautwald S (2012). Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81:751–761 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.