Abstract
Targeting areas of inflammation offers potential therapeutic and diagnostic benefits by maximizing drug and imaging marker on-target effects while minimizing systemic exposure that can be associated with adverse side effects. This strategy is particularly beneficial in the management of inflammatory bowel disease (IBD). Here we describe an inflammation-targeting (IT) approach based on heparin-coated human serum albumin nanoparticles (HEP-HSA NPs) that utilize the increased intestinal permeability and changes in electrostatic interaction at the site of intestinal inflammation. Using small-molecule and biologic drugs as a model for drug combination, the HEP-HSA NPs demonstrate the capacity to load both drugs simultaneously; the dual-drug loaded HEP-HSA NPs exhibit a higher anti-inflammatory effect than both of the single-drug loaded NPs in vitro and selectively bind to inflamed intestine after enema administration in vivo in a murine model of colitis. Importantly, analysis of the physicochemical characteristics and targeting capacities of these NPs indicate that HEP coating modulates NP binding to the inflamed intestine, providing a foundation for future IT-NP formulation development.
Keywords: drug delivery, nanoparticles, drug combination, intestinal inflammation
Table of Contents:
Targeting areas of inflammation stands to transform the capacity to effectively treat inflammatory bowel disease while minimizing systemic side effects. This report describes heparin-coated human serum albumin nanoparticles that can encapsulate drug combination, suppress inflammation in macrophages in vitro, and selectively bind to inflamed intestine in vivo in a murine model of acute colitis.
Graphical Abstract

Inflammatory bowel disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic, idiopathic inflammatory set of conditions that affect the gastrointestinal tract.[1] The etiology of IBD appears to be multifactorial, with genetic predisposition, immune dysregulation, environmental triggers, and microbial exposure contributing to disease development.[2] Currently, there is no cure for IBD; life-long medication and surgical interventions are often required. Existing treatment options for IBD fail to control symptoms adequately in a large number of patients;[3] major side effects, including opportunistic infections, autoimmunity, and malignancies, are associated with the treatment, potentially due to frequent dosing and systemic exposure of high concentration of active drugs.[4] Given the rising incidence of IBD globally and its negative impact on quality of life,[5] there is a critical need for improved therapies applying highly effective drugs or drug combinations without adverse side effects. Targeted drug delivery stands to transform our capacity to effectively treat the disease while minimizing side effects.[6]
Inflammation-targeting (IT) drug delivery exploits the pathophysiological characteristics at the inflamed mucosa. One critical layer often affected by intestinal inflammation is the mucus barrier, which becomes thinner or depleted in active UC; the thickness of the mucus layer has been found to inversely correlate with disease severity.[7] Another characteristic of intestinal inflammation is the increased intestinal permeability, where tight junctions between epithelial cells are disrupted upon activation of proinflammatory cytokines,[8] allowing passive deposition of nano-sized drug carriers at the damaged epithelium. Inflammation of the colonic mucosa is also accompanied by an in situ secretion and accumulation of positively charged proteins, including bactericidal/permeability-increasing protein, antimicrobial peptides, and transferrin at the site of inflammation.[9] The buildup of positively charged proteins at the damaged mucosa, where the mucus layer is thinner and/or depleted, provides instructive cues for negatively charged drug carriers to preferentially adhere to the inflamed intestine.[6a, 9c]
Nanoparticles (NPs) can be tailored with versatile surface chemistry to leverage the pathophysiological changes at the site of inflammation for selective delivery.[10] Protein-based NPs are advantageous compared to synthetic NPs, owing to their naturally bestowed biocompatibility, degradability, and low immunogenicity and toxicity.[11] Human serum albumin (HSA) is a highly stable and abundant protein in the serum, and a natural transport protein serving as a depot and courier of endogenous and exogenous compounds.[12] HSA has been widely used in preclinical evaluations as an attractive candidate for drug delivery systems, and several HSA-based carriers have been approved for clinical applications, mainly for cancer treatment.[13] HSA NPs can be formulated in aqueous solutions under mild conditions;[14] therefore, it is beneficial for drug encapsulation, particularly for biologics. Additionally, the availability of functional moieties on HSA, including amines and carboxylates, is amenable to further modification.[11]
Heparin (HEP) is a heavily sulfated polyanionic carbohydrate (glycosaminoglycan, GAG) that bears the highest negative charge density among all naturally derived biomolecules,[15] which can be harnessed to interact with the accumulated positively charged proteins at the inflamed mucosa.[6a] HEP has been reported to show therapeutic benefits in UC treatment,[16] due to its anticoagulant properties that inhibit microvascular thromboses, and its immuno-modulating and anti-inflammatory properties that suppress neutrophil recruitment [17] and reduce proinflammatory cytokines/signaling molecules.[18] HEP can also restore the high-affinity receptor binding of basic fibroblast growth factor, aiding in healing of the ulcerated mucosa.[16b] HEP and HEP-like GAGs are known to interact with proteins to form complexes, arising from the interaction between proteins and the negative charge on GAGs; it has been reported that albumin could bind to HEP and form complexes.[19] Hence, we choose HEP to bind to HSA to increase negative charge on NPs to target the accumulated positively charged proteins at the inflamed mucosa and for the potential beneficial effects of HEP in IBD.
Based on biological functions and physical properties of therapeutics, we choose both small-molecule drugs and biologics to be encapsulated in HSA NPs, including budesonide (BUD), an anti-inflammatory steroid that has been widely used for IBD treatment;[20] vancomycin (Vanco), an antibiotic that modifies gut microbiome and treats bacterial infections in colitis;[21] and granulocyte macrophage colony-stimulating factor (GM-CSF), a cytokine that regulates the innate immune response and epithelial regeneration [22] and helps to achieve remission in IBD.[23] BUD and Vanco, respectively, represent hydrophobic and hydrophilic small-molecule drugs, which require different encapsulation methods; while GM-CSF exemplifies most biologics that necessitate targeted delivery to reduce systemic exposure. Importantly, drug combination provides the potential of tackling IBD through different pathways and has been shown to support improved outcomes in treating IBD.[24] As one example of drug combination, here we choose to encapsulate BUD and GM-CSF simultaneously in the NPs; however, different combinations of these drugs can be formulated using the methods reported here.
Here we show HEP-coated HSA (HEP-HSA) NPs are capable of loading drug combinations and targeting the inflamed colon in a murine model of acute colitis. As a widely studied delivery system, HSA NPs have only recently been studied for drug delivery in IBD, in which HSA was chemically conjugated to an anti-inflammatory drug, and glutaraldehyde was used as a crosslinker to form NPs.[25] On the other hand, HEP has been encapsulated as a drug in synthetic cationic NPs for colitis treatment;[26] it has not been studied as a coating layer on NPs to interact with the inflamed mucosa. Here we utilize a facile method to load drug combination in HEP-HSA NPs for drug delivery in IBD and study the effect of HEP coating on NP targeting the inflamed intestine compared to healthy control. We set out to characterize the physicochemical properties of the HEP-HSA NPs that modulate binding to the inflamed mucosa ex vivo and in vivo, for the development of optimal IT-NP formulations that are preferentially retained in the inflamed intestine.
The HSA NPs were formulated by using L-glutathione (GSH) as a cleavage reagent followed by ethanol addition as a desolvation agent.[14] HEP was then coated on the HSA NPs to form HEP-HSA NPs and convert the surface charge from positive to negative (Figure 1a). The scanning electron microscopic image confirmed the spherical shape of the mostly uniform HEP-HSA NPs (Figure 1b). The size of HSA NPs can be tuned by varying HSA concentrations for NP preparation, resulting in NPs of approximately 120 nm, 180 nm, and 250 nm in diameter using HSA of 40, 80, and 120 mg mL−1, respectively; all had a polydispersity index (PDI) less than 0.2 (Figure 1c). Albumin has been reported to bind to HEP through electrostatic interaction to form complexes, during which the interaction was increasing with a reduction in pH and/or ionic strength.[19] In our studies, the HSA NPs were slightly positively charged after dialysis against water, and the coating was also conducted in water to increase the attractive interaction between HSA and HEP. We evaluated HSA NPs in different buffers and various HEP concentration for coating and finalized on 1.0 mg mL−1 of HEP in water as the optimal coating condition, since it yielded HEP-HSA NPs with lower PDI and comparable negative charge (Figure S1, S2). After HEP coating, the zeta potential of NPs dramatically changed from about +10 mV before coating to −38, −43, and −44 mV, correspondingly (Figure 1d). The coating efficiency of HEP was about 50% for NPs prepared from 120 mg mL−1 HSA, while about 30% for NPs from 40 and 80 mg mL−1 HSA (Figure 1e), which was probably due to more HSA in the former to interact with HEP than the latter two.
Figure 1. Synthesis and characterization of heparin-coated human serum albumin nanoparticles (HEP-HSA NPs).
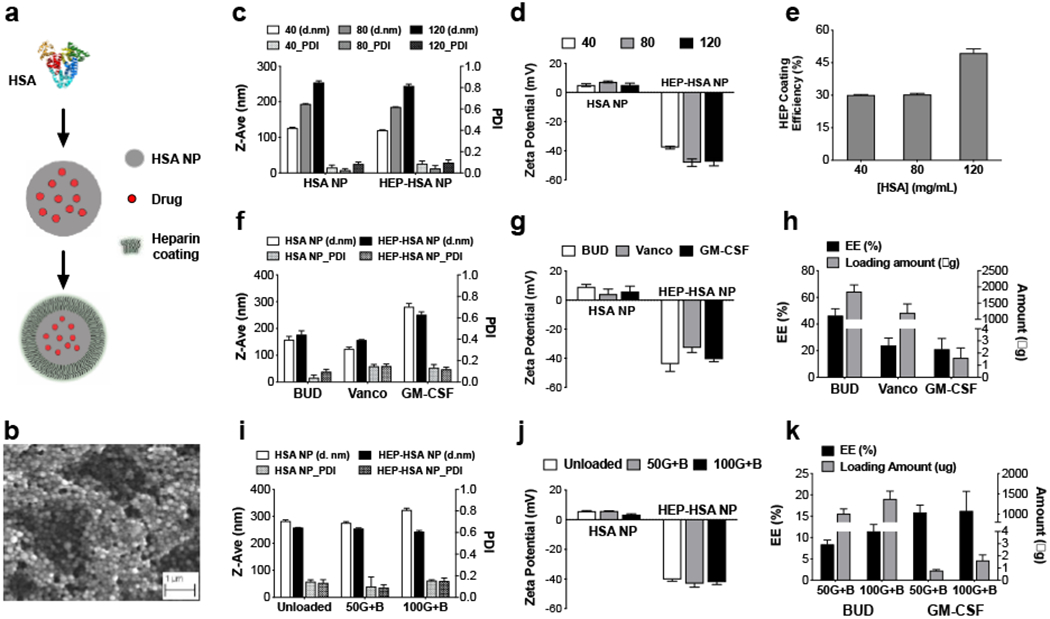
(a) Schematic outlining the formation of drug-loaded HEP-HSA NPs. (b) A scanning electron microscopic image of HEP-HSA NPs. (c, d) Size, polydispersity index (PDI), and zeta potential measurement of NPs prepared from 40, 80, and 120 mg mL−1 of HSA, before and after HEP coating. (e) Coating efficiency (%) of 1.0 mg mL−1 HEP in water on different HSA NPs. (f, g) Size, PDI, and zeta potential of HSA NPs loaded with different drugs, before and after HEP coating. BUD: budesonide; Vanco: vancomycin; GM-CSF: granulocyte macrophage colony-stimulating factor. (h) Encapsulation efficiency (EE) and the amount of drug loaded in HSA NPs. (i, j) Size, PDI, and zeta potential of unloaded and dual-drug loaded HSA NPs with BUD (3 mg mL−1) and GM-CSF (50 and 100 μg mL−1, respectively), before and after HEP coating. (k) EE and the amount of BUD and GM-CSF in the dual-drug loaded HSA NPs. Data are presented as mean ± SD from a representative experiment (n = 3; independent experiment was performed at least twice).
As an example, small-molecule drugs BUD and Vanco were loaded in HSA NPs prepared from 80 mg mL−1 HSA, respectively, and a biologic drug GM-CSF was loaded in HSA NPs prepared from 120 mg mL−1 HSA (Figure 1f, g). To determine the encapsulation efficiency (EE) of each drug, HSA NPs were digested by GSH and the amount of drug was quantified by high performance liquid chromatography (HPLC) for BUD and Vanco, and by ELISA for GM-CSF. For these single-drug loaded NP formulations, our result showed an EE of 46 ± 5%, 24 ± 5%, and 21 ± 8% for BUD, Vanco, and GM-CSF, respectively (Figure 1h). Next, we chose to load drug combination of BUD and GM-CSF as a model of combining small-molecule drugs and biologics in NPs. We first optimized the drug EE in each single-drug loaded NPs and determined on the concentrations of HSA and BUD for dual-drug loaded NP preparation (Figure S3); we then evaluated the concentration of GM-CSF for dual-drug loading (Figure 1i–k), and finalized on 100 μg mL−1 of GM-CSF and 3.0 mg mL−1 BUD for dual-drug loaded NPs prepared from 120 mg mL−1 HSA, which led to higher EE for both drugs and were used for the following studies.
To evaluate the anti-inflammatory effect, we studied the single- and dual-drug loaded HEP-HSA NPs in RAW264.7 macrophages with lipopolysaccharide (LPS) activation to simulate the inflammatory condition. First, we characterized the size, PDI, and zeta potential of each formulation: the unloaded (UNP), BUD-loaded (B/NP), GM-CSF-loaded (G/NP), and BUD- and GM-CSF-loaded (G+B/NP) NPs, before and after HEP coating. All NPs were prepared from 120 mg mL−1 HSA, and depending on the formulation, the size ranged from 250 to 350 nm in diameter and the PDI was all below 0.2 (Figure 2a). The zeta potential of all HEP-HSA NPs was between −38 and −42 mV (Figure 2b). Next, the EE of BUD and GM-CSF in the single- and dual-drug loaded NPs was quantified (Figure 2c, d), respectively, and used for preparation of the same amount of each drug in NP formulations before adding them to cells. Untreated, non-activated macrophages served as Negative Control (NC), indicating the baseline of inflammatory mediators; while the LPS-activated macrophages without NP treatment served as Positive Control (PC), exhibiting an upper threshold of these secretions. Our results showed that the (G+B)/NP reduced tumor necrosis factor-α (TNF-α) expression to a much lower level than the B/NP and the G/NP; both (G+B)/NP and B/NP significantly reduced TNF-α expression compared to the PC (P = 0.0012 and P = 0.0472, respectively), with the (G+B)/NP being 34% and the B/NP being 16% in reduction (Figure 2e). Furthermore, all NP treated groups displayed significantly lower nitric oxide (NO) expression than the PC (P <0.001), and the (G+B)/NP demonstrated the lowest NO expression among all treated groups, which was even a 3-fold reduction compared to the B/NP and the G/NP treatments (Figure 2f). Overall, the (G+B)/NP exhibited the highest anti-inflammatory effect against TNF-α and NO expressions compared to the B/NP and the G/NP treatments in LPS-activated macrophages.
Figure 2. Dual-drug loaded HEP-HSA NPs demonstrate higher anti-inflammatory effect than single-drug loaded HEP-HSA NPs evaluated in vitro.

(a, b) Size, PDI, and zeta potential measurement of unloaded (UNP), single-drug loaded (B/NP, G/NP), and dual-drug loaded (G+B/NP) HSA NPs, before and after HEP coating. (c, d) EE and the amount of BUD (c) and GM-CSF (d) in single-drug loaded NPs (B/NP, G/NP) and dual-drug loaded NPs (G+B/NP). (e) Comparison of different NP formulations on the inhibition of TNF-α expression by RAW264.7 macrophages. NC: Untreated, non-activated macrophages as negative control; PC: LPS-activated macrophages without NP treatment as positive control. * p = 0.0472 for B/HNP with PC, ** p = 0.0012 for G+B/NP with PC, and p = 0.4775 for G/NP and UNP with PC (Holm-Sidak’s multiple comparison test). (f) Comparison of different NP formulations on the inhibition of NO expression by RAW264.7 macrophages. All NP formulations significantly reduced NO expression compared with PC. **** p < 0.0001 for G+B/NP compared with PC (Holm-Sidak’s multiple comparison test). Data are presented as mean ± SD from a representative experiment (n = 3; independent experiment was performed at least twice).
Interestingly, the unloaded HEP-HSA NPs (UNP) also dramatically reduced NO expression compared to the PC (4.7 vs. 18.3 μM) (Figure 2f), supporting additive effects of HEP coating on the suppression of inflammation. NO overproduction by intestinal epithelium has been consistently associated with IBD, and the role of NO in promoting the immune response during colitis has been previously established.[27] Several studies have found that NO production is increased in human IBD tissues and the increased NO production correlates well with the severity of inflammation in UC.[28] The inhibitory effect of HEP on NO production in murine macrophages[29] and endothelial cells[30] has been reported, in which HEP might react directly with NO or reduce the transcription of Nos 3 and endothelial NO synthase protein expression, leading to a decrease in bioactive NO. Additionally, we observed a dose- and time-dependent uptake of (G+B)/NP by RAW264.7 macrophages (Figure S4 a–c). This is expected because HEP, as a polyanion, is recognized as a ligand for class A and B scavenger receptors that are preferentially taken up by phagocytic cells, especially macrophages;[29a] HEP is also known to enhance the uptake of HEP/protein complexes, such as platelet factor 4 and HEP,[31] by monocytes and macrophages. The accompanied Live/Dead stain indicated ~100% RAW264.7 macrophage viability for all volumes of NPs tested. Furthermore, the NP cytotoxicity was evaluated using human colonic epithelial cell lines, HT29 and Caco-2; both indicated lack of cytotoxicity for HEP-HSA NPs under the conditions studied (Figure S4 d, e).
Next, the effects of size and surface charge/chemistry on NP targeting the inflamed colon ex vivo was studied using four groups of Alexa Fluor®680-labeled NPs (G1 - G4), by comparing NP binding to colons excised from colitic mice with colons from healthy mice. Here we used the dextran sulfate sodium (DSS)-induced acute colitis, which is a widely used animal model of IBD due to its simplicity, wide applicability, and similarities to human disease.[32] The colitis induction was confirmed by body weight loss percentage (%) and shortened colon length in mice compared with healthy controls (Figure S5a, b). G1, G2, and G3 were HEP-HSA NPs of varying sizes, and G4 was HSA NPs. The size of G1 - G4 NPs was 113.5 nm, 144.6 nm, 242.5 nm, and 202.7 nm; the zeta potential was −39.4, −42.4, −36.4, and +4.0 mV, respectively (Figure 3a–c). NPs from G1 - G4 were incubated with the dissected colon and washed before IVIS imaging (Figure 3d). Our results showed that G1 (113.5 nm, −39.4 mV), G2 (144.6 nm, −42.4 mV), and G4 (202.7 nm, +4.0 mV) did not exhibit preferential binding to the inflamed colon compared to healthy controls; instead, G1 showed significantly higher retention in healthy colon (P = 0.0107) and both G2 and G4 displayed no significant difference in binding to the inflamed colon compared to healthy colon (P = 0.2080 and P = 0.1476, respectively) (Figure 3e). When the size of HEP-HSA NPs increased, G3 (242.5 nm, −36.4 mV) exhibited significantly higher binding to the inflamed colon than healthy colon (Figure 3e). The binding of G3 was also evaluated at 10, 30, and 60 min incubation time ex vivo, during which the binding occurred at as early as 10 min after incubation and maintained preferential binding to the inflamed colon throughout the time points studied (Figure S5c, S6).
Figure 3. Larger HEP-HSA NPs, not smaller or uncoated ones, exhibited preferential binding to the inflamed colon evaluated ex vivo.
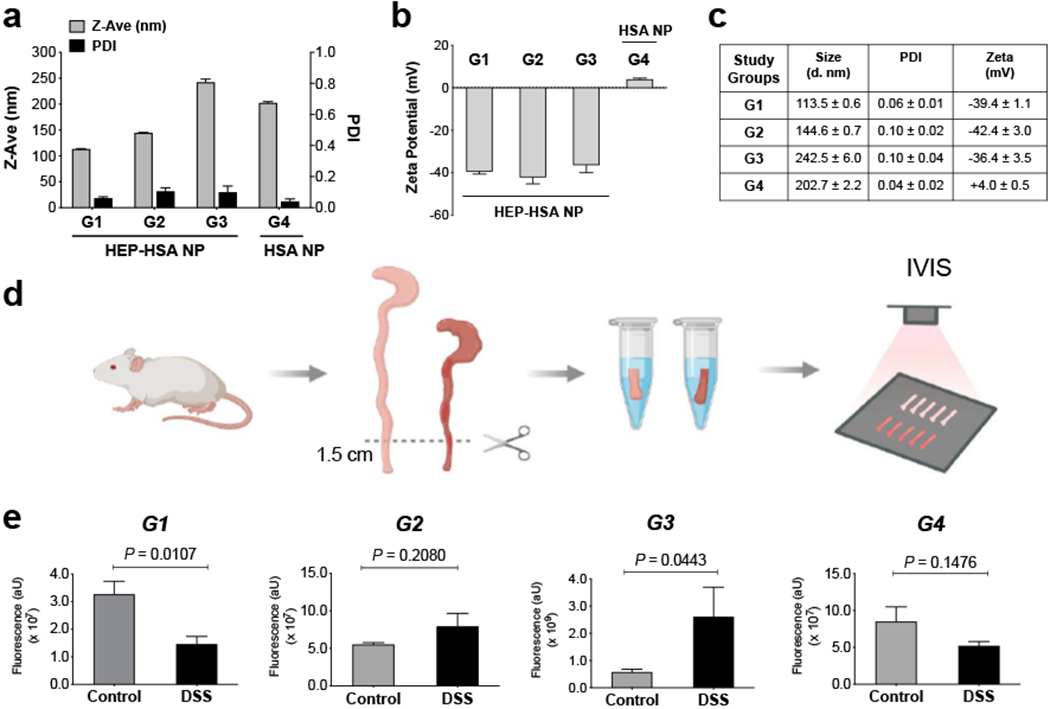
(a - c) Size, PDI, and zeta potential of different Alexa Fluor®680-labeled NP formulations (G1, G2, G3, and G4) for the ex vivo evaluation. (d) Schematic outlining the experimental workflow. The distal colons of mice with dextran sulfate sodium (DSS)-induced colitis and healthy controls were incubated with NPs under 37°C for 30 min, washed, and imaged by IVIS. (e) IVIS fluorescence intensity of G1, G2, G3, and G4 NPs evaluated with mouse colons ex vivo. Control: colons from healthy mice; DSS: colons from mice with DSS-induced colitis. The total fluorescence intensity was determined in a standard-sized region of interest (ROI) around the individual colon pieces with background fluorescence subtracted. Data are presented as mean ± SD (n = 3) in (a - c) and presented as mean ± SEM (n = 3 - 5 mice per group) in (e); P-values were determined by Student’s t test.
Our initial expectation was that smaller, more negatively charged NPs leading to higher binding to the inflamed colon. To our surprise, negatively charged HEP-HSA NPs of G1 and G2 with size less than 200 nm did not exhibit preferential binding to the inflamed colon; instead, G1, the smallest in size, showed significantly higher fluorescence retention in healthy colon than the inflamed colon. Presumably, this observation could be attributed to the dense mucus layer in the healthy colon that is known to entrap small-sized NPs;[33] the entrapment might surpass the electrostatic interaction between HEP and the accumulated positively charged proteins at the inflamed colon where the mucus layer was thinner or depleted. Another factor could be the formation of HEP-mucin complexes through a HEP-binding C-terminal tail in the MUC2 mucin,[34] leading to higher retention of the smaller sized HEP-HSA NPs in the healthy colon. However, as the size of HEP-HSA NPs increased, G3 preferentially bound to the inflamed colon evaluated at different incubation time (10, 30, and 60 min). Therefore, HEP-HSA NPs of larger size seemed to be able to escape from the entrapment by the dense mucus network;[33] additionally, the electrostatic attraction between HEP and the positively charged proteins at the inflamed mucosa, together with the enhanced uptake of HEP and NPs of larger size by phagocytic cells,[29a, 35] might exceed the formation of HEP-mucin complexes in the healthy colon, thereby preferentially binding to the inflamed colon. For G4, without HEP coating, it did not exhibit higher binding to the inflamed colon than the healthy colon and that was expected. However, when we studied the NP stability in simulated gastric fluids (SGF) and simulated intestinal fluids (SIF) over a 24-h period (Figure S7), we found that there was a marked difference in the surface charge of HSA NPs, which was positive in SGF while being negative in SIF; on the other hand, the HEP-HSA NPs remained negatively charged in both SGF and SIF (Figure S7b, d). Therefore, although G4 might become negatively charged under the ex vivo evaluation, it still did not preferentially bind to the inflamed colon at a size of 200 nm without HEP coating.
Based on the ex vivo results, we prepared Alexa Fluor®680-conjugated, dual-drug loaded HEP-HSA NPs for in vivo evaluation, which had a diameter of 278.2 ± 2.4 nm and surface charge of −41.9 ± 4.7 mV (Figure 4a, b). Each mouse from the healthy and colitic groups received a single enema of the prepared HEP-HSA NPs and was sacrificed at 3h after the treatment (Figure 4c). We considered several factors necessitating a reasonable short duration of 3h for evaluation, including the nature of protein-based NPs susceptible to degradation in vivo, an increased turnover of colonic epithelial cells affected by colitis,[36] as well as a potential detection limit of the dye in vivo due to the HEP-HSA NPs primarily composed of unmodified HSA with a small amount of dye-conjugated HSA. The 3-cm distal colon from mice was dissected for IVIS imaging (Figure 4d), and the result showed significantly higher fluorescence retention in the inflamed colon compared to healthy control (Figure 4e). This observation confirmed the ex vivo evaluation that HEP-HSA NPs of larger size (around 250 nm or larger) with negative charge preferentially bound to the inflamed colon. We also compared the body weight loss% and colon length of mice to confirm colitis induction in the DSS-treated mice (Figure S8). Hence, our in vivo study on the HEP-HSA NPs (278 nm, −42 mV) supported such IT-NPs’ preferential binding to the inflamed colon.
Figure 4. Larger HEP-HSA NPs loaded with drug combinations displayed preferential binding to the inflamed colon in mice in vivo.

(a, b) Size, PDI, and zeta potential measurement of dual-drug loaded, Alexa Fluor®680-labeled HSA NPs, before and after HEP coating. (c) Schematic of colitis induction in mice and treatment regimen. (d) IVIS imaging of mouse colons dissected from healthy controls (Control) and mice with DSS-induced colitis (DSS) 3h after the enema treatment. (e) IVIS quantification of the fluorescence intensity in (d). The total fluorescence intensity was determined in a standard-sized ROI around the individual colon pieces with background fluorescence subtracted. Data are presented as mean ± SD (n = 3) in (a - b) and presented as mean ± SEM (n = 7 mice per group) in (e); P-values were determined by Student’s t test.
To further understand the effect of size and surface charge/chemistry on NP binding to the inflamed mucosa, we investigated two more NP formulations that possessed similar characteristics to the ones evaluated ex vivo (G1 and G4, respectively), for their binding to the inflamed mucosa in vivo. We prepared HEP-HSA NPs (126.0 nm, −41.4 mV) and HSA NPs (230.2 nm, 21.5 mV) (Figure 5a, b) and followed the same treatment schedule outlined in Figure 4c for in vivo evaluation via enema treatment. After 3h, the 3-cm distal colon was excised for IVIS imaging. The inflamed mucosal binding of these two NPs was found statistically insignificant compared to healthy colon (P = 0.1251 and P = 0.1216, respectively) (Figure 5a, b), suggesting the consistency between the in vivo and ex vivo evaluations. We also assessed an inflammatory marker myeloperoxidase (MPO) activity and histology scores of the colonic tissues that were used for IVIS imaging by dividing them equally into 1-cm pieces (Figure 5c, d; the 3rd piece was used for fluorescence quantification after homogenization). The MPO activity is proportional to the number of neutrophils in the intestinal mucosa; the higher the MPO activity, the more severe the colitis in the colon.[37] Both MPO activity and histology assessment, together with the body weight loss% and colon length (Figure S9), indicated colitis induction in the DSS-treated mice, compared to healthy controls.
Figure 5. Smaller HEP-HSA NPs and uncoated HSA NPs did not exhibit preferential binding to the inflamed colon in mice in vivo.

(a, b) Size, PDI, and zeta potential measurement of Alexa Fluor®680-labeled (a) HEP-HSA NPs prepared from 40 mg mL−1 HSA and (b) uncoated HSA NPs prepared from 120 mg mL−1 HSA for in vivo evaluation, respectively. The treatment regimen was the same as in Figure 4c. The 3-cm distal colons from healthy mice (Control) and colitic mice (DSS) were excised for IVIS imaging (middle) and quantification (right) for (a) and (b), respectively. (c, d) Individual mouse colons after IVIS imaging were divided equally into three 1-cm pieces for fluorescence quantification (left) and myeloperoxidase (MPO) activity (middle) after homogenization, as well as for histology assessment (right) for (a) and (b), respectively. Data are presented as mean ± SD (n = 3) in (a - b) and all data on fluorescence intensity, MPO activity, and histology scores are presented as mean ± SEM (n = 6 - 7 mice per group) in (a - d); P-values were determined by Student’s t test.
One possible issue in determining NP targeting the intestinal mucosa is whether smaller NPs could penetrate into the submucosa or deeper, thereby preventing the fluorescence signals from being detected due to tissue thickness. A previous study investigated how the size of poly(lactic-co-glycolic acid) (PLGA) particles affected their localization at the inflamed colonic mucosa in human using fluorescent endoscope, and found a pronounced accumulation of larger particles (3 μm), not smaller ones (300 nm), in rectal ulcers of a patient with IBD.[38] The subsequent analysis of colonic biopsies from the patient by Ussing chambers in vitro showed that, under inflammation, the majority of 300 nm PLGA particles translocated from the mucosa to the serosa, thereby not being detected. To understand whether NPs penetrated into the submucosa or deeper in our study, we homogenized one of the 1-cm pieces of the distal colon after IVIS imaging, quantified the fluorescence intensity in the homogenate by a microplate reader (TECAN), and normalized against total amount of protein in that piece of tissue. Similar patterns of fluorescence intensity in the homogenized colon were observed, compared to the IVIS quantification, where there was no significant difference for NP retention between the inflamed colon and the healthy control for both NP formulations evaluated (P = 0.1574 and P = 0.2110, respectively) (Figure 5c, d). Therefore, unlike the reported observation in human colonic biopsies, we did not observe a difference in fluorescence signals due to NP translocation in mouse colons with DSS-induced colitis; this may be attributed to the difference in thickness of colonic tissues between humans and mice.
In our hands, the HEP-HSA NPs exhibited an inverse correlation between the inflamed mucosal binding and NP diameter, where larger NPs demonstrated preferential binding to the inflamed mucosa; this has been observed ex vivo and in vivo. It has been reported that larger NPs exhibited higher retention at the inflamed mucosa in colitis, compared to healthy controls, for synthetic poly(ethylene glycol)-conjugated polystyrene NPs (200 nm vs. 100 nm) in vivo in mice.[39] We did not normalize the direct IVIS quantification against colon tissue weight, however, the colon tissue was measured out at 3cm for a uniform length for comparison; additionally, our analysis of the homogenized colon pieces, in which the fluorescence intensity was normalized against the total protein, showed a similar pattern to the direct IVIS quantification (Figure 5). HSA NPs have recently been investigated for drug delivery in IBD, in which HSA was chemically conjugated to 5-aminosalicylic acid and crosslinked by glutaraldehyde to form NPs; however, the retention of these NPs in the inflamed intestine, compared to healthy controls, was not studied.[25] Without HEP coating, our HSA NPs of 230 nm did not exhibit preferential binding to the inflamed mucosa evaluated at 3h after the enema treatment. Presumably, the overexpressed proteolytic enzymes at the site of inflammation [40] in colitis might cause degradation of these HSA NPs, thereby reducing their retainment at the inflamed vicinity. In this regard, the HEP coating, in addition to providing negative charge to interact with the accumulated positively charged proteins at the inflamed mucosa, could protect HSA NPs from being degraded and extend their localization at the inflamed intestine. NP targeting the inflamed intestine in vivo was evaluated at 3h after administration, approximating prior time scales previously reported at around 2h for the evaluation of NP retention in vivo in animal models and human patients with IBD.[38–39] Our future studies aim to expand our understanding of the dynamics of retention of HEP-HSA NPs in vivo for longer durations and with various labeling techniques for comparison.
We selected HSA and porcine intestinal mucosal HEP for NP formulation and evaluation considering potential human translation of the system. Here we exploited HSA as a potential drug delivery platform and harnessed the high negative charge density of HEP for NP coating to interact with positively charged proteins at the inflamed mucosa. An important consideration was that the incorporation of small-molecule drugs and biologics could potentially benefit from selective targeting to the inflamed mucosa to minimize possible side effects otherwise associated with untargeted drugs. It is known that albumin and HEP are available from various mammalian species [11, 41] and they may differ in physicochemical properties depending on their origins; hence, differences in NP formulation parameters, size, and surface charge may be expected. Additionally, we employed HEP mainly of 17 - 19 kDa for HSA NP coating, which would be different in NP physicochemical characteristics as compared to low-molecular-weight-HEP (~5 kDa).[42] Furthermore, the potential benefit and mechanisms of action of HEP in IBD treatment have been observed to be associated with its molecular weight;[43] therefore, differences in biological interactions of HEP with the mucosal components may also be expected when using HEP of different molecular weight for NP coating.
We selected the DSS-induced murine model of acute colitis to study NP targeting the inflamed intestine due to the rapid onset of inflammation and the relative straightforward experimental procedure in the generation of the colitis model.[32] Although DSS-induced acute colitis is a widely used animal model in IBD studies, the nature of chemically induced colitis is known to be heterogeneous.[44] Evaluation of therapeutic efficacy in DSS-induced colitis is complex and affected by multiple factors, including the choice of drugs, dosage, treatment regimen, and the intestinal microflora.[45] Importantly, this self-resolving acute colitis model is limited in disease duration as the colitis recovers naturally. Chronically developed colitis in the DSS model or adoptive T-cell transfer model, [46] as well as spontaneous models of IBD [47], may be more relevant to study therapeutic efficacy in vivo. Furthermore, we use HSA as drug carriers, which potentially necessitates humanized mouse models to fully understand the degradation of HSA for efficacy evaluation. Based on current understanding of intestinal inflammation and components in our NPs, we expect that, once the HEP-HSA NPs localize on the inflamed intestine, several biological events may occur sequentially or simultaneously, including cellular uptake of NPs by phagocytes or endothelial cells at the site of inflammation, NP degradation by HEP lyase enzymes and proteases in the vicinity,[16b, 48] and possible interaction of NPs with the microflora.[49] Our future studies will determine whether the preferential binding of HEP-HSA NPs (278 nm, −42 mV) to the inflamed mucosa may lead to improved drug localization at ulcers for IBD treatment in a diverse set of IBD models using NPs loaded with various drug combinations.
In summary, we have developed a new IT drug delivery system with relevance for IBD treatment. We introduce here HEP-HSA NPs capable of loading drug combinations and targeting the inflamed colon in DSS-induced colitis in mice. We show that HEP-HSA NPs can be loaded with both small-molecule drugs and biologics simultaneously. The dual-drug loaded HEP-HSA NPs demonstrate higher anti-inflammatory effect than both of the single-drug loaded NPs, evaluated in RAW264.7 macrophages in vitro. By modulating the NP characteristics, the dual-drug loaded HEP-HSA NPs (278 nm, −42 mV) can be preferentially retained in the inflamed colon in DSS-treated mice, compared to healthy controls. Interestingly, an inverse correlation between inflamed mucosal binding and HEP-HSA NP diameter has been found, where larger NPs preferentially bind to the inflamed colon. Our study suggests that specific physicochemical properties modulate the inflamed mucosal binding of NPs and provides further understanding on the underlying interactions between HEP/albumin-based NPs and the biological interface in colitis.
Experimental Section
Formulation and characterization of synthesized HEP-HSA NPs.
HSA solution of different concentrations (40, 80, and 120 mg mL−1 in water) was used to formulate NPs based on a previously reported method[14] with modification. Briefly, 225 µL of HSA solution was added to 225 µL of 0.1 M GSH. The mixture was stirred at 800 rpm under 37ºC. After 1h, 50µL of water was added to the mixture under room temperature (R.T.) and stirred for 5 min, which was followed by dropwise addition of 2 mL of 100% ethanol. Note that all volumes were doubled for the formulation using 120 mg mL−1 HSA. The mixture was then stirred at 1200 rpm for 10 min under 37ºC. The so formed HSA NPs were dialyzed (MWCO 3.5 kDa) against water under 4°C overnight. To formulate HEP-HSA NPs, 375 µL of 1.0 mg mL−1 HEP in water was added dropwise to 300 µL of the dialyzed HSA NPs. The mixture was shaking gently under R.T. for 1 h. The HEP-HSA NPs were dialyzed (MWCO 100 kDa) under R.T. against water (x5). The size and zeta potential of all NPs were measured using 10 µL of NPs diluted in 1 mL of 1 mM of NaCl (Malvern Zetasizer Nanoseries®). To observe NP morphology, 5 µL of NPs was added onto a clean silica surface and dried under R.T. with natural convection. The prepared sample was then imaged by a high-resolution scanning electron microscope (Zeiss Merlin).
Quantification of drug encapsulation.
The encapsulation efficiency (EE) of BUD and Vanco was analyzed by HPLC (Agilent 1260 Infinity), and the EE of GM-CSF was quantified by ELISA (R&D Systems). Chromatographic separation was carried out on a 50-mm x 4.6-mm EC-C18 Agilent Poroshell 120 analytical column with 2.7-µm spherical particles. Unloaded HSA NPs were used as control. EE% = [(The amount of drug in NPs)/(Total drug input)]*100%. (1) BUD. The BUD-loaded HSA NPs were prepared by dissolving BUD in 100% ethanol for dropwise addition. To quantify the EE, 500 µL of the BUD-loaded HSA NPs and 500 µL of 0.1 M GSH were mixed under R.T. for overnight shaking. Next morning 1.0 mL of 100% ethanol was added, and the mixture was sonicated for 5 min before HPLC analysis. The mobile phase consisted of a gradient elution of acetonitrile: buffer (pH 3.5 adjusted with 0.1% formic acid) (0 min, 10:90 v/v; 3.5 min, 10:90; 5.5 min, 70:30; 8.5 min, 10:90; 10 min, 10:90) at a flow rate of 1.0 mL min−1 over a 10-min run time. The column temperature was maintained at 30°C. The injection volume was 20 µL and the UV detection wavelength was 244 nm. (2) Vanco. The Vanco-loaded HSA NPs were prepared by dissolving Vanco in 0.1 M GSH before mixing. For HPLC analysis, the Vanco-loaded NPs were processed the same way as described above, except that 1.0 mL of water, instead of 100% ethanol, was added to the mixture after overnight shaking. The mobile phase consisted of acetonitrile: buffer (pH 3.5 adjusted with 0.1% formic acid) (6:94, v/v) at a flow rate of 0.8 mL min−1 over an 8-min run time. The column temperature was maintained at 40°C. The injection volume was 20 µL and the UV detection wavelength was 280 nm. (3) GM-CSF. The GM-CSF-loaded HSA NPs were prepared by adding the GM-CSF solution to the mixture of HSA and GSH after the 1h incubation. The formed NPs were dialyzed (MWCO 100 kDa) against water under 4°C overnight. To quantify the EE, 500 µL of GM-CSF-loaded NPs were mixed with 500 µL of 0.1 M GSH under R.T. for overnight shaking. The amount of GM-CSF was then analyzed by ELISA according to manufacturer’s instructions. (4) GM-CSF+BUD. To formulate BUD and GM-CSF dual-drug loaded HSA NPs, 450 µL of 120 mg mL−1 HSA solution was added to 450 µL of 0.1 M GSH. The mixture was stirred for 1h at 800 rpm under 37ºC. The GM-CSF solution (different concentrations in water) was then added and stirred for 5min under R.T. at 800 rpm. Next, 4.0 mL of 3.0 mg mL−1 of BUD in 100% ethanol was added dropwise and stirred at 1200 rpm for another 10 min under 37°C. The formed NPs was first dialyzed (MWCO 3.5 kDa) against water overnight under 4°C and then repeated (MWCO 100 kDa). The dual-drug loaded NPs were processed for EE quantification according to procedures described above for BUD and GM-CSF, respectively.
Formulation of dye-conjugated HSA NPs.
Briefly, 100 μL of 10 mg mL−1 Alexa Fluor®680 NHS ester in DMSO was added to 10 mL of 10 mg mL−1 HSA in 0.1 M bicarbonate buffer (pH = 8.3) and incubated under R.T. for 1h, protected from light. The mixture was dialyzed (MWCO 12-14 kDa) against 0.1 M phosphate buffer (pH = 5.8, x3) followed by against water (x2). The dye-conjugated HSA was then lyophilized. To formulate the dye-conjugated HSA NPs, the Alexa Fluor®680-labeled HSA was mixed with unmodified HSA at a mass ratio of 1:11 and the NPs were prepared using the same procedure described above.
Anti-inflammatory effect of drug-loaded HEP-HSA NPs in vitro.
RAW 264.7 macrophages were seeded in a 96-well plate (15,000 cells/well) in DMEM containing 10% heat-inactivated fetal bovine serum (HI FBS) under 37°C with 5% CO2. After 24h, 10 µL of 10 ng mL−1 LPS was added to cells for activation, while 10 µL of medium was added to non-activated cells. NPs were concentrated or diluted to achieve the same amount of drug in each single- and dual-drug loaded formulation (BUD at 13.3 μg mL−1 and GM-CSF at 14.8 ng mL−1). Four NP formulations were evaluated: (i) Unloaded (UNP), (ii) BUD-loaded (B/NP), (iii) GM-CSF-loaded (G/NP), and (iv) (GM-CSF+BUD)-loaded (G+B/NP). After 4h of LPS activation, 10 µL of each NPs were added to cells in triplicate. The expression of TNF-α and NO by macrophages were evaluated at 24h after NP treatment, for which the supernatant of macrophages was collected and analyzed by TNF-α ELISA (R&D Systems) and NO assays (Promega Corp.).
Colitis model.
All animal experiments were performed in accordance with protocols approved by the Committee on Animal Care at Massachusetts Institute of Technology and the institutional and National Institutes of Health (NIH) guidelines for humane use of animals. Wild-type female BALB/c mice at the age of 7-week-old were purchased from the Jackson Laboratory. The colitis was induced by feeding mice with 3% DSS (ca. 40 kDa) in drinking water ad libitum for 5 days, and then switched to regular water for another 2 days prior to experiments.[6a] For imaging studies, mice were fed with purified diet (AIN-93G) one week before colitis induction to reduce autofluorescence background in the intestine. Mice without DSS treatment were healthy controls. All mice were fasted overnight before the enema treatment.
Evaluation of NP targeting the inflamed colon ex vivo.
On day 7, mice with colitis and healthy controls were sacrificed and colons were cut between the cecum and the anus, measured, and rinsed with PBS. The 1.5-cm distal colons were collected and inverted to expose the mucosa (flipped inside out). Four Alexa Fluor®680-conjugated NP formulations (G1 - G4), in which G1, G2, and G3 were HEP-HSA NPs of different sizes and G4 was HSA NPs, were used for the ex vivo evaluation. Briefly, 500 µL of NPs were incubated with the inverted 1.5-cm distal colon for 30 min under 37ºC on a rotary mixer.[6a] The colons were then washed with PBS (x3) and cut open longitudinally with luminal side facing up for IVIS imaging (PerkinElmer). The fluorescence intensity was quantified by Living Image 4.5 (PerkinElmer) in a standard-sized region of interest (ROI) drawn around individual colon pieces. Background fluorescence intensity was determined as the average of four ROIs not containing any colon tissue and was subtracted from all specimens.
Evaluation of NP targeting the inflamed colon in vivo.
After overnight fasting, each mouse from the two groups, healthy controls and mice with colitis, received an enema of 100 µL of the dual-drug loaded, Alexa Fluor®680-conjugated HEP-HSA NPs prepared from 120 mg mL−1 of HSA. Briefly, individual mouse was anesthetized and a 20-gauge sterile flexible feeding needle (Cadence Science, Inc.) was advanced into the rectum 3-cm past the anus, NPs were administered, the catheter was removed, and the mouse was kept vertically upside down for 1 min before back in the cage. After 3h, mice were sacrificed and the colon length from the cecum to the anal verge was measured. The distal 3-cm colon was removed, cut open longitudinally with luminal side facing up and imaged freshly without wash by IVIS. In another set of experiment, two NP formulations, HSA NPs prepared from 120 mg mL−1 HSA and HEP-HSA NPs prepared from 40 mg mL−1 HSA, were evaluated for targeting the inflamed colon in vivo by IVIS. After imaging, the 3-cm distal colon was divided into three of 1-cm pieces; two of them were homogenized for fluorescence quantification by a microplate reader (TECAN, Ex = 680 nm, Em = 720 nm) and MPO assay,[6a] respectively, and one piece was processed for histopathology assessment. The 1-cm colon piece was fixed in 10% neutral-buffered formalin, and the segments were embedded in paraffin, sectioned (5 μm), stained with hematoxylin and eosin (H&E). Histopathology of colonic tissues was scored by a pathologist blinded to study group assignment. The histology score was a sum-score composed of 4 features – extent of: inflammation (0-3), crypt damage (0-3), edema (0-3), and accumulation of inflammatory cells (0-3) with a maximum of 12 points.
Statistical analysis.
Statistical analysis and graphing were performed with Prism 8 (Graphpad Software). The Student’s t test was used to compare differences between two experimental groups. In experiments with multiple groups, Holm-Sidak’s multiple comparison test and one-way analysis of variance (ANOVA) were used. A value of p < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
The authors thank Dr. Robert Langer (R.L.) for his review of the manuscript and helpful suggestions. This work was supported by a Research Fellowship award from the Crohn’s & Colitis Foundation (to S.Z.) and in part by an NIH Grant 2R01EB000244-39 (G.T.) and an NIH Grant No. R01 CA225655 (to J.K.L.). The authors also thank Dr. Thomas von Erlach for helpful discussions. The authors acknowledge the Animal Imaging Core facility and the Histology Core facility at the Koch Institute Swanson Biotechnology Center at the Massachusetts Institute of Technology for their help with the animal studies. The graphical Table of Contents (TOC) and the schematic in Figure 3d were created with Biorender.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
S.Z., J.R.K. and G.T. are co-inventors on a provisional patent application encompassing the technology described in this manuscript.
References
- [1].Kaser A, Zeissig S, Blumberg RS, Annu Rev Immunol 2010, 28, 573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].(a) Cho JH, Nat Rev Immunol 2008, 8, 458–466; [DOI] [PubMed] [Google Scholar]; (b) Chassaing B, Darfeuille-Michaud A, Gastroenterology 2011, 140, 1720–1728. [DOI] [PubMed] [Google Scholar]
- [3].(a) Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE, Am J Gastroenterol 2018, 113, 481–517; [DOI] [PubMed] [Google Scholar]; (b) Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD, Am J Gastroenterol 2019, 114, 384–413. [DOI] [PubMed] [Google Scholar]
- [4].(a) Her M, Kavanaugh A, J Allergy Clin Immunol 2016, 137, 19–27; [DOI] [PubMed] [Google Scholar]; (b) Stallmach A, Hagel S, Bruns T, Best Pract Res Clin Gastroenterol 2010, 24, 167–182. [DOI] [PubMed] [Google Scholar]
- [5].(a) Hoivik ML, Moum B, Solberg IC, Cvancarova M, Hoie O, Vatn MH, Bernklev T, Group IS, Inflamm Bowel Dis 2012, 18, 1540–1549; [DOI] [PubMed] [Google Scholar]; (b) Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Lancet 2018, 390, 2769–2778. [DOI] [PubMed] [Google Scholar]
- [6].(a) Zhang S, Ermann J, Succi MD, Zhou A, Hamilton MJ, Cao B, Korzenik JR, Glickman JN, Vemula PK, Glimcher LH, Traverso G, Langer R, Karp JM, Sci Transl Med 2015, 7, 300ra128; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N, Nat Mater 2010, 9, 923–928; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Schoellhammer CM, Schroeder A, Maa R, Lauwers GY, Swiston A, Zervas M, Barman R, DiCiccio AM, Brugge WR, Anderson DG, Blankschtein D, Langer R, Traverso G, Sci Transl Med 2015, 7, 310ra168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Strugala V, Dettmar PW, Pearson JP, Int J Clin Pract 2008, 62, 762–769. [DOI] [PubMed] [Google Scholar]
- [8].Tolstanova G, Deng X, French SW, Lungo W, Paunovic B, Khomenko T, Ahluwalia A, Kaplan T, Dacosta-Iyer M, Tarnawski A, Szabo S, Sandor Z, Lab Invest 2012, 92, 9–21. [DOI] [PubMed] [Google Scholar]
- [9].(a) Monajemi H, Meenan J, Lamping R, Obradov DO, Radema SA, Trown PW, Tytgat GN, Van Deventer SJ, Gastroenterology 1996, 110, 733–739; [DOI] [PubMed] [Google Scholar]; (b) Ramasundara M, Leach ST, Lemberg DA, Day AS, J Gastroenterol Hepatol 2009, 24, 202–208; [DOI] [PubMed] [Google Scholar]; (c) Tirosh B, Khatib N, Barenholz Y, Nissan A, Rubinstein A, Mol Pharm 2009, 6, 1083–1091. [DOI] [PubMed] [Google Scholar]
- [10].(a) Zhang S, Langer R, Traverso R, Nano Today 2017, 16, 82–96; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Takedatsu H, Mitsuyama K, Torimura T, World J Gastroenterol 2015, 21, 11343–11352; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lamprecht A, Nat Rev Gastroenterol Hepatol 2010, 7, 311–312. [DOI] [PubMed] [Google Scholar]
- [11].Karimi M, Bahrami S, Ravari SB, Zangabad PS, Mirshekari H, Bozorgomid M, Shahreza S, Sori M, Hamblin MR, Expert Opin Drug Deliv 2016, 13, 1609–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fasano M, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, Notari S, Ascenzi P, IUBMB Life 2005, 57, 787–796. [DOI] [PubMed] [Google Scholar]
- [13].Larsen MT, Kuhlmann M, Hvam ML, Howard KA, Mol Cell Ther 2016, 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang W, Huang Y, Zhao S, Shao T, Cheng Y, Chem Commun (Camb) 2013, 49, 2234–2236. [DOI] [PubMed] [Google Scholar]
- [15].Paluck SJ, Nguyen TH, Maynard HD, Biomacromolecules 2016, 17, 3417–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].(a) Prajapati DN, Newcomer JR, Emmons J, Abu-Hajir M, Binion DG, Inflamm Bowel Dis 2002, 8, 192–195; [DOI] [PubMed] [Google Scholar]; (b) Papa A, Danese S, Gasbarrini A, Gasbarrini G, Aliment Pharmacol Ther 2000, 14, 1403–1409; [DOI] [PubMed] [Google Scholar]; (c) Ang YS, Mahmud N, White B, Byrne M, Kelly A, Lawler M, McDonald GS, Smith OP, Keeling PW, Aliment Pharmacol Ther 2000, 14, 1015–1022. [DOI] [PubMed] [Google Scholar]
- [17].Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, Bevilacqua MP, Blood 1993, 82, 3253–3258. [PubMed] [Google Scholar]
- [18].White B, Ang YS, Mahmud N, Keeling PW, Smith OP, Lancet 1999, 354, 1122–1123. [DOI] [PubMed] [Google Scholar]
- [19].Hattori T, Kimura K, Seyrek E, Dubin PL, Anal Biochem 2001, 295, 158–167. [DOI] [PubMed] [Google Scholar]
- [20].Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S, I. B. D. S. o. t. B. S. o. Gastroenterology, Gut 2011, 60, 571–607. [DOI] [PubMed] [Google Scholar]
- [21].Sovran B, Planchais J, Jegou S, Straube M, Lamas B, Natividad JM, Agus A, Dupraz L, Glodt J, Da Costa G, Michel ML, Langella P, Richard ML, Sokol H, Microbiome 2018, 6, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].(a) Bernasconi E, Favre L, Maillard MH, Bachmann D, Pythoud C, Bouzourene H, Croze E, Velichko S, Parkinson J, Michetti P, Velin D, Inflamm Bowel Dis 2010, 16, 428–441; [DOI] [PubMed] [Google Scholar]; (b) Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M, Science 2014, 343, 1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Korzenik JR, Dieckgraefe BK, Valentine JF, Hausman DF, Gilbert MJ, Sargramostim G in Crohn’s Disease Study, N Engl J Med 2005, 352, 2193–2201. [DOI] [PubMed] [Google Scholar]
- [24].(a) Panaccione R, Ghosh S, Middleton S, Marquez JR, Scott BB, Flint L, van Hoogstraten HJ, Chen AC, Zheng H, Danese S, Rutgeerts P, Gastroenterology 2014, 146, 392–400 e393; [DOI] [PubMed] [Google Scholar]; (b) Xiao B, Zhang Z, Viennois E, Kang Y, Zhang M, Han MK, Chen J, Merlin D, Theranostics 2016, 6, 2250–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iwao Y, Tomiguchi I, Domura A, Mantaira Y, Minami A, Suzuki T, Ikawa T, Kimura SI, Itai S, Eur J Pharm Biopharm 2018, 125, 141–147. [DOI] [PubMed] [Google Scholar]
- [26].Yazeji T, Moulari B, Beduneau A, Stein V, Dietrich D, Pellequer Y, Lamprecht A, Drug Deliv 2017, 24, 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].(a) Kolios G, Valatas V, Ward SG, Immunology 2004, 113, 427–437; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cross RK, Wilson KT, Inflamm Bowel Dis 2003, 9, 179–189; [DOI] [PubMed] [Google Scholar]; (c) Boughton-Smith NK, Evans SM, Hawkey CJ, Cole AT, Balsitis M, Whittle BJ, Moncada S, Lancet 1993, 342, 338–340. [DOI] [PubMed] [Google Scholar]
- [28].(a) Rachmilewitz D, Eliakim R, Ackerman Z, Karmeli F, Am J Gastroenterol 1998, 93, 409–412; [DOI] [PubMed] [Google Scholar]; (b) Kimura H, Hokari R, Miura S, Shigematsu T, Hirokawa M, Akiba Y, Kurose I, Higuchi H, Fujimori H, Tsuzuki Y, Serizawa H, Ishii H, Gut 1998, 42, 180–187; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Middleton SJ, Shorthouse M, Hunter JO, Lancet 1993, 341, 465–466. [DOI] [PubMed] [Google Scholar]
- [29].(a) Otsuka M, Tsuchiya S, Aramaki Y, Biol Pharm Bull 2006, 29, 499–502; [DOI] [PubMed] [Google Scholar]; (b) Matsuno R, Aramaki Y, Arima H, Tsuchiya S, Biochem Biophys Res Commun 1997, 237, 601–605. [DOI] [PubMed] [Google Scholar]
- [30].Upchurch GR Jr., Goodman DG, Willoughby SR, Zhang YY, Welch GN, Freedman JE, Ye S, Costello CE, Loscalzo J, J Cardiovasc Pharmacol Ther 2001, 6, 163–173. [DOI] [PubMed] [Google Scholar]
- [31].Joglekar M, Khandelwal S, Cines DB, Poncz M, Rauova L, Arepally GM, J Thromb Haemost 2015, 13, 1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF, Nat Protoc 2017, 12, 1295–1309. [DOI] [PubMed] [Google Scholar]
- [33].Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J, Proc Natl Acad Sci U S A 2007, 104, 1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Xu G, Forstner GG, Forstner JF, Glycoconj J 1996, 13, 81–90. [DOI] [PubMed] [Google Scholar]
- [35].Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, Brown D, Alkilany AM, Farokhzad OC, Mahmoudi M, Chem Soc Rev 2017, 46, 4218–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].(a) Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F, Am J Physiol Gastrointest Liver Physiol 2001, 281, G216–228; [DOI] [PubMed] [Google Scholar]; (b) Gibson PR, van de Pol E, Barratt PJ, Doe WF, Gut 1988, 29, 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].(a) Masoodi I, Kochhar R, Dutta U, Vaishnavi C, Prasad KK, Vaiphei K, Kaur S, Singh K, J Gastroenterol Hepatol 2009, 24, 1768–1774; [DOI] [PubMed] [Google Scholar]; (b) Krawisz JE, Sharon P, Stenson WF, Gastroenterology 1984, 87, 1344–1350. [PubMed] [Google Scholar]
- [38].Schmidt C, Lautenschlaeger C, Collnot EM, Schumann M, Bojarski C, Schulzke JD, Lehr CM, Stallmach A, J Control Release 2013, 165, 139–145. [DOI] [PubMed] [Google Scholar]
- [39].Maisel K, Ensign L, Reddy M, Cone R, Hanes J, J Control Release 2015, 197, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sorokin L, Nat Rev Immunol 2010, 10, 712–723. [DOI] [PubMed] [Google Scholar]
- [41].Kouta A, Jeske W, Hoppensteadt D, Iqbal O, Yao Y, Fareed J, Clin Appl Thromb Hemost 2019, 25, 1076029619889406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Weitz JI, N Engl J Med 1997, 337, 688–698. [DOI] [PubMed] [Google Scholar]
- [43].Day R, Forbes A, Lancet 1999, 354, 62–65. [DOI] [PubMed] [Google Scholar]
- [44].(a) Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M, Curr Protoc Immunol 2014, 104, 15.25.1–15.25.14; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Perse M, Cerar A, J Biomed Biotechnol 2012, 2012, 718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].(a) Chai JN, Peng Y, Rengarajan S, Solomon BD, Ai TL, Shen Z, Perry JSA, Knoop KA, Tanoue T, Narushima S, Honda K, Elson CO, Newberry RD, Stappenbeck TS, Kau AL, Peterson DA, Fox JG, Hsieh CS, Sci Immunol 2017, 2; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sann H, Erichsen J, Hessmann M, Pahl A, Hoffmeyer A, Life Sci 2013, 92, 708–718; [DOI] [PubMed] [Google Scholar]; (c) Ocon B, Aranda CJ, Gamez-Belmonte R, Suarez MD, Zarzuelo A, Martinez-Augustin O, Sanchez de Medina F, Biochem Pharmacol 2016, 116, 73–88. [DOI] [PubMed] [Google Scholar]
- [46].Mottet C, Uhlig HH, Powrie F, J Immunol 2003, 170, 3939–3943. [DOI] [PubMed] [Google Scholar]
- [47].(a) Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH, Cell 2007, 131, 33–45; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Scheinin T, Butler DM, Salway F, Scallon B, Feldmann M, Clin Exp Immunol 2003, 133, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Key NS, Platt JL, Vercellotti GM, Arterioscler Thromb 1992, 12, 836–842. [DOI] [PubMed] [Google Scholar]
- [49].(a) Watanabe M, Tsuda H, Yamada S, Shibata Y, Nakamura T, Sugahara K, J Biochem 1998, 123, 283–288; [DOI] [PubMed] [Google Scholar]; (b) Riley TV, J Clin Pathol 1987, 40, 384–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


