Abstract
To investigate the possible influence of head rotation on the results of salivary gland scintigraphy, a phantom study was designed to simulate clinical salivary gland scintigraphy. The quantitative accuracy of regional activity counts was compared for two data acquisition methods involving head rotation: (i) an anterior planar projection-only (ANT) method and (ii) a geometric mean (GM) method using both the anterior and posterior planar projections. The roles and limitations of the GM and ANT methods when used at different head rotation angles were examined. Parallel planar projections of a head phantom with four salivary gland simulators, containing 3.7 MBq 99mTc-sodium pertechnetate, at various rotational settings were acquired using a dual-head gamma camera. The difference between the standard activity counts (no phantom rotation) and the activity counts affected by the phantom rotation was calculated and defined as the rotational bias that decreased the accuracy of activity quantification. For small-angle rotation (≤10°), use of the GM method decreased the bias for all salivary gland simulators. In contrast, the bias of large-angle rotation (>10°) between four salivary gland simulators became conspicuous and complex in both methods. This bias may reflect different attenuation effects caused by displacement of the structures. Our data suggest that the GM method can be used when the head rotation angle is small (≤10°); however, when the head rotation angle is >10°, the non-negligible influence of head rotation should be considered during image acquisition.
Keywords: 99mTc-pertechnetate, salivary gland scintigraphy, rotation, phantom, motion correction
INTRODUCTION
Salivary gland hypofunction can result from autoimmune diseases and medical therapy, such as medication and radiation therapy, and usually presents as the clinical symptom of xerostomia or the subjective sensation of dry mouth [1]. The diagnosis of salivary gland hypofunction is based on medical history, imaging results and specialized examination [2]. Most imaging modalities such as CT, MRI and sonography provide information about anatomy rather than function and are used in the diagnosis of salivary gland tumours and sialolithiasis. Specialized examinations of salivary gland function, such as sialometry, sialography and sialendoscopy, require complicated or invasive procedures. In contrast to these imaging and specialized examinations, salivary gland scintigraphy is non-invasive and well tolerated by patients, and can be used to quantify both the parenchymal function and excretion of all salivary glands [2–5].
Nerveless salivary gland scintigraphy appears to be an ideal method for objectively evaluating salivary gland function. As in other nuclear imaging methods, motion by the object can produce artefacts that affect the accuracy of the quantitative activity counts. Such motion can be limited or prevented with the use of straps, pillows or other restraints, as suggested by the International Atomic Energy Agency [6]. However, given the rounded shape of the head, head rotation (defined as rotation of the head around the fixed axis along the cervical spine) can still occur during acquisition of planar imaging even when translational motion (i.e. displacement) is prevented.
Only a few studies have examined the influence of head rotation on the accuracy of quantitative data in planar imaging used with salivary gland scintigraphy. Angelis et al. reported that using the geometric mean (GM) obtained from parallel planar projections improved the accuracy of quantitative data from mouse phantoms with rotation [7]. Yapar et al. also used the GM to improve the accuracy of 99mTc-dimercaptosuccinic acid (DMSA) renal cortical scanning by correcting for the asymmetrical depth of both kidneys [8]. This analysis produced different distances and tissue attenuation, which may be important for similar situations involving asymmetric locations and limited range of rotation.
During image acquisition in salivary gland scintigraphy, voluntary head rotation can occur as a combination of any number of stationary projections along different angles of head rotation around the long axis of the cervical spine (referred to here as ‘rotation status’). To understand better the influence of head rotation on the accuracy of salivary gland scintigraphy, we designed a phantom study in which we simulated the standard position (no phantom rotation) and altered rotation status (stationary position of the phantom after rotation to a specified position at different angles).Our aims here were to investigate (i) the influence of head rotation (change in head position relative to the long axis of the spine) during acquisition and (ii) whether the GM method using both anterior and posterior planar projections could reduce the influence of head rotation in quantitative studies compared with the anterior planar projection-only (ANT) method.
MATERIALS AND METHODS
Imaging of a head phantom with salivary gland simulators
An anthropomorphic head phantom (5220 RS900T, Radiology Support Devices, Inc., Long Beach, CA, USA) was used [9, 10]. Soft-tissue and skeletal moulds of the phantom were matched for anatomic fidelity. The soft-tissue materials comprise transparent tissue-equivalent materials, and the skeletal materials met the radiation interaction properties standardized by the International Commission on Radiation Units and Measurements. We simulated the positions of the salivary glands by attaching four cotton balls sealed in thin plastic bags to the surface of the head phantom in positions as anatomically appropriate as possible (Fig. 1). Adding 3.7 MBq of 99mTc-sodium pertechnetate to each cotton ball produced acquired counts of planar projections that mimicked those obtained by imaging normally functioning salivary glands. To simulate the rotation of a patient’s head, we used laser guidance to place the head phantom in the standard position, which we defined as the starting position without phantom rotation (0°) (Fig. 2A) and rotation status for different angles of clockwise rotation around the long axis of the cervical spine, from a view superior to the top of the phantom in the prone position (Fig. 2B). In the clinical scenario, a patient head can rotate to the right (clockwise) or left (anti-clockwise), and the influence should be symmetrical for the two possible directions of rotation. To simplify and standardize this condition, we used only one direction of rotation (clockwise). Because of the clockwise rotation, the right side of the salivary gland simulators was defined as the rotary side, which included the rotary-side parotid gland simulator (RPS) and rotary-side submandibular gland simulator (RSMS), and the left side (anti-rotary-side) included the anti-rotary-side parotid gland simulator (ARPS) and anti-rotary-side submandibular gland simulator (ARSMS).
Fig. 1.

Head phantom with salivary gland simulators.
Fig. 2.

Angles used with the phantom under guidance of a laser from a gamma camera. (A) Standard position (no phantom rotation) and (B) rotation status (stationary position of the phantom after rotation) for clockwise rotation. The arrow indicates the clockwise direction of rotation of the phantom head.
Imaging procedure
The phantom study was conducted using a dual-head gamma camera (Discovery 670 NM/CT, GE Healthcare, Waukesha, WI, USA) in the Nuclear Medicine Department at Tri-Service General Hospital, Taipei, Taiwan. The camera was fitted with a low-energy, all-purpose parallel collimator. The opposing planar projections were acquired for a 256 × 256 matrix and lasted for 4 min. The head phantom was fastened to the patient table using a belt and was rotated in a clockwise direction in angular increments of 0, 5, 10, 20 and 30° to acquire the planar projections. This procedure was repeated 12 times to obtain the average activity counts to reduce the potential for operator bias.
Data analysis
The planar projections and data processing were performed by an experienced operator using a nuclear medicine workstation (Xeleris™ 4.0, GE Healthcare). Regions of interest (ROIs) around the salivary gland simulators were defined in both the anterior and posterior images (Fig. 3A and B), and the counts within these regions were recorded by parallel detectors for the different rotational angles of the phantom. The anterior planar acquisition without phantom rotation collected ~67 000 total counts for the parotid gland simulators and ~41 000 total counts for the submandibular gland simulators, which are close to those acquired in the clinical scenario. All datasets were corrected for isotope decay and normalized to standard activity counts (no phantom rotation) in each ROI. The percentage absolute difference between rotational and standard planar normalized counts was considered to represent the bias related to the phantom rotation in the acquisition methods of salivary scintigraphy and was calculated using equation (1)1.
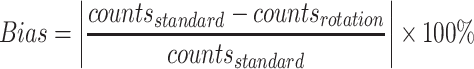 |
(1) |
Fig. 3.

Representative images from the gamma camera with a planar detector capable of visualizing all salivary gland simulators: (A) anterior view and (B) posterior view. SPECT/CT fusion images: (C) parotid gland simulators and (D) submandibular gland simulators.
We then compared the bias using the following two data processing methods: (i) only using the anterior planar counts method and (ii) using the GM of both the anterior and posterior planar counts method, which are hereafter called the ANT method and GM method, respectively.
RESULTS
The activity counts for the four ROIs of planar projections (bilateral parotid gland simulators and submandibular gland simulators) were extracted at different angles of rotation using the ANT and GM methods. In the standard position (no phantom rotation), the counts for RPS and RSMS were virtually equal to those of ARPS and ARSMS, respectively. The counts were higher for the parotid gland simulators than the submandibular gland simulators, which matches the natural condition of the salivary glands. The absolute difference in normalized counts between the rotational and standard planar projections was considered to reflect the bias of the acquisition method caused by the phantom rotation. The bias values are presented in a bar chart (Fig. 4).
Fig. 4.

Absolute difference in normalized regional activity counts extracted from the planar projections between the GM and ANT acquisition methods. The bias values for four regions were plotted for different angles of rotation: (A) rotary parotid gland simulator, (B) anti-rotary parotid gland simulator, (C) rotary submandibular gland simulator, and (D) anti-rotary submandibular gland simulator. The counts were normalized to the standard counts (0°) for each region.
The bias was smaller for the GM method than for the ANT method in both the salivary gland simulators for small-angle rotation (≤10°) of the phantom around the rotational centre of the neck. In contrast, the relationship between the bias for the GM and ANT methods was complex for large-angle rotation. The bias seemed to result from displacement of structures and changes in the distance between the salivary gland simulators and detectors (Fig. 5).
Fig. 5.

Attenuation of photons emitted from the simulators by the skull and neck. (A) Parotid gland simulators for non-rotation, (B) submandibular gland simulators for non-rotation, (C) parotid gland simulators for large-angle rotation, and (D) submandibular gland simulators for large-angle rotation.
Small rotation ranges (≤10°)
The bias was larger for the RPS than for other simulators. The largest bias values for the GM method were 15.1, 0.9, 3.8 and 1.6% for the RPS, ARPS, RSMS and ARSMS, respectively. The largest bias values for the ANT method were 29.6, 6.1, 4.2 and 3.0% for the RPS, ARPS, RSMS and ARSMS, respectively.
Large rotation ranges (>10°)
For the RPS, the counts for both methods showed severe reduction. In the GM method, the normalized values decreased by 51.9% at 20° and 57.8% at 30°. This decrease in normalized values was smaller for the GM than the ANT method: 77.8% at 20° and 83.0% at 30°. For the ARPS, the bias in the anti-rotary side was markedly larger for the GM method than for the ANT method; the normalized values decreased by 11.7% at 20° and 28.8% at 30° for the GM method and increased by 7.1% at 20° and 7.4% at 30° for the ANT method.
For the RSMS, the bias was markedly larger for the GM method than for the ANT method. The normalized values increased by 32.9% at 20° and 88.6% at 30° for the GM method and decreased by 9.6% at 20° and 24.9% at 30° for the ANT method.
Similar to the ARPS, the bias for the ARSMS was also larger for the GM than for the ANT method. The normalized values decreased by 7.6% at 20° and 15.4% at 30° for the GM method and increased by 3.9% at 20° and 5.1% at 30° for the ANT method.
Attenuation between large ranges of rotation and non-rotation
For planar projection acquisition without phantom rotation, photons emitted from the parotid gland simulators were not attenuated by the neck and skull at both the anterior and posterior detectors. However, photons from the submandibular gland simulators were attenuated by the neck at the posterior detector but not the anterior detector (Fig. 5A and B). For large-angle rotation, photons were attenuated by the skull when travelling from the ARPS to the posterior detector but not when travelling to the anterior detector (Fig. 5C). Photons were attenuated by the skull when travelling from the RPS to the anterior detector but not when travelling to the posterior detector. Photons emitted from the ARSMS travelling to the posterior detector encountered increasing attenuation effects caused by the longer distance in the neck than in the pathway from the margin area to the central area of the neck (Fig. 5D). However, photons reached the anterior detector without any such obstruction. For the RSMS, the photons were not blocked from travelling to either the anterior or posterior detector.
DISCUSSION
Salivary gland scintigraphy is a useful tool for diagnosing salivary gland disease and providing quantitative data to assist clinical evaluation. The uptake of 99mTc-pertechnetate in the salivary glands is based on both 1-fold negative charge and the similar diameter to chloride, which yield information about the corresponding physicochemical characteristics and may reflect the function and condition of the salivary glands. However, the detection of photons emitted from 99mTc-pertechnetate in the salivary glands can be affected by many factors, particularly head motion. Compared with transmission photon imaging (such as CT and X-ray), the acquisition of emission photon images requires longer periods for biodistribution and in vivo radiopharmaceutical kinetics.
Because head motion degrades the spatial resolution and quantitative reliability, motion should be prevented or limited during imaging. Although the International Atomic Energy Agency suggests using straps, pillows or other restraints to restrict head motion [6], the round shape of the head means that head rotation can still occur in clinical practice even when translational motion is prevented. Theoretically, rotation results in variations in the distance and attenuation of the structure between the salivary glands and planar detectors. These factors are probably similar to the situation in the study by Yapar et al., which investigated the effects of differences in asymmetrical distance and attenuation between the kidneys [8]. In that study, use of the GM method improved the accuracy of the relative renal function results in 99mTc-DMSA scintigraphy for different groups of kidney diseases and conditions. Although methods using only the anterior view are most frequently used in image acquisition, some studies, such as that of Olmos et al., used the GM method from parallel detectors to reduce the effects of absorption by human tissues and to correct the attenuation effect [11–14].
Angelis et al. also used the GM method to correct for rotational motion in their mouse phantom study and found that improved accuracy of the normalized activity was extracted from target regions [7]. They demonstrated a large reduction in measured activity in the kidney (up to 17%) for small-angle rotation (≤10°) and 24% for large-angle rotation (>10°). After correcting for attenuation using the GM method, the bias decreased to 3–4% for small-angle rotation and up to 12% for larger-angle rotation. Angelis et al. suggested using the GM method to increase the accuracy of quantitative data.
Although these studies discussed the improvement in accuracy obtained by using the GM method, there are limited data about the application of the GM method to salivary gland scintigraphy. Hence, we designed a prototype head phantom with salivary gland simulators to simulate clinical scenarios and measured the influence of rotation at different angles (5, 10, 20 and 30°) and using different acquisition methods (GM method and ANT method). For small-angle rotation, the bias was less for the GM method than for the ANT method for all salivary glands. This is similar to that reported in previous studies, especially the study by Angelis et al. [7]. For large-angle rotation, the bias became larger, and the results for the two methods for each salivary gland simulator were complex and different. This might reflect a change in the attenuation effect when photons pass through different materials during rotation. Although Angelis et al. used small organs, including the striatum, heart and kidneys, and found no distinct changes in the attenuation effect [7], the radiation of the radioisotope contained in the salivary gland simulators recorded by parallel detectors should be affected by different attenuation effects caused by changes in the position of the head and neck structures; these changes would be reflected in changes in the detection of radiation, i.e. the activity counts of the salivary gland.
For planar projection acquisition of salivary gland simulators during head rotation, the main obstructions to the parotid gland simulators and submandibular gland simulators were the skull and neck, respectively. For small-angle rotation, the accuracy benefits of the GM method were noted for the regional activity counts. That is, the difference in attenuation of the skull and neck should be much smaller for small-angle rotation than for larger-angle rotation. Therefore, small changes in position (i.e. small displacement of a structure’s position) in small-angle rotation should not influence image acquisition, and the small change in the distance and attenuation of other materials could be corrected. Conversely, the effects of attenuation and distance between the detectors and simulators play important roles in large-angle rotation and produced different effects in different regions when measured using the two acquisition methods. For RPS with large-angle rotation (Fig. 5C), the high attenuation coefficient suggested that the photons emitted from the RPS were blocked from going through the material to the anterior detector, and the decreasing counts may represent attenuation caused by the skull.
The attenuation effect was greater when using the GM method rather than the ANT method because the ANT method used only the counts from the anterior detector. For the ARPS (Fig. 5C), the bias was significantly larger for the GM method than for the ANT method. The counts extracted by the GM method were reduced because the skull of the phantom blocked the photons emitted from the ARPS going to the posterior detector. In contrast, the ANT method was not influenced by the attenuation effect of the phantom skull, and the ARPS was positioned closer to the anterior detector at larger ranges of rotation. Hence, the counts detected by the ANT method showed an increasing trend.
Compared with the parotid gland simulators, the detected counts for the submandibular simulators were affected more by the neck than the skull, which suggests that the photons emitted from the RSMS were attenuated by the neck before detection by the posterior detector in the standard condition (Fig. 5B). For RSMS in larger-angle rotation (Fig. 5D), attenuation by the neck became smaller and the posterior planar counts displayed an increasing trend. The attenuation effect of the neck considerably increased the bias in the GM method, whereas the bias in the ANT method increased only slightly for shorter distances. Similar to the ARPS, the bias for the ARSMS was also larger for the GM method and smaller for the ANT method. The decreasing values in the GM method may be explained by the displacement of structures in the projection path that influenced the attenuation effect. For large-angle rotation, the photons from the ARSMS to the posterior detector were significantly affected by high-density obstacles, such as the cervical spine phantom, and the longer pathway in the neck part of the phantom. The ANT method was not affected by the blocking effect of the neck and had slightly increasing values for a shorter distance, and the influence of head rotation should similar between the ARSMS and ARPS.
Although the accuracy of the ANT method was affected only by the change in anterior planar projection counts, that of the GM method was affected by any change in both the anterior and posterior planar projection counts. The GM method did not improve the accuracy of the quantification of regional activity in salivary scintigraphy and yielded less accurate results than the ANT method, except for the RPS with any angle of rotation and for all salivary gland simulators with small-angle rotation. This does not mean that the ANT method can deal with the influence of rotation more accurately than the GM method at large angles because the activity counts for the PRS were also affected in the ANT method, which may produce false positives. At any angle, the reduction in accuracy, especially for quantification of regional activity, resulting from head rotation cannot be eliminated by either the GM or ANT method, and head rotation should be restricted as much as possible. However, in contrast to large-angle rotation, small-angle rotation is difficult to avoid completely because of the round shape of the head. This problem of small-angle rotation of the head might be solved by using the GM method because the influence of small-angle rotation is markedly less than that of large-angle rotation and this method had less of a reduction in accuracy than the ANT method.
In conclusion, because of the influence of rotation, head rotation should be restricted during scanning as much as possible to avoid decreasing the accuracy of quantitative activity measured with scintigraphy of the salivary glands. If small-angle rotation cannot be avoided in clinical practice, the GM method can be used to reduce the influence of rotation. However, in cases involving a head rotation angle >10°, the non-negligible influence of head rotation should be considered during image acquisition.
FUNDING
The work in this paper was supported by the Tri-Service General Hospital Public Medical Treatment Fund (TSGH-C108–084).
CONFLICT OF INTEREST
We declare that we do not have any commercial or associated interest that represents a conflict of interest in connection with the work submitted.
References
- 1. Grisius MM. Salivary gland dysfunction: A review of systemic therapies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;92:156–62. [DOI] [PubMed] [Google Scholar]
- 2. Loutfi I, Nair MK, Ebrahim AK. Salivary gland scintigraphy: The use of semiquantitative analysis for uptake and clearance. J Nucl Med Technol 2003;31:81–5. [PubMed] [Google Scholar]
- 3. Klutmann S, Bohuslavizki KH, Kroger S. et al. Quantitative salivary gland scintigraphy. J Nucl Med Technol 1999;27:20–6. [PubMed] [Google Scholar]
- 4. Hermann GA, Vivino FB, Shnier D. et al. Variability of quantitative scintigraphic salivary indices in normal subjects. J Nucl Med 1998;39:1260–3. [PubMed] [Google Scholar]
- 5. Vivino FB, Hermann GA. Role of nuclear scintigraphy in the characterization and management of the salivary component of Sjogren’s syndrome. Rheum Dis Clin North Am 2008;34:973–86. [DOI] [PubMed] [Google Scholar]
- 6. International Atomic Energy Agency Quantitative Nuclear Medicine Imaging: Concepts, Requirements and Methods. Human Health Reports No. 9. Vienna, Austria: International Atomic Energy Agency, 2014. [Google Scholar]
- 7. Angelis GI, Ryder WJ, Gillam JE. et al. Rigid motion correction of dual opposed planar projections in single photon imaging. Phys Med Biol 2017;62:3923–43. [DOI] [PubMed] [Google Scholar]
- 8. Yapar AF, Aydin M, Reyhan M. et al. The conditions for which the geometric mean method revealed a more accurate calculation of relative renal function in 99mTc-DMSA scintigraphy. Nucl Med Commun 2005;26:141–6. [DOI] [PubMed] [Google Scholar]
- 9. White DR, Booz J, Griffith RV. et al. Tissue substitutes in radiation dosimetry and measurement. ICRU Report, J ICRU 1989;44, 23:188. [Google Scholar]
- 10. Stodilka RZ, Kemp BJ, Prato FS. et al. Importance of bone attenuation in brain SPECT quantification. J Nucl Med 1998;39:190–7. [PubMed] [Google Scholar]
- 11. Hamilton DI. Diagnostic Nuclear Medicine. A Physics Perspective. Berlin: Springer, 2004. [Google Scholar]
- 12. Acker F, Flamen P, Lambin P. et al. The utility of SPECT in determining the relationship between radiation dose and salivary gland dysfunction after radiotherapy. Nucl Med Commun 2001;22:225–31. [DOI] [PubMed] [Google Scholar]
- 13. Delpassand ES, Zare F, Broussard W. et al. Functional ratios of normal salivary glands using dual detector gamma camera and geometric mean. Clin Nucl Med 1997;22:200. [Google Scholar]
- 14. Valdes Olmos RA, Keus RB, Takes RP. et al. Scintigraphic assessment of salivary function and excretion response in radiation-induced injury of the major salivary glands. Cancer 1994;73:2886–93. [DOI] [PubMed] [Google Scholar]


