Abstract
Small cell lung cancer (SCLC) has been a devastating actuality in clinic and the molecular mechanisms underlying this disease remain unclear. The epigenetic alterations located in the promoter region of human telomerase reverse transcriptase (hTERT) have been demonstrated as one of the most prevalent non-coding genomic modifications in multiple cancers. However, alteration of hTERT promoter methylation in SCLC and the subsequently induced change in tumor cell behavior remains unclear. In this research, we hypothesized that abnormal methylation of hTERT promotor enhanced the progression of SCLC and the outcome of radiotherapy resistance. Quantitative real-time PCR and western blot assays were performed to evaluate the RNA and protein levels of hTERT and enhancer of zeste homolog 2 (EZH2), respectively. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to estimate the viability and X-ray sensitivity of H20 and H446 cell lines. Functionally, upregulation of hTERT promoted the proliferation and migration of H20 and H446 cells, and the high-level of methylation in the promoter region of hTERT induced by radiation caused radio-resistance in SCLC. Mechanically, methylation of hTERT promoter enhanced the progression and radio-resistance of SCLC through upregulating the expression of its downstream effector EZH2.
Keywords: human telomerase reverse transcriptase (hTERT), small cell lung cancer (SCLC), radiotherapy resistance, methylation, promoter
INTRODUCTION
Small cell lung cancer (SCLC) has become one of the most malignant and deadly cancers around the world, with more than 30 000 newly diagnosed cases in the USA annually [1–3]. The heterogeneity and diversity of tumor cells composed of SCLC lead to the frequent acquirement of radiotherapy resistance and poor prognosis, with an estimated 5-year survival rate of <7% in North America [4, 5].
Telomeres are composed of special non-coding DNA cascade repeats (TTAGGG) and a DNA binding protein complex, shelterin, located at the terminals of eukaryotic chromosomes [6, 7]. Telomeres function as ‘caps’ to protect eukaryotic chromosomes from DNA damage and degradation [8, 9]. The shortening of telomeres caused by incomplete replication during cell division and the attack of nucleases and other factors tends to elicit the DNA damage response, thus resulting in cell senescence and apoptosis [10, 11].
Human telomerase is a type of RNA-dependent DNA polymerase that is responsible for the protection and elongation of telomeres [12, 13]. The human telomerase reverse transcriptase (hTERT) is a rate-limiting subunit of telomerase, which functions as a catalyst for the synthesis of telomere DNA templated by human telomerase RNA (hTER) [14]. Accumulating evidence has demonstrated that hTERT is essential for the development of several cancers, and genome-wide association studies have suggested a strong connection between single nucleotide polymorphism in the promoter region of hTERT and risk of tumor [15, 16].
Epigenetics modulates hereditary alteration by DNA methylation, genomic imprinting, RNA editing etc., without any diversification of genomic sequence [17–19]. Aberrant methylation in the promoter region of hTERT causes abnormal overexpression of hTERT and thus increases cancer risk [20], and hypermethylation of the promoter of hTERT has been reported to promote progression and migration in gastrointestinal cancer and pituitary adenomas [20, 21].
In this research, we reported that increased methylation level in the promoter region of hTERT caused its upregulation and thus contributed to the progression, migration and radiotherapy resistance of SCLC. Mechanically, abnormal hTERT upregulation promoted the aberrant overexpression of enhancer of zeste homolog 2 (EZH2), which regulated transcriptional alteration by inducing methylation of histone H3 lysine 27 (H3K27) in SCLC cells. Our results might provide a new biomarker for the diagnosis and treatment of SCLC in clinic.
MATERIALS AND METHODS
Patients and tissue samples
Tumor tissue samples and their adjacent normal tissues were collected form patients with SCLC in the Central Hospital of Zibo of Shandong Province from 2017 to 2018. Our research was approved by the Ethics Commitment of the Central Hospital of Zibo of Shandong Province. The acquired samples were stored at −80°C for subsequent research. All the patients participating in the study signed informed consent forms.
Cell culture and irradiation treatment
H20 and H446 human SCLC cell lines were purchased from the China Center for Type Culture Collection. All the cell lines were cultured in RPMI 1640 (GIBCO-BRL) medium supplemented with 10% fetal bovine serum (Gibco, Melbourne, Australia), 100 U/ml penicillin and 100 μg/ml of streptomycin in a cell incubator that provided humidified air with 5% CO2 at 37°C. The cells were treated with a gradient dose of 5 Gy using a Varian Clinac 21Ex at 6 Gy/min. To detect the mRNA and methylation status, the cells were collected 24 h after irradiation.
Quantitative real-time polymerase chain reaction (PCR)
RNA from H20 and H446 cell lines was extracted by TRIzol regent (Invitrogen Life Technologies, Carlsbad, CA, USA) under the vendor’s instructions. Reverse transcription was then performed with 2 μg of RNA of each kind of cell and Reverse Transcription Kits (Fermentas, St. Leon-Rot, Germany) to produce cDNA. To establish the 20 μL real-time PCR system, 2 μg of cDNA and 0.5 μM of each primer were used, and the mixtures were detected by an S1000 PCR Thermal cycler. β-Actin served as the endogenous control.
The primers used in this assay were as follows. hTERT sense: 5′-CCGATTGTGAACATGGACTACG-3′, antisense: 5′-CACGCTGAACAGTGCCTTC-3′. EZH2 sense: 5′-GGACCACAGTGTTACCAGCAT-3′, antisense: 5′-GTGGGGTCTTTATCCGCTCAG-3′. Occludin (OCLN) sense: 5′-CGGCGAGTCCTGTGATGAG-3′, antisense: 5′-TCTTGTATTCCTGTAGGCCAGT-3′. Junction Plakoglobin (JUP) sense: 5′-CTCTGTGCGTCTCAACTATGG-3′, antisense: 5′-GATCAAGCCGATGGTTGCCT-3′. Zinc Finger E-Box Binding Homeobox (ZEB)1 sense: 5′-CAGCTTGATACCTGTGAATGGG-3′, antisense: 5′-TATCTGTGGTCGTGTGGGACT-3′. ZEB2 sense: 5′-GCGATGGTCATGCAGTCAG-3′, antisense: 5′-CAGGTGGCAGGTCATTTTCTT-3′. TWIST1 sense: 5′-GTCCGCAGTCTTACGAGGAG-3′, antisense: 5′-GCTTGAGGGTCTGAATCTTGCT-3′. FN1 sense: 5′-GAGAATAAGCTGTACCATCGCAA-3′, antisense: 5′-CGACCACATAGGAAGTCCCAG-3′. β-Actin sense: 5′-AAGACCTGTACGCCAACAC-3′, antisense: 5′-GTCATACTCCTGCTTGCTGAT-3′.
Western blot assay
Protein from H20 and H446 SCLC cell lines was extracted by radioimmunoprecipitation and loading buffer and then boiled for 10 min until the samples were denatured. A 10% SDS-polyacrylamide gel was applied to separate the protein. Then the protein was transferred onto a nitrocellulose membrane (Life technologies, Carlsbad, CA, USA), and 5% milk in Tris-buffered saline containing 0.1% (v/v) Tween-20 (TBST) was used to block the membrane for 1 h at 37°C. Anti-hTERT antibody, anti-EZH2 antibody (Sigma, St. Louis, USA) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) a ntibody (Sigma, St. Louis, USA), diluted by TBST at a concentration of 1:1000, were incubated with the membrane overnight at 4°C. The horse radish peroxidase secondary antibody (Sigma, St. Louis, USA), diluted by TBST at a concentration of 1:10 000, was incubated with the membrane after it was washed three times with TBST. An exposer (Thermo Fisher Labsystems, Waltham, MA, USA) was then used to display the protein bands. GAPDH served as the endogenous control.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was utilized to estimate the response of the SCLC cell lines to radiation. The cells were digested in 0.5% trypsin (Gibco, Melbourne, Australia) and made into a suspension. Then the cells were seeded in 96-well plates. MTT solution was then added into the medium and the cells were incubated for 16 h. DMSO was utilized to dissolve crystals.
Bisulfide sequencing
The methylation level of the promoter region of hTERT was estimated by bisulfide sequencing assay. Approximately 2 μg of DNA extracted from H20 and H446 cell lines was diluted with deuterium depleted water, and 5.5 μL of NaOH was added into the mixture. Then the mixture was incubated in a 42°C water bath. Hydroquinone (30 μL, 10 mM) was then added to the mixture after incubation in the water bath until the solution turned yellow. Sodium hydrogen sulfite (3.6 M; Sigma, S9000) was added into the mixture and it was incubated in a 50°C water bath in the dark for 16 h. The methylation status of the hTERT promoter was assessed by PCR using methyl-specific primers: hTERT_F: 5′-GGGTTATTTTATAGTTTAGGT-3′, hTERT_R: 5′-AATCCCCAATCCCTC-3′.
Transwell assay
All cells and Transwell chambers were incubated at 37°C. The cells to be tested were cultured to logarithmic growth phase and digested and washed successively with PBS and serum-free medium. The cells were suspended in serum-free medium and the concentration was adjusted to 2 × 105 cells/ml. A 600–800 μL volume of medium containing 10% serum was added to the lower chamber (in the bottom of the 24-well plate), and 100–150 μL of cell suspension was added into the chamber and incubated for 24 h in the incubator. The chamber was carefully removed with tweezers and the upper chamber fluid was eliminated. The cells on the chamber were fixed in the pre-added methanol at room temperature for 30 min. The chamber was then transferred into the pre-added Giemsa stain and stained at room temperature for 30 min.
Statistical analysis
All data were examined by Student’s t test, and one- or two-way ANOVA analysis followed by a Tukey’s post hoc test. Data are shown as mean ± S.D. Experiments were repeated at least three times. P < 0.05 indicated that there was a statistical significance in the divergence. The analysis and calculations were performed by GraphPad Prism 5000.
RESULTS
The promoter of hTERT was methylated and correlated with radiotherapy outcome in SCLC
To investigate the alteration of hTERT in SCLC tissues and their adjacent normal tissues, quantitative PCR (qPCR) assay was utilized to estimate the RNA levels of hTERT in acquired tissue samples. As shown in Fig. 1A, hTERT was dramatically upregulated in cancer tissues compared to para-cancer tissues. In view of the fact that these tumor tissues came from patients who had received radiotherapy and those who had not, we divided the tumor samples into two types: radiotherapy and non-radiotherapy, and compared the expression levels of hTERT and the methylation of the hTERT promoter in these samples. qPCR assay was then performed to demonstrate the role that hTERT played in radiotherapy resistance in SCLC. As shown in Fig. 1B, radiotherapy induced the upregulation of hTERT in SCLC cancer tissues compared to the normal and non-radiotherapy groups. Similarly, the methylation levels of the promoter region of hTERT only increased in the radiation-treated SCLC tissues compared to the normal and non-radiotherapy groups (Fig. 1C and D). The correlation between hTERT and DNA methyltransferase 3 B (DNMT3B) was analyzed by the Cancer Genome Atlas (TCGA) database. As shown in Fig. 1E, the positive correlation between DNMT3B and hTERT promoter methylation indicated that this methylation was regulated by DNMT3B. To verify our conclusion, short hairpin RNA (shRNA) targeting DNMT3B was used to knock-down DNMT3B expression in H20 cells. Down-regulation of DNMT3B decreased hTERT promoter methylation level significantly, suggesting that DNMT3B played a crucial role in the regulation of hTERT promoter methylation (Fig. 1F and G).
Fig. 1.
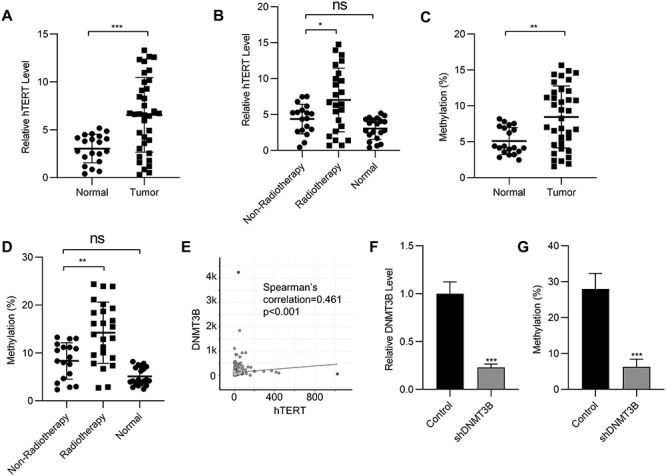
The promoter of hTERT is methylated and correlates with radiotherapy in SCLC. (A) mRNA levels of hTERT in normal and SCLC tissues. (B) mRNA levels of hTERT in normal tissues and SCLC patients with or without radiotherapy. (C) Methylation status of the hTERT promoter in normal and SCLC tissues. (D) Methylation status of the hTERT promoter in normal tissues and SCLC patients with or without radiotherapy. (E) The correlation of hTERT and DNMT3B was analyzed in the TCGA lung cancer dataset. (F) mRNA expression of DNMT3B in H20 cells transfected with DNMT3B shRNA was determined by qPCR. (G) Methylation status of the hTERT promoter in H20 cells transfected with DNMT3B shRNA. Data are shown as mean ± S.D. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant (Student’s t-test).
hTERT promoter methylation promoted SCLC proliferation
To investigate the efficacy of pcDNA 3.1-hTERT, qPCR and western blot assays were performed to estimate the expression level of hTERT after transfection. As shown in Fig. 2A and B, the RNA and protein levels of hTERT increased significantly after the transfection of pcDNA 3.1-HA/hTERT. The viability of SCLC cells with hTERT overexpression was demonstrated by flow cytometry (Fig. 2C and D) and MTT assays (Fig. 2E and F). hTERT upregulation enhanced the proliferation of H20 and H446 cells. Compared to the normal pulmonary epithelium BEAS-2B cells, methylation of the hTERT promoter region increased in the H20 and H446 cells (Fig. 2G). 5-Aza-2′-deoxycytidine (5-Aza) is a methyltransferase inhibitor and was able to decrease the methylation levels of the hTERT promoter region in both H20 and H446 cell lines (Fig. 2H). As a result, the expression of hTERT was inhibited by treatment with 5-Aza (Fig. 2I). The viability of H20 and H446 cells treated with 5-Aza and hTERT was demonstrated by flow cytometry. 5-Aza inhibited the growth of the SCLC cells, while the combined treatment of 5-Aza and hTERT rescued the viability of SCLC cells (Fig. 2J and K). In addition, knock-down of hTERT by shRNA in H20 and H446 cells inhibited their proliferation ability significantly (Fig. 2L and M). These results together suggested that methylation of the hTERT promoter and overexpression of hTERT were crucial for the viability of SCLC.
Fig. 2.

The methylation of hTERT promotes SCLC proliferation. (A) mRNA expression of hTERT in H20 cells transfected with hTERT expression plasmid was determined by qPCR. (B) Protein expression of hTERT in H20 cells transfected with hTERT expression plasmid was determined by western blot. Cell viability of H20 cells (C) or H446 cells (D) transfected with hTERT expression plasmid was determined by cell count assay. Cell viability of H20 cells (E) or H446 cells (F) transfected with hTERT expression plasmid was determined by MTT assay. (G) Methylation status of the hTERT promoter in the normal pulmonary epithelium BEAS-2B cells or SCLC cells H20 and H446. (H) Methylation status of the hTERT promoter in H20 and H446 cells treated with 5-Aza. (I) mRNA expression of hTERT in H20 and H446 cells treated with 5-Aza. Cell viability of H20 cells (J) or H446 cells (K) treated with 5-Aza and transfected with or without hTERT expression plasmid was determined by cell count assay. (L) mRNA expression of hTERT in H20 cells transfected with hTERT shRNA was determined by qPCR. (M) Cell viability of H20 and H446 cells transfected with hTERT shRNA was determined by cell count assay. Data are shown as mean ± S.D. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant (ANOVA test in E and G, others Student’s t-test).
hTERT promoter methylation promoted SCLC migration and invasion
To investigate whether the upregulation of hTERT could enhance the migration and invasion of SCLC cells, a transwell assay was performed. As shown in Fig. 3A and B, the migration and invasion of H20 cells increased dramatically after the transfection of pcDNA 3.1-HA/hTERT. qPCR assays were performed to demonstrate the expression levels of epithelial to mesenchymal transition (EMT) markers following the overexpression of hTERT. As shown in Fig. 3C, hTERT overexpression enhanced the transcription of several EMT markers such as OCLN, JUP and ZEB and thus promoted the migration of SCLC cells. To further confirm this conclusion, 5-Aza was utilized to inhibit the expression of hTERT. As shown in Fig. 3D and E, the migration and invasion of H20 cells decreased with the downregulation of hTERT. Similarly, the expression of EMT markers was also reduced by treatment with 5-Aza (Fig. 3F).
Fig. 3.

The methylation of hTERT promotes SCLC migration and invasion. (A) Transwell assay of migration and invasion of H20 cells transfected with hTERT expression plasmid or vector. (B) Statistical results of the Transwell assay in (A). (C) qPCR analysis of the expression of epithelioid markers OCLN and JUP or the mesenchymal markers ZEB1, ZEB2, TWIST1 and FN1 in H20 cells transfected with hTERT expression plasmid or vector. (D) Transwell assay of migration and invasion of H20 cells treated with 5-Aza. (E) Statistical results of the Transwell assay in (D). (F) qPCR analysis of the expression of epithelioid markers OCLN and JUP or the mesenchymal markers ZEB1, ZEB2, TWIST1 and FN1 in H20 cells treated with 5-Aza. Data are shown as mean ± S.D. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant (Student’s t-test).
hTERT promoter methylation promoted SCLC radiotherapy resistance
The methylation levels in the promoter region of hTERT were assessed by bisulfide sequencing assay, and we observed hyper-methylation in H20 and H446 cells following radiation of 5Gy (Fig. 4A and B). In addition, radiation treatment induced the upregulation of hTERT in H20 and H446 cells (Fig. 4C and D). To demonstrate the regulatory role of hTERT in the radiotherapy resistance of SCLC cells, MTT assays were performed. As shown in Fig. 4E and F, the upregulation of hTERT promoted radio-resistance in H20 and H446 cells. Similarly, treatment with 5-Aza induced sensitivity to radiation in SCLC cells by inhibiting the expression of hTERT in H20 and H446 cells (Fig. 4G and H).
Fig. 4.

The methylation of hTERT promotes SCLC radiotherapy resistance. Methylation status of the hTERT promoter in H20 (A) and H446 (B) cells treated with a single dose of 5 Gy irradiation. mRNA level of hTERT in H20 (C) and H446 (D) cells treated with a single dose of 5 Gy irradiation. Cell viability of H20 cells (E) or H446 cells (F) transfected with hTERT expression plasmid and treated with a gradient dose of irradiation. Cell viability of H20 cells (G) or H446 cells (H) treated with 5-Aza and treated with a gradient dose of irradiation. Data are shown as mean ± S.D. ***P < 0.001; ns, not significant (ANOVA test in (E) and (H), others Student’s t-test).
EZH2 acted as the downstream effector of hTERT
To investigate the mechanism of hTERT in regulating the progression of SCLC, the correlation of hTERT with other genes was analyzed in the TCGA database. The correlation coefficient between EZH2 and hTERT is one of the highest in the TCGA database (Fig. 5A). EZH2 was the main component of Polycomb repressive complex 2 (PRC2), which epigenetically regulated gene expression by modifying their promoter methylation. Therefore, we chose EZH2 as the downstream effector of hTERT. To demonstrate the regulatory effect of hTERT on EZH2, the RNA level of EZH2 was estimated following the overexpression of hTERT. As shown in Fig. 5B, the overexpression of hTERT promoted the expression of EZH2. Similarly, treatment with 5-Aza inhibited the expression of EZH2, and the rescue experiment verified that hTERT was an important intermediate molecule in this inhibition (Fig. 5C). To identify the efficacy of pcDNA 3.1-HA/EZH2, qPCR assay was performed, and EZH2 was upregulated with transfection of the plasmid in H20 cells (Fig. 5D). The viability of SCLC cells with the overexpression of EZH2 was then assessed by flow cytometry and MTT assays. As shown in Fig. 5E and F, the proliferation of SCLC cells was enhanced by the upregulation of EZH2. In addition, the migration and invasion of SCLC cells also increased with the upregulation of EZH2 (Fig. 5G), and the radio-resistance of SCLC cells was enhanced by the overexpression of EZH2 (Fig. 5H).
Fig. 5.

EZH2 acted as the downstream effector of hTERT. (A) Correlation of hTERT and EZH2 analysed in the TCGA lung cancer dataset. (B) mRNA levels of EZH2 in H20 or H446 cells transfected with hTERT expression plasmid were determined by qPCR. (C) mRNA levels of EZH2 in H20 or H446 cells treated with 5-Aza and transfected with or without hTERT expression plasmid were determined by qPCR. (D) mRNA levels of EZH2 in H20 cells transfected with EZH2 expression plasmid were determined by qPCR. (E) Cell viability of H20 cells transfected with EZH2 expression plasmid was determined by cell count assay. (F) Cell viability of H20 cells transfected with EZH2 expression plasmid was determined by MTT assay. (G) Transwell assay of migration and invasion of H20 cells transfected with EZH2 expression plasmid or vector. (H) Cell viability of H20 cells transfected with EZH2 expression plasmid and treated with a gradient dose of irradiation. Data are shown as mean ± S.D. **P < 0.01; ***P < 0.001; ns, not significant (ANOVA test in F and H, others Student’s t-test).
DISCUSSION
SCLC is a neuroendocrine tumor with extreme aggressiveness and high growth fraction [22]. The high degree of metastasis and rapid development of SCLC greatly reduces the 5-year survival rate of SCLC patients, making it the sixth most common cause of cancer-related death [23]. Radiation therapy has been widely used since 1992. However, accumulating evidence has revealed that radiotherapy resistance is usually acquired soon after the treatment, and the corresponding additional irradiation dose often leads to unacceptable toxicity [24, 25]. It is urgent for researchers to identify the regulatory mechanism for the metastasis of SCLC cells and reveal the molecular mechanisms underlying acquired radiotherapy resistance. In this research, we first reported that hTERT was upregulated in SCLC cells with radiation treatment.. We found that hTERT enhanced the proliferation and migration of SCLC cells and hTERT also participated in acquired radiotherapy resistance in SCLC. Mechanically, the upregulation of hTERT in SCLC cells promoted the expression of EZH2 to enhance the viability and radiotherapy resistance of tumor cells.
hTERT functions as a stabilizer of chromosomal DNA during cell mitosis and aging through elongating the telomere templated by hTER [26]. Accumulating evidence has revealed the close correlation between the upregulation of hTERT and cancer development and metastasis [27]. For instance, previous research has demonstrated that hTERT induces the upregulation of Caudal Type Homeobox 2 and thus enhances the viability of gastric cancer cells [28]. hTERT has also been reported to promote the ubiquitination of FOXO3a and expression of ITGB1 and thus enhance the metastasis of gastric cancer cells [29]. It was also reported that upregulation of hTERT promoted the progression of pediatric intracranial ependymoma and induced the resistance to chemotherapy of intracranial ependymoma cells [30]. In addition, the special role that hTERT played in T cells and the link between the alteration of hTERT and tumor immunity have also drawn great attention [31]. An increasing amount of research has also focused on the relationship between the hTERT and non-small cell lung cancer. It has been reported that repression of hTERT promoted the prognosis of early stage non-small cell lung cancer [32]. However, understanding of the mechanism underlying hTERT involvement in the regulation of SCLC remains unclear to the best of our knowledge [33]. In our research, we first demonstrated the upregulation of hTERT in SCLC cells. We identified that radiation therapy could induce the overexpression of hTERT in SCLC cells. hTERT dramatically promoted the proliferation and invasion of SCLC cells, and methyltransferase inhibitors such as 5-Aza could inhibit the viability and metastasis of SCLC cells by downregulating hTERT, which could provide us with a potential diagnosis and therapy target for SCLC in clinic.
The abnormal alteration of transcription of certain genes is a special characteristic of multiple cancer types [34, 35]. The disordered and excessive transcription initiation of abundant oncogenes promote the proliferation and metastasis of tumor cells. The heterogeneity of the promoter region of hTERT has been reported to be involved in the progression of different cancers. For example, the polymorphism in the promoter of hTERT has been suggested to be associated with alteration of the expression of epidermal growth factor receptor, thus regulating the proliferation of non-small cell lung cancer [36]. It has been reported that methylation of the hTERT promoter enhanced the upregulation of hTERT in pituitary adenoma cells thus promoting the progression of pituitary adenomas in clinic [20]. Besides, mutations in the promoter region of hTERT such as C228T and 1p19q have been identified as crucial biomarkers in several cancer types [37]. Similarly to previous studies, we also discovered that methylation of the hTERT promoter induced its own upregulation and promoted the progression of SCLC. We also identified that upregulation of hTERT promoted the expression of epidermal factors such as ZEB1, JUN and OCLN and thus enhanced the metastasis of SCLC cells.
EZH2 is the catalytic component of PRC2 that participates in the methylation of histone H3K27 and alters the gene expression levels by changing the chromatin structure [38]. Accumulating evidence has revealed the close correlation between EZH2 and the progression of several cancer types. For instance, it has been reported that circular RNA promoted the viability of non-small cell lung cancer cells by sponge microRNA, thus upregulating the expression of EZH2 [39]. Prostate cancer-associated transcript 1 (PCAT-1) has been indicated to recruit EZH2 to induce cisplatin resistance in gastric cancer by silencing Phosphatase And Tensin Homolog [40]. In addition, EZH2 repressed the expression of FBXO32 by inducing the methylation of the FBXO32 promoter region and thus enhanced acquired fluorouracil resistance in gastric cancer cells [41]. Similarly to this previous research, we identified that EZH2 promoted the proliferation and migration of SCLC cells. In addition, we identified that EZH2 induced radiotherapy resistance in SCLC. We also demonstrated that the expression of EZH2 could be promoted by the upregulation of hTERT, which could be a possible regulatory mechanism responsible for the progression of SCLC.
CONCLUSION
In conclusion, in this study we reported that hTERT promoter methylation promoted the progression and radiotherapy resistance of SCLC cells. The upregulation of hTERT promoted the proliferation and invasion of SCLC cells. We identified that radiation therapy utilized in the treatment of SCLC enhanced the upregulation of hTERT, which could subsequently induce radiotherapy resistance. Mechanically, we demonstrated that hTERT promoted the upregulation of EZH2 to function as an accelerator for the progression of SCLC. Our results have the potential to provide a new therapy target for the treatment of SCLC in clinic.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1. Raphael J, Batra A, Boldt G et al. Predictors of survival benefit from immune checkpoint inhibitors in patients with advanced non-small-cell lung cancer: A systematic review and meta-analysis. Clin Lung Cancer 2020;21:106–13 e5. [DOI] [PubMed] [Google Scholar]
- 2. Wu, Wang J, Zhao L et al. The prognosis of non-small cell lung cancer combined with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Clin Respir J 2020;14:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Govindan R, Page N, Morgensztern D et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539–44. [DOI] [PubMed] [Google Scholar]
- 4. Kanaji N, Watanabe N, Kita N et al. Paraneoplastic syndromes associated with lung cancer. World J Clin Oncol 2014;5:197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baldini E, Tibaldi C, Delli Paoli C. Chemo-radiotherapy integration in unresectable locally advanced non-small-cell lung cancer: A review. Clin Transl Oncol 2020. doi: 10.1007/s12094-020-02326-6 . [DOI] [PubMed] [Google Scholar]
- 6. Zvereva MI, Shcherbakova DM, Dontsova OA. Telomerase: Structure, functions, and activity regulation. Biochemistry (Mosc) 2010;75:1563–83. [DOI] [PubMed] [Google Scholar]
- 7. Stogbauer L, Stummer W, Senner V et al. Telomerase activity, TERT expression, hTERT promoter alterations, and alternative lengthening of the telomeres (ALT) in meningiomas - a systematic review. Neurosurg Rev 2020;43:903–10. [DOI] [PubMed] [Google Scholar]
- 8. Lawlor RT, Veronese N, Pea A et al. Alternative lengthening of telomeres (ALT) influences survival in soft tissue sarcomas: A systematic review with meta-analysis. BMC Cancer 2019;19:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olsson M, Wapstra E, Friesen CR. Evolutionary ecology of telomeres: A review. Ann N Y Acad Sci 2018;1422:5–28. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Wang X, Flores ER et al. Dysfunctional telomeres induce p53-dependent and independent apoptosis to compromise cellular proliferation and inhibit tumor formation. Aging Cell 2016;15:646–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang F, Sheng JF, Cai L et al. The telomerase and alternative lengthening of telomeres mechanisms regulate laryngeal cancer cell apoptosis via the PI3K/Akt pathway. ORL J Otorhinolaryngol Relat Spec 2018;80:227–37. [DOI] [PubMed] [Google Scholar]
- 12. Recagni M, Bidzinska J, Zaffaroni N et al. The role of alternative lengthening of telomeres mechanism in cancer: Translational and therapeutic implications. Cancers (Basel) 2020;12:949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kent T, Gracias D, Shepherd S et al. Alternative lengthening of telomeres in pediatric cancer: Mechanisms to therapies. Front Oncol 2019;9:1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garrels W, Kues WB, Herrmann D et al. Ectopic expression of human telomerase RNA component results in increased telomerase activity and elongated telomeres in bovine blastocysts. Biol Reprod 2012;87:95. [DOI] [PubMed] [Google Scholar]
- 15. Jady BE, Richard P, Bertrand E et al. Cell cycle-dependent recruitment of telomerase RNA and Cajal bodies to human telomeres. Mol Biol Cell 2006;17:944–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hosgood HD 3rd, Cawthon R, He X et al. Genetic variation in telomere maintenance genes, telomere length, and lung cancer susceptibility. Lung Cancer 2009;66:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castelo-Branco P, Choufani S, Mack S et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: An integrative genomic and molecular study. Lancet Oncol 2013;14:534–42. [DOI] [PubMed] [Google Scholar]
- 18. Yi X, Shay JW, Wright WE. Quantitation of telomerase components and hTERT mRNA splicing patterns in immortal human cells. Nucleic Acids Res 2001;29:4818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee S, Borah S, Bahrami A. Detection of aberrant TERT promoter methylation by combined bisulfite restriction enzyme analysis for cancer diagnosis. J Mol Diagn 2017;19:378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miyake Y, Adachi J, Suzuki T et al. TERT promoter methylation is significantly associated with TERT upregulation and disease progression in pituitary adenomas. J Neurooncol 2019;141:131–8. [DOI] [PubMed] [Google Scholar]
- 21. Huang DS, Wang ZH, He XJ et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur J Cancer 2015;51:969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shepherd FA, Crowley J, Van Houtte P et al. The international association for the study of lung cancer - lung cancer staging project: Proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067–77. [DOI] [PubMed] [Google Scholar]
- 23. Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E86. [DOI] [PubMed] [Google Scholar]
- 24. Liu RF, Wei SH, Zhang QN et al. Epidermal growth factor receptor tyrosine kinase inhibitors combined with thoracic radiotherapy or chemoradiotherapy for advanced or metastatic non-small cell lung cancer: A systematic review and meta-analysis of single-arm trials. Medicine 2019;98:e16427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang SL, Sun X, Sun L et al. Benefits of postoperative thoracic radiotherapy for small cell lung cancer subdivided by lymph node stage: A systematic review and meta-analysis. J Thorac Dis 2017;9:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aubert G, Baerlocher GM, Vulto I et al. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet 2012;8:e1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Batista LFZ, Pech M, Zhong FL et al. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature 2011;474:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen BJ, Zeng S, Xie R et al. hTERT promotes gastric intestinal metaplasia by upregulating CDX2 via NF-kappa B signaling pathway. Oncotarget 2017;8:26969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu CJ, Ni ZH, Li BS et al. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut 2017;66:31–42. [DOI] [PubMed] [Google Scholar]
- 30. Li J, Zhang NN, Zhang R et al. CDC5L promotes hTERT expression and colorectal tumor growth. Cell Physiol Biochem 2017;41:2475–88. [DOI] [PubMed] [Google Scholar]
- 31. Zhdanov DD, Vasina DA, Grachev VA et al. Alternative splicing of telomerase catalytic subunit hTERT generated by apoptotic endonuclease EndoG induces human CD4(+) T cell death. Eur J Cell Biol 2017;96:653–64. [DOI] [PubMed] [Google Scholar]
- 32. Zalewska-Ziob M, Dobija-Kubica K, Biernacki K et al. Clinical and prognostic value of hTERT mRNA expression in patients with non-small-cell lung cancer. Acta Biochim Pol 2017;64:641–6. [DOI] [PubMed] [Google Scholar]
- 33. Qin Y, Chen WB, Xiao Y et al. RFPL3 and CBP synergistically upregulate hTERT activity and promote lung cancer growth. Oncotarget 2015;6:27130–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu S, Ge YL, Huang LQ et al. BRG1, the ATPase subunit of SWI/SNF chromatin remodeling complex, interacts with HDAC2 to modulate telomerase expression in human cancer cells. Cell Cycle 2014;13:2869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biegel JA, Busse TM, Weissman BE. SWI/SNF chromatin remodeling complexes and cancer. Am J Med Genet C 2014;166:350–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ludlow AT, Wong MS, Robin JD et al. NOVA1 regulates hTERT splicing and cell growth in non-small cell lung cancer. Nat Commun 2018;9:3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arita H, Narita Y, Fukushima S et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol 2013;126:267–76. [DOI] [PubMed] [Google Scholar]
- 38. Cao R, Wang LJ, Wang HB et al. Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science 2002;298:1039–43. [DOI] [PubMed] [Google Scholar]
- 39. Niu YC, Ma F, Huang WM et al. Long non-coding RNA TUG1 is involved in cell growth and chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2. Mol Cancer 2017;16:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang J, Deng GR, Liu TM et al. Long noncoding RNA PCAT-1 acts as an oncogene in osteosarcoma by reducing p21 levels. Biochem Bioph Res Co 2018;495:2622–9. [DOI] [PubMed] [Google Scholar]
- 41. Wang CY, Li XW, Zhang JJ et al. EZH2 contributes to 5-FU resistance in gastric cancer by epigenetically suppressing FBXO32 expression. Oncotargets Ther 2018;11:7853–64. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


