Abstract
During tissue morphogenesis, mechanical forces are propagated across tissues, resulting in tissue shape changes. These forces in turn can influence cell behaviour, leading to a feedback process that can be described as self-organizing. Here, I discuss cytoskeletal self-organization and point to evidence that suggests its role in directing force during morphogenesis. During Drosophila mesoderm invagination, the shape of the region of cells that initiates constriction creates a mechanical pattern that in turn aligns the cytoskeleton with the axis of greatest resistance to contraction. The wild-type direction of the force controls the shape and orientation of the invaginating mesoderm. Given the ability of the actomyosin cytoskeleton to self-organize, these types of feedback mechanisms are likely to play important roles in a range of different morphogenetic events.
This article is part of the discussion meeting issue ‘Contemporary morphogenesis'.
Keywords: self-organization, actomyosin, cytoskeleton
1. Introduction
One of the most intriguing features of a developing embryo is the extent to which its constituent components can self-organize to form structures. For the purpose of my argument, I define self-organize as the components composing a system (i.e. cells or molecular motors) organizing themselves into a pattern without a clear template or scaffold. Self-organization has fascinated and mystified biologists for at least half a century.
In developmental biology, one of the most impressive examples of self-organization is cell sorting, where like cells separate from non-like cells. In embryos, this phenomenon was characterized by Townes & Holtfreter in classic reconstitution assays [1]. In these assays, the cells of a developing embryo were dissociated, thoroughly mixed together in a random configuration, and allowed to reassociate. The reassociating cells sorted into layers according to their original fate and the authors used the term ‘tissue affinity' to explain this sorting behaviour. Later it was shown that different adhesive interactions between cells or even different levels of the same adhesive interaction could explain this behaviour [2,3]. More recently, a variety of factors, such as cell cortical tension, hydrostatic pressure, extracellular matrix and directed migration, have been shown to influence cell sorting [4–8].
Proteins in cells are also capable of feats of self-organization. The cytoskeleton exhibits a remarkable degree of self-organization. For example, the mitotic spindle, composed of microtubules, does not require any template or even a plasma membrane to assemble. Bipolar mitotic spindles can self-assemble, through the action of microtubule-based motors, around DNA-coated beads in mitotic extracts [9]. The actin cytoskeleton also exhibits striking self-organization. Indeed, different types of actin-based structures (i.e. branched networks and bundles) dynamically respond to the underlying cell-substrate adhesive pattern and cell shape [10]. Modelling studies have illustrated how the collective behaviour of actin filaments and myosin II motors can give rise to behaviours like stiffness sensing [11,12]. These studies showed that ‘stiffness sensing', in theory, is an emergent property of cytoskeletal dynamics without the need for signalling feedback. One question that results from these examples is, can groups of cells harness this cytoskeletal self-organization to work collectively, such as in a tissue undergoing morphogenesis?
Here, I synthesize works by my laboratory and others that suggest a role for cytoskeletal self-organization in generating a mechanical pattern during Drosophila gastrulation. This is not meant to be a comprehensive review of Drosophila gastrulation, tissue morphogenesis or tissue mechanics, but I start with a brief overview of Drosophila mesoderm invagination for readers to understand the possible role of cytoskeletal self-organization. For reviews on tissue morphogenesis and mechanics, I recommend other excellent work [13,14]. For a comprehensive review on mechanosensing, I recommend the work of Hannezo & Heisenberg [15].
2. The tissue-scale mechanics of Drosophila mesoderm invagination
Gastrulation is the process by which a single-layered embryo is converted into multiple germ layers, which are referred to as the ectoderm, mesoderm and endoderm. The mesoderm and the endoderm internalize from an outer layer and then spread on the underside of the ectoderm. In Drosophila, mesoderm cells internalize as a coherent sheet of cells that only lose apical–basal polarity and dissociate after the cells have internalized [16,17]. During Drosophila gastrulation, the presumptive mesoderm is initially located on the outside of the ventral-most part of the embryo [18].
The Drosophila mesoderm is specified by transcription factors (Twist, Snail and Dorsal), which are expressed or most active in the presumptive mesoderm [19]. The nuclear localization and activity of Dorsal (NF-κB) forms a gradient with highest nuclear Dorsal in the ventral-most cells [20–22]. Dorsal in turn activates the expression of two other genes, twist and snail, which regulate the expression of additional genes [23]. It is this transcriptional patterning process that specifies the mesoderm and sets the stage for subsequent coordinated cell and tissue shape changes.
The mesoderm invaginates by folding to form a tube-like structure [24,25]. Mesoderm invagination and other tissue folding events are associated with a cell shape change called apical constriction, which involves epithelial cells constricting their apical (in this case outer) surface [26]. Apical constriction initially results in a furrow that extends along the anterior–posterior axis of the embryo, called the ventral furrow [24,25]. However, when cells shorten along their apical–basal axis to adopt a wedge-shaped morphology, the presumptive mesoderm invaginates, resulting in an epithelial tube [27,28]. Several pieces of evidence argue that apical constriction is important to drive furrow formation and invagination. First, most of the mutants that disrupt mesoderm invagination have at least some effect on apical constriction [29–33]. In addition, optogenetic activation of apical myosin contractility in an ectopic location can result in invagination, though the shape does not necessarily resemble that of the invaginating mesoderm [34].
In addition to apical constriction, it is clear that other physical factors are important for Drosophila mesoderm invagination. Apical constriction is initially associated with basal-ward hydrodynamic flow and cell lengthening in the apical–basal axis [35,36]. In silico models of invagination require a force (i.e. stiffness) shortening the height between apical and basal surfaces (i.e. lateral cell cortex tension) [28,37]. Experimentally, it has been shown that there is lateral cortex tension and that this is important for folding [38]. Apical–basal cell shortening coincides with when myosin accumulation at the basal ends of the mesoderm cells is reduced [28]. In addition, experiments directed at misregulating basal myosin II showed that basal relaxation is important for the final invagination [27].
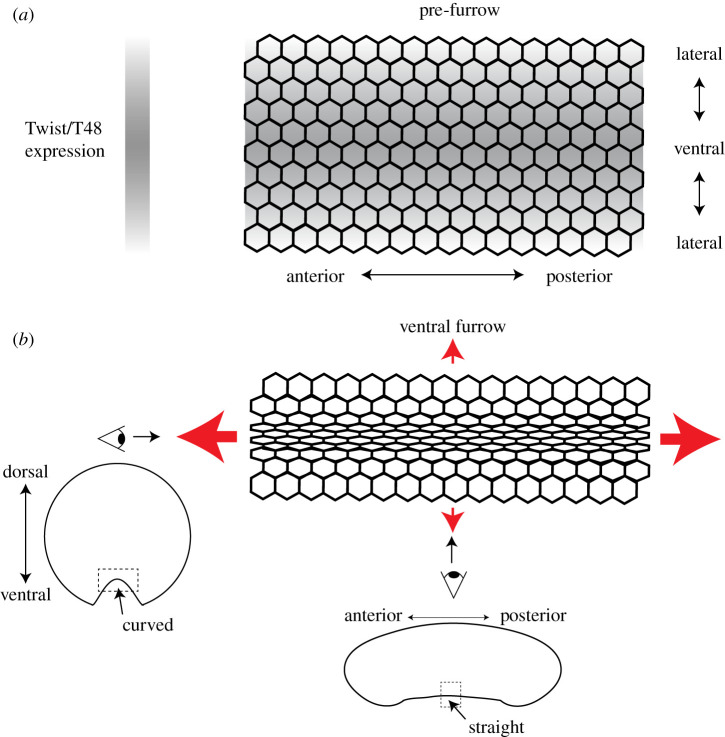
During mesoderm invagination, there is a pattern of apical constriction. Cells closest to the ventral midline constrict their apices the most and there is a gradient of constriction along the ventral–lateral axis [39] (figure 1b). This pattern of apical constriction has been shown to result from a gradient in the activity of transcription factors that specify the mesoderm [40–42]. Live imaging of the transcriptional response of mesoderm cells demonstrated that the transcription of a Twist target gene (T48) starts at the ventral midline and progresses dorsally, which leads to a gradient in accumulated T48 transcripts [42] (figure 1a).
Figure 1.
The mechanical pattern and pattern of apical constriction during mesoderm invagination. (a) The gradient in the expression of the transcription factor Twist and its target T48 before apical constriction and furrow formation. Expression is highest at the ventral midline and drops towards the sides of the embryo. (b) Apical constriction is coordinated so that 4–6 cells at the ventral midline constrict the most and there is a gradient of constriction extending towards the sides of the embryo. Red arrows indicate tension. Eyes indicate different views of the furrow, a dorsal–ventral cross section (left) and mid-sagittal section (bottom).
Because the T48 protein recruits a positive regulator of contractility to the apical surface [33], graded T48 expression leads to a gradient in apical myosin II levels [39–41]. This apical recruitment of myosin II generates epithelial tension across the tissue [43]. Tension is predominantly directed along the anterior–posterior axis of the embryo (figure 1b). Because tension is not equal in all directions, it is called anisotropic. Anisotropic tension is associated with the formation of a supracellular actomyosin network, which is integrated between cells [43,44]. The resulting anisotropic tension influences cell and tissue shape, apparently by limiting or constraining constriction [45]. Higher anterior–posterior tension makes it harder for cells to constrict (i.e. less compliant) along this axis. Thus, despite their preference to constrict isotropically when separated from the surrounding tissue, mesoderm cells in the intact embryo constrict anisotropically along the ventral–lateral axis [25,40]. This directional constriction results in greater curvature along the ventral–lateral axis rather than the anterior–posterior axis, which results in a long, narrow furrow (figure 1b).
3. Cytoskeletal dynamics as an example of self-organization at the cell level
Apical constriction of Drosophila mesoderm cells is driven by discrete contractile events called actin and myosin II pulses [46]. Actin and myosin II pulses were previously observed in other organisms, such as the Caenorhabditis elegans zygote [47]. The myosin II motor is a hexamer of two identical heavy chains, two essential light chains and two regulatory light chains that polymerizes into a bipolar filament in a manner that depends on phosphorylation [48]. Actomyosin pulses require turnover of the myosin II motor (i.e. phosphorylation and dephosphorylation), suggesting that actomyosin pulses involve cycles of activation and inactivation [49,50]. Indeed, quantitative imaging with single molecule resolution revealed that myosin pulses in C. elegans exhibit net myosin II assembly and then disassembly [51].
Pulsing in many systems also requires the dynamic regulation of the small GTPase RhoA, which regulates myosin II phosphorylation through its effector, Rho-kinase [52]. In the Drosophila mesoderm, the RhoGAP, Cumberland-GAP (also known as RhoGAP71E) is required for actomyosin pulsing [53]. Cumberland-GAP is also required for actomyosin dynamics associated with secretory vesicle release in the Drosophila salivary glands [54]. In the C. elegans zygote, the Rho GAPs RGA-3/4 are required for the observed actomyosin pulsing [51]. Thus, actomyosin pulses appear to result from dynamic regulation of actin and myosin II by RhoA.
A common observation in many of these systems is that disrupting myosin II activation interferes with the dynamics of the signalling system. For example, actin inhibitors decrease the amount of Rho-kinase associated with a pulse [49]. In Drosophila mesoderm cells, acute administration of Rho-kinase inhibitor or actin drugs results in the rapid disruption of Rho-kinase localization, suggesting a feedback loop [44]. In addition, the optogenetic activation of Rho can induce pulsing, even when the input is continuous light [34]. Thus, myosin II contractility involves a complicated feedback loop where the activity of the actomyosin cortex influences the organization of its regulators. One important question is what the importance of these dynamics is in the context of an organism or how we can test this given that interrupting the feedback will clearly interfere with myosin II.
4. Self-organization promoting cytoskeletal alignment in an embryo
Here, I speculate that one role for these apical actomyosin cytoskeleton dynamics is to enable collective behaviour, such as self-organization in response to mechanical cues in the tissue. A feature of the supracellular actomyosin network in the Drosophila mesoderm is that apical actomyosin cables preferentially orient with the anterior–posterior axis of the embryo, which is the axis of highest tension [45] (figure 2a, left). This cytoskeletal orientation can be quantified by analysing the supracellular actomyosin network structure, which reveals both a higher proportion of anterior–posterior (versus ventral–lateral) connections and straighter anterior–posterior connections [55]. In sum, this argues that there is an embryonic polarity to the apical actomyosin meshwork during Drosophila mesoderm invagination.
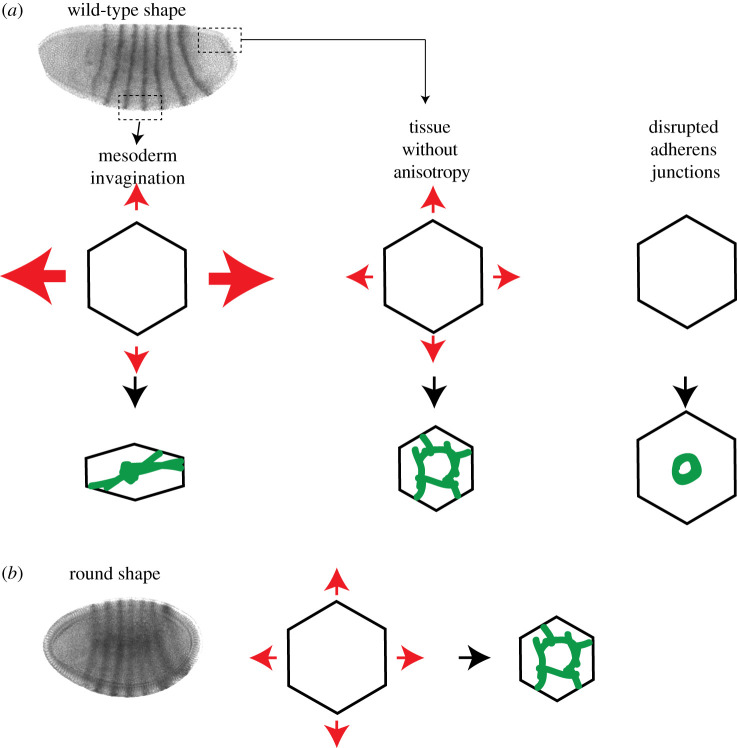
Figure 2.
Tissue tension and surrounding constraints to contraction influence cytoskeletal organization. (a) Differing constraints that influence cytoskeletal organization in normally shaped embryos. Red arrows indicate tension. Green illustrates the organization of apical myosin II. (b) The effect of changing embryo shape. Round embryos have different constraints to contraction resulting in cells having a different cytoarchitecture. Images are embryos stained for eve expression, from [45].
As opposed to signalling mechanisms that establish the planar cell polarity of cytoskeletal cables in the Drosophila germband [56], the cytoskeletal orientation in the mesoderm appears to result from a combination of cytoskeletal self-organization and an existing mechanical anisotropy. The mechanical constraints that influence tissue contraction appear to result from the shape of the contractile domain and the pattern of contractility. For example, modelling studies showed that having an anisotropic domain of contracting cells exhibiting a ventral–lateral contractility gradient will lead to anisotropy in apical cell shape [39]. Experiments have also shown that the shape of the contractile domain affects cell shape [45,57]. Thus, in principle, the shape of the contractile domain leads to mechanical constraints to how cells constrict their apices. Other effects, such as the curvature of the embryo around the poles, have yet to be determined.
The self-organization of this system arises from the fact that the mechanical constraints on contraction affect the organization of the machine that is trying to contract. Changing the mechanical constraints from anisotropic resistance to isotropic resistance changes actomyosin organization. Anisotropic resistance to contraction results in an alignment of actomyosin cables with the axis of highest resistance (i.e. the anterior–posterior axis) and a Rho-kinase spot in the middle of the apical surface (figure 2a, left). By contrast, isotropic resistance to contraction results in individual cells exhibiting actomyosin rings across the apical surface and a loss in the orientation bias of myosin II cables in the supracellular meshwork [45,55]. Actomyosin rings result in tissues that normally exhibit isotropic resistance to contraction, such as the posterior midgut invagination, or when tissues that exhibit anisotropic tension are cut, to reduce the anisotropy [45] (figure 2a, middle). Furthermore, uncoupling actomyosin networks between mesoderm cells by disrupting adherens junctions results in contraction into a ring-like structure, suggesting that in the absence of restraining forces a ring is the default cytoskeletal organization [43] (figure 2a, right). Because adherens junctions are required for myosin II-driven forces to be propagated, the failure to exhibit a proper organization in the absence of adherens junctions suggests that this process is self-organized.
All of these results suggest that the orientation of apical actomyosin meshworks is the result of mechanical constraints in the tissue that cause the cytoskeleton to self-organize with a directional bias. Modelling actomyosin contraction has shown that two- to fivefold anisotropy in compliance is sufficient to orient force generation by the cytoskeleton along the least compliant axis [45]. Consistent with cytoskeletal orientation resulting from global constraints to constriction, changing the shape of the embryo from ellipsoidal to spherical causes defective actomyosin organization (i.e. myosin II and Rho-kinase rings) [45] (figure 2b).
5. Outstanding questions
In summary, the pattern of Twist transcriptional activity appears to create an anisotropy in the resistance to constriction. This anisotropy leads to anisotropic cell constriction such that the long axes of cell apices are aligned with the axis of most resistance. There is also alignment of actomyosin fibres with this axis, which modelling suggests might reflect greater actin and myosin II filament translation and rotation towards the axis of most resistance [45]. The consequence of this system is that tension and possibly stiffness are greatest along the anterior–posterior axis, which prevents incorrectly oriented furrows [55].
There are two aspects to the transcriptional pattern that could cause the anisotropy in compliance. First, is that the shape of the Twist expression domain, regardless of Twist levels, is asymmetric, being rectangular (ca 18 cells in d–v x 70 cells in a–p). Second, is that the graded expression of Twist and its target genes results in a ventral–lateral gradient in apical constriction. So is it the shape or the gradient? Experimental perturbations using laser ablation or optogenetics suggest that shape is, at least, important [45,57]. Furthermore, changing embryo shape from ellipsoidal to spherical, which would not a priori be expected to affect the gradient, also reduces the anisotropy [45] (figure 2b). However, the relative contributions of shape versus the gradient have not been explored, which would be an area of productive study.
Another critical question is how the dynamics of the cytoskeleton play a role in this process. This is a tricky question to address given that the process is self-organized and disrupting cytoskeletal dynamics is likely to affect the ability of the cytoskeleton to produce force. One interesting possibility is to compare morphogenetic processes that have different extents of self-organization. I predict that processes requiring cytoskeletal self-organization would need to be dynamic and exhibit feedback, whereas processes that have a more directed, signalling-based mechanism for establishing polarity would require less dynamics and feedback. Therefore, it will be important to more extensively characterize variations in morphogenetic mechanisms across different processes and species.
Acknowledgements
I thank past and present members of the Martin lab and members of the field for their helpful comments when discussing these ideas. I also thank the anonymous reviewers for their suggestions to improve this manuscript.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Townes PL, Holtfreter J. 1955. Directed movements and selective adhesion of embryonic amphibian cells. J. Exp. Zool. 128, 53–120. ( 10.1002/jez.1401280105) [DOI] [PubMed] [Google Scholar]
- 2.Foty RA, Steinberg MS. 2005. The differential adhesion hypothesis: a direct evaluation. Dev. Biol. 278, 255–263. ( 10.1016/j.ydbio.2004.11.012) [DOI] [PubMed] [Google Scholar]
- 3.Steinberg MS. 1963. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 141, 401–408. ( 10.1126/science.141.3579.401) [DOI] [PubMed] [Google Scholar]
- 4.Krens SFG, Veldhuis JH, Barone V, Capek D, Maitre JL, Brodland GW, Heisenberg CP. 2017. Interstitial fluid osmolarity modulates the action of differential tissue surface tension in progenitor cell segregation during gastrulation. Development 144, 1798–1806. ( 10.1242/dev.144964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. 2008. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 10, 429–436. ( 10.1038/ncb1705) [DOI] [PubMed] [Google Scholar]
- 6.Rohani N, Canty L, Luu O, Fagotto F, Winklbauer R. 2011. EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol. 9, e1000597 ( 10.1371/journal.pbio.1000597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winklbauer R. 2015. Cell adhesion strength from cortical tension – an integration of concepts. J. Cell Sci. 128, 3687–3693. ( 10.1242/jcs.174623) [DOI] [PubMed] [Google Scholar]
- 8.Winklbauer R. 2019. Dynamic cell–cell adhesion mediated by pericellular matrix interaction – a hypothesis. J. Cell Sci. 132, 231597 ( 10.1242/jcs.231597) [DOI] [PubMed] [Google Scholar]
- 9.Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. 1996. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425. ( 10.1038/382420a0) [DOI] [PubMed] [Google Scholar]
- 10.Vignaud T, Galland R, Tseng Q, Blanchoin L, Colombelli J, Thery M. 2012. Reprogramming cell shape with laser nano-patterning. J. Cell Sci. 125, 2134–2140. ( 10.1242/jcs.104901) [DOI] [PubMed] [Google Scholar]
- 11.Borau C, Kim T, Bidone T, Garcia-Aznar JM, Kamm RD. 2012. Dynamic mechanisms of cell rigidity sensing: insights from a computational model of actomyosin networks. PLoS ONE 7, e49174 ( 10.1371/journal.pone.0049174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etienne J, Fouchard J, Mitrossilis D, Bufi N, Durand-Smet P, Asnacios A. 2015. Cells as liquid motors: mechanosensitivity emerges from collective dynamics of actomyosin cortex. Proc. Natl Acad. Sci. USA 112, 2740–2745. ( 10.1073/pnas.1417113112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillot C, Lecuit T. 2013. Mechanics of epithelial tissue homeostasis and morphogenesis. Science 340, 1185–1189. ( 10.1126/science.1235249) [DOI] [PubMed] [Google Scholar]
- 14.Herrera-Perez RM, Kasza KE. 2018. Biophysical control of the cell rearrangements and cell shape changes that build epithelial tissues. Curr. Opin. Genet. Dev. 51, 88–95. ( 10.1016/j.gde.2018.07.005) [DOI] [PubMed] [Google Scholar]
- 15.Hannezo E, Heisenberg CP. 2019. Mechanochemical feedback loops in development and disease. Cell 178, 12–25. ( 10.1016/j.cell.2019.05.052) [DOI] [PubMed] [Google Scholar]
- 16.Sun J, Stathopoulos A. 2018. FGF controls epithelial-mesenchymal transitions during gastrulation by regulating cell division and apicobasal polarity. Development 145, dev161927 ( 10.1242/dev.161927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weng M, Wieschaus E. 2017. Polarity protein Par3/Bazooka follows myosin-dependent junction repositioning. Dev. Biol. 422, 125–134. ( 10.1016/j.ydbio.2017.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leptin M. 2005. Gastrulation movements: the logic and the nuts and bolts. Dev. Cell. 8, 305–320. ( 10.1016/j.devcel.2005.02.007) [DOI] [PubMed] [Google Scholar]
- 19.Chopra VS, Levine M. 2009. Combinatorial patterning mechanisms in the Drosophila embryo. Brief. Funct. Genom. 8, 243–249. ( 10.1093/bfgp/elp026) [DOI] [PubMed] [Google Scholar]
- 20.Roth S, Stein D, Nusslein-Volhard C. 1989. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell 59, 1189–1202. ( 10.1016/0092-8674(89)90774-5) [DOI] [PubMed] [Google Scholar]
- 21.Rushlow CA, Han K, Manley JL, Levine M. 1989. The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell 59, 1165–1177. ( 10.1016/0092-8674(89)90772-1) [DOI] [PubMed] [Google Scholar]
- 22.Steward R. 1989. Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell 59, 1179–1188. ( 10.1016/0092-8674(89)90773-3) [DOI] [PubMed] [Google Scholar]
- 23.Leptin M. 1991. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 5, 1568–1576. ( 10.1101/gad.5.9.1568) [DOI] [PubMed] [Google Scholar]
- 24.Leptin M, Grunewald B. 1990. Cell shape changes during gastrulation in Drosophila. Development 110, 73–84. [DOI] [PubMed] [Google Scholar]
- 25.Sweeton D, Parks S, Costa M, Wieschaus E. 1991. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development 112, 775–789. [DOI] [PubMed] [Google Scholar]
- 26.Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B. 2010. Apical constriction: a cell shape change that can drive morphogenesis. Dev. Biol. 341, 5–19. ( 10.1016/j.ydbio.2009.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krueger D, Tardivo P, Nguyen C, De Renzis S. 2018. Downregulation of basal myosin-II is required for cell shape changes and tissue invagination. EMBO J. 37, e100170 ( 10.15252/embj.2018100170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polyakov O, He B, Swan M, Shaevitz JW, Kaschube M, Wieschaus E. 2014. Passive mechanical forces control cell-shape change during Drosophila ventral furrow formation. Biophys. J. 107, 998–1010. ( 10.1016/j.bpj.2014.07.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett K, Leptin M, Settleman J. 1997. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91, 905–915. ( 10.1016/S0092-8674(00)80482-1) [DOI] [PubMed] [Google Scholar]
- 30.Costa M, Wilson ET, Wieschaus E. 1994. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell 76, 1075–1089. ( 10.1016/0092-8674(94)90384-0) [DOI] [PubMed] [Google Scholar]
- 31.Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. 2005. Folded gastrulation, cell shape change and the control of myosin localization. Development 132, 4165–4178. ( 10.1242/dev.01938) [DOI] [PubMed] [Google Scholar]
- 32.Hacker U, Perrimon N. 1998. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 12, 274–284. ( 10.1101/gad.12.2.274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolsch V, Seher T, Fernandez-Ballester GJ, Serrano L, Leptin M. 2007. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315, 384–386. ( 10.1126/science.1134833) [DOI] [PubMed] [Google Scholar]
- 34.Izquierdo E, Quinkler T, De Renzis S. 2018. Guided morphogenesis through optogenetic activation of Rho signalling during early Drosophila embryogenesis. Nat. Commun. 9, 2366 ( 10.1038/s41467-018-04754-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelbart MA, He B, Martin AC, Thiberge SY, Wieschaus EF, Kaschube M. 2012. Volume conservation principle involved in cell lengthening and nucleus movement during tissue morphogenesis. Proc. Natl Acad. Sci. USA 109, 19 298–19 303. ( 10.1073/pnas.1205258109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He B, Doubrovinski K, Polyakov O, Wieschaus E. 2014. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 508, 392–396. ( 10.1038/nature13070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conte V, Ulrich F, Baum B, Munoz J, Veldhuis J, Brodland W, Miodownik M. 2012. A biomechanical analysis of ventral furrow formation in the Drosophila melanogaster embryo. PLoS ONE 7, e34473 ( 10.1371/journal.pone.0034473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gracia M, Theis S, Proag A, Gay G, Benassayag C, Suzanne M. 2019. Mechanical impact of epithelial–mesenchymal transition on epithelial morphogenesis in Drosophila. Nat. Commun. 10, 2951 ( 10.1038/s41467-019-10720-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spahn P, Reuter R. 2013. A vertex model of Drosophila ventral furrow formation. PLoS ONE 8, e75051 ( 10.1371/journal.pone.0075051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heer NC, Miller PW, Chanet S, Stoop N, Dunkel J, Martin AC. 2017. Actomyosin-based tissue folding requires a multicellular myosin gradient. Development 144, 1876–1886. ( 10.1242/dev.146761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim B, Levine M, Yamazaki Y. 2017. Transcriptional pre-patterning of Drosophila gastrulation. Curr. Biol. 27, 610 ( 10.1016/j.cub.2017.01.067) [DOI] [PubMed] [Google Scholar]
- 42.Rahimi N, Averbukh I, Carmon S, Schejter ED, Barkai N, Shilo BZ. 2019. Dynamics of Spaetzle morphogen shuttling in the Drosophila embryo shapes pattern. Development 146, dev181487 ( 10.1242/dev.181487) [DOI] [PubMed] [Google Scholar]
- 43.Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. 2010. Integration of contractile forces during tissue invagination. J. Cell Biol. 188, 735–749. ( 10.1083/jcb.200910099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coravos JS, Martin AC. 2016. Apical sarcomere-like actomyosin contracts nonmuscle Drosophila epithelial cells. Dev. Cell. 39, 346–358. ( 10.1016/j.devcel.2016.09.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chanet S, Miller CJ, Vaishnav ED, Ermentrout B, Davidson LA, Martin AC. 2017. Actomyosin meshwork mechanosensing enables tissue shape to orient cell force. Nat. Commun. 8, 15014 ( 10.1038/ncomms15014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin AC, Kaschube M, Wieschaus EF. 2009. Pulsed contractions of an actin–myosin network drive apical constriction. Nature 457, 495–499. ( 10.1038/nature07522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munro E, Nance J, Priess JR. 2004. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424. ( 10.1016/j.devcel.2004.08.001) [DOI] [PubMed] [Google Scholar]
- 48.Heissler SM, Manstein DJ. 2013. Nonmuscle myosin-2: mix and match. Cell. Mol. Life Sci. 70, 1–21. ( 10.1007/s00018-012-1002-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munjal A, Philippe JM, Munro E, Lecuit T. 2015. A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 524, 351–355. ( 10.1038/nature14603) [DOI] [PubMed] [Google Scholar]
- 50.Vasquez CG, Tworoger M, Martin AC. 2014. Dynamic myosin phosphorylation regulates contractile pulses and tissue integrity during epithelial morphogenesis. J. Cell Biol. 206, 435–450. ( 10.1083/jcb.201402004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michaux JB, Robin FB, McFadden WM, Munro EM. 2018. Excitable RhoA dynamics drive pulsed contractions in the early C. elegans embryo. J. Cell Biol. 217, 4230–4252. ( 10.1083/jcb.201806161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20 246–20 249. ( 10.1074/jbc.271.34.20246) [DOI] [PubMed] [Google Scholar]
- 53.Mason FM, Xie S, Vasquez CG, Tworoger M, Martin AC. 2016. RhoA GTPase inhibition organizes contraction during epithelial morphogenesis. J. Cell Biol. 214, 603–617. ( 10.1083/jcb.201603077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segal D, Zaritsky A, Schejter ED, Shilo BZ. 2018. Feedback inhibition of actin on Rho mediates content release from large secretory vesicles. J. Cell Biol. 217, 1815–1826. ( 10.1083/jcb.201711006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yevick HG, Miller PW, Dunkel J, Martin AC. 2019. Structural redundancy in supracellular actomyosin networks enables robust tissue folding. Dev. Cell. 50, 586–598. ( 10.1016/j.devcel.2019.06.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pare AC, Vichas A, Fincher CT, Mirman Z, Farrell DL, Mainieri A, Zallen JA. 2014. A positional Toll receptor code directs convergent extension in Drosophila. Nature 515, 523–527. ( 10.1038/nature13953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guglielmi G, Barry JD, Huber W, De Renzis S. 2015. An optogenetic method to modulate cell contractility during tissue morphogenesis. Dev. Cell. 35, 646–660. ( 10.1016/j.devcel.2015.10.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.