Abstract
Dynamic rearrangements of epithelial cells play central roles in shaping tissues and organs during development. There are also scenarios, however, in which epithelial cell movements synergize with the secretion of extracellular matrix to build rigid, acellular structures that persist long after the cells are gone. The formation of the Drosophila micropyle provides an elegant example of this epithelial craftsmanship. The micropyle is a cone-shaped projection of the eggshell through which the sperm will enter to fertilize the oocyte. Though simple on the surface, both the inner structure and construction of the micropyle are remarkably complex. In this review, I first provide an overview of egg development, focusing on the key events required to understand micropyle formation. I then describe the structure of the micropyle, the cellular contributions to its morphogenesis and some interesting open questions about this process. There is a brief discussion of micropyle formation in other insects and fish to highlight the potential for comparative studies. Finally, I discuss how new studies of micropyle formation could reveal general mechanisms that epithelia use to build complex extracellular structures.
This article is part of a discussion meeting issue ‘Contemporary morphogenesis'.
Keywords: epithelium, morphogenesis, extracellular matrix, eggshell, micropyle, Drosophila
1. Introduction
Dynamic rearrangements of epithelial cells play central roles in shaping tissues and organs during development. There are also scenarios, however, in which epithelial cell movements synergize with the secretion of extracellular matrix (ECM) to build rigid, acellular structures that persist long after the cells are gone. Although less studied, this type of morphogenesis is all around us—from the mantle epithelium that sculpts the spiral shell of a mollusc, to the highly structured epithelial pockets that create the unique shapes of each one of our teeth [1,2]. In these examples, the epithelial cells are both the supplier of the extracellular material and the artisans that mould it into a functional form, but how the cells integrate their secretory and morphogenetic programmes to build elaborate extracellular structures is poorly understood.
In this review, I describe an underappreciated yet elegant example of this epithelial craftsmanship—the formation of the micropyle in the eggshell of Drosophila melanogaster (hereafter Drosophila). The micropyle is a cone-shaped projection through which the sperm will enter to fertilize the oocyte. Though simple on the surface, both the inner structure and construction of the micropyle are remarkably complex, requiring input from at least four cell types. Most of what we know about micropyle formation comes from studies performed in the 1980s and 1990s that revealed the main roles each cell type plays in shaping the cone and the ultrastructure of the ECM they secrete. However, we still know very little about the cellular dynamics and molecular mechanisms that give rise to this structure. Modern genetic tools and fluorescent reporters now provide the means to probe these aspects of micropyle morphogenesis. It is with the hope of inspiring new studies of micropyle formation that I am reviewing the older literature on this topic.
Below, I first provide an overview of egg development (oogenesis) in Drosophila, with a focus on the key events required to understand micropyle formation. I then describe the structure of the mature micropyle, the cellular contributions to its morphogenesis, and some interesting open questions about this process. This is followed by a brief discussion of micropyle formation in other insects and fish to highlight the potential for comparative studies. The piece concludes with some thoughts on why this is the right time to revisit the study of micropyle formation with modern genetic tools.
2. Structure and formation of the Drosophila egg
The Drosophila egg is a marvel of biological design that consists of an oocyte surrounded by an elaborate eggshell [3–5]. In this section, I first describe the structure of the main body of the eggshell, as well as the structure and function of four specialized eggshell regions. I then cover the key aspects of egg development that are required to understand the discussion of micropyle formation that occurs in the next section.
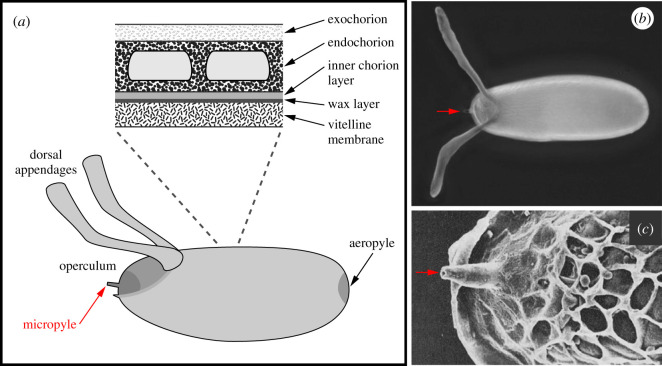
Because the embryo develops outside the mother, the eggshell must be strong enough to protect the embryo from physical assaults, yet flexible enough to permit fertilization, gas exchange and larval escape. The main body of the eggshell is a highly structured ECM with two main layers—an internal vitelline membrane layer and an external chorion layer (figure 1) [3–5]. The chorion can be further divided into a wax layer, inner chorion layer, endochorion and exochorion. There are also four specialized regions of the eggshell that each serves a specific function (figure 1). At the posterior pole, the flattened rosette-like structure of the aeropyle mediates gas exchange between the embryo and the environment [4]. At the anterior pole, two prominent extensions of the chorion called dorsal appendages also mediate gas exchange, particularly when the embryo is submerged in liquid [6,7]. Below the dorsal appendages, a flattened region known as the operculum forms a trap door through which the larva will exit when embryonic development is complete [4]. The operculum is surrounded by a thick ridge called the collar, and the micropyle sits in a ventral region of the operculum just above the collar.
Figure 1.
Structure of the Drosophila eggshell. In all images, anterior is to the left. (a) Illustration highlighting the layers and specialized regions of the eggshell. Illustration is adapted from reference [3]. (b) Light micrograph of the eggshell, dorsal view. The red arrow points to the micropyle. (c) Scanning electron micrograph of an anterior region of the eggshell, dorsal view. The red arrow points to the micropyle. Image is reprinted from reference [4] with permission from Journal of Cell Science.
Although the oocyte is the only cell left when oogenesis is complete, it develops within an ovarian follicle (egg chamber) of approximately 850 cells [8,9]. Egg chamber formation begins with the division of a germline stem cell to produce a cystoblast. The cystoblast then undergoes four rounds of division with incomplete cytokinesis, such that the 16 daughter germ cells remain connected to one another through large cytoplasmic bridges called ring canals. One of these cells becomes the oocyte and the others become nurse cells, which provide nutrients, organelles, and other maternal gene products to the oocyte. The germ cells are encapsulated by a somatic epithelium called the follicle cells, with the oocyte localized at the posterior. Together, the germ cell cyst and its somatic covering form an egg chamber, which progresses through 14 developmental stages on its way to becoming an egg.
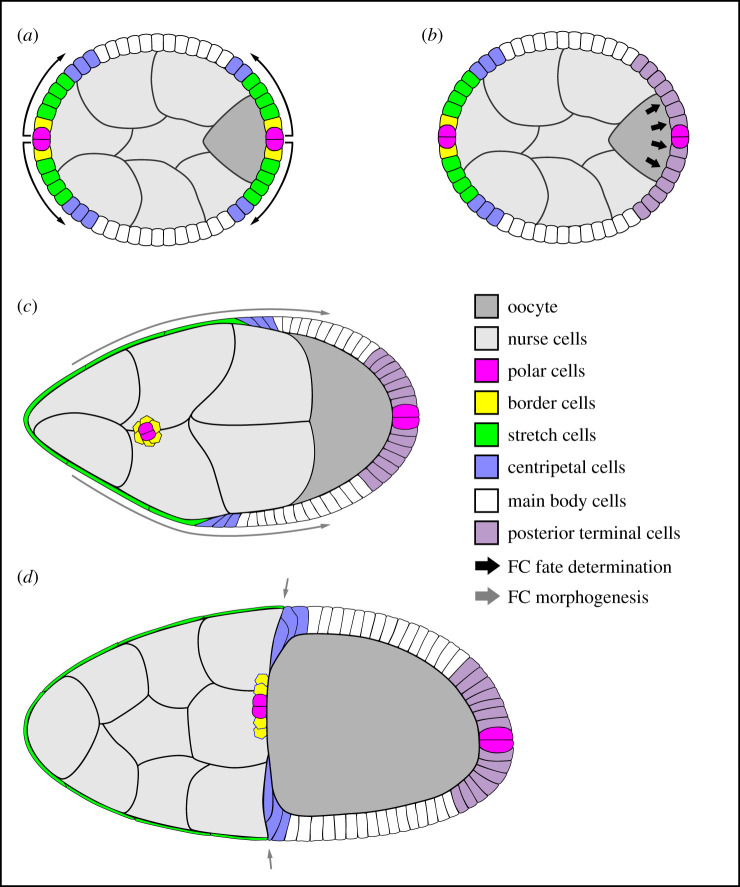
The eggshell is built during stages 10–14 by the follicle cells. To accomplish this feat, the follicle cells must first differentiate into multiple cell types that can each execute distinct morphogenetic and secretory programmes (figure 2). The first cell type to develop is the polar cells; there are exactly two of these cells at the anterior of the egg chamber and two at the posterior. The polar cells then induce the formation of the border, stretch and centripetal cells by secreting ligands for the Jak-Stat pathway, Unpaired (Upd) and Unpaired 3 (Upd3), in a concentration gradient from this point source [10–12]. These three cell types are initially specified at both egg chamber poles; however, a second signal from the oocyte overrides the posterior Upd/Upd3 signal to produce a fifth cell type called the posterior terminal cells. The follicle cells near the egg chamber's centre that do not receive these signals are called main body cells.
Figure 2.
Illustrations of select stages of egg chamber development. In all images, anterior is to the left. (a) Signals from the polar cells induce the nearby follicle cells (FCs) to adopt a variety of cell fates. (b) A second signal from the oocyte creates a new cell fate at the egg chamber's posterior. (a,b) Both signalling events occur before stage 6. (c) A combination of oocyte growth and epithelial cell shape changes/migrations that occur during stage 9 brings the bulk of the follicle cells into contact with the oocyte. (d) Migration of the centripetal cells between the oocyte and nurse cells late in stage 10 allows them to meet up with the border and polar cells to form a continuous epithelium around the oocyte's anterior. Illustrations are adapted from references [9,10].
Following their differentiation, the follicle cells undergo a series of cell shape changes and migrations to encapsulate the oocyte [8,9] (figure 2). Early in oogenesis, most of the follicle cells contact the nurse cells. This relationship shifts, starting at stage 8, when the oocyte begins to grow by importing the yolk proteins and lipids that will nourish the embryo. At stage 9, the stretch cells flatten to cover the nurse cells, while the remaining follicle cells become columnar cells over the oocyte. The one exception is the six to eight border cells, which delaminate from the epithelium and migrate through the nurse cells to the oocyte, carrying the anterior polar cells with them on their journey [13]. Late in stage 10, the centripetal cells, which now form the anterior border of the columnar cells, dive between the oocyte and nurse cells to begin covering the oocyte's anterior. The growth of the oocyte is largely complete at stage 11, when the nurse cells rapidly transfer their cytoplasm into the oocyte through the ring canals by a process called nurse cell dumping. The centripetal cells then complete their migration, joining with the border and polar cells to form a continuous epithelium around the anterior of the oocyte.
The construction of the eggshell around the oocyte requires precisely controlled waves of protein secretion and additional follicle cell rearrangements. The main body of the eggshell is built by the columnar follicle cells, which synthesize and secrete the proteins for each eggshell layer over time, depositing the vitelline membrane first and the exochorion last (figure 1) [3,11]. The specialized regions of the eggshell are constructed in a similar way by distinct follicle cell populations that execute cell-specific secretory programmes. For example, the aeropyle is built by a subset of the posterior terminal cells and the operculum is built by a subset of the centripetal cells [3–5]. The dorsal appendages represent another remarkable case of epithelial construction. Here, two new populations of follicle cells, roof and floor cells, emerge from a dorso-anterior region of the main body cells during stage 10, and then rearrange to form two blind tubes, into which chorionic proteins are secreted [6,7]. The micropyle forms in a similar way to the dorsal appendages, but it requires the contributions of four cell types instead of two: the anterior polar cells, border cells, proximally located centripetal cells and the oocyte [14–17]. Below, I first describe the structure of the mature micropyle, and then discuss how these four cell types work in concert to build it.
3. Structure of the mature Drosophila micropyle
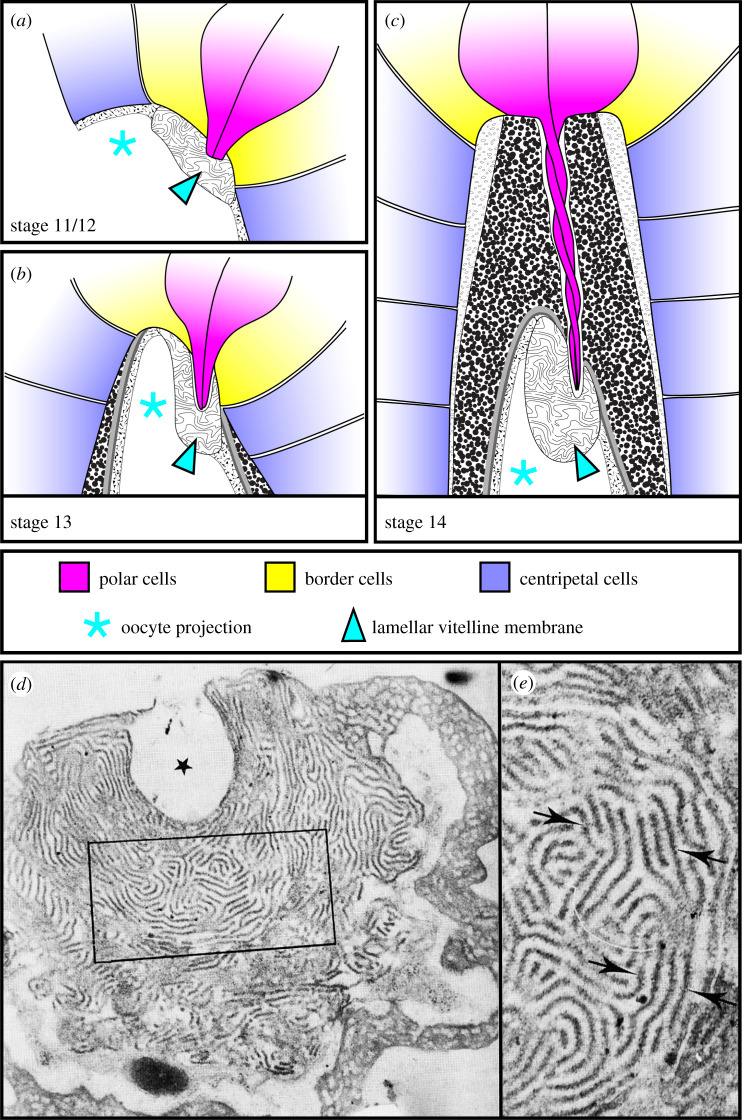
At the end of oogenesis, the micropyle sits in a dorso-anterior region of the eggshell, adjacent to the oocyte nucleus—a position that allows for rapid congression of the maternal and paternal chromosomes at fertilization [18]. The external portion of the cone is 20 µm long and 10 µm wide at its base. The oocyte protrudes into the base of the cone, forming a nipple-like projection that is 12 µm long and 6 µm wide. More distally, the cone has a hollow channel that is 0.8 µm in diameter, just wide enough to permit the entry of a single sperm [17]. Like the main body of the eggshell, the extracellular portion of the cone composed of two main layers—vitelline membrane and chorion, with the chorion having four sublayers. However, the structure and organization of each of layer are distinct from those seen in the main body [16,17]. These differences are most apparent in the vitelline membrane. Near the tip of the oocyte projection, the vitelline membrane has an intricate, maze-like, lamellar structure (figure 3), whereas the lateral sides of the projection are covered by vitelline membrane with a spongy appearance [14,16,17]. Figure 3c shows the structure of the mature micropyle right before the cells that build it are sloughed away at the end of oogenesis.
Figure 3.
Micropyle morphogenesis and structure. In all images, anterior is to the top. (a–c) Illustrations of micropyle formation adapted from references [16,17]. (a) At stage 11/12, the centripetal and border cells are secreting the spongy and lamellar vitelline membrane, respectively, and the polar cells have extended stout protrusions. (b) At stage 13, the centripetal cells have deformed the oocyte and are beginning to secrete the chorion layers. (c) At stage 14, micropyle morphogenesis is complete, and the polar cell protrusions twist around one another inside the channel. (d,e) Transmission electron micrographs of a thin section taken through the lamellar vitelline membrane within the mature micropyle of an unfertilized egg. (d) The maze-like organization of the lamellae and the ‘pocket’ (asterisk) made by the tips of the polar cell protrusions are both visible. (e) Blowup of the boxed region in (d). Arrows point to individual lamellae. Images are reproduced from reference [17], © Canadian Science Publishing or its licensors.
Fertilization and egg activation both induce changes in the initial structure of the micropyle. Upon fertilization, the ooplasmic projection is withdrawn from the micropylar cone, leaving the nipple-like protrusion of the vitelline membrane behind [17]. Egg activation occurs independently of fertilization in Drosophila and is instead induced by mechanical pressure on the egg from the female reproductive tract [19]. When the egg is activated, both the lamellar and the spongy regions of the vitelline membrane condense [17], causing them to become indistinguishable from the vitelline membrane associated with the main body of the eggshell.
4. Cellular contributions to micropyle morphogenesis
Micropyle formation begins when the border cells complete their migration and come to lie against a dorso-anterior region of the oocyte. They then secrete the lamellar portion of the vitelline membrane during stages 10 and 11 [14,16,17] (figure 3). There has been some speculation that the oocyte may also contribute protein to this ornately structured ECM, but this assertion remains to be verified [14]. By contrast, the border cells play only a minor role in building the chorion. When the border cells are prevented from reaching the oocyte, the chorionic portion of the micropyle forms relatively normally; however, closer inspection reveals two defects [20]. First, the tip of the cone lacks its characteristic rectangular morphology, suggesting that the border cells shape the chorion at the tip. Second, the channel for sperm entry is missing. From this observation and others, it was long thought that the border cells make the channel. Later work revealed, however, that it is the non-migratory polar cells at the centre of the border cell cluster that play this critical role in micropyle formation [21,22].
The polar cells make the channel by extending long, cytoplasmic protrusions around which the lamellar vitelline membrane and chorionic cone are secreted [14–17] (figures 3 and 4). Upon reaching the oocyte, each of the two polar cells forms a stout protrusion; these protrusions are tightly apposed, and their ends contact the oocyte. As the vitelline membrane is deposited by the border cells, the entire cluster (including the polar cells) is pushed away from the oocyte and the stout protrusions become embedded within the vitelline deposits to form a ‘pocket’ that will become the blind end of the sperm-entry channel. Simultaneously, a thin filopodium emerges from each stout protrusion that seems to maintain continuous contact between the polar cells and the oocyte through the vitelline deposits [16] (figure 4). During stages 13 and 14, the centripetal cells build the chorionic cone (discussed below). Throughout this process, the tips of the protrusions remain embedded in the vitelline pocket. By contrast, the polar cell bodies move away from the oocyte, as they sit at the tip of the chorionic cone throughout its construction. This causes the initially stout protrusions to lengthen dramatically between these two anchor points. By the end of stage 14, the polar cell protrusions are 14 µm long [16], filled with parallel arrays of microtubules [14,15,17], and wind around one another in a helical manner [16]. When the construction of the chorionic cone is complete, the protrusions are withdrawn, leaving an open channel in their wake. Continued secretion of Upd/Upd3 may be required for the polar cells to extend the channel-forming protrusions, as some eggs from upd3 mutant females lack the micropylar channel even though border cell migration occurs normally [11].
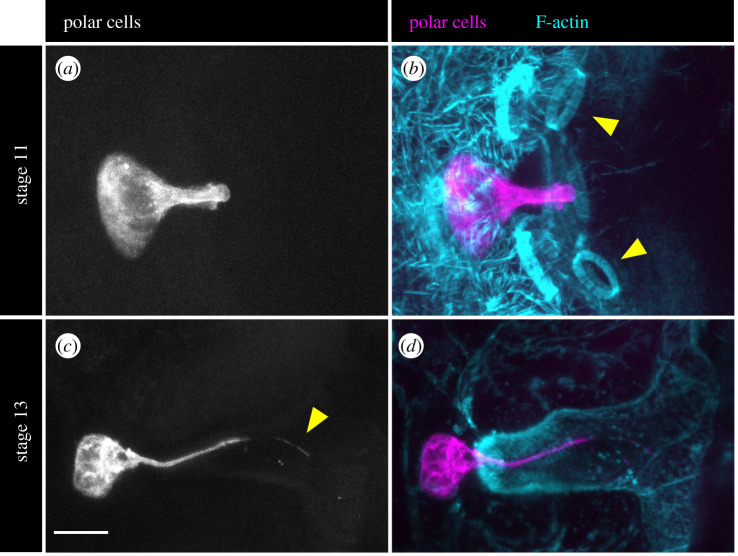
Figure 4.
Modern views of micropyle formation. In all images, anterior is to the left. (a–d) Upd-Gal4 expresses UAS-mCD8-GFP in the polar cells, revealing the morphology of their protrusions. Scale bar, 10 µm. (a,b) Stage 11. (b) Yellow arrowheads point to two of the four ring canals that connect the nurse cells to the oocyte. (c,d) Stage 13. (c) Yellow arrowhead points to the filopodium at the end of one polar cell protrusion. (d) Intense F-actin staining at the apical surfaces of the border and centripetal cells reveals the shape of the chorionic cone that is formed around the polar cell protrusions.
The centripetal cells make the largest contribution to micropyle morphogenesis [14,16,17] (figure 3). Following nurse cell dumping, the centripetal cells have linked up with the border and polar cells to form a continuous epithelium around the oocyte's anterior. The most proximal centripetal cells then undergo further rearrangement to sculpt the micropylar cone. First, they deform the oocyte to produce the nipple-like projection and secrete the spongy vitelline membrane around it [16,17]. Together, the oocyte projection and polar cell protrusions occupy the innermost regions of the growing cone, as shown in figure 3. The centripetal cells then construct the chorionic portion of the cone, starting from its base. When micropyle formation is complete, 36 centripetal cells surround the cone in four distinct rings [17]. The cell movements that produce these rings have not yet been defined, but we do have some information about the molecular control of this morphogenesis. Disrupting Jun N-terminal kinase (Jnk) signalling in the centripetal cells causes a dramatic shortening of the micropyle without perturbing the earlier movement of the centripetal cells between the oocyte and nurse cells [23]. The TGF-beta homologue Decapentaplegic (Dpp) has also been implicated in micropyle formation, where it appears to function independently of Jnk [24].
5. Open questions about micropyle formation in Drosophila
The early studies of micropyle formation described above revealed its intricate final structure, some remarkable cell biology that goes into its construction, and hints about the molecular control of this process. Below, I highlight some particularly interesting questions that remain about how the micropyle forms that could provide the basis for future studies of this process.
The lamellar vitelline membrane is an ECM of exceptional beauty (figure 3). How does the maze-like pattern of lamellae form? Zarani & Margaritis likened this structure to the cholesteric liquid crystals formed by some proteins and nucleic acids in vitro [17]. In this model, the vitelline membrane proteins secreted by the border cells would have distinct biochemical properties from those in the main body of the eggshell that allow them to self-assemble into lamellae in the extracellular space. Theses authors also noted, however, that the spacing of the lamellae is wider than what is typically seen for cholesteric liquid crystals, calling this model into question. Another possibility is that the lamellae are templated by the border cells during the secretion process. Some mucosal epithelia extended actin-based protrusions called microridges from their apical surfaces that organize into similar maze-like patterns [25–27]. If the border cells have microridges during micropyle formation, the maze-like pattern in the vitelline membrane could reflect the imprints of their apical surfaces on this ECM. Ultrastructural studies have revealed microvilli on the border cells during vitelline membrane secretion [14]; in thin sections, microridges would resemble microvilli. The function of the lamellar vitelline membrane is almost as mysterious as its formation. Since the sperm must penetrate this ECM to reach the oocyte, it is likely that the lamellae facilitate this process. However, testing this idea will require knowledge of how the lamellae form so that their structure can be altered in a predictable way.
There are several interesting questions relating to the channel-forming protrusions of the polar cells (figures 3 and 4). First, how do these protrusions come to wind around one another at the end of micropyle formation? The twisting could arise if the tips of the protrusions are held fast within the vitelline membrane pocket and the entire assemblage of polar, border and centripetal cells rotates as the chorionic cone is built. Alternatively, the cell bodies might remain fixed as the protrusions autonomously wind around each other as they extend. Live imaging of micropyle formation should distinguish between these possibilities. Second, what is the function of the fine filopodia at the protrusion tips? Since the filopodia seem to maintain constant contact between the polar cells and the oocyte, they may be cytonemes or tunnelling nanotubes that mediate signalling between these cells [28]. If so, what is the nature of these signals and how do they control micropyle morphogenesis? Given that the molecular underpinnings of filopodia formation are relatively well understood, it may be possible to eliminate the filopodia without disrupting their host protrusions as an initial approach to probing their function.
Finally, the dynamic movements of the centripetal cells that deform the oocyte and shape the chorionic cone are also of great interest (figure 3). How do these cells go from forming a relatively flat sheet over the oocyte's anterior to being organized into four, distinct rings around the micropylar cone? This transformation is likely to occur through a circular convergent extension-type mechanism like those used to elongate epithelial tubes. Once again, live imaging will be the key to mapping these cell rearrangements. An equally interesting question is whether continued secretion of Upd ligands by the polar cells is required for this morphogenesis to occur. Precedent for this idea comes from the Drosophila hindgut, where a point source of Upd directs the cellular movements that lengthen the tube [29]. Finally, there appear to be two distinct cell fates within the population of centripetal cells that build the micropyle, as Dpp is only expressed in two of the four rings of cells surrounding the completed micropylar cone [24]. Future work may reveal that Dpp-positive cells make different contributions to micropyle formation compared with Dpp-negative cells, indicating that the cellular contributions to micropyle formation are even more complex than originally thought.
6. Micropyle formation in other insects and fish
Insect eggs come in a variety of shapes and sizes [30–32], and their micropyles are similarly diverse. Moreover, fish eggs have micropyles that form through a mechanism that is remarkably like that of insects. In this section, I highlight the diversity found in insect micropyles and the recent interest in studying micropyle formation in fish, with an eye toward identifying both diverse and conserved strategies that secretory epithelia employ to build extracellular assemblies.
Among insect eggs, the number, location and shape of the micropyles can vary dramatically from what is seen in Drosophila. For example, the ant lion Euroleon nostras has identical micropyles at both the anterior and posterior poles of the egg [33], whereas the grasshopper Eyprepocnemis plorans has 40 micropyles concentrated solely at the posterior pole [34]. The number of micropyles can also vary among individuals within a species [35,36]. Moreover, a single chorionic projection can have multiple sperm-entry channels, and sperm-entry channels can form in the absence of an obvious chorionic projection [33,36–38]. In the handful of species where the development of the micropyle has been studied, the sperm-entry channels are typically made by follicle cell protrusions, similar to the situation in Drosophila [33,36,38–43]. However, the identity of the channel-forming cells can differ, as polar and border cells are only found in brachyceran flies [44,45]. The almond wasp Eurytoma amygdali builds its micropylar channel in a manner that does not depend on follicle cell protrusions [46]. In these micropyles, the chorionic projection is a stunning 130 µm long (compared with 20 µm in Drosophila), which may necessitate the different mode of channel formation. Thus, insect micropyles offer a rich system for comparative studies that could reveal new ways that secretory epithelia build extracellular structures. For a more complete description of micropyle diversity among insects, please see the book Biology of insect eggs by H. E. Hinton [32].
The micropyles of fish eggs represent a remarkable case of convergent evolution with insects. Fish embryos develop within a chorionic envelope, and this chorion is secreted by follicular epithelial cells that surround the developing oocyte. One or more micropylar channels are formed in the chorion by individual follicle cells that extend a microtubule-filled protrusion around which the chorionic proteins are secreted. In the medaka Oryzias latipes, this protrusion even has a twisted morphology like the channel-forming protrusions in Drosophila [47]. Three papers recently reported that specification of the micropyle-forming cell in zebrafish requires the Hippo pathway effector Taz [48–50]. Given that Taz is a transcription factor, RNA sequencing on Taz mutant cells could reveal the molecular logic for micropyle formation and the extent to which this programme is shared with insects.
7. Conclusion and perspective
The egg chamber is a powerful system for the study of epithelial dynamics, and yet the formation of the micropyle has received surprisingly little attention. The classic studies of this process performed in the 1980s and 1990s identified the three epithelial cell types that build the micropylar cone and offered clues about their morphogenetic and secretory functions. With modern genetic tools, however, we can now probe the cellular dynamics of micropyle formation with a higher degree of sophistication. The wealth of new fluorescent cytoskeletal and plasma membrane markers will facilitate live imaging of the winding of the polar cell protrusions and the cell movements that generate the four rings of centripetal cells around the cone. Moreover, there are Gal4 drivers that can now be used to selectively alter gene expression in each cell type, and thereby decipher the molecular control of these movements. An example of this is shown in figure 4, where Upd-Gal4 is used to express UAS-mCD8-GFP exclusively in the polar cells to highlight the morphology of their protrusions. Finally, fluorescent tags on eggshell proteins will reveal how the cells' secretory programme is integrated with the morphogenetic programme to build the extracellular portions of the cone [51]. With these approaches, modern studies of micropyle formation are likely to identify general strategies that epithelial cells use to build functional extracellular structures in a variety of contexts.
Acknowledgements
I am deeply grateful to the talented group of trainees with whom I worked during the 2019 Embryology Course at the Marine Biological Laboratory in Woods Hole, MA: Alexander Bulgakov, Allan M. Carrillo-Baltodano, Marina Martinez-Bartolome, Fernanda Palominos and Jenna Perry. It was their unbridled enthusiasm for exploration and teamwork that allowed us to all watch the micropyle forming live for the first time (https://www.youtube.com/watch?v=O4AcL_46E1g). They are the inspiration for this review. I am also grateful to Nick Badovinac for the illustrations in figures 1–3 and to Kristin Sherrard for the fluorescence micrographs in figure 4 and for helpful comments on the manuscript.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
Work in the Horne-Badovinac Laboratory is funded by the National Institutes of Health, grant no. R01-GM126047.
References
- 1.Marin F, Le Roy N, Marie B. 2012. The formation and mineralization of mollusk shell. Front. Biosci. 4, 1099–1125. ( 10.2741/s321) [DOI] [PubMed] [Google Scholar]
- 2.Yu T, Klein OD. 2020. Molecular and cellular mechanisms of tooth development, homeostasis and repair. Development 147, dev184754 ( 10.1242/dev.184754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavaliere V, Bernardi F, Romani P, Duchi S, Gargiulo G. 2008. Building up the Drosophila eggshell: first of all the eggshell genes must be transcribed. Dev. Dyn. 237, 2061–2072. ( 10.1002/dvdy.21625) [DOI] [PubMed] [Google Scholar]
- 4.Margaritis LH, Kafatos FC, Petri WH. 1980. The eggshell of Drosophila melanogaster. I. Fine structure of the layers and regions of the wild-type eggshell. J. Cell Sci. 43, 1–35. [DOI] [PubMed] [Google Scholar]
- 5.Waring GL. 2000. Morphogenesis of the eggshell in Drosophila. Int. Rev. Cytol. 198, 67–108. ( 10.1016/s0074-7696(00)98003-3) [DOI] [PubMed] [Google Scholar]
- 6.Berg CA. 2008. Tube formation in Drosophila egg chambers. Tissue Eng. A 14, 1479–1488. ( 10.1089/ten.tea.2008.0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osterfield M, Berg CA, Shvartsman SY. 2017. Epithelial patterning, morphogenesis, and evolution: Drosophila eggshell as a model. Dev. Cell 41, 337–348. ( 10.1016/j.devcel.2017.02.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duhart JC, Parsons TT, Raftery LA. 2017. The repertoire of epithelial morphogenesis on display: progressive elaboration of Drosophila egg structure. Mech. Dev. 148, 18–39. ( 10.1016/j.mod.2017.04.002) [DOI] [PubMed] [Google Scholar]
- 9.Horne-Badovinac S, Bilder D. 2005. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev. Dyn. 232, 559–574. ( 10.1002/dvdy.20286) [DOI] [PubMed] [Google Scholar]
- 10.Denef N, Schupbach T. 2003. Patterning: JAK-STAT signalling in the Drosophila follicular epithelium. Curr. Biol. 13, R388–R390. ( 10.1016/s0960-9822(03)00317-8) [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Sexton TR, Venard C, Giedt M, Guo Q, Chen Q, Harrison DA. 2014. Pleiotropy of the Drosophila JAK pathway cytokine Unpaired 3 in development and aging. Dev. Biol. 395, 218–231. ( 10.1016/j.ydbio.2014.09.015) [DOI] [PubMed] [Google Scholar]
- 12.Xi R, McGregor JR, Harrison DA. 2003. A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Dev. Cell 4, 167–177. ( 10.1016/s1534-5807(02)00412-4) [DOI] [PubMed] [Google Scholar]
- 13.Montell DJ, Yoon WH, Starz-Gaiano M. 2012. Group choreography: mechanisms orchestrating the collective movement of border cells. Nat. Rev. Mol. Cell Biol. 13, 631–645. ( 10.1038/nrm3433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishop DL, King RC. 1984. An ultrastructural study of ovarian development in the otu7 mutant of Drosophila melanogaster. J. Cell Sci. 67, 87–119. [DOI] [PubMed] [Google Scholar]
- 15.Margaritis LH. 1984. Microtubules during formation of the micropylar canal in Drosophila melanogaster. Cell Biol. Int. Rep. 8, 317–321. ( 10.1016/0309-1651(84)90158-9) [DOI] [PubMed] [Google Scholar]
- 16.Zarani FE, Margaritis LH. 1991. The eggshell of Drosophlla melanogaster: VII. Formation of the micropylar canal and the role of the paracrystalline structure. Roux Arch. Dev. Biol. 200, 95–103. ( 10.1007/BF00637189) [DOI] [PubMed] [Google Scholar]
- 17.Zarani FE, Margaritis LH. 1986. The eggshell of Drosophila melanogaster. V. Structure and morphogenesis of the micropylar apparatus. Can. J. Zool. 64, 2509–2519. ( 10.1139/z86-372) [DOI] [Google Scholar]
- 18.Loppin B, Dubruille R, Horard B. 2015. The intimate genetics of Drosophila fertilization. Open Biol. 5, 150076 ( 10.1098/rsob.150076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sartain CV, Wolfner MF. 2013. Calcium and egg activation in Drosophila. Cell Calcium 53, 10–15. ( 10.1016/j.ceca.2012.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montell DJ, Rorth P, Spradling AC. 1992. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell 71, 51–62. ( 10.1016/0092-8674(92)90265-e) [DOI] [PubMed] [Google Scholar]
- 21.Edwards KA, Demsky M, Montague RA, Weymouth N, Kiehart DP. 1997. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev. Biol. 191, 103–117. ( 10.1006/dbio.1997.8707) [DOI] [PubMed] [Google Scholar]
- 22.Han DD, Stein D, Stevens LM. 2000. Investigating the function of follicular subpopulations during Drosophila oogenesis through hormone-dependent enhancer-targeted cell ablation. Development 127, 573–583.10631178 [Google Scholar]
- 23.Suzanne M, Perrimon N, Noselli S. 2001. The Drosophila JNK pathway controls the morphogenesis of the egg dorsal appendages and micropyle. Dev. Biol. 237, 282–294. ( 10.1006/dbio.2001.0384) [DOI] [PubMed] [Google Scholar]
- 24.Twombly V, Blackman RK, Jin H, Graff JM, Padgett RW, Gelbart WM. 1996. The TGF-beta signaling pathway is essential for Drosophila oogenesis. Development 122, 1555–1565. [DOI] [PubMed] [Google Scholar]
- 25.Depasquale JA. 2018. Actin microridges. Anat. Rec. 301, 2037–2050. ( 10.1002/ar.23965) [DOI] [PubMed] [Google Scholar]
- 26.Pinto CS, Khandekar A, Bhavna R, Kiesel P, Pigino G, Sonawane M. 2019. Microridges are apical epithelial projections formed of F-actin networks that organize the glycan layer. Scient. Rep. 9, 12191 ( 10.1038/s41598-019-48400-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Loon AP, Erofeev IS, Maryshev IV, Goryachev AB, Sagasti A. 2020. Cortical contraction drives the 3D patterning of epithelial cell surfaces. J. Cell Biol. 219, e201904144 ( 10.1083/jcb.201904144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita YM, Inaba M, Buszczak M. 2018. Specialized intercellular communications via cytonemes and nanotubes. Annu. Rev. Cell Dev. Biol. 34, 59–84. ( 10.1146/annurev-cellbio-100617-062932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen KA, Iwaki DD, Lengyel JA. 2003. Localized JAK/STAT signaling is required for oriented cell rearrangement in a tubular epithelium. Development 130, 135–145. ( 10.1242/dev.00202) [DOI] [PubMed] [Google Scholar]
- 30.Church SH, Donoughe S, de Medeiros BAS, Extavour CG. 2019. Insect egg size and shape evolve with ecology but not developmental rate. Nature 571, 58–62. ( 10.1038/s41586-019-1302-4) [DOI] [PubMed] [Google Scholar]
- 31.Church SH, Donoughe S, de Medeiros BAS, Extavour CG. 2019. A dataset of egg size and shape from more than 6,700 insect species. Scient. Data 6, 104 ( 10.1038/s41597-019-0049-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinton HE. 1977. Biology of insect eggs Oxford, UK: Pergamon Press. [Google Scholar]
- 33.Kubrakiewicz JJ, Szymanska I, Bilinski B, Biliński SM. 2005. Micropyle in neuropterid insects. Structure and late stages of morphogenesis. Arthropod Struct. Dev. 34, 179–188. ( 10.1016/j.asd.2005.02.001) [DOI] [Google Scholar]
- 34.Viscuso R, Longo G, Giuffrida A. 1990. Ultrastructural features of chorion and micropyles in eggs of Eyprepocnemis plorans (Orthoptera, Acrididae). Ital. J. Zool. 57, 303–308. ( 10.1080/11250009009355712) [DOI] [Google Scholar]
- 35.Lossa G, Gage MJ, Eady PE. 2016. Micropyle number is associated with elevated female promiscuity in Lepidoptera. Biol. Lett. 12, 20160782 ( 10.1098/rsbl.2016.0782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamauchi H, Yoshitake N. 1984. Formation and ultrastructure of the micropylar apparatus in Bombyx mori ovarian follicles. J. Morphol. 179, 47–58. ( 10.1002/jmor.1051790106) [DOI] [PubMed] [Google Scholar]
- 37.Mouzaki DG, Zarani FE, Margaritis LH. 1991. Structure and morphogenesis of the eggshell and micropylar apparatus in the olive fly, Dacus oleae (Diptera: Tephritidae). J. Morphol. 209, 39–52. ( 10.1002/jmor.1052090105) [DOI] [PubMed] [Google Scholar]
- 38.Wenzel F, Gutzeit HO, Zissler D. 1990. Morphogenesis of the micropylar apparatus in ovarian follicles of the fungus gnat Bradysia tritici (syn. Sciara ocellaris). Roux Arch. Dev. Biol. 199, 146–155. ( 10.1007/BF01681487) [DOI] [PubMed] [Google Scholar]
- 39.Kubrakiewicz J, Jablonska A, Mazurkiewicz M, Bilinski SM. 2003. Differentiation and diversification of the follicular cells in flies: insight from the studies of the lower brachycerans’ ovaries. Genesis 36, 214–224. ( 10.1002/gene.10222) [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi H, Yoshitake N. 1984. Developmental stages of ovarian follicles of the silkworm, Bombyx mori L. J. Morphol. 179, 21–31. ( 10.1002/jmor.1051790104) [DOI] [PubMed] [Google Scholar]
- 41.Zarani FE, Margaritis LH. 1991. Ultrastructural features and formation of the micropylar apparatus in the cherry fly Rhagoletis cerasi. J. Morphol. 208, 205–214. ( 10.1002/jmor.1052080206) [DOI] [PubMed] [Google Scholar]
- 42.Zarani FE, Margaritis LH. 1991. Fine structure and morphogenesis of the micropylar apparatus in the medfly Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Int. J. Insect Morphol. Embryol. 20, 127–139. ( 10.1016/0020-7322(91)90004-S) [DOI] [Google Scholar]
- 43.Zawadzka M, Jankowska W, Bilinski SM. 1997. Egg shells of mallophagans and anoplurans (Insecta: Phthiraptera): morphogenesis of specialized regions and the relation to F-actin cytoskeleton of follicular cells. Tissue Cell 29, 665–673. ( 10.1016/s0040-8166(97)80042-0) [DOI] [PubMed] [Google Scholar]
- 44.Jaglarz MK, Krzeminski W, Bilinski SM. 2008. Structure of the ovaries and follicular epithelium morphogenesis in Drosophila and its kin. Dev. Genes Evol. 218, 399–411. ( 10.1007/s00427-008-0233-0) [DOI] [PubMed] [Google Scholar]
- 45.Jaglarz MK, Kubrakiewicz J, Bilinski SM. 2010. A novel pattern of follicular epithelium morphogenesis in higher dipterans. Zoology 113, 91–99. ( 10.1016/j.zool.2009.07.002) [DOI] [PubMed] [Google Scholar]
- 46.Zarani FE, Margaritis LH. 1994. The eggshell of the almond wasp Eurytoma amygdali (Hymenoptera, Eurytomidae) - 2. The micropylar appendage. Tissue Cell 26, 569–577. ( 10.1016/0040-8166(94)90009-4) [DOI] [PubMed] [Google Scholar]
- 47.Nakashima S, Iwamatsu T. 1989. Ultrastructural changes in micropylar cells and formation of the micropyle during oogenesis in the medaka Oryzias latipes. J. Morphol. 202, 339–349. ( 10.1002/jmor.1052020304) [DOI] [PubMed] [Google Scholar]
- 48.Dingare C, Niedzwetzki A, Klemmt PA, Godbersen S, Fuentes R, Mullins MC, Lecaudey V. 2018. The Hippo pathway effector Taz is required for cell morphogenesis and fertilization in zebrafish. Development 145, dev167023 ( 10.1242/dev.167023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia P, Gutl D, Zheden V, Heisenberg CP. 2019. Lateral inhibition in cell specification mediated by mechanical signals modulating TAZ activity. Cell 176, 1379–1392. ( 10.1016/j.cell.2019.01.019) [DOI] [PubMed] [Google Scholar]
- 50.Yi X, et al. 2019. The effector of Hippo signaling, Taz, is required for formation of the micropyle and fertilization in zebrafish. PLoS Genet. 15, e1007408 ( 10.1371/journal.pgen.1007408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarov M, et al. 2016. A genome-wide resource for the analysis of protein localisation in Drosophila. eLife 5, e12068 ( 10.7554/eLife.12068) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.