Abstract
In this review, we address the function of immunoglobulin superfamily cell adhesion molecules (IgCAMs) in epithelia. Work in the Drosophila model system in particular has revealed novel roles for calcium-independent adhesion molecules in the morphogenesis of epithelial tissues. We review the molecular composition of lateral junctions with a focus on their IgCAM components and reconsider the functional roles of epithelial lateral junctions. The epithelial IgCAMs discussed in this review have well-defined roles in the nervous system, particularly in the process of axon guidance, suggesting functional overlap and conservation in mechanism between that process and epithelial remodelling. We expand on the hypothesis that epithelial occluding junctions and synaptic junctions are compositionally equivalent and present a novel hypothesis that the mechanism of epithelial cell (re)integration and synaptic junction formation are shared. We highlight the importance of considering non-cadherin-based adhesion in our understanding of the mechanics of epithelial tissues and raise questions to direct future work.
This article is part of the discussion meeting issue ‘Contemporary morphogenesis’.
Keywords: epithelial junctions, cell–cell adhesion, immunoglobulin superfamily
1. Introduction
Epithelial tissues are sheets of cells that compose organs and line animal body compartments. Their component cells adhere to one another via interactions in trans of cell–cell adhesion proteins. Because epithelia form mechanical and permeability barriers, these interactions are integral to tissue function.
Epithelial cadherin (E-cad) is considered the principal cell–cell adhesion molecule (CAM) in epithelia. E-cad molecules use their extracellular domains to form calcium-dependent homophilic adhesions and are linked to the cytoskeletal machinery via catenin-containing complexes [1]. An ample body of literature documents the biology of cadherin and its role in epithelial tissue organization and tissue dynamics [1–6]. Despite its essential role in maintaining epithelial integrity and regulating morphogenetic cell behaviours, E-cad is only one of the intercellular adhesion systems present in epithelia. Immunoglobulin superfamily domain cell adhesion molecules (IgCAMs), among others, also mediate adhesion at epithelial cell–cell contacts. The contribution of these calcium-independent adhesion molecules to the formation and maintenance of epithelial tissue architecture and epithelial cell morphology has received less attention. Recent evidence, particularly from the Drosophila model, has revealed that IgCAMs play important roles in the cell behaviours that drive epithelial morphogenesis. The genetic tractability of the Drosophila model, combined with the fast developmental pace and abundant methodologies for tissue imaging, makes it a uniquely strong animal system for the investigation of the molecular machinery that drives tissue morphogenesis.
2. Immunoglobulin superfamily domain proteins
The immunoglobulin (Ig) superfamily proteins (IgSFs) constitute one of the largest and most diverse protein superfamilies [7]. IgSFs function in antigen recognition, growth factor binding, signal transduction and adhesion. The extracellular region of IgCAMs includes at least one Ig homology (Ig-like) domain and forms homophilic or heterophilic interactions in trans to mediate cell–cell adhesions. These interactions are mediated by Ig-like domains, which are generally found at the most N-terminal region. The number of Ig-like domains is proposed to correlate with the specificity of interaction [8]. IgCAMs commonly also contain a number of fibronectin (FN) domains in their extracellular regions. FN domains may function as spacers to extend the position of the Ig extracellular binding region, thereby facilitating interaction specificity through a ‘size exclusion’ mechanism [9]; interactions between IgCAMs with longer extracellular domains may prevent trans interactions of IgCAMs with shorter extracellular domains by restricting opposed cell membranes from coming into close contact. This mechanism is hypothesized to define interaction specificity of transmembrane proteins (including IgCAMS) at the immune synapse [10,11]. FN domains may also contribute to cis interactions to assist the organization of IgCAMs at the cell membrane, affecting the plasticity and stability of adhesion complexes [12,13].
IgCAMs are significantly better understood for their neural roles than for their epithelial functions, in large part because IgCAM mutants exhibit obvious and quantifiable defects in the nervous system. Most epithelial IgCAMs have well-defined roles in the nervous system, where they participate in axon outgrowth and fasciculation, neuronal migration and survival, synaptic plasticity, and regeneration after trauma (well-reviewed in [14]). IgCAMs define synaptic interactions during neuronal development and are present at the leading edge of growth cones during axon guidance [15–18].
At the point of axon–axon interaction, diverse interactions between the extracellular domains of CAMs presented at the growth cone surface allow a specific ‘zip code’ to direct interaction specificity to construct complex and robust network architecture [19]. IgCAM interactions bring apposed synaptic cell membranes into contact. Following contact initiation, the expansion of trans interactions at the contact surface and intracellular interactions with the adaptor molecules facilitate the formation of an intercellular signalling platform [20,21].
3. Epithelial cell junctions
Epithelia are defined by the polarized architecture of their component cells. The lateral surfaces of epithelial cells are characterized by multiple types of cell–cell junction, each considered to play a distinct function: cell–cell adhesion, maintenance of tissue impermeability and connection between the cytoplasm of adjacent cells. Much of the cellular machinery that makes up these junctions, and the apical–basal cell polarity network that establishes and maintains their positions, is evolutionarily conserved.
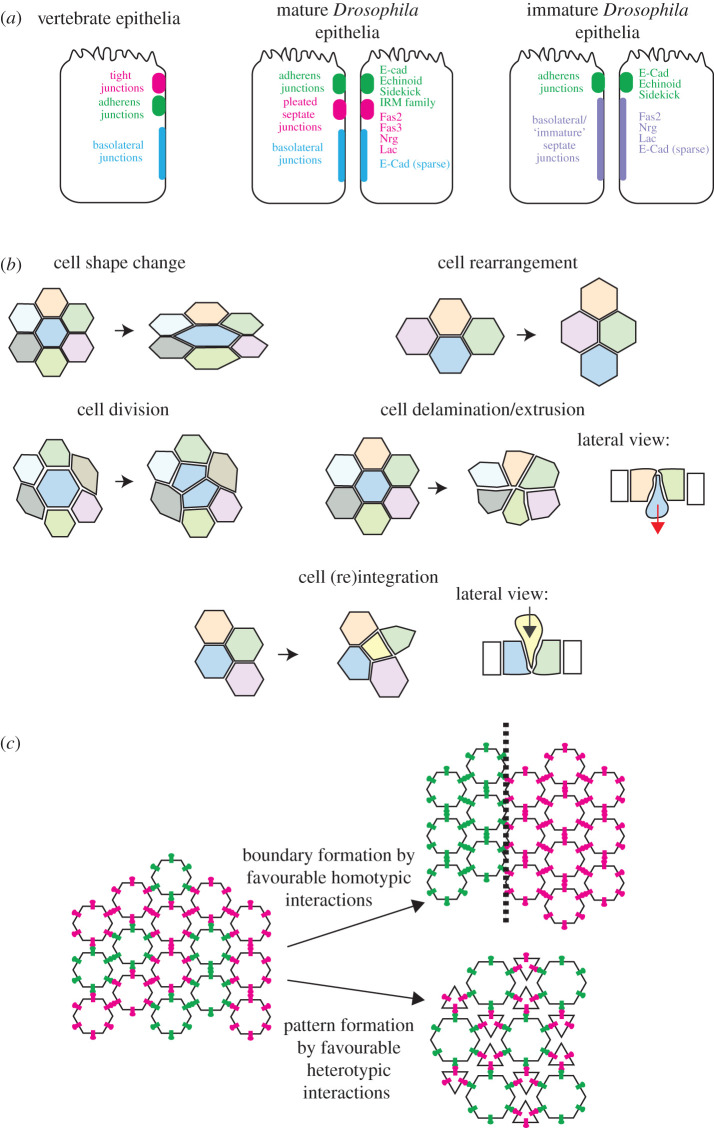
Epithelial adherens junctions (AJs) are considered the primary junctions that mediate cell–cell adhesion and mechanical coupling between cells (figure 1a). Cadherin/catenin complexes at these junctions are mechanically linked to a circumferential belt of actin and myosin filaments. Adherens junctions therefore couple adhesion and contractility, mechanically linking the contractile cortices of neighbouring cells and facilitating the passage of supracellular, tissue-level force across the tissue [4]. While cadherin-based adhesion complexes are the defining components of AJs, these junctions also include IgCAMs (such as nectins in vertebrates, and Echinoid and Sidekick in Drosophila) [22,23].
Figure 1.
(a) The junctional arrangement of epithelial cells in various epithelial types. The localization of specific IgCAMs discussed in this review are shown. (b) The five cell behaviours driving epithelial morphogenesis. (c) Differential adhesion can drive patterning in epithelial morphogenesis.
Occluding junctions (OJs) are thought to regulate tissue permeability by restricting paracellular transport across epithelia. The ultrastructure and composition of OJs vary according to the physiological and permeability needs of the tissue [24,25]. In vertebrates, OJs take the form of tight junctions (TJs). TJs are the most apical cell–cell junction found in vertebrate epithelia, meaning they are at the ‘top’ of the lateral surface. The OJs in invertebrates are septate junctions (SJs). Two types of SJ, pleated and smooth (pSJs and sSJs), are observed in arthropods (reviewed in [26,27]). Their names derive from their differential structural appearance in electron microscopy (EM) images. pSJs are found in epithelia derived from ectoderm, whereas sSJs are found in endoderm-derived epithelia, which lack AJs [28]. Unlike TJs, pSJs are found immediately basolateral to AJs in most arthropod epithelia (a notable exception being the Drosophila midgut [29]). Despite their distinct structure and composition, a commonality between vertebrate TJs and invertebrate SJs is the presence of proteins from four families: claudin/claudin-like (tetraspan transmembrane proteins), MAGUKs (scaffolding proteins that contain GUK and PSD-95/Discs large/ZO-1 (PDZ) domains), neurexins (single-pass transmembrane proteins) and IgCAMs [30]. Although OJs are considered to fulfil the function of regulating permeability, many of the transmembrane protein components of OJs also mediate cell–cell adhesion. In Drosophila, the SJ components Bark beetle/Anakonda, and the IgCAMs Fasciclin 2 (Fas2) and Fasciclin 3 (Fas3 named for its dynamic expression on fasciculating axons in the arthropod central nervous system (CNS), rather than any structural similarity to other Fas molecules) all mediate cell–cell adhesion, suggesting that SJs are important for the mechanical coupling of cells and remodelling during morphogenesis [31–35].
Multiple epithelia in Drosophila exhibit ‘immature’ SJs [30]. Immature SJs appear to lack several proteins that make up the extracellular occluding protein complex, including claudins, but retain key SJ protein components of the MAGUK and IgCAM families [36]. Immature SJs typically extend further ‘down’ (in the basal direction) lateral surfaces than mature SJs, which are relatively restricted [37]. The molecular components of immature SJs also exhibit greater mobility, suggesting that they are more ‘plastic’ and amenable to remodelling [30,37]. The early embryonic ectoderm (prior to stage 14) and the follicular epithelium that surrounds developing egg chambers in the female ovary exhibit immature SJs for extended periods, suggesting that this state is physiologically important for the function of these tissues [28,38–40]. The Drosophila neuroepithelium may also contain immature SJs, as key SJ proteins are not restricted at lateral junctions, but the junctional composition of this tissue remains to be characterized [41,42]. The retention of only a subset of SJ components in immature SJs suggests that these molecules are important for functions distinct from occlusion. In support of this possibility, the loss of the molecular components of SJs retained in immature SJs leads to defects in cell signalling, cell polarity and other polarized cell processes such as the orientation of the cell division apparatus. For example, the loss of the MAGUK protein Discs large is implicated in a disruption of cell proliferation [43,44].
Junctions resembling immature SJs are found in the CNS of mammals at synapses and the nodes of Ranvier (paranodal junctions between axons and myelinated glial cells) and at Drosophila neuromuscular junctions [45–49]. It has been noted that many synaptic scaffolding proteins resemble epithelial SJ components, suggesting that neuronal synapses derived from pSJs [30,40,47,50,51]. IgCAM family members are notably conserved between these junctions. Harden et al. [30] proposed the hypothesis that neuronal synaptic junction and immature SJs in epithelia both allow the plasticity in structure that is required for synaptic wiring and epithelial morphogenesis.
4. Looking beyond cadherin in epithelial morphogenesis
Developing epithelial tissues undergo dramatic remodelling events as they acquire their mature shape. Morphogenesis involves any combination of five cell-level behaviours: (i) cell shape changes; (ii) cell intercalation (junctional exchange); (iii) cell division; (iv) cell delamination/extrusion; and (v) cell integration (figure 1b). All of these morphogenetic cell behaviours require changes in cell–cell adhesion and remodelling of lateral junctions. Epithelial cells acquire and retain their shape in the context of the tissue through a force balance between tension, originating from the energetically favourable binding of adhesion proteins, and contractile forces generated by the actomyosin-rich cortex.
The contribution of non-cadherin-based adhesion to the mechanics of epithelial morphogenesis remains largely unaddressed on account of technical considerations. Conventional confocal microscopy is limited in the depth of data acquisition in tissues, owing to light scattering caused by the variability in refractive index of biological tissue. Live imaging, integral to the study of epithelial morphogenesis, has largely been restricted to the apical tissue surface and therefore focused primarily on cadherin-based adhesions. Modelling of epithelial morphogenesis has also been historically limited to two dimensions, both to limit complexity and to use and test available in vivo data. Only recently has the challenge of understanding the mechanical contribution of lateral epithelial cell junctions been tackled, as advancements in imaging technology (particularly two-photon and light sheet techniques) have allowed improvements in resolution, both over time and in tissue depth [52–54]. Furthermore, improvements in computational power permit the acquisition and analysis of large datasets generated by live microscopy of developing tissues, as well as the use of in silico cell tracking and tissue modelling tools.
A confounding factor in the study of the role of IgCAMs in epithelial morphogenesis is the absence of obvious ‘dramatic’ tissue phenotypes, like those observed in the absence of the E-cad/catenin AJ complex. This may be explained by functional redundancy between these proteins, meaning that obvious phenotypes may only be uncovered when more than one IgCAM is removed. Further complicating the assessment of the mechanical role played by IgCAMs is the question of whether these proteins contribute cell adhesion directly, or rather act primarily through the recruitment of other junctional factors, such as scaffolds, signalling activators and polarity regulators. Although current evidence suggests that most IgCAMs do not play an essential role in epithelial tissue integrity and adhesion, recent studies have revealed that they do regulate the cell behaviours of morphogenesis, and their contribution should not be overlooked [42,55–57].
In the following sections, we will review what is currently known about the role of IgCAMs in morphogenetic behaviours from the Drosophila model system.
5. Pattern formation in epithelia is driven by the differential expression of immunoglobulin superfamily cell adhesion molecules
An important aspect of epithelial morphogenesis is patterning and compartment definition (figure 1c). This is driven by differences in cell identity, which in part is defined by each cell's expression complement of CAMs. Morphogenetic cell behaviours are triggered in response to the interaction of CAMs at cell–cell boundaries, leading to changes in tissue shape and pattern formation. In many cases, CAM-driven epithelial patterning can be explained by the concept of differential adhesion. The differential adhesion hypothesis, presented in the 1960s by Malcolm Steinberg after the experimental work of Townes and Holtfreter, states that cells of similar adhesive strength rearrange to be adjacent in order to maximize the bonding strength between cells to produce a more energetically favourable architecture [58,59]. Our current understanding of the differential adhesion hypothesis takes a broad interpretation of ‘adhesiveness’, taking into account several aspects of material association [60]. It is now understood that not only protein–protein adhesion as measured by dimerization dissociation constant (KD), but many biophysical properties—including cell–cell tensile adhesion, cortical tension and elasticity—co-operate and feed back to generate the forces required for morphogenetic cell behaviours driving tissue shape and patterning [61].
CAM interactions can lead to complex tissue structures, such as the insect compound eye [62]. One patterning mechanism is the segregation of cells into homotypic compartments, via the preferential adhesion of cells expressing the same complement of CAMs (figure 1c). However, CAM interaction strength is not necessarily higher upon homotypic binding. Heterotypic binding can drive complex pattern formation through cells of distinct types rearranging and changing shape to maximize energetic favourability (figure 1c). Compartment boundaries between CAM-defined compartments in epithelial tissues are commonly defined and maintained by supracellular actomyosin cables [63]. One example of this is the Drosophila embryonic germband, where differential transmembrane protein expression between cells leads to cell-level asymmetric myosin II enrichment and thus supracellular tissue-level actomyosin cables [63–65].
(a). Echinoid
The Drosophila IgCAM Echinoid (Ed) is required for cell sorting that drives the morphogenesis of several epithelial tissues [66,67]. Ed is required for the morphogenetic processes of dorsal closure and head involution during embryogenesis and appendage tube morphogenesis during oogenesis [66,67]. Ed can mediate cell–cell adhesion, either through homotypic binding or by interacting heterotypically with Neuroglian (Nrg), another IgCAM [68,69]. However, the molecular mechanism by which Ed functions to regulate epithelial morphogenesis in vivo remains unclear; current evidence suggests that Ed homophilic binding serves a recognition function, rather than a role in directly mediating cell–cell adhesion, to induce planar polarized actomyosin localization and activity [66,67,70,71].
ed mutant cells sort and segregate from wild-type (wt) cells in clonal experiments via differential adhesion [22,66,71]. Ed also associates with the unconventional myosin VI motor Jaguar (Jar), and it is suggested that Jar may act as an anchor molecule to link homophilic CAMs like Ed and E-cad of AJs to actin filaments [67]. Planar polarized expression of Ed induces actomyosin assembly and contraction at the boundary between cells defined by differential levels of ed expression [22,66,67]. The recruitment of supracellular actomyosin cable at the boundary of ed mutant cell clone boundaries leads to a straightening of the compartment boundary [71].
Dorsal closure is a well-characterized morphogenetic event in the embryo; the two lateral epidermal cell sheets on either side of the embryo close a dorsal hole filled with extra-embryonic amnioserosa cells by circumferentially elongating [72]. ed mutants exhibit defects in two actin-based structures that are responsible for dorsal closure and segmental alignment of the two sides of the epidermis: (i) ed mutants fail to form supracellular actomyosin cables at the leading edge of the two lateral epidermal cell sheets [67,70]. These cables form a so-called ‘purse string’ which is important to generate tension to promote closure. ed mutants exhibit defects in the recruitment of actin regulators at the leading edge [70]. (ii) ed mutants exhibit defects in the formation of actin-rich filopodia, which are important to align the epidermal sheets during closure [67].
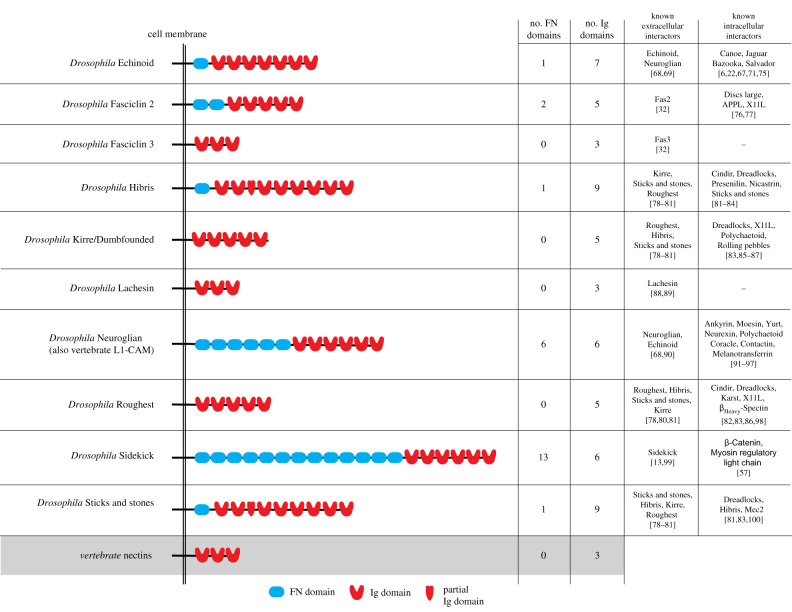
Vertebrate nectins drive mosaic patterning in auditory and olfactory epithelia by differential adhesion [73,74]. Drosophila Ed has been described as a nectin orthologue, but this identification is based on its ability to drive patterning via differential adhesion, its subcellular localization at adherens junctions, and the identification of intracellular binding partners shared with vertebrate nectins, namely afadin/Canoe and Par-3/Bazooka (vertebrate/fly), rather than sequence or structural similarity; Ed has seven Ig domains and a fibronectin domain in its C-terminal cytoplasmic region whereas the nectin family of IgCAMs are defined by an extracellular region containing three extracellular Ig domains (figure 2; [6,22,71]). One possibility is that intracellular functions in IgCAMs have rearranged over evolutionary time.
Figure 2.
The protein structures and interactors of the IgCAMs discussed in this review.
(b). Fasciclin 3
Drosophila Fas3 is an IgCAM with an extracellular domain containing three Ig domains, suggesting it is a member of the nectin family (figure 2; [32]). The asymmetric distribution of Fas3 adhesion is important for shaping the Drosophila gut (33). Cells making up the inside of the hindgut localize Fas3 along their full lateral cell–cell contact lengths. In the absence of JAK/STAT signalling-activated Fas3 lateralization, the gut fails to form the correct curvature. The mechanism of action for Fas3-driven fold/curve formation is thought to be increased preferential adhesion between Fas3-expressing cells causing changes to local tissue tension (33).
Fas3 is also implicated in mediating differential adhesion during Drosophila cardiogenesis [101]. Cardioblasts of distinct identities express a unique expression set of CAMs on pioneer filopodial protrusions. The Drosophila heart is composed of two contralateral rows of cardioblasts that collectively migrate and meet with their partners to form a tube structure [102]. The differential expression of Fas3 in cardioblasts regulates filopodia binding affinity and hence cell identity ‘matching’ and organ patterning [101].
(c). Irre cell-recognition module family immunoglobulin superfamily cell adhesion molecules
Four IgCAMs of the Drosophila Irre cell-recognition module (IRM) family regulate pattern formation epithelia via differential adhesion [103]. These are the nephrin-like proteins Sticks and stones (Sns) and Hibris (Hbs) and the Neph-like proteins Roughest (Rst, also known as Irregular chiasm C) and Kin of irre (Kirre, also called Dumbfounded). The nephrin-like proteins (Sns and Hbs) interact with Neph-like proteins (Rst and Kirre) hetero- and homophilically in trans to generate complex cell patterns (figure 2; [78,103,104]). IRM proteins localize to cell–cell junctions in multiple Drosophila epithelia, with specific AJ localization in the wing and eye imaginal discs [105,106].
Differential adhesion between IRM proteins drives the formation of repeating patterns in the Drosophila wing and eye [79,103,105,107–111]. In both tissues, preferential adhesion between cells expressing different IRM proteins drives the regular spacing of specialized cells to form complex, repeating tissue patterns. Loss of any one IRM protein has little phenotypic effect on adhesion or patterning in these tissues as their functions are partially redundant [79,105,108]. The role of IRM proteins in patterning the ommatidial units of the eye is not limited to adhesion alone. Rst regulates signalling pathways including the Decapentaplegic/BMP pathway, which leads to downstream regulation of transcription and junctional organization, and may regulate apoptotic pathways to ensure the proper elimination of excess inter-ommatidial cells [112,113].
IRM proteins function in a number of other cell identity matching processes that drive organogenesis in Drosophila, including renal tubule and muscle development [85,104,114–117]. As with most of the IgCAMs discussed in this review, the Drosophila IRM proteins also drive cell matching in axon guidance during neurogenesis [118–120].
6. Intercalation and mechanosensing
(a). Irre cell-recognition module family
As discussed, the differential expression of IRM proteins drives the sorting of cells in ommatidial morphogenesis. Cell sorting to achieve the mature ommatidial pattern requires the directional intercalation of inter-ommatidial precursor cells, a process that relies on actin-based cellular extension [110]. It has been proposed that the Rst–Hbs interaction regulates the activity of the GTPase activating protein Arf6 through the adaptor protein Cindr to inhibit cellular extensions at Rst–Hbs defined cell–cell junctions. Intercalation of these cells is independent of myosin II activity in intercalating cells [100]. Computational modelling predicts that tissue-contractile forces are required for intercalation.
(b). Sidekick
Sidekick proteins are highly conserved throughout Metazoa and best known for their role in specifying synaptic interactions in the retina [121–124]. Three parallel studies have recently identified the Drosophila Sidekick (Sdk) as a mechanosensitive protein and a regulator of junctional rearrangements in the embryonic ectoderm during germband extension, during tracheal branching in the embryo and male genitalia, and during retinal development in the pupa [56,57,125]. Proteins of the Sidekick family possess large extracellular domains composed of six Ig-like domains and 13 FN domains and can mediate homotypic cell aggregation in cell culture experiments (figure 2) [13,99].
Sdk exhibits a unique localization in Drosophila epithelial tissues, concentrating at epithelial vertices—points where three or more cells meet—at the level of AJs in most epithelia [56,57,125,126]. Sdk is unique in its localization to vertices at AJs, as all other known vertex-specific proteins localize at specialized OJs at vertices known as tricellular junctions (TCJs). Drosophila Sdk is the first protein found in any species to localize at tricellular adherens junctions (tAJs). This includes epithelia of diverse origins and morphologies and epithelia with both immature and mature SJs [125]. Epithelial vertices have widely been suggested to be important for regulating and sensing epithelial tension [127,128]. Drosophila Sdk vertex enrichment is modified when tissue tension is experimentally perturbed, becoming less enriched at vertices when tension is reduced, and more enriched when it is increased [56]. This finding suggests that Sdk localization is mechanosensitive.
sdk mutants exhibit abnormal epithelial cell shapes [56,57,125]. The dynamics of junction remodelling are abnormal in the absence of Sdk [56,57,125]. The molecular mechanism of Sdk's role in junction remodelling is difficult to decipher owing to the complex relationship between adhesion and contractility in this process. Disruption of one causes compensatory changes to the other [129]. Furthermore, the phenotypes are subtle, implying that other machinery compensates in the absence of sdk. Immunoprecipitation of Sdk pulls down the AJ component β-catenin and the myosin regulatory light chain, demonstrating a link to the cadherin/catenin AJ machinery and the actomyosin cytoskeleton [57].
Four observations hint at the molecular role of Sdk in epithelia: (i) Sdk and E-cad exhibit a mostly non-overlapping localization at AJs, suggesting an inhibitory relationship between them [23,57]; (ii) persistent holes in adhesion appear at AJs in the embryonic ectoderm in sdk mutants, which could mean that adhesion is compromised, or that tension is abnormal at apical junctions in the absence of Sdk [125]; (iii) Sdk is necessary for the accumulation of myosin, Canoe (Cno), Polychaetoid (Pyd) and actin at tAJs, but Cno and Pyd are not required for Sdk localization at tAJs; (iv) strikingly similar phenotypes are observed in Cno and Pyd mutant embryos [130]. An attractive hypothesis is that Sdk is a ‘hub’ that organizes a tAJ-specific protein complex that maintains adhesion and modulates the actomyosin cytoskeleton at these vertex junctions, which are important sites for the anchoring of the cytoskeleton and experience high tension. This work demonstrates an important, but previously overlooked, role for IgCAMs in regulating the cell behaviours that drive well-understood epithelial morphogenetic behaviours.
7. Reintegration
(a). Neuroglian and Fasciclin 2
Cell reintegration is a relatively recently identified morphogenetic cell behaviour that appears to be a fundamental, conserved morphogenetic process [42]. Through live imaging and genetic studies in Drosophila epithelial tissues, it was shown that daughter cells born apically or basally displaced from a tissue layer are able to (re)integrate back into the layer [42,131]. The IgCAMs Nrg and Fas2 are regulators of cell reintegration in Drosophila epithelia [42]. Genetic disruption of Nrg or Fas2 cause reintegration to fail, resulting in the appearance of ‘popped out’ cells situated apically to the tissue layer [42,132].
Neuroglian (Nrg) is a member of the L1 family of IgCAMs and is essential for paracellular barrier formation [91]. Members of the L1 family of IgCAMs are characterized by an extracellular region with six Ig domains, between three and five FN domains, a single transmembrane segment and an intracellular domain containing an ankyrin-binding region (figure 2; [133–136]). The vertebrate homologue of Nrg, L1-CAM, is functionally conserved in the developing nervous system [19]. Nrg is considered a central component of pSJs [91].
Fas2 is a member of the N-CAM family of proteins based on its extracellular domain structure of five Ig domains and two FN domains. Fas2 is implicated in epithelial polarity organization and cell motility [137,138]. It has been extensively studied for its role in axon guidance and neuronal development [76,139–142]. Fas2 is considered to be an exclusively homophilic adhesion molecule and mediates homophilic cell aggregation in an in vitro S2 cell assay (figure 2; [32]).
Nrg and Fas2 are highly enriched along lateral cell–cell contacts in mitotically active epithelia with immature SJs that exhibit reintegration behaviour [42]. Reintegration is not prevented by disruption of AJs [42]. Reintegration has only been described in Drosophila epithelia tissues that possess immature SJs. Together, this suggests reintegration specifically requires lateral adhesion of immature SJ components [42].
Cell reintegration is proposed to be driven by the energy-favourable tendency to maximize cell–cell adhesion along the lateral surface [42,131]. This model to explain reintegration can be pictured as ‘zipping up’, where the rapid expansion of cell–cell contact is driven by the formation of homophilic adhesions between IgCAM molecules, acting as the teeth of a zipper. Biochemical analysis of the structure of the vertebrate homologue of Fas2, N-CAM, lends evidence to the ‘zipping up’ hypothesis of cell (re)integration. N-CAM homophilic binding is proposed to occur via the formation of ‘zippers’. The two N-terminal-most Ig domains are proposed to mediate dimerization of N-CAM molecules situated on the same cell surface (in cis), whereas the third Ig domain mediates interactions between N-CAM molecules expressed on the surface of opposing cells (in trans) through simultaneous binding to the first two Ig domains [143]. This arrangement results in two perpendicular zippers forming a double zipper-like adhesion complex. This demonstrates the importance of cis interactions between IgCAMs in the formation of cell–cell interactions and suggests that trans interactions catalyse the formation of expansion of cell–cell contact areas.
Several lines of evidence suggest that the vertebrate homologue of Nrg L1-CAM might likewise mediate reintegration. L1-CAM is expressed in monolayered epithelia in the intestine and kidney, where it localizes to lateral cell–cell contacts [144–146]. Though its function in these tissues is unclear, L1-CAM has been functionally characterized in antigen-presenting dendritic cells, in which it promotes transmigration through endothelial walls [147]. Transmigration is mediated by L1-CAM expressed both at endothelial cell–cell contacts and on the dendritic cell surface, akin to ‘zipping up’ [147].
(b). Axon guidance and reintegration: a common molecular mechanism driving distinct processes?
IgCAM interactions facilitate adhesion between axons and the motility of axons along neural pathways. IgCAM interactions also lead to cytoskeletal remodelling, which is required for axonal pathfinding and connection maturation [19]. Synaptic junctions and immature SJs are orthologous in structure and composition [30,40,47,50,51]. We suggest that the molecular mechanism of IgCAM-mediated axon guidance/synaptic connection and cell reintegration may be conserved (figure 3).
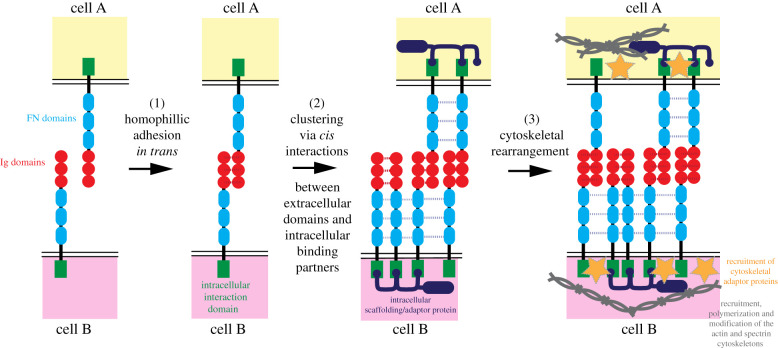
Figure 3.
A common multi-step molecular mechanism may drive axon guidance/paranodal junction formation in the nervous system and epithelial cell reintegration. (1) Trans interactions mediated by extracellular domains of CAMs initially form cell–cell interactions; (2) CAMs cluster at the cell membrane by the interaction via cis interactions; (3) and intracellular signalling scaffolding platform is built (figure inspired by model put forward in Siegenthaler et al. [20]).
Siegenthaler et al. [20] dissected the molecular mechanism of Nrg in guiding neuronal paths via the mediation of axon–axon interactions in the Drosophila mushroom body. The interaction of Nrg with the membrane–cytoskeleton linker proteins Ankyrin 2 (which binds to spectrin) and Moesin (which binds Actin) are required for axon guidance [20]. This strongly implicates spectrin and actin-based cytoskeletons as important to stabilize axonal interactions.
It is currently unclear if intracellular factors are required for cell reintegration. The molecules that drive axon guidance and cell reintegration are shared and we therefore propose that a shared molecular mechanism is likely to drive these two processes (figure 3). When cells of the same ‘type’ come into contact, homophilic adhesion can occur in trans between IgCAMs on different cells. Following contact initiation, initial contact sites enlarge via a ‘zippering’ mechanism, whereby the contact area between cells is expanded by the rapid expansion of trans interactions at the contact surface, facilitating the formation of an intercellular signalling platform. Cell lipid membranes exhibit domains of specialization in composition—so-called ‘microdomains’—which can lead to localized clustering of transmembrane proteins owing to changes in their ability to laterally diffuse [148,149]. Microdomain formation may be a result of FN interactions with the lipid membrane, interactions between the extracellular domains of the IgCAMs, and changes to the juxtaposed intracellular actin- or spectrin-based cytoskeletons.
(c). Lachesin: a candidate for future study
Drosophila Lachesin (Lac) localizes to SJs and is required for the late stages of tracheal epithelial morphogenesis [88]. Defects are present in the shape, width and path of tracheal branches [88]. Lac is expressed in early embryos prior to the formation of mature SJs, suggesting that similarly to Fas2, Fas3 and Nrg, its function is not restricted to OJ function and organization. Lac promotes homophilic binding in bead and cell-culture aggregation assays [88,89]. The composition of the extracellular domain of Lac, which contains three Ig domains, suggests that it is a nectin orthologue (figure 2). Little is known about the molecular function of Lac and it is an exciting candidate for further exploration.
8. Conclusion and perspectives
IgCAMs shape and pattern animal tissues through development. A commonality in IgCAM-mediated processes is the importance of cell–cell identity recognition. Although IgCAMs are thought of as adhesion molecules, the mechanical contribution of IgCAMs in adhering cells together remains to be quantitatively addressed in most cases. The Drosophila model in particular has revealed that IgCAMs regulate morphogenetic cell behaviours in diverse epithelial tissues.
Recent imaging-based studies of epithelial morphogenesis in Drosophila have revealed three novel observations: (i) epithelial cells can exhibit significantly different junctional arrangements along their lateral junctions, with cell conformations being drastically different at apical versus basal junctions [52,125,150]; (ii) the tension that drives morphogenetic cell shape change can originate from lateral cell–cell junctions that are distinct from AJs [52,54]; (iii) cell reintegration, a morphogenetic cell behaviour, is mediated by lateral junctions [42]. These novel insights raise several fundamental questions: What are the adhesion proteins maintaining adhesion and facilitating mechanical coupling along lateral cell–cell junctions? How are changes in adhesion and cell–cell contact remodelling mechanically transmitted apico-basally along lateral cell surfaces? While AJs have long been thought to be the driving force of morphogenetic cell behaviours, emerging evidence shows that SJ components are also important to modulate morphogenesis [33,34,151–158]. IgCAMs in particular are intriguing candidates as proteins that could regulate lateral junction-driven behaviours. Consistent with this, it has been proposed that immature SJs, which retain IgCAM SJ components, are plastic and may be kept in the immature state to facilitate the dynamic cell behaviours of epithelial morphogenesis [30].
Moving forward, IgCAM-based adhesion should no longer be ignored in our consideration of the mechanics of epithelial morphogenesis. The substantial body of work elucidating the mechanical role of IgCAMs in epithelia in Drosophila provides numerous candidates to direct future investigations in vertebrates for IgCAMs that regulate epithelial morphogenesis.
Data accessibility
This article has no additional data.
Authors' contributions
T.M.F. wrote the manuscript with guidance and edits from D.T.B.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by NIH grant no. R01GM125839.
References
- 1.Shapiro L, Weis WI. 2009. Structure and biochemistry of cadherins and catenins. Cold Spring Harb. Perspect. Biol. 1, a003053 ( 10.1101/cshperspect.a003053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeichi M. 1991. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251, 1451–1455. ( 10.1126/science.2006419) [DOI] [PubMed] [Google Scholar]
- 3.Yap AS, Brieher WM, Gumbiner BM. 1997. Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13, 119–146. ( 10.1146/annurev.cellbio.13.1.119) [DOI] [PubMed] [Google Scholar]
- 4.Lecuit T, Yap AS. 2015. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 17, 533–539. ( 10.1038/ncb3136) [DOI] [PubMed] [Google Scholar]
- 5.Harris TJ. C, Tepass U. 2010. Adherens junctions: from molecules to morphogenesis. Nat. Rev. Mol. Cell Biol. 11, 502–514. ( 10.1038/nrm2927) [DOI] [PubMed] [Google Scholar]
- 6.Röper K. 2015. Integration of cell-cell adhesion and contractile actomyosin activity during morphogenesis. Curr. Top. Dev. Biol. 112, 103–127. ( 10.1016/bs.ctdb.2014.11.017) [DOI] [PubMed] [Google Scholar]
- 7.Halaby DM, Mornon JP. 1998. The immunoglobulin superfamily: an insight on its tissular, species, and functional diversity. J. Mol. Evol. 46, 389–400. ( 10.1007/pl00006318) [DOI] [PubMed] [Google Scholar]
- 8.Volkmer H, Schreiber J, Rathjen FG. 2013. Regulation of adhesion by flexible ectodomains of IgCAMs. Neurochem. Res. 38, 1092–1099. ( 10.1007/s11064-012-0888-9) [DOI] [PubMed] [Google Scholar]
- 9.Schmid EM, Bakalar MH, Choudhuri K, Weichsel J, Ann H, Geissler PL, Dustin ML, Fletcher DA. 2016. Size-dependent protein segregation at membrane interfaces. Nat. Phys. 12, 704–711. ( 10.1038/nphys3678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Springer TA. 1990. Adhesion receptors of the immune system. Nature 346, 425–434. ( 10.1038/346425a0) [DOI] [PubMed] [Google Scholar]
- 11.Cartwright ANR, Griggs J, Davis DM. 2014. The immune synapse clears and excludes molecules above a size threshold. Nat. Commun. 5, 5479 ( 10.1038/ncomms6479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunz B, Lierheimer R, Rader C, Spirig M, Ziegler U, Sonderegger P. 2002. Axonin-1/TAG-1 mediates cell-cell adhesion by a cis-assisted trans-interaction. J. Biol. Chem. 277, 4551–4557. ( 10.1074/jbc.M109779200) [DOI] [PubMed] [Google Scholar]
- 13.Tang H, Chang H, Dong Y, Guo L, Shi X, Wu Y, Huang Y, He Y. 2018. Architecture of cell–cell adhesion mediated by sidekicks. Proc. Natl Acad. Sci. USA 115, 9246–9251. ( 10.1073/pnas.1801810115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maness PF, Schachner M. 2007. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat. Neurosci. 10, 19–26. ( 10.1038/nn1827) [DOI] [PubMed] [Google Scholar]
- 15.Walsh FS, Doherty P. 1997. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu. Rev. Cell Dev. Biol. 13, 425–456. ( 10.1146/annurev.cellbio.13.1.425) [DOI] [PubMed] [Google Scholar]
- 16.Dityatev A, Bukalo O, Schachner M. 2008. Modulation of synaptic transmission and plasticity by cell adhesion and repulsion molecules. Neuron Glia Biol. 4, 197–209. ( 10.1017/S1740925X09990111) [DOI] [PubMed] [Google Scholar]
- 17.Zinn K, Özkan E. 2017. Neural immunoglobulin superfamily interaction networks. Curr. Opin. Neurobiol. 45, 99–105. ( 10.1016/j.conb.2017.05.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron S, McAllister AK. 2018. Immunoglobulin-like receptors and their impact on wiring of brain synapses. Annu. Rev. Genet. 52, 567–590. ( 10.1146/annurev-genet-120417-031513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamiguchi H. 2007. The role of cell adhesion molecules in axon growth and guidance. In Axon growth and guidance (ed. Bagnard D.), pp. 95–102. New York, NY: Springer; ( 10.1007/978-0-387-76715-4_7) [DOI] [PubMed] [Google Scholar]
- 20.Siegenthaler D, Enneking E-M, Moreno E, Pielage J. 2015. L1CAM/Neuroglian controls the axon–axon interactions establishing layered and lobular mushroom body architecture. J. Cell Biol. 208, 1003–1018. ( 10.1083/jcb.201407131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Südhof TC. 2018. Towards an understanding of synapse formation. Neuron 100, 276–293. ( 10.1016/j.neuron.2018.09.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei S-Y, et al. 2005. Echinoid is a component of adherens junctions that cooperates with DE-cadherin to mediate cell adhesion. Dev. Cell 8, 493–504. ( 10.1016/j.devcel.2005.03.015) [DOI] [PubMed] [Google Scholar]
- 23.Lye CM, Naylor HW, Sanson B. 2014. Subcellular localisations of the CPTI collection of YFP-tagged proteins in Drosophila embryos. Development 141, 4006–4017. ( 10.1242/dev.111310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farquhar MG, Palade GE. 1963. Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412. ( 10.1083/jcb.17.2.375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claude P. 1973. Fracture faces of zonulae occludentes from ‘tight’ and ‘leaky’ epithelia. J. Cell Biol. 58, 390–400. ( 10.1083/jcb.58.2.390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izumi Y, Furuse M. 2014. Molecular organization and function of invertebrate occluding junctions. Semin. Cell Dev. Biol. 36, 186–193. ( 10.1016/j.semcdb.2014.09.009) [DOI] [PubMed] [Google Scholar]
- 27.Jonusaite S, Donini A, Kelly SP. 2016. Occluding junctions of invertebrate epithelia. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 186, 17–43. ( 10.1007/s00360-015-0937-1) [DOI] [PubMed] [Google Scholar]
- 28.Tepass U, Hartenstein V. 1994. The development of cellular junctions in the Drosophila embryo. Dev. Biol. 161, 563–596. ( 10.1006/dbio.1994.1054) [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Sayadian A-C, Lowe N, Lovegrove HE, St Johnston D. 2018. An alternative mode of epithelial polarity in the Drosophila midgut. PLoS Biol. 16, e3000041 ( 10.1371/journal.pbio.3000041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harden N, Wang SJH, Krieger C. 2016. Making the connection – shared molecular machinery and evolutionary links underlie the formation and plasticity of occluding junctions and synapses. J. Cell Sci. 129, 3067–3076. ( 10.1242/jcs.186627) [DOI] [PubMed] [Google Scholar]
- 31.Snow PM, Bieber AJ, Goodman CS. 1989. Fasciclin III: a novel homophilic adhesion molecule in Drosophila. Cell 59, 313–323. ( 10.1016/0092-8674(89)90293-6) [DOI] [PubMed] [Google Scholar]
- 32.Grenningloh G, Bieber AJ, Rehm EJ, Snow PM, Traquina ZR, Hortsch M, Patel NH, Goodman CS. 1990. Molecular genetics of neuronal recognition in Drosophila: evolution and function of immunoglobulin superfamily cell adhesion molecules. Cold Spring Harb. Symp. Quant. Biol. 55, 327–340. ( 10.1101/sqb.1990.055.01.034) [DOI] [PubMed] [Google Scholar]
- 33.Wells RE, Barry JD, Warrington SJ, Cuhlmann S, Evans P, Huber W, Strutt D, Zeidler MP. 2013. Control of tissue morphology by fasciclin III-mediated intercellular adhesion. Development 140, 3858–3868. ( 10.1242/dev.096214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byri S, et al. 2015. The triple-repeat protein Anakonda controls epithelial tricellular junction formation in Drosophila. Dev. Cell 33, 535–548. ( 10.1016/j.devcel.2015.03.023) [DOI] [PubMed] [Google Scholar]
- 35.Halberg KA, Rainey SM, Veland IR, Neuert H, Dornan AJ, Klämbt C, Davies S-A, Dow JAT. 2016. The cell adhesion molecule fasciclin2 regulates brush border length and organization in Drosophila renal tubules. Nat. Commun. 7, 11266 ( 10.1038/ncomms11266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulte J, Tepass U, Auld VJ. 2003. Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila. J. Cell Biol. 161, 991–1000. ( 10.1083/jcb.200303192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oshima K, Fehon RG. 2011. Analysis of protein dynamics within the septate junction reveals a highly stable core protein complex that does not include the basolateral polarity protein Discs large. J. Cell Sci. 124, 2861–2871. ( 10.1242/jcs.087700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahowald AP. 1972. Ultrastructural observations on oogenesis in Drosophila. J. Morphol. 137, 29–48. ( 10.1002/jmor.1051370103) [DOI] [PubMed] [Google Scholar]
- 39.Müller HA. 2000. Genetic control of epithelial cell polarity: lessons from Drosophila. Dev. Dyn. 218, 52–67. () [DOI] [PubMed] [Google Scholar]
- 40.Tepass U, Tanentzapf G, Ward R, Fehon R. 2001. Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 35, 747–784. ( 10.1146/annurev.genet.35.102401.091415) [DOI] [PubMed] [Google Scholar]
- 41.Moyer KE, Jacobs JR. 2008. Varicose: a MAGUK required for the maturation and function of Drosophila septate junctions. BMC Dev. Biol. 8, 99 ( 10.1186/1471-213X-8-99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergstralh DT, Lovegrove HE, St Johnston D. 2015. Lateral adhesion drives reintegration of misplaced cells into epithelial monolayers. Nat. Cell Biol. 17, 1497–1503. ( 10.1038/ncb3248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woods DF, Bryant PJ. 1991. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 66, 451–464. ( 10.1016/0092-8674(81)90009-x) [DOI] [PubMed] [Google Scholar]
- 44.Schulte J, Charish K, Que J, Ravn S, MacKinnon C, Auld VJ. 2006. Gliotactin and Discs large form a protein complex at the tricellular junction of polarized epithelial cells in Drosophila. J. Cell Sci. 119, 4391–4401. ( 10.1242/jcs.03208) [DOI] [PubMed] [Google Scholar]
- 45.Hortsch M, Margolis B. 2003. Septate and paranodal junctions: kissing cousins. Trends Cell Biol. 13, 557–561. ( 10.1016/j.tcb.2003.09.004) [DOI] [PubMed] [Google Scholar]
- 46.Poliak S, Peles E. 2003. The local differentiation of myelinated axons at nodes of Ranvier. Nat. Rev. Neurosci. 4, 968–980. ( 10.1038/nrn1253) [DOI] [PubMed] [Google Scholar]
- 47.Banerjee S, Sousa AD, Bhat MA. 2006. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem. Biophys. 46, 65–77. ( 10.1385/CBB:46:1:65) [DOI] [PubMed] [Google Scholar]
- 48.Nans A, Einheber S, Salzer JL, Stokes DL. 2011. Electron tomography of paranodal septate-like junctions and the associated axonal and glial cytoskeletons in the central nervous system. J. Neurosci. Res. 89, 310–319. ( 10.1002/jnr.22561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganot P, Zoccola D, Tambutté E, Voolstra CR, Aranda M, Allemand D, Tambutté S. 2015. Structural molecular components of septate junctions in cnidarians point to the origin of epithelial junctions in eukaryotes. Mol. Biol. Evol. 32, 44–62. ( 10.1093/molbev/msu265) [DOI] [PubMed] [Google Scholar]
- 50.Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. 1997. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J. Cell Biol. 139, 1495–1506. ( 10.1083/jcb.139.6.1495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McLachlan IG, Heiman MG. 2013. Shaping dendrites with machinery borrowed from epithelia. Curr. Opin. Neurobiol. 23, 1005–1010. ( 10.1016/j.conb.2013.06.011) [DOI] [PubMed] [Google Scholar]
- 52.Sun Z, Amourda C, Shagirov M, Hara Y, Saunders TE, Toyama Y. 2017. Basolateral protrusion and apical contraction cooperatively drive Drosophila germ-band extension. Nat. Cell Biol. 120, 827 ( 10.1038/ncb3497) [DOI] [PubMed] [Google Scholar]
- 53.Tang VW. 2018. Cell-cell adhesion interface: orthogonal and parallel forces from contraction, protrusion, and retraction. F1000Research 7, 1544 ( 10.12688/f1000research.15860.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sui L., et al. 2018. Differential lateral and basal tension drive folding of Drosophila wing discs through two distinct mechanisms. Nat. Commun. 9, 4620 ( 10.1038/s41467-018-06497-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finegan TM, Hervieux N, Nestor-Bergmann A, Fletcher AG, Blanchard GB, Sanson B. 2019. The tricellular vertex-specific adhesion molecule Sidekick facilitates polarised cell intercalation during Drosophila axis extension. PLoS Biol. 17, e3000522 ( 10.1371/journal.pbio.3000522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Letizia A, He D, Astigarraga S, Colombelli J, Hatini V, Llimargas M, Treisman JE. 2019. Sidekick is a key component of tricellular adherens junctions that acts to resolve cell rearrangements. Dev. Cell 50, 313–326. ( 10.1016/j.devcel.2019.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uechi H, Kuranaga E. 2019. The tricellular junction protein Sidekick regulates vertex dynamics to promote bicellular junction extension. Dev. Cell 50, 327–338. ( 10.1016/j.devcel.2019.06.017) [DOI] [PubMed] [Google Scholar]
- 58.Townes PL, Holtfreter J. 1955. Directed movements and selective adhesion of embryonic amphibian cells. J. Exp. Zool. 128, 53–120. ( 10.1002/jez.1401280105) [DOI] [PubMed] [Google Scholar]
- 59.Steinberg MS. 2003. Cell adhesive interactions and tissue self-organization. In Origination of organismal form: beyond the gene in developmental and evolutionary biology (eds Müller GB, Newman SA), pp. 137–163. Cambridge, MA: MIT Press. [Google Scholar]
- 60.Steinberg MS. 2007. Differential adhesion in morphogenesis: a modern view. Curr. Opin. Genet. Dev. 17, 281–286. ( 10.1016/j.gde.2007.05.002) [DOI] [PubMed] [Google Scholar]
- 61.Foty RA, Steinberg MS. 2013. Differential adhesion in model systems. Wiley Interdis. Rev. Dev. Biol. 2, 631–645. ( 10.1002/wdev.104) [DOI] [PubMed] [Google Scholar]
- 62.Carthew RW. 2007. Pattern formation in the Drosophila eye. Curr. Opin. Genet. Dev. 17, 309–313. ( 10.1016/j.gde.2007.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monier B, Pélissier-Monier A, Brand AH, Sanson B. 2010. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat. Cell Biol. 12, 60–69. ( 10.1038/ncb2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zallen JA, Wieschaus E. 2004. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell 6, 343–355. ( 10.1016/S1534-5807(04)00060-7) [DOI] [PubMed] [Google Scholar]
- 65.Tetley RJ, Blanchard GB, Fletcher AG, Adams RJ, Sanson B. 2016. Unipolar distributions of junctional myosin II identify cell stripe boundaries that drive cell intercalation throughout Drosophila axis extension. eLife 5, 967 ( 10.7554/eLife.12094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laplante C, Nilson LA. 2006. Differential expression of the adhesion molecule Echinoid drives epithelial morphogenesis in Drosophila. Development 133, 3255–3264. ( 10.1242/dev.02492) [DOI] [PubMed] [Google Scholar]
- 67.Lin H-P, Chen H-M, Wei S-Y, Chen L-Y, Chang L-H, Sun Y-J, Huang S-Y, Hsu J-C. 2007. Cell adhesion molecule Echinoid associates with unconventional myosin VI/Jaguar motor to regulate cell morphology during dorsal closure in Drosophila. Dev. Biol. 311, 423–433. ( 10.1016/j.ydbio.2007.08.043) [DOI] [PubMed] [Google Scholar]
- 68.Islam R, Wei S-Y, Chiu W-H, Hortsch M, Hsu J-C. 2003. Neuroglian activates Echinoid to antagonize the Drosophila EGF receptor signaling pathway. Development 130, 2051–2059. ( 10.1242/dev.00415) [DOI] [PubMed] [Google Scholar]
- 69.Spencer SA, Cagan RL. 2003. Echinoid is essential for regulation of Egfr signaling and R8 formation during Drosophila eye development. Development 130, 3725–3733. ( 10.1242/dev.00605) [DOI] [PubMed] [Google Scholar]
- 70.Laplante C, Nilson LA. 2011. Asymmetric distribution of Echinoid defines the epidermal leading edge during Drosophila dorsal closure. J. Cell Biol. 192, 335–348. ( 10.1083/jcb.201009022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang L-H, et al. 2011. Differential adhesion and actomyosin cable collaborate to drive Echinoid-mediated cell sorting. Development 138, 3803–3812. ( 10.1242/dev.062257) [DOI] [PubMed] [Google Scholar]
- 72.Kiehart DP, Crawford JM, Aristotelous A, Venakides S, Edwards GS. 2017. Cell sheet morphogenesis: dorsal closure in Drosophila melanogaster as a model system. Annu. Rev. Cell Dev. Biol. 33, 169–202. ( 10.1146/annurev-cellbio-111315-125357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Togashi H, Kominami K, Waseda M, Komura H, Miyoshi J, Takeichi M, Takai Y. 2011. Nectins establish a checkerboard-like cellular pattern in the auditory epithelium. Science 333, 1144–1147. ( 10.1126/science.1208467) [DOI] [PubMed] [Google Scholar]
- 74.Togashi H. 2016. Differential and cooperative cell adhesion regulates cellular pattern in sensory epithelia. Front Cell Dev Biol 4, 104 ( 10.3389/fcell.2016.00104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yue T, Tian A, Jiang J. 2012. The cell adhesion molecule Echinoid functions as a tumor suppressor and upstream regulator of the Hippo signaling pathway. Dev. Cell 22, 255–267. ( 10.1016/j.devcel.2011.12.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kohsaka H, Takasu E, Nose A. 2007. In vivo induction of postsynaptic molecular assembly by the cell adhesion molecule fasciclin2. J. Cell Biol. 179, 1289–1300. ( 10.1083/jcb.200705154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ashley J, Packard M, Ataman B, Budnik V. 2005. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J. Neurosci. 25, 5943–5955. ( 10.1523/JNEUROSCI.1144-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galletta BJ, Chakravarti M, Banerjee R, Abmayr SM. 2004. SNS: adhesive properties, localization requirements and ectodomain dependence in S2 cells and embryonic myoblasts. Mech. Dev. 121, 1455–1468. ( 10.1016/j.mod.2004.08.001) [DOI] [PubMed] [Google Scholar]
- 79.Bao S, Fischbach K-F, Corbin V, Cagan RL. 2010. Preferential adhesion maintains separation of ommatidia in the Drosophila eye. Dev. Biol. 344, 948–956. ( 10.1016/j.ydbio.2010.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dworak HA, Charles MA, Pellerano LB, Sink H. 2001. Characterization of Drosophila hibris, a gene related to human nephrin. Development 128, 4265–4276. [DOI] [PubMed] [Google Scholar]
- 81.Shelton C, Kocherlakota KS, Xhuang S, Abmayr SM. 2009. The immunoglobulin superfamily member Hbs functions redundantly with Sns in interactions between founder and fusion-competent myoblasts. Development 136, 1159–1168. ( 10.1242/dev.026302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson RI, Bao S, Cagan RL. 2012. Interactions between Drosophila IgCAM adhesion receptors and cindr, the Cd2ap/Cin85 ortholog. Dev. Dyn. 241, 1933–1943. ( 10.1002/dvdy.23879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaipa BR, et al. 2013. Dock mediates Scar- and WASp-dependent actin polymerization through interaction with cell adhesion molecules in founder cells and fusion-competent myoblasts. J. Cell Sci. 126, 360–372. ( 10.1242/jcs.113860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh J, Mlodzik M. 2012. Hibris, a Drosophila nephrin homolog, is required for presenilin-mediated Notch and APP-like cleavages. Dev. Cell 23, 82–96. ( 10.1016/j.devcel.2012.04.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, Ruiz-Gómez M, Skaer H, Denholm B. 2009. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457, 322–326. ( 10.1038/nature07526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vishnu S, Hertenstein A, Betschinger J, Konblich JA, Gert de Couet H, Fischbach K-F. 2006. The adaptor protein X11 Lα/Dmint1 interacts with the PDZ-binding domain of the cell recognition protein Rst in Drosophila. Dev. Biol. 289, 296–307. ( 10.1016/j.ydbio.2005.09.016) [DOI] [PubMed] [Google Scholar]
- 87.Chen EH, Olson EN. 2001. Antisocial, an intracellular adaptor protein, is required for myoblast fusion in Drosophila. Dev. Cell 1, 705–715. ( 10.1016/S1534-5807(01)00084-3) [DOI] [PubMed] [Google Scholar]
- 88.Llimargas M, Strigini M, Katidou M, Karagogeos D, Casanova J. 2004. Lachesin is a component of a septate junction-based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system. Development 131, 181–190. ( 10.1242/dev.00917) [DOI] [PubMed] [Google Scholar]
- 89.Strigini M, Cantera R, Morin X, Bastiani MJ, Bate M, Karagogeos D. 2006. The IgLON protein Lachesin is required for the blood–brain barrier in Drosophila. Mol. Cell. Neurosci. 32, 91–101. ( 10.1016/j.mcn.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 90.Hortsch M, Wang YE, Marikar Y, Bieber AJ. 1995. The cytoplasmic domain of the Drosophila cell adhesion molecule neuroglian is not essential for its homophilic adhesive properties in S2 cells. J. Biol. Chem. 270, 18 809–18 817. ( 10.1074/jbc.270.32.18809) [DOI] [PubMed] [Google Scholar]
- 91.Genova JL, Fehon RG. 2003. Neuroglian, gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J. Cell Biol. 161, 979–989. ( 10.1083/jcb.200212054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hortsch M, Homer D, Malhotra JD, Chang S, Frankel J, Jefford G, Dubreuil RR. 1998. Structural requirements for outside-in and inside-out signaling by Drosophila neuroglian, a member of the L1 family of cell adhesion molecules. J. Cell Biol. 142, 251–261. ( 10.1083/jcb.142.1.251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Enneking E-M, Kudumala SR, Moreno E, Stephan R, Boerner J, Godenschwege TA, Pielage J. 2013. Transsynaptic coordination of synaptic growth, function, and stability by the L1-type CAM Neuroglian. PLoS Biol. 11, e1001537 ( 10.1371/journal.pbio.1001537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Faivre-Sarrailh C, Banerjee S Li J, Hortsch M, Laval M, Bhat MA. 2004. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development 131, 4931–4942. ( 10.1242/dev.01372) [DOI] [PubMed] [Google Scholar]
- 95.Goossens T, Kang YY, Wuytens G, Zimmermann P, Callaerts-Végh Z, Pollarolo G, Islam R, Hortsch M, Callaerts P. 2011. The Drosophila L1CAM homolog Neuroglian signals through distinct pathways to control different aspects of mushroom body axon development. Development 138, 1595–1605. ( 10.1242/dev.052787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laprise P, et al. 2009. Yurt, Coracle, Neurexin IV and the Na+,K+-ATPase form a novel group of epithelial polarity proteins. Nature 459, 1141–1145. ( 10.1038/nature08067) [DOI] [PubMed] [Google Scholar]
- 97.Tiklová K, Senti K-A, Wang S, Gräslund A, Samakovlis C. 2010. Epithelial septate junction assembly relies on melanotransferrin iron binding and endocytosis in Drosophila. Nat. Cell Biol. 12, 1071–1077. ( 10.1038/ncb2111) [DOI] [PubMed] [Google Scholar]
- 98.Lee H-G, Zarnescu DC, MacIver B, Thomas GH. 2010. The cell adhesion molecule Roughest depends on βHeavy-spectrin during eye morphogenesis in Drosophila. J. Cell Sci. 123, 277–285. ( 10.1242/jcs.056853) [DOI] [PubMed] [Google Scholar]
- 99.Goodman KM, et al. 2016. Molecular basis of sidekick-mediated cell-cell adhesion and specificity. eLife 5, 213 ( 10.7554/eLife.19058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blackie L, Tozluoglu M, Trylinski M, Walther RF, Mao Y, Schweisguth F, Pichaud F. 2019. Neph/nephrin-like adhesion and tissue level pulling forces regulate cell intercalation during Drosophila retina development. bioRxiv 564708 ( 10.1101/564708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang S, Amourda C, Garfield D, Saunders TE. 2018. Selective filopodia adhesion ensures robust cell matching in the Drosophila heart. Dev. Cell 46, 189–203. ( 10.1016/j.devcel.2018.06.015) [DOI] [PubMed] [Google Scholar]
- 102.Vogler G, Bodmer R. 2015. Cellular mechanisms of Drosophila heart morphogenesis. J. Cardiovasc. Dev. Dis. 2, 2–16. ( 10.3390/jcdd2010002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fischbach K-F, Linneweber GA, Andlauer TFM, Hertenstein A, Bonengel B, Chaudhary K. 2009. The irre cell recognition module (IRM) proteins. J. Neurogenet. 23, 48–67. ( 10.1080/01677060802471668) [DOI] [PubMed] [Google Scholar]
- 104.Bour BA, Chakravarti M, West JM, Abmayr SM. 2000. Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev. 14, 1498–1511. ( 10.1101/gad.14.12.1498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Linneweber GA, Winking M, Fischbach K-F. 2015. The cell adhesion molecules Roughest, Hibris, Kin of Irre and Sticks and Stones are required for long range spacing of the Drosophila wing disc sensory sensilla. PLoS ONE 10, e0128490 ( 10.1371/journal.pone.0128490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valer FB, Machado MCR, Junior RMPS, Ramos RGP. 2018. Expression of Hbs, Kirre, and Rst during Drosophila ovarian development. Genesis 56, e23242 ( 10.1002/dvg.23242) [DOI] [PubMed] [Google Scholar]
- 107.Reddy GV, Reiter C, Shanbhag S, Fischbach K-F, Rodrigues V. 1999. Irregular chiasm-C-roughest, a member of the immunoglobulin superfamily, affects sense organ spacing on the Drosophila antenna by influencing the positioning of founder cells on the disc ectoderm. Dev. Genes Evol. 209, 581–591. ( 10.1007/s004270050292) [DOI] [PubMed] [Google Scholar]
- 108.Bao S, Cagan R. 2005. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev. Cell 8, 925–935. ( 10.1016/j.devcel.2005.03.011) [DOI] [PubMed] [Google Scholar]
- 109.Hill BKG, Wolff T. 2009. Dynamic cell shapes and contacts in the developing Drosophila retina are regulated by the Ig cell adhesion protein hibris. Dev. Dyn. 238, 2223–2234. ( 10.1002/dvdy.21981) [DOI] [PubMed] [Google Scholar]
- 110.Johnson RI, Sedgwick A, D'Souza-Schorey C, Cagan RL. 2011. Role for a Cindr–Arf6 axis in patterning emerging epithelia. Mol. Biol. Cell 22, 4513–4526. ( 10.1091/mbc.e11-04-0305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bao S. 2014. Notch controls cell adhesion in the Drosophila eye. PLoS Genet. 10, e1004087 ( 10.1371/journal.pgen.1004087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reiter C, Schimansky T, Nie Z, Fischbach KF. 1996. Reorganization of membrane contacts prior to apoptosis in the Drosophila retina: the role of the IrreC-rst protein. Development 122, 1931–1940. [DOI] [PubMed] [Google Scholar]
- 113.Cordero JB, Larson DE, Craig CR, Hays R, Cagan R. 2007. Dynamic Decapentaplegic signaling regulates patterning and adhesion in the Drosophila pupal retina. Development 134, 1861–1871. ( 10.1242/dev.002972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ruiz-Gómez M, Coutts N, Price A, Taylor MV, Bate M. 2000. Drosophila Dumbfounded: a myoblast attractant essential for fusion. Cell 102, 189–198. ( 10.1016/S0092-8674(00)00024-6) [DOI] [PubMed] [Google Scholar]
- 115.Strünkelnberg M, Bonengel B, Moda LM, Hertenstein A, de Couet HG, Ramos RGP, Fischbach K-F.. 2001. rst and its paralogue kirre act redundantly during embryonic muscle development in Drosophila. Development 128, 4229–4239. ( 10.1093/nar/25.17.3389) [DOI] [PubMed] [Google Scholar]
- 116.Gildor B, Schejter ED, Shilo B-Z. 2012. Bidirectional Notch activation represses fusion competence in swarming adult Drosophila myoblasts. Development 139, 4040–4050. ( 10.1242/dev.077495) [DOI] [PubMed] [Google Scholar]
- 117.Denholm B, Sudarsan V, Pasalodos-Sanchez S, Artero R, Lawrence P, Maddrell S, Baylies M, Skaer H. 2003. Dual Origin of the renal tubules in Drosophila: mesodermal cells integrate and polarize to establish secretory function. Curr. biol. 13, 1052–1057. ( 10.1016/S0960-9822(03)00375-0) [DOI] [PubMed] [Google Scholar]
- 118.Ramos RG, Igloi GL, Lichte B, Baumann U, Maier D, Schneider T, Brandstätter JH, Fröhlich A, Fischbach KF. 1993. The irregular chiasm C-roughest locus of Drosophila, which affects axonal projections and programmed cell death, encodes a novel immunoglobulin-like protein. Genes Dev. 7, 2533–2547. ( 10.1101/gad.7.12b.2533) [DOI] [PubMed] [Google Scholar]
- 119.Schneider T, Reiter C, Eule E, Bader B, Lichte B, Nie Z, Schimansky T, Ramos RGP, Fischbach K-F. 1995. Restricted expression of the IrreC-rst protein is required for normal axonal projections of columnar visual neurons. Neuron 15, 259–271. ( 10.1016/0896-6273(95)90032-2) [DOI] [PubMed] [Google Scholar]
- 120.Boschert U, Ramos RGP, Tix S, Technau GM, Fischbach K-F. 2009. Genetic and developmental analysis of irreC, a genetic function required for optic chiasm formation in Drosophila. J. Neurogenet. 6, 153–171. ( 10.3109/01677069009107107) [DOI] [PubMed] [Google Scholar]
- 121.Yamagata M, Weiner JA, Sanes JR. 2002. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell 110, 649–660. ( 10.1016/S0092-8674(02)00910-8) [DOI] [PubMed] [Google Scholar]
- 122.Yamagata M, Sanes JR. 2008. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature 451, 465–469. ( 10.1038/nature06469) [DOI] [PubMed] [Google Scholar]
- 123.Krishnaswamy A, Yamagata M, Duan X, Hong YK, Sanes JR. 2015. Sidekick 2 directs formation of a retinal circuit that detects differential motion. Nature 524, 466–470. ( 10.1038/nature14682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Astigarraga S, Douthit J, Tarnogorska D, Creamer MS, Mano O, Clark DA, Meinertzhagen IA, Treisman JE. 2018. Drosophila Sidekick is required in developing photoreceptors to enable visual motion detection. Development 145, dev158246 ( 10.1242/dev.158246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Finegan TM, Hervieux N, Nestor-Bergmann A, Fletcher AG, Blanchard GB, Sanson B. 2019. The tricellular vertex-specific adhesion molecule Sidekick facilitates polarised cell intercalation during Drosophila axis extension. bioRxiv 704932 ( 10.1101/704932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lye CM, Blanchard GB, Naylor HW, Muresan L, Huisken J, Adams RJ, Sanson B. 2015. Mechanical coupling between endoderm invagination and axis extension in Drosophila. PLoS Biol. 13, e1002292 ( 10.1371/journal.pbio.1002292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Higashi T, Miller AL. 2017. Tricellular junctions: how to build junctions at the TRICkiest points of epithelial cells. Mol. Biol. Cell 28, 2023–2034. ( 10.1091/mbc.E16-10-0697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bosveld F, Wang Z, Bellaiche Y. 2018. Tricellular junctions: a hot corner of epithelial biology. Curr. Opin. Cell Biol. 54, 80–88. ( 10.1016/j.ceb.2018.05.002) [DOI] [PubMed] [Google Scholar]
- 129.Vasquez CG, Martin AC. 2016. Force transmission in epithelial tissues. Dev. Dyn. 245, 361–371. ( 10.1002/dvdy.24384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Manning LA, Perez-Vale KZ, Schaefer KN, Sewell MT, Peifer M. 2019. The Drosophila Afadin and ZO-1 homologues Canoe and Polychaetoid act in parallel to maintain epithelial integrity when challenged by adherens junction remodeling. Mol. Biol. Cell 30, 1938–1960. ( 10.1091/mbc.E19-04-0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilson TJ, Bergstralh DT. 2017. Cell reintegration: stray epithelial cells make their way home. Bioessays 39, 1600248 ( 10.1002/bies.201600248) [DOI] [PubMed] [Google Scholar]
- 132.Wei J, Hortsch M, Goode S. 2004. Neuroglian stabilizes epithelial structure during Drosophila oogenesis. Dev. Dyn. 230, 800–808. ( 10.1002/dvdy.20108) [DOI] [PubMed] [Google Scholar]
- 133.Davis JQ, Bennett V. 1994. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J. Biol. Chem. 269, 27 163–27 166. [PubMed] [Google Scholar]
- 134.Hortsch M. 2000. Structural and functional evolution of the L1 family: are four adhesion molecules better than one? Mol. Cell. Neurosci. 15, 1–10. ( 10.1006/mcne.1999.0809) [DOI] [PubMed] [Google Scholar]
- 135.Dickson TC, Mintz CD, Benson DL, Salton SRJ. 2002. Functional binding interaction identified between the axonal CAM L1 and members of the ERM family. J. Cell Biol. 157, 1105–1112. ( 10.1083/jcb.200111076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen L, Zhou S. 2010. ‘CRASH'ing with the worm: insights into L1CAM functions and mechanisms. Dev. Dyn. 239, 1490–1501. ( 10.1002/dvdy.22269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Szafranski P, Goode S. 2004. A Fasciclin 2 morphogenetic switch organizes epithelial cell cluster polarity and motility. Development 131, 2023–2036. ( 10.1242/dev.01097) [DOI] [PubMed] [Google Scholar]
- 138.Szafranski P, Goode S. 2007. Basolateral junctions are sufficient to suppress epithelial invasion during Drosophila oogenesis. Dev. Dyn. 236, 364–373. ( 10.1002/dvdy.21020) [DOI] [PubMed] [Google Scholar]
- 139.Harrelson AL, Goodman CS. 1988. Growth cone guidance in insects: fasciclin II is a member of the immunoglobulin superfamily. Science 242, 700–708. ( 10.1126/science.3187519) [DOI] [PubMed] [Google Scholar]
- 140.Grenningloh G, Rehm EJ, Goodman CS. 1991. Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell 67, 45–57. ( 10.1016/0092-8674(91)90571-f) [DOI] [PubMed] [Google Scholar]
- 141.Schuster CM, Davis GW, Fetter RD, Goodman CS. 1996. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17, 641–654. ( 10.1016/s0896-6273(00)80197-x) [DOI] [PubMed] [Google Scholar]
- 142.Mao Y, Freeman M. 2009. Fasciclin 2, the Drosophila orthologue of neural cell-adhesion molecule, inhibits EGF receptor signalling. Development 136, 473–481. ( 10.1242/dev.026054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Soroka V, et al. 2003. Structure and interactions of NCAM Ig1–2–3 suggest a novel zipper mechanism for homophilic adhesion. Structure 11, 1291–1301. ( 10.1016/j.str.2003.09.006) [DOI] [PubMed] [Google Scholar]
- 144.Thor G, Probstmeier R, Schachner M. 1987. Characterization of the cell adhesion molecules L1, N-CAM and J1 in the mouse intestine. EMBO J. 6, 2581–2586. ( 10.1002/j.1460-2075.1987.tb02548.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Debiec H, Christensen EI, Ronco PM. 1998. The cell adhesion molecule L1 Is developmentally regulated in the renal epithelium and is involved in kidney branching morphogenesis. J. Cell Biol. 143, 2067–2079. ( 10.1083/jcb.143.7.2067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nolte C, Moos M, Schachner M. 1999. Immunolocalization of the neural cell adhesion molecule L1 in epithelia of rodents. Cell Tissue Res. 298, 261–273. ( 10.1007/s004419900063) [DOI] [PubMed] [Google Scholar]
- 147.Maddaluno L, Verbrugge SE, Martinoli C, Matteoli G, Chiavelli A, Zeng Y, Williams ED, Rescigno M, Cavallaro U. 2009. The adhesion molecule L1 regulates transendothelial migration and trafficking of dendritic cells. J. Exp. Med. 206, 623–635. ( 10.1084/jem.20081211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kusumi A, Sako Y, Yamamoto M. 1993. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys. J. 65, 2021–2040. ( 10.1016/S0006-3495(93)81253-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maxfield FR. 2002. Plasma membrane microdomains. Curr. Opin. Cell Biol. 14, 483–487. ( 10.1016/S0955-0674(02)00351-4) [DOI] [PubMed] [Google Scholar]
- 150.Gómez-Gálvez P, et al. 2018. Scutoids are a geometrical solution to three-dimensional packing of epithelia. Nat. Commun. 9, 2960 ( 10.1038/s41467-018-05376-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Behr M, Riedel D, Schuh R. 2003. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev. Cell 5, 611–620. ( 10.1016/s1534-5807(03)00275-2) [DOI] [PubMed] [Google Scholar]
- 152.Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. 2004. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J. Cell Biol. 164, 313–323. ( 10.1083/jcb.200309134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu VM, Yu MH, Paik R, Banerjee S, Liang Z, Paul SM, Bhat MA, Beitel GJ. 2007. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development 134, 999–1009. ( 10.1242/dev.02785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nelson KS, Furuse M, Beitel GJ. 2010. The Drosophila Claudin Kune-kune is required for septate junction organization and tracheal tube size control. Genetics 185, 831–839. ( 10.1534/genetics.110.114959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bätz T, Förster D, Luschnig S. 2014. The transmembrane protein Macroglobulin complement-related is essential for septate junction formation and epithelial barrier function in Drosophila. Development 141, 899–908. ( 10.1242/dev.102160) [DOI] [PubMed] [Google Scholar]
- 156.Hall S, Bone C, Oshima K, Zhang L, McGraw M, Lucas B, Fehon RG, Ward RE. 2014. Macroglobulin complement-related encodes a protein required for septate junction organization and paracellular barrier function in Drosophila. Development 141, 889–898. ( 10.1242/dev.102152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hildebrandt A, Pflanz R, Behr M, Tarp T, Riedel D, Schuh R. 2015. Bark beetle controls epithelial morphogenesis by septate junction maturation in Drosophila. Dev. Biol. 400, 237–247. ( 10.1016/j.ydbio.2015.02.008) [DOI] [PubMed] [Google Scholar]
- 158.Hall S, Ward RE. 2016. Septate junction proteins play essential roles in morphogenesis throughout embryonic development in Drosophila. G3 6, 2375–2384. ( 10.1534/g3.116.031427) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.