Abstract
Sonic Hedgehog (Shh) Is a critical protein in vertebrate development, orchestrating patterning and growth in many developing systems. First described as a classic morphogen that patterns tissues through a spatial concentration gradient, subsequent studies have revealed a more complex mechanism, in which Shh can also regulate proliferation and differentiation. While the mechanism of action of Shh as a morphogen is well understood, it remains less clear how Shh might integrate patterning, proliferation and differentiation in a given tissue, to ultimately direct its morphogenesis. In tandem with experimental studies, mathematical modelling can help gain mechanistic insights into these processes and bridge the gap between Shh-regulated patterning and growth, by integrating these processes into a common theoretical framework. Here, we briefly review the roles of Shh in vertebrate development, focusing on its functions as a morphogen, mitogen and regulator of differentiation. We then discuss the contributions that modelling has made to our understanding of the action of Shh and highlight current challenges in using mathematical models in a quantitative and predictive way.
This article is part of a discussion meeting issue ‘Contemporary morphogenesis’.
Keywords: Sonic Hedgehog, patterning, growth, morphogenesis, developmental biology, mathematical modelling
1. Introduction
Sonic Hedgehog (Shh) is a secreted glycoprotein encoded by the Shh gene. First cloned in the 1990s, on the basis of its high conservation with the Drosophila hedgehog (hh) gene [1–6], Shh is the best studied ligand of the hedgehog family and plays a key role in vertebrate development and organogenesis. Initial studies (described below) revealed the importance of Shh in patterning the embryonic ventral neural tube and posterior limb bud. These studies were followed swiftly by those indicating that Shh plays an important role in directing a vast array of developmental processes in the embryo, including development of the somites [7], foregut [8], lung [9] and brain [10–12], as well as craniofacial development [13–16], and is instrumental in directing cell proliferation in particular embryonic populations [17]. Further investigations showed Shh has diverse functional roles in fetal and postnatal life, in circuit wiring and in stem cell regulation [18–22].
Here, we briefly summarize the current state of knowledge regarding the roles of Shh as a morphogen and a mitogen in the embryo, before focusing on the utility of mathematical modelling in dissecting the complexity of Shh activity. Through illustrative examples, we discuss how different modelling approaches have allowed mechanistic insights into Shh-controlled gene expression at the cellular level, as well as the actions of Shh as a mitogen and a morphogen at the tissue scale. We conclude by discussing how mathematical modelling could help future efforts to study the multifunctional nature of Shh signalling throughout development.
2. Sonic Hedgehog as a morphogen
Turing introduced the concept of a morphogen [23] and subsequent studies [24,25] led to its conventional definition: a molecule that diffuses through cells and tissues to establish a concentration gradient that evokes discrete cell responses at particular threshold concentrations to confer position identity and pattern cell/tissue fields. Classic grafting studies in the chick had suggested that the dorsoventral (DV) axis of the posterior neural tube (the future spinal cord) and anterior–posterior (AP) axis of the limb bud are patterned through the activity of a morphogen deriving from ventral midline cells of the notochord and floor plate [26], and the posterior zone of polarizing activity (ZPA) ([27], respectively. The cloning of Shh provided insight into the molecular identity of the morphogen: Shh showed restricted expression to the notochord, floor plate and ZPA [1–4,6].
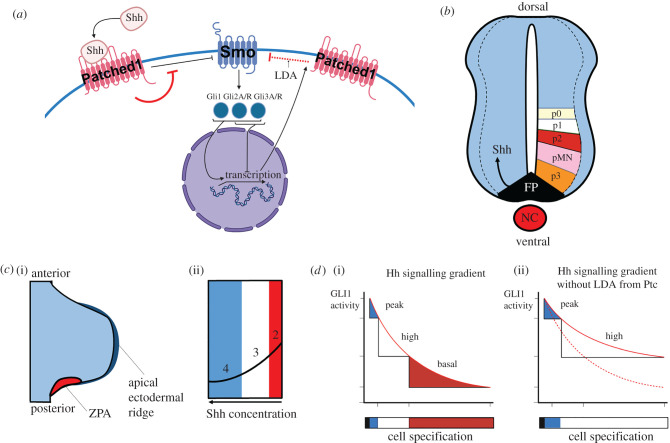
The canonical Shh signalling pathway involves effector zinc-finger transcription factors of the Gli family: Gli1, Gli2 and Gli3. Gli1 exists only as an activator, whereas Gli2 and Gli3 can be converted from repressor (GliR) to activator (GliA) forms (reviewed in [28]). The signalling pathway is initiated when secreted Shh binds Patched (Ptc) at the surface of a responsive cell. Binding relieves inhibition of the transmembrane protein Smoothened (Smo) and ultimately triggers the activation of the Gli transcription factors. This in turn results in activation of Shh target genes, including Ptc, forming a negative feedback loop (figure 1a).
Figure 1.
Key features of the Shh pathway. (a) Simplified schematic of the ‘canonical’ Shh pathway. Shh binds to the membrane-bound receptor Patched1, relieving Patched1's constitutive inhibition of Smoothened (Smo). Upon Shh signalling, Smo is thus able to interact with Gli transcription factors, which initiate transcription of Shh target genes such as the gene encoding Patched1. This gives rise to ligand-dependent antagonism (LDA; red dotted flathead arrow), whereby Shh network activity stimulates the expression of its own repressor (Ptc). Red flathead arrows indicate effects that occur in the presence of Shh binding. (b) Simplified schematic of neural tube patterning by Shh. Shh is expressed by the notochord (NC), and movement of Shh into the floor plate (FP) induces Shh expression in the FP. From here, Shh forms a concentration gradient from ventral to dorsal in the developing neural tube, specifying neural progenitors (p0–3, pMN). Dotted lines indicates progenitor region. (c) Simplified schematic of limb bud digit patterning by Shh. Shh is secreted from the zone of polarizing activity (ZPA) and travels through the posterior limb bud, specifying posterior identity through Gli1 and Gli3 induction. (d) Experimental evidence for Patched1-dependent ligand-dependent antagonism on developing hair follicles, adapted from [29]. (i) The levels of Gli1 activity resulting from a proximo-distal Shh concentration gradient. (ii) In a Patched1 genetic knockout, the Hedgehog gradient is no longer attenuated via ligand-dependent antagonism, so high concentration-dependent cell identities are found more distally. Red dotted line is the shape of the wild-type Shh gradient.
Loss-of-function and gain-of-function studies of Shh, or components of its signalling pathway, indicated that Shh acts as a stereotypical morphogen in both the neural tube and the limb bud, i.e. establishing a concentration gradient that is translated into a GliA–GliR gradient that patterns these tissues and instruct cell fates (although note that the precise regulatory role of each Gli is not fully elucidated) [3,30]. Thus in the neural tube, Shh is secreted from the notochord and floor plate and diffuses through ventral regions of the neural tube, converting Gli2 and Gli3 to GliA forms, and inducing Gli1 (a GliA) [31]. In turn, this leads to the establishment of different progenitor cells types along the DV axis (figure 1b). In the developing limb bud, Shh is secreted from the ZPA, travels through the posterior limb bud and confers posterior identity through canonical induction of Gli1 and prevention of Gli3R [32] (figure 1c). Thus in both systems, the relative levels of GliA–GliR, and the balance between activation and repression of target genes, are the pivotal mechanism by which cells translate a gradient of Shh ligand into a discrete set of cell identities (reviewed in [31,33,34]). The mechanisms through which Shh becomes spatially distributed in both the neural tube and limb bud remain poorly understood (reviewed in [33,34]). Candidate mechanisms include diffusion that is free [24] or diffusion that is modified by lipid modification of Shh, or its binding to proteoglycans in the extracellular matrix [35].
Importantly, the timing of exposure of cells to Shh—in particular the length of their exposure—is critical to a cell's development: cells are capable of measuring their own exposure to Shh and integrating this as meaningful information over periods of time [36,37]. This challenges the conventional definition as a morphogen, as it means that absolute levels of Shh are not directly translated into a spatial ‘positional’ value [38]. This is perhaps most well characterized in the embryonic chick. Here, in both the neural tube and the limb bud, Shh-responsive cells integrate Shh levels over time and transiently progress through progenitor identities, promoting to sequential ventral, or posterior identities [39,40]. Furthermore, cells can become refractory to Shh over time, an event that is triggered by the Shh-induced negative feedback loop described above. Thus, the higher the amount of Shh signalling over time, the more the pathway is suppressed, a mechanism termed ligand-dependent antagonism (reviewed in [41]) (figure 1d). These dynamic responses indicate that to understand Shh's action as a morphogen it is critical to study its effects in both space and time. As we will discuss, such advanced mechanistic understanding greatly benefits from mathematical modelling.
3. Sonic Hedgehog functions as a mitogen and regulates the cell cycle
A less well-characterized role for Shh is as a mitogen (reviewed in [41]), a role that it orchestrates by altering cell cycle kinetics. First described as being critical for proliferation of granule neuron progenitors in the developing cerebellum [17,22,42], Shh signalling is now known to govern cell proliferation in many tissues. In the chick limb bud, classic studies suggested an integration of growth and patterning [43], and more recently Shh's influence on the cell cycle has been characterized: Shh signalling first stimulates ZPA cell proliferation via Cyclin D2 before downregulating proliferation in the ZPA through control of BMP2 signalling [44]. This fine control of proliferation by Shh ensures the correct number of digits form in the limb. In spinal cord progenitors, Shh signalling regulates the length of the G1 phase of the cell cycle, decreases cell cycle length and increases expression of Cyclin D1 and N-myc, to expand specific progenitor pools [45,46]. In the brain, Shh induces progenitor cell proliferation and the maintenance or growth of progenitor cell populations [47]; similarly, Shh has a proliferative role in retinal, hypothalamic and telencephalic progenitors [48–50] (see reviews by [46,47]). The specific effectors regulating the mitogenic activity of Shh are likely to vary across tissues [51]: for example, the phosphatase Eya1 is known to lie upstream of Shh-controlled symmetrical cell divisions in the granule precursor cells [52]. Moreover, an important feature of Shh control of proliferation is timing, as Shh first promotes cell cycle progression but then inhibits it [37,44], and in this manner, can play a role in cell cycle exit.
Indeed, in many systems, Shh also governs cell differentiation (reviewed in [53]). Studies suggest that this can occur as Shh triggers the transcription of signalling pathways that feedback to promote cell cycle arrest, or to antagonize Shh signalling [54]. In the hypothalamus, Shh may ultimately upregulate p57, driving cell cycle exit [49]; in the developing thymus gland, auto-repression of Shh signalling by Gli3 stimulates differentiation [55]. It remains unclear whether these events are context-dependent or are a common mechanism of differentiation regulation by Shh. Other cell behaviours, directly governed by Shh, may likewise govern cell differentiation. Increasing numbers of studies reveal that Shh can control the plane of cell division [52], as well as cell orientation and migration [56,57], each of which could intrinsically direct an exit from the cell cycle.
In summary, our understanding of the action of Shh has undergone a dramatic change in recent decades. Shh does not simply provide positional information by establishing a spatial morphogenic field, but instead triggers complex downstream effects that control the entire process of morphogenesis: patterning, proliferation, growth and differentiation. The open questions that remain, however, are: how are patterning, proliferation, growth and differentiation integrated, and what properties do their integration confer that we cannot understand by taking each alone?
Traditionally, the actions of Shh have been dissected through genetic or pharmacological interventions, conducted and analysed at specific time-points. While these approaches have enormous merit and are highly tractable, consideration needs to be given to the idea that such studies will inevitably miss many dynamic events, given that Shh is operating under tight timescales and has strong positive and negative feedback loops. Further, it is simply not feasible to conduct interventions at repeated time-points during development. As will be discussed below, a potential solution to this issue is the analysis of Shh in development via mathematical modelling, an approach that lends itself to the analysis of the multifunctional Shh pathway in a systematic, quantitative and predictive manner.
4. A role for mathematical modelling
Mathematical modelling refers to the use of mathematical language to describe the behaviour of a system and is becoming an increasingly important component of the developmental biologist's ‘toolbox’ [58,59]. Mathematics has several uses as a tool for understanding complex scientific phenomena. First, mathematics enables formalization: early biological examples include Fisher's interpretation of Darwin's theory of natural selection [60] and Turing's general mechanism of pattern formation through diffusion-driven instability [23]. Second, the precision of mathematics allows us to obtain a quantitative and predictive understanding of specific phenomena: an early biological example is Hodgkin & Huxley's modelling of action potentials in neurons and cardiomyocytes [61]. The scale at which developmental processes can be modelled has undergone a step-change in recent years, as large-scale data collection methodologies, advances in genetics, manipulation of cellular behaviours and light microscopy are allowing quantitative descriptions and analyses down to the nanoscale [62].
When constructing a mathematical model of a biological system, it is usual to adopt a ‘modular’ approach, where the component parts of the model are chosen on the basis of existing experimental data and the questions being asked of the system [63]. Such an approach may be compared with the experimental investigation of developmental processes through modular perturbations such as genetic knockouts. Thus, a useful starting point is to construct a simple or generic model that neglects fine-grained details but helps improve our qualitative understanding of the mechanisms that can give rise to important features of development such as robustness [64]. We can then refine a model in the light of new experimental data and increase the level of complexity, such as including molecular details or spatial effects. For the remainder of the review, we will discuss how mathematical approaches have given insights into the actions of Shh, broadly following this concept of increasing complexity; for a more detailed discussion of the underlying mathematics, see e.g. [65].
5. Modelling Sonic Hedgehog-controlled gene expression
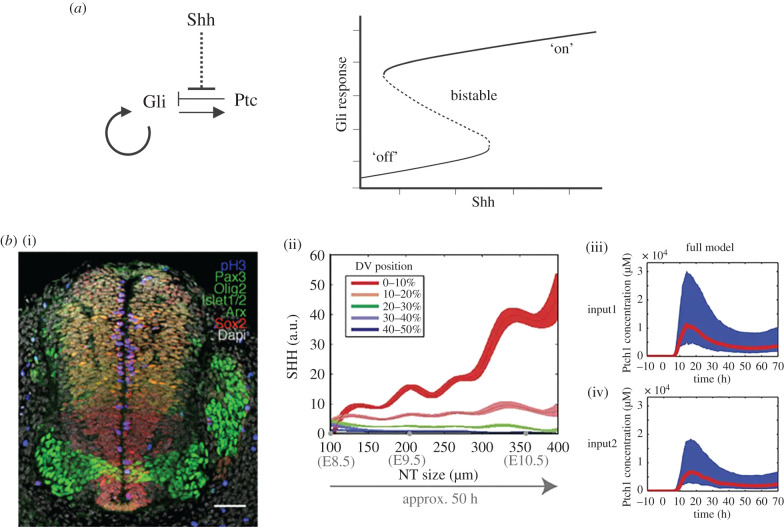
A major focus of mathematical modelling to date has been to understand how Shh effects changes in signal transduction and gene regulation. Lai et al. [66] proposed the first mathematical model of the gene regulatory network downstream of Shh, focusing on how this network can switch fate choices at a threshold Shh concentration. Adopting the ‘start simple’ approach outlined above, the authors simplify the network by considering only the receptor Ptc and the transcription factors Gli1, Gli3 and Gli3R, and—to reduce complexity and because the activities of Gli1 and Gli2 are deemed by the authors to overlap—a lumped term ‘Gli’ representing the effects of both these factors (figure 2a). Their model comprises a set of coupled ordinary differential equations (ODEs), which describe how the concentrations of the network components change smoothly over time owing to Shh binding to Ptc, the basal and inducible activities of the gli and ptc promoters, and the constitutive degradation of each component.
Figure 2.
Modelling Shh-controlled gene expression. (a) (i) Key feedbacks present in the model in [66]. (ii) Bifurcation analysis of the model in [66]. The model exhibits bistability, demonstrating that a graded input of Shh can result in a binary response. (b) (i) Example of transverse section of the developing neural tube, molecularly labelled for genes that indicate DV identity, from [67]. (ii) Experimental measurements of Shh levels from [68]. (iii, iv) Fitting of the data to the mathematical model in [68], with high (upper) and low (lower) expression of Shh. a.u., arbitrary units.
By mathematically analysing the qualitative behaviour of this model, the authors find that the network's ability to function as a genetic switch is due to a tight combination of positive feedback (Gli upregulates its own expression) and negative feedback (Gli upregulates its repressor, Ptc) (figure 2a). In the language of systems biology, this behaviour is called a toggle switch [69] and is one of the common network motifs found in nature. Unlike irreversible switches underlying ‘points of no return’, such as apoptosis [70], the system can switch from the low Gli state to the high Gli state and back again, if the Shh concentration is increased and decreased enough. To explore whether fluctuations in Shh concentration could undermine the genetic switch, Lai et al. [66] used a stochastic modelling approach, which accounts for intrinsic noise in transcriptional processes. Through stochastic simulation of their model, the authors identified a role for the negative Ptc feedback loop in dampening Gli fluctuations, thereby reducing the likelihood for such back-and-forth switching to occur.
This work highlights how mathematical modelling can refine our mechanistic understanding of Shh-controlled gene expression. In addition, the model is capable of generating experimentally testable predictions. For example, parameter sensitivity analysis revealed that an increase in the maximal rate of inducible Gli transcription can lead to the genetic switch becoming irreversible: if the Shh concentration increases above a threshold, the system reaches and stays in a high Gli state, no matter how much the Shh concentration is later decreased. Such behaviour is predicted in cells with mutations resulting in constitutively active Smo.
While useful, the simplified and qualitative nature of the above model prevents more detailed predictions from being made. A more recent example that addressed these limitations is provided by Cohen et al. [68], who made use of approximate Bayesian computation (ABC) to inform a mathematical model of gene expression in the developing neural tube (figure 2b). This demonstrates how we can estimate the values of model parameters such as the rate that Shh binds Ptc, and quantify our uncertainty in these estimates, based on in vivo measurements. In other work, Cohen et al. [71] modelled the transcriptional network downstream of Shh. This model differs from [66], in that the investigation into the Shh-regulated transcriptional network is done in the biological context of the developing neural tube. Additionally, Cohen et al. fitted their model to biological data of gene expression domains, using wild-type and mutants to provide this basis. This model is more complex and attempts to incorporate more biological features of the Shh network. The network of downstream transcription factors analysed in this model are able, through their combinatorial activity, to produce a sharp output response around a neutral point; either side of this point the produced effect is opposite. Taken together the above two studies provide a useful framework to better understand how graded morphogenetic signals can produce sharp and distinct responses, adding to our understanding of how distinct progenitor domains are formed at different axial levels in developing tissues (figure 1b,c).
6. Modelling Sonic Hedgehog as a morphogen or mitogen
Another focus of modelling has been to understand how Shh acts as a morphogen within a developing tissue. As outlined in §2, the conventional definition of a morphogen is a molecule that provides positional information by inducing distinct cell types/cell signatures in a concentration-dependent manner. Perhaps the most well-known conceptual description of positional information is the ‘French flag model’, which refers to the autonomous formation of a spatial morphogen gradient within a tissue, with individual cells in the tissue interpreting the local concentration gradient to inform their fate. This elegant idea emerged through studies of the vertebrate limb and became a core concept in developmental biology [72].
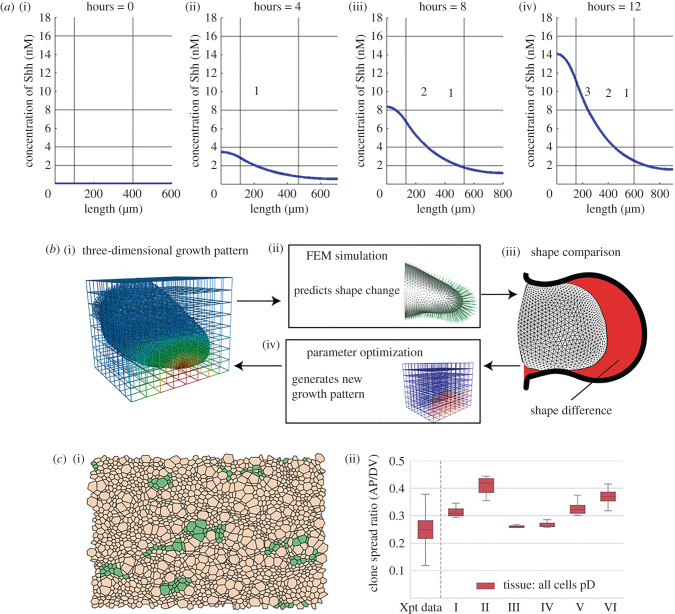
An example of how the mechanisms of Shh in development may be modelled mathematically is provided by Woolley et al. [73], who considered the Shh gradient formation and digit specification in a one-dimensional domain representing the chick wing bud. This domain was split into three distinct regions (posterior polarizing region where Shh is produced, digit-forming field and anterior region). The authors use a partial differential equation (PDE) approach to describe how the concentration of Shh evolves in time and space, owing to localized production, diffusion and decay of Shh within a uniformly growing domain. By prescribing a tissue growth rate the authors neglect any mitogenic activity of Shh, instead treating growth as an independent process which serves to dilute the Shh concentration. They found that, under the assumption that digit number is specified by the size of the digit-forming domain, this model could reproduce the temporal specification of the identities of the three chick wing digits (figure 3a). Furthermore, if digits were allowed to form from the polarizing region, this model could reproduce the four-digit pattern found in the chick leg. However, the model could not be extended in a straightforward manner to reproduce the five-digit pattern found in the mouse limb, suggesting that additional mechanisms must be present.
Figure 3.
Modelling Shh in patterning and growth. (a) Simulation of chick wing digit specification by a Shh gradient in a uniformly linearly growing domain. Vertical black lines delineate the domain into three sections; horizontal black lines illustrate the thresholds required for each digit identity. Reproduced with permission from [73]. (b) Schematic illustrating how parameters in a three-dimensional model of the developing limb are fitted by comparing simulated shape dynamics to in vivo data. FEM, finite element model. Reproduced with permission from [74]. (c) Simulation of progenitor proliferation and differentiation in the developing neuroepithelium. (i) Simulated clones reveal a shape bias in the DV direction (dorsal progenitor cells are brown, green represents clones of these dorsal progenitor cells). (ii) Comparison of clone shape bias for six different parameter sets with in vivo data (Xpt data). pD, dorsal progenitor. Reproduced with permission from [75].
The study by Woolley et al. [73] illustrates how mathematical modelling can help to shed light on the maximal contribution of a specific mechanism to a developmental behaviour. In addition, the authors made modelling predictions that pave the way for future experimental validation and, in turn, model refinement. For example, the model assumes no movement of Shh outside of the limb bud and neglects known important components of the Shh network in the limb bud, such as the effects of BMP signalling (discussed in §3) [76] or intracellular signalling feedback events: these assumptions could be revised if needed, as informed by experimental evidence.
Alongside the French flag model, a second major theory for morphogen-directed pattern formation is that of diffusion-driven instability, whereby an initially homogeneous state breaks symmetry owing to the diffusion of, and reactions between, two chemical morphogens [23]. Originally developed in a general setting by Turing, subsequent studies made this theory more accessible by assigning specific characteristics to these morphogens: one being an ‘activator’ inducing positive feedback, the other being an ‘inhibitor’ inducing negative feedback [77]. Using such a model, Economou et al. [78] showed how development of the mammalian palate occurs through a Turing-type mechanism, with FGF10 as the activator and Shh as the inhibitor interacting to pattern the system. Subsequently, Menshykau et al. [79] also showed a similar mechanism occurred during the development of the embryonic lung. More recently, Menshykau et al. [80] demonstrated a similar potential role for Turing patterning in kidney morphogenesis. In contrast with Woolley et al. [73], this model requires the inclusion of the Shh receptor Ptc to successfully reproduce observed behaviour, in this case wild-type and mutant branching phenotypes.
Mathematical modelling has also been used to explore how a spatially and temporally evolving gradient of Shh signalling can be interpreted by the regulatory logic of the downstream transcriptional network. For example, in the context of neural tube development, Balaskas et al. [40] used an ODE model to interrogate and explain in vivo measurements and manipulations of the Shh network. Their model describes the temporal dynamics in expression of three neural tube genes downstream of Shh signalling (Pax6, Olig2, Nkx2.2), due to synthesis, degradation and cross-repressive interactions between these genes, and in response to Shh signalling. These three genes are used as a readout of the positional information the cell is receiving from Shh-induced Gli activity. Conducting a sensitivity analysis of the model, the authors found that with comparable degradation rates and repression levels, the behaviour of the system was robust. Low levels of Shh signalling coincided with increased Pax6 expression, moderate levels of Shh with increased Olig2 expression, and highest levels of Shh with high Nkx2.2 levels. The authors captured the presence of a Shh spatial gradient implicitly by analysing the model's response to different temporally varying Shh inputs. This work illustrates how mathematical modelling can help us to understand how morphogen interpretation emerges from the dynamical behaviour of complex transcriptional networks, rather than an intrinsic difference in individual gene responses to the morphogen.
While the above models neglect cell behaviours ‘downstream’ of Shh signalling, it is instructive to note the experimental demonstration by Xiong et al. [81] that active cell sorting of neural progenitors corrects any inconsistencies in Shh patterning to form sharp domains. This work indicates an uncoupling of cells' specification and their location and helps to explain how morphogen gradients can be scaled and operate over long distances. The mechanism described here confers robustness on the developing system, building upon the Shh signalling network that (e.g. through the modelling work by Lai et al. [66] described above) is known to be resilient to perturbation. We further discuss the inclusion of ‘downstream’ cell behaviours in mathematical models below.
Mathematical modelling has also been applied to help understand the role(s) of Shh as a mitogen. For example, Leffler et al. [82] developed a model to understand the action of Shh in the developing cerebellum [22,52,54]. This model consists of a set of coupled ODEs that describe how the numbers of proliferating granule cell precursors and differentiated granule cells change over time owing to symmetric division and differentiation of the former and exit of the latter from the external granule layer of the cerebellum. Their model showed interesting temporal features, such as a 2 day delay between highest number of proliferating granule progenitor cells in the outer and inner layers of the granule cell layer. This result predicts a regulatory mechanism in vivo—currently unknown—which is effecting this delay. Additionally Leffler et al. [82] ran simulations to examine growth dynamics in diseases, such as paediatric brain tumours, where too many granule cell precursors are generated. Thus, a promising avenue for future developmental biology and mathematical modelling studies is to examine and test our knowledge and assumptions about the relationship between patterning and growth in developmental disease.
7. Coupling growth and patterning in models of Sonic Hedgehog
A salient question, which is still not satisfactorily answered, is how patterning and growth couple to form coherent developmental structures. Considering that Shh functions as a morphogen and as a mitogen, it is thus a good candidate for answering this question mathematically. The models described in §6 focused on patterning and thus, for computational simplicity, tissue growth is an imposed rather than an emergent phenomenon in these models [73,80,83]: in each study, the authors prescribe tissue growth rates based on empirical measurements, instead of deriving equations for growth due to regulated cell proliferation, differentiation and death. An early model by Dillon & Othmer [84] including an explicit description of growth (i.e. so that growth is coupled to patterning, rather than being pre-determined) described the development of the vertebrate limb bud. Here, the growing tissue was described mathematically by a viscous fluid, whose volume increased over time owing to cell division. The rate of cell division was then assumed to be regulated by interactions between Shh and FGF signalling. The ability of the model to reproduce limb bud development was assessed through qualitative comparison of fluid particle trajectories to in vivo fate maps. A key insight of this work is that the explicit coupling of growth to patterning results in the predicted dynamics of a cell's exposure to the morphogens being much more heavily dependent on the cell's initial position in the early limb bud than would be predicted by a model with imposed growth.
While providing useful qualitative insights, the model by Dillon & Othmer [84] was proposed when there were limited quantitative data on growth dynamics. More recent work by Guerrero et al. [75] illustrates how such models can be placed on a quantitative footing. The authors develop and analyse a mechanical model of the developing neural tube, where every cell is represented by a polygon connected by vertices [85]. The neural tube shows anisotropic tissue growth: the DV axis grows significantly more than the anteroposterior axis (figure 3c). Through systematic comparison of their model using different parameter sets and mechanistic assumptions with in vivo measurements of tissue growth and cellular clone sizes and shapes, the authors found that such anisotropic growth can be attributed to a difference in differentiation rate between different progenitor domains specified by Shh signalling. This iterative process of building upon previous models to understand the modularity of development provides a blueprint for future growth-pattern-coupled models.
Further work has considered the role of growth in more complex three-dimensioal space in mathematical models. For example, Boehm et al. [74] used a computational model to examine whether differential cell proliferation rates were responsible for the shape changes seen in limb bud morphogenesis (figure 3b). As embryos change shape, the activity of morphogenetic signals can change, depending on the relative timescales of patterning and morphogenesis. In this first three-dimensional growth model of limb bud morphogenesis, the authors found, through parameter fitting, that purely proliferation was a possible explanation for limb bud outgrowth, but unlikely given the observed data and the acceptable parameter space in which the model could explain the data. This elegant study shows that there is a much greater complexity to morphogen-/mitogen-controlled development than a simple proliferation gradient. While this model did not explicitly account for Shh, a future incorporation to see how Shh would affect the results of the model would be interesting. This study represents a good example of an experiment–model–experiment cycle, as, after the authors discounted proliferation, they investigated whether oriented cell activities such as the axis of cell division were responsible for the outgrowth that is observed in vivo. Future work in this area could also include the effects of the ectoderm in shaping the developing limb bud.
Finally, Hiscock & Megason [86] discussed how in a Turing-type patterning system of activator and inhibitor there are three areas of control that determine the robust formation of patterns: gradients of activator/inhibitor (morphogen gradients), gradients of parameters (rates of differentiation) and tissue anisotropies (rates of growth). This work highlights the need to understand development at all these points of control and to try to integrate these three aspects into models attempting to further our understanding of morphogenesis.
8. Outlook
Shh has greatly informed our understanding of developmental processes, but also is a great case study for the construction of useful mathematical models of development. In particular, the roles of Shh as a morphogen and mitogen allow for mathematical models of both patterning and growth. As discussed above, these models have largely focused on either patterning or growth. Yet, in cases where Shh can act as a morphogen and mitogen, we require integrative models of patterning and growth.
One such example is the hypothalamus. The hypothalamus is of enormous importance, as it centrally regulates all core homeostatic mechanisms. These include sleep cycles, circadian rhythms and reproduction (reviewed in [87]). Since the year of its discovery, it has been known that Shh is involved in the development of the prospective hypothalamus [1]. Despite this, only recently has there been a clearer understanding of how Shh governs the development and growth of the hypothalamus. It has now been shown that, after acting as a morphogen to pattern the DV axis, Shh-expressing ventral hypothalamic progenitors produce progenitors that populate much of the basal hypothalamus through anisotropic growth [49] (reviewed in [88]). The key insight from this work is that hypothalamic progenitor cells concurrently specify as they grow and migrate anisotropically. This complexity is difficult to probe experimentally, owing to the narrow time windows and the complex regulatory networks involved. This remains a salient area of further research and a promising problem for modern techniques. A combined model of patterning and growth in the hypothalamus would need to account for the complex spatio-temporal dynamics of Shh and other key morphogens (Fgf10, BMP) in this tissue, as well as the dynamic and transient changes in tissue shape due to differential rates of proliferation/differentiation across the tissue. A key obstacle to calibrating such a model is the detailed experimental quantification of expression domains and characterization of developmental stages.
With regard to the future of mathematical models of Shh activity, there are promising avenues. For example, no model of Shh has included cell death in morphogenesis, let alone Shh-mediated cell death. This is relevant, as cell death has been suggested to play a key role in Shh-mediated developmental systems, such as the developing wing bud [89]. Additionally, new methods of obtaining and analysing biological data will make mathematical models more quantitative and provide opportunities to elucidate more subtle mechanisms and/or effects. Single cell RNA sequencing and super-resolution microscopy should provide the necessary basis on which to perform more detailed mathematical analyses and model calibration. Finally, with the advances made in modelling software and techniques, the field is now beginning to appreciate the importance of tissue geometry and shape in mathematical models of developing embryonic structures [90]. Further advances that focus on the role of cell/tissue shape in developing systems are likely to yield more exciting results.
We have reviewed the functions of Shh as a morphogen, a mitogen and a regulator of progenitor cell differentiation, focusing on the use of mathematical modelling to gain mechanistic insights. We conclude by highlighting the salient challenge in developmental biology: that of understanding how signalling ligands ultimately affect appropriate cellular behaviours and morphogenesis. The well-characterized activities of Shh, tools that enable exquisitely precise manipulation of its activity, and recent technological advances in light microscopy, live imaging, and tools to interpret, analyse, and model data, mean that Shh is an ideal candidate to investigate and overcome this challenge.
Data accessibility
This article has no additional data.
Authors' contributions
I.G., M.P. and A.G.F. contributed to the development of the ideas presented in the paper and wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Wellcome Trust (grant no. 212247/Z/18/Z to M.P.); and the Engineering and Physical Sciences Research Council (PhD studentship to I.G.).
References
- 1.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. 1993. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–1430. ( 10.1016/0092-8674(93)90627-3) [DOI] [PubMed] [Google Scholar]
- 2.Krauss S, Concordet JP, Ingham PW. 1993. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75, 1431–1444. ( 10.1016/0092-8674(93)90628-4) [DOI] [PubMed] [Google Scholar]
- 3.Riddle RD, Johnson RL, Laufer E, Tabin C. 1993. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75, 1401–1416. ( 10.1016/0092-8674(93)90626-2) [DOI] [PubMed] [Google Scholar]
- 4.Roelink H, et al. 1994. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell 76, 761–775. ( 10.1016/0092-8674(94)90514-2) [DOI] [PubMed] [Google Scholar]
- 5.Marigo V, et al. 1995. Cloning, expression, and chromosomal location of SHH and IHH: two human homologues of the Drosophila segment polarity gene hedgehog. Genomics 28, 44–51. ( 10.1006/geno.1995.1104) [DOI] [PubMed] [Google Scholar]
- 6.Chang DT, Lopez A, Von Kessler DP, Chiang C, Simandl BK, Zhao R, Seldin MF, Fallon JF, Beachy PA. 1994. Products, genetic linkage and limb patterning activity of a murine hedgehog gene. Development 120, 3339–3353. [DOI] [PubMed] [Google Scholar]
- 7.Fan CM, Tessier-Lavigne M. 1994. Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell 79, 1175–1186. ( 10.1016/0092-8674(94)90009-4) [DOI] [PubMed] [Google Scholar]
- 8.Litingtung Y, Lei L, Westphal H, Chiang C. 1998. Sonic hedgehog is essential to foregut development. Nat. Genet. 20, 58–61. ( 10.1038/1717) [DOI] [PubMed] [Google Scholar]
- 9.Pepicelli CV, Lewis PM, McMahon AP. 1998. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol. 8, 1083–1086. ( 10.1016/S0960-9822(98)70446-4) [DOI] [PubMed] [Google Scholar]
- 10.Ericson J, Muhr J, Placzek M, Lints T, Jessel TM, Edlund T. 1995. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell 81, 747–756. ( 10.1016/0092-8674(95)90536-7) [DOI] [PubMed] [Google Scholar]
- 11.Hynes M, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A. 1995. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron 15, 35–44. ( 10.1016/0896-6273(95)90062-4) [DOI] [PubMed] [Google Scholar]
- 12.Dahmane N, Ruiz I Altaba A. 1999. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 126, 3089–3100. [DOI] [PubMed] [Google Scholar]
- 13.Haworth KE, Wilson JM, Grevellec A, Cobourne MT, Healy C, Helms JA, Sharpe PT, Tucker AS. 2007. Sonic hedgehog in the pharyngeal endoderm controls arch pattern via regulation of Fgf8 in head ectoderm. Dev. Biol. 303, 244–258. ( 10.1016/j.ydbio.2006.11.009) [DOI] [PubMed] [Google Scholar]
- 14.Nagase T, Nagase M, Osumi N, Fukuda S, Nakamura S, Ohsaki K, Harii K, Asato H, Yoshimura K. 2005. Craniofacial anomalies of the cultured mouse embryo induced by inhibition of Sonic hedgehog signaling: an animal model of holoprosencephaly. J. Craniofac. Surg. 16, 80–88. ( 10.1097/00001665-200501000-00016) [DOI] [PubMed] [Google Scholar]
- 15.Dubourg C, Lazaro L, Pasquier L, Bendavid C, Blayau M, Le Duff F, Durou M, Odent S, David V. 2004. Molecular screening of SHH, ZIC2, SIX3, and TGIF genes in patients with features of holoprosencephaly spectrum: mutation review and genotype-phenotype correlations. Hum. Mutat. 24, 43–51. ( 10.1002/humu.20056) [DOI] [PubMed] [Google Scholar]
- 16.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. 1996. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413. ( 10.1038/383407a0) [DOI] [PubMed] [Google Scholar]
- 17.Rowitch DH, St-Jacques B, Lee SMK, Flax JD, Snyder EY, McMahon AP. 1999. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J. Neurosci. 19, 8954–8965. ( 10.1523/JNEUROSCI.19-20-08954.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, Ling LE, Karanu FN, Bhatia M. 2001. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat. Immunol. 2, 172–180. ( 10.1038/84282) [DOI] [PubMed] [Google Scholar]
- 19.Palma V, et al. 2005. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 132, 335–344. ( 10.1242/dev.01567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Álvarez-Buylla A, Ihrie RA. 2014. Sonic hedgehog signaling in the postnatal brain. Semin. Cell Dev. Biol. 33, 105–111. ( 10.1016/j.semcdb.2014.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia ADR, Han YG, Triplett JW, Farmer WT, Harwell CC, Ihrie RA. 2018. The elegance of Sonic Hedgehog: emerging novel functions for a classic morphogen. J. Neurosci. 38, 9338–9345. ( 10.1523/JNEUROSCI.1662-18.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wechsler-Reya RJ, Scott MP. 1999. Control of neuronal precursor proliferation in the cerebellum by Sonic hedgehog. Neuron 22, 103–114. ( 10.1016/S0896-6273(00)80682-0) [DOI] [PubMed] [Google Scholar]
- 23.Turing AM. 1952. The chemical basis of morphogenesis. Phil. Trans. R. Soc. Lond. B 237, 37–72. ( 10.1098/rstb.1952.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crick F. 1970. Diffusion in embryogenesis. Nature 225, 420–422. ( 10.1038/225420a0) [DOI] [PubMed] [Google Scholar]
- 25.Wolpert L. 1969. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 25, 1–47. ( 10.1016/S0022-5193(69)80016-0) [DOI] [PubMed] [Google Scholar]
- 26.Yamada T, Placzek M, Tanaka H, Dodd J, Jessell TM. 1991. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell 64, 635–647. ( 10.1016/0092-8674(91)90247-V) [DOI] [PubMed] [Google Scholar]
- 27.Tickle C, Summerbell D, Wolpert L. 1975. Positional signalling and specification of digits in chick limb morphogenesis. Nature 254, 199–202. ( 10.1038/254199a0) [DOI] [PubMed] [Google Scholar]
- 28.Carballo GB, Honorato JR, de Lopes GPF, Spohr TdS. 2018. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 16, 11 ( 10.1186/s12964-018-0220-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adolphe C, Junker JP, Lyubimova A, van Oudenaarden A, Wainwright B. 2017. Patched receptors sense, interpret, and establish an epidermal Hedgehog signaling gradient. J. Investig. Dermatol. 137, 179–186. ( 10.1016/j.jid.2016.06.632) [DOI] [PubMed] [Google Scholar]
- 30.Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. 1996. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell 87, 661–673. ( 10.1016/S0092-8674(00)81386-0) [DOI] [PubMed] [Google Scholar]
- 31.Briscoe J, Thérond PP. 2013. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 14, 418–431. ( 10.1038/nrm3598) [DOI] [PubMed] [Google Scholar]
- 32.Marigo V, Johnson RL, Vortkamp A, Tabin CJ. 1996. Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Dev. Biol. 180, 273–283. ( 10.1006/dbio.1996.0300) [DOI] [PubMed] [Google Scholar]
- 33.Tickle C, Towers M. 2017. Sonic hedgehog signaling in limb development. Front. Cell Dev. Biol. 5, 14. ( 10.3389/fcell.2017.00014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Placzek M, Briscoe J. 2018. Sonic hedgehog in vertebrate neural tube development. Int. J. Dev. Biol. 62, 221–230. ( 10.1387/ijdb.170293jb) [DOI] [PubMed] [Google Scholar]
- 35.Saha K, Schaffer DV. 2006. Signal dynamics in Sonic hedgehog tissue patterning. Development 133, 889–900. ( 10.1242/dev.02254) [DOI] [PubMed] [Google Scholar]
- 36.Kohtz JD, Baker DP, Corte G, Fishell G. 1998. Regionalization within the mammalian telencephalon is mediated by changes in responsiveness to Sonic Hedgehog. Development 125, 5079–5089. [DOI] [PubMed] [Google Scholar]
- 37.Chinnaiya K, Tickle C, Towers M. 2014. Sonic hedgehog-expressing cells in the developing limb measure time by an intrinsic cell cycle clock. Nat. Commun. 5, 4230 ( 10.1038/ncomms5230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briscoe J, Small S. 2015. Morphogen rules: design principles of gradient-mediated embryo patterning. Development 142, 3996–4009. ( 10.1242/dev.129452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Mackem S. 2011. Analysis of mutants with altered shh activity and posterior digit loss supports a biphasic model for shh function as a morphogen and mitogen. Dev. Dyn. 240, 1303–1310. ( 10.1002/dvdy.22637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balaskas N, Ribeiro A, Panovska J, Dessaud E, Sasai N, Page KM, Briscoe J, Ribes V. 2012. Gene regulatory logic for reading the Sonic Hedgehog signaling gradient in the vertebrate neural tube. Cell 148, 273–284. ( 10.1016/j.cell.2011.10.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dessaud E, McMahon AP, Briscoe J. 2008. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135, 2489–2503. ( 10.1242/dev.009324) [DOI] [PubMed] [Google Scholar]
- 42.Kenney AM, Rowitch DH. 2000. Sonic hedgehog promotes G1 cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol. Cell. Biol. 20, 9055–9067. ( 10.1128/MCB.20.23.9055-9067.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Towers M, Mahood R, Yin Y, Tickle C. 2008. Integration of growth and specification in chick wing digit-patterning. Nature 452, 882–886. ( 10.1038/nature06718) [DOI] [PubMed] [Google Scholar]
- 44.Pickering J, Chinnaiya K, Towers M. 2019. An autoregulatory cell cycle timer integrates growth and specification in chick wing digit development. eLife. 8, e47626 ( 10.7554/eLife.47625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cayuso J, Ulloa F, Cox B, Briscoe J, Marti E. 2006. The Sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development 133, 517–528. ( 10.1242/dev.02228) [DOI] [PubMed] [Google Scholar]
- 46.Ulloa F, Briscoe J. 2007. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle 6, 2640–2649. ( 10.4161/cc.6.21.4822) [DOI] [PubMed] [Google Scholar]
- 47.Donovan SL, Dyer MA. 2005. Regulation of proliferation during central nervous system development. Sem. Cell Dev. Biol. 16, 407–421. ( 10.1016/j.semcdb.2005.02.012) [DOI] [PubMed] [Google Scholar]
- 48.Wilson SL, Wilson JP, Wang C, Wang B, Mcconnell SK. 2012. Primary cilia and Gli3 activity regulate cerebral cortical size. Dev. Neurobiol. 72, 1196–1212. ( 10.1002/dneu.20985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu T, Towers M, Placzek MA. 2017. Fgf10+ progenitors give rise to the chick hypothalamus by rostral and caudal growth and differentiation. Development 144, 3278–3288. ( 10.1242/dev.153379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Q, Wonders CP, Anderson SA. 2005. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development 132, 4987–4998. ( 10.1242/dev.02090) [DOI] [PubMed] [Google Scholar]
- 51.Schuller U, Zhao Q, Godinho SA, Heine VM, Medema RH, Pellman D, Rowitch DH. 2007. Forkhead transcription factor FoxM1 regulates mitotic entry and prevents spindle defects in cerebellar granule neuron precursors. Mol. Cell. Biol. 27, 8259–8270. ( 10.1128/MCB.00707-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merk DJ, et al. 2019. The Eya1 phosphatase mediates Shh-driven symmetric cell division of cerebellar granule cell precursors. bioRxiv, 668277 ( 10.1101/668277) [DOI]
- 53.Fuccillo M, Joyner AL, Fishell G. 2006. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat. Rev. Neurosci. 7, 772–783. ( 10.1038/nrn1990) [DOI] [PubMed] [Google Scholar]
- 54.Yang H, Zhu Q, Cheng J, Wu Y, Fan M, Zhang J, Wu H. 2019. Opposite regulation of Wnt/β-catenin and Shh signaling pathways by Rack1 controls mammalian cerebellar development. Proc. Natl. Acad. Sci. USA 116, 4661–4670. ( 10.1073/pnas.1813244116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solanki A, Yanez DC, Ross S, Lau CI, Papaioannou E, Li J, Saldaña JI, Crompton T. 2018. Gli3 in fetal thymic epithelial cells promotes thymocyte positive selection and differentiation by repression of Shh. Development 145, dev146910 ( 10.1242/dev.146910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riaz SK, Ke Y, Wang F, Kayani MA, Malik MFA. 2019. Influence of SHH/GLI1 axis on EMT mediated migration and invasion of breast cancer cells. Scient. Rep. 9, 6620 ( 10.1038/s41598-019-43093-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin J, Saunders TE. 2019. Shh induces symmetry breaking in the presomitic mesoderm by inducing tissue shear and orientated cell rearrangements. bioRxiv, 589549 ( 10.1101/589549) [DOI]
- 58.Umulis DM, Othmer HG. 2015. The role of mathematical models in understanding pattern formation in developmental biology. Bull. Math. Biol. 77, 817–845. ( 10.1007/s11538-014-0019-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Möbius W, Laan L. 2015. Physical and mathematical modeling in experimental papers. Cell 163, 1577–1583. ( 10.1016/j.cell.2015.12.006) [DOI] [PubMed] [Google Scholar]
- 60.Fisher R. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press. [Google Scholar]
- 61.Hodgkin AL, Huxley AF. 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544. ( 10.1113/jphysiol.1952.sp004764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pargett M, Umulis DM. 2013. Quantitative model analysis with diverse biological data: applications in developmental pattern formation. Methods 62, 56–67. ( 10.1016/j.ymeth.2013.03.024) [DOI] [PubMed] [Google Scholar]
- 63.Tomlin CJ, Axelrod JD. 2007. Biology by numbers: mathematical modelling in developmental biology. Nat. Rev. Genet. 8, 331–340. ( 10.1038/nrg2098) [DOI] [PubMed] [Google Scholar]
- 64.Von Dassow G, Meir E, Munro EM, Odell GM. 2000. The segment polarity network is a robust developmental module. Nature 406, 188–192. ( 10.1038/35018085) [DOI] [PubMed] [Google Scholar]
- 65.Calvo J. 2018. On Sonic Hedgehog morphogenic action and finite propagation speed models. SeMA J. 75, 173–195. ( 10.1007/s40324-017-0128-y) [DOI] [Google Scholar]
- 66.Lai K, Robertson MJ, Schaffer DV. 2004. The Sonic Hedgehog signaling system as a bistable genetic switch. Biophys. J. 86, 2748–2757. ( 10.1016/S0006-3495(04)74328-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kicheva A, Bollenbach T, Ribeiro A, Pérez Valle H, Lovell-Badge R, Episkopou V, Briscoe J. 2014. Coordination of progenitor specification and growth in mouse and chick spinal cord. Science 345, 1254927 ( 10.1126/science.1254927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen M, Kicheva A, Ribeiro A, Blassberg R, Page KM, Barnes CP, Briscoe J. 2015. Ptch1 and Gli regulate Shh signalling dynamics via multiple mechanisms. Nat. Commun. 6, 6709 ( 10.1038/ncomms7709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tyson JJ, Chen KC, Novak B. 2003. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr. Opin. Cell Biol. 15, 221–231. ( 10.1016/S0955-0674(03)00017-6) [DOI] [PubMed] [Google Scholar]
- 70.Cho KH, Joo JI, Shin D, Kim D, Park SM. 2016. The reverse control of irreversible biological processes. WIREs Syst. Biol. Med. 8, 366–377. ( 10.1002/wsbm.1346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen M, Page KM, Perez-Carrasco R, Barnes CP, Briscoe J. 2014. A theoretical framework for the regulation of Shh morphogen-controlled gene expression. Development 141, 3868–3878. ( 10.1242/dev.112573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolpert L, Hornbruch A. 1981. Positional signalling along the anteroposterior axis of the chick wing. The effect of multiple polarizing region grafts. J. Embryol. Exp. Morphol. 63, 145–159. [PubMed] [Google Scholar]
- 73.Woolley TE, Baker RE, Tickle C, Maini PK, Towers M. 2014. Mathematical modelling of digit specification by a sonic hedgehog gradient. Dev. Dyn. 243, 290–298. ( 10.1002/dvdy.24068) [DOI] [PubMed] [Google Scholar]
- 74.Boehm B, Westerberg H, Lesnicar-Pucko G, Raja S, Rautschka M, Cotterell J, Swoger J, Sharpe J. 2010. The role of spatially controlled cell proliferation in limb bud morphogenesis. PLoS Biol. 8, e1000420 ( 10.1371/journal.pbio.1000420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerrero P, Perez-Carrasco R, Zagorski M, Page D, Kicheva A, Briscoe J, Page KM. 2019. Neuronal differentiation influences progenitor arrangement in the vertebrate neuroepithelium. Development 146, dev176297 ( 10.1242/dev.176297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Badugu A, Kraemer C, Germann P, Menshykau D, Iber D. 2012. Digit patterning during limb development as a result of the BMP-receptor interaction. Scient. Rep. 2, 991 ( 10.1038/srep00991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gierer A, Meinhardt H. 1972. A theory of biological pattern formation. Kybernetik. 12, 30–39. ( 10.1007/BF00289234) [DOI] [PubMed] [Google Scholar]
- 78.Economou AD, Ohazama A, Porntaveetus T, Sharpe PT, Kondo S, Basson MA, Gritli-Linde A, Cobourne MT, Green JB. 2012. Periodic stripe formation by a Turing mechanism operating at growth zones in the mammalian palate. Nat. Gen. 44, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menshykau D, Blanc P, Unal E, Sapin V, Iber D. 2014. An interplay of geometry and signaling enables robust lung branching morphogenesis. Development 141, 4526–4536. ( 10.1242/dev.116202) [DOI] [PubMed] [Google Scholar]
- 80.Menshykau D, Michos O, Lang C, Conrad L, McMahon AP, Iber D. 2019. Image-based modeling of kidney branching morphogenesis reveals GDNF-RET based Turing-type mechanism and pattern-modulating WNT11 feedback. Nat. Commun. 10, 239 ( 10.1038/s41467-018-08212-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiong F, et al. 2013. Specified neural progenitors sort to form sharp domains after noisy Shh signaling. Cell 153, 550–561. ( 10.1016/j.cell.2013.03.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leffler SR, Legué E, Aristizábal O, Joyner AL, Peskin CS, Turnbull DH. 2016. A mathematical model of granule cell generation during mouse cerebellum development. Bull. Math. Biol. 78, 859–878. ( 10.1007/s11538-016-0163-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menshykau D, Kraemer C, Iber D. 2012. Branch mode selection during early lung development. PLoS Comput. Biol. 8, e1002377 ( 10.1371/journal.pcbi.1002377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dillon R, Othmer HG. 1999. A mathematical model for outgrowth and spatial patterning of the vertebrate limb bud. J. Theor. Biol. 197, 295–330. ( 10.1006/jtbi.1998.0876) [DOI] [PubMed] [Google Scholar]
- 85.Fletcher AG, Osterfield M, Baker RE, Shvartsman SY. 2014. Vertex models of epithelial morphogenesis. Biophys. J. 106, 2291–2304. ( 10.1016/j.bpj.2013.11.4498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hiscock TW, Megason SG. 2015. Orientation of Turing-like patterns by morphogen gradients and tissue anisotropies. Cell Syst. 1, 408–416. ( 10.1016/j.cels.2015.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le Tissier P, Campos P, Lafont C, Romanò N, Hodson DJ, Mollard P. 2017. An updated view of hypothalamic–vascular–pituitary unit function and plasticity. Nat. Rev. Endocrinol. 13, 257–267. ( 10.1038/nrendo.2016.193) [DOI] [PubMed] [Google Scholar]
- 88.Fu T, Pearson C, Towers M, Placzek M. 2019. Development of the basal hypothalamus through anisotropic growth. J. Neuroendocrinol. 31, e12727 ( 10.1111/jne.12727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanz-Ezquerro JJ, Tickle C. 2000. Autoregulation of Shh expression and Shh induction of cell death suggest a mechanism for modulating polarising activity during chick limb development. Development 127, 4811–4823. [DOI] [PubMed] [Google Scholar]
- 90.Umulis DM, Othmer HG. 2012. The importance of geometry in mathematical models of developing systems. Curr. Opin. Genet. Dev. 22, 547–552. ( 10.1016/j.gde.2012.09.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.