Abstract
Cell intercalation is a key topological transformation driving tissue morphogenesis, homeostasis and diseases such as cancer cell invasion. In recent years, much work has been undertaken to better elucidate the fundamental mechanisms controlling intercalation. Cells often use protrusions to propel themselves in between cell neighbours, resulting in topology changes. Nevertheless, in simple epithelial tissues, formed by a single layer of densely packed prism-shaped cells, topology change takes place in an astonishing fashion: cells exchange neighbours medio-laterally by conserving their apical–basal architecture and by maintaining an intact epithelial layer. Medio-lateral cell intercalation in simple epithelia is thus an exemplary case of both robustness and plasticity. Interestingly, in simple epithelia, cells use a combinatory set of mechanisms to ensure a topological transformation at the apical and basal sides.
This article is part of the discussion meeting issue ‘Contemporary morphogenesis’.
Keywords: junction remodelling, actomyosin tension, E-cadherin adhesion, actomyosin flow
1. Introduction
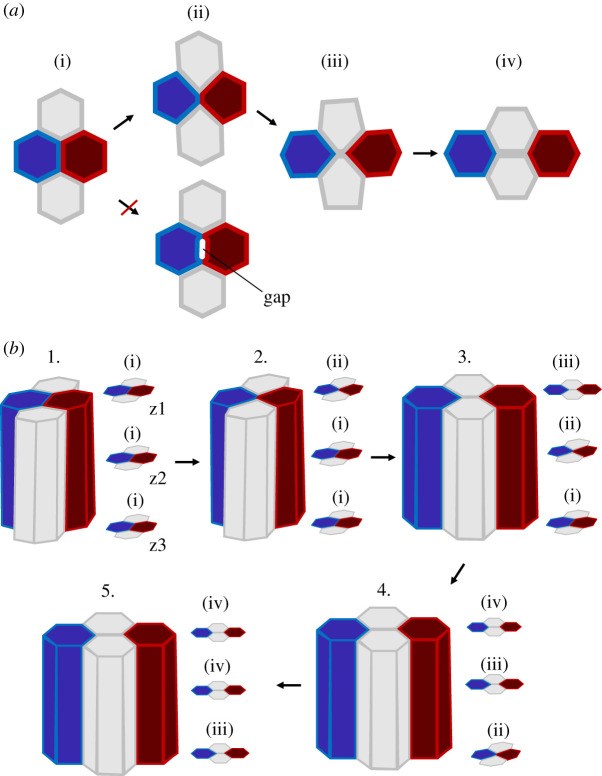
A fascinating feature of cells, in epithelia sheets, is their capacity to change topology: topology change occurs when a cell loses or gains new contact with neighbouring cells. One important topological change is cell intercalation (referred to as type 1 transition, T1, in foam physics). Intercalation results from the gain of new contact between two or more cells and the eventual complete loss of contact between adjacent cells, leading to cell neighbour exchange (i.e. cell rearrangement). Gain and complete loss of contact are inextricable in an imaginary 2D space (figure 1a, from (ii) to (iii)) but not in the real 3D space, where new contact gain between two cells can occur without complete loss of contact with neighbouring cells (figure 1b, from 1. to 3.).
Figure 1.
Cell intercalation in 2D and 3D. (a) Two-dimensional representation of cell intercalation. During intercalation a junction shrinks and a new junction forms and extends. During this process no gaps are formed. (b) Three-dimensional representation of cell intercalation. Cells start to intercalate from one end (e.g. from the apical side) forming scutoid shapes. Cell neighbour exchange then propagates from one end to the other, resolving cell intercalation. (Online version in colour.)
Cell intercalation has been shown to play a key role in both tissue homeostasis and morphogenesis. For instance, cell rearrangement ensures tissue homeostasis in more mature epithelia that do not undergo extensive remodelling, forming robust barriers. Mature epithelia can be subject to stresses caused by intrinsic (e.g. cell division and cell delamination) and extrinsic (boundary condition variations) forces. Cell intercalation plays the role of stress releaser by providing the fluidity required to ensure tissue integrity [1]. Under these conditions, cell neighbour exchange is not polarized, can be reversible, has no effect on global or local shape and is driven by stochastic local fluctuations of the cell cytoskeleton, and it is predicted to eventually decrease local topological disorder [1,2].
Cell intercalation has been shown to also play an important role in competitive environments. For instance, in cell crowding conditions, cell neighbour exchange can facilitate cell extrusion [3], while during cell invasion, it promotes winner–loser cell contact increase, which can lead to loser cell apoptosis [4].
Cell intercalation is a key process during embryonic epithelial morphogenesis and more specifically during embryo gastrulation. To drive tissue shape changes, intercalation has to be (i) polarized to orient tissue deformation and (ii) irreversible to impose morphogenetic progression resulting in a net displacement of cellular masses driving local, and eventually global, tissue shape changes. While intercalation polarity is a key morphogenetic driver, recent studies have shown that non-polarized cell neighbour exchange can also promote epithelial morphogenesis. During chick gastrulation, stochastic cell rearrangements, mediated by cell division in tandem with primitive streak contraction, favour polarized rotatory cell flows (i.e. ‘Polonaise movements’) [5]. This supports the idea that stochastic cell intercalation can function as a tissue fluidizing process not only during tissue homeostasis but also during morphogenesis, eventually preventing cell jamming [6,7].
Cells can rearrange in different ways. In simple epithelia (tissue constituted by a single cellular layer), cells can intercalate medio-laterally: cell rearrangement occurs within the plane of the epithelium. Planar polarized medio-lateral intercalation promotes tissue convergence and extension. One exemplary case is the convergence–extension of the Drosophila embryo germband during gastrulation [8]. If intercalation polarity is centrosymmetric, medio-lateral cell rearrangement can also facilitate tissue folding: a classic example is archenteron elongation in the gastrulating sea urchin embryo [9] or, as presented in a much more recent study, salivary gland tube formation in the Drosophila embryo [10]. Polarized cell intercalation associated with planar cell-shape chirality can instead facilitate tissue rotation, for instance during genitalia development in the Drosophila pupa [11]. In multi-stratified epithelia, cells can intercalate radially: cells belonging to one layer rearrange with cells of a second layer driving extension in the plane of and thinning along the axis perpendicular to the multi-stratified epithelium [12–14]. A review by Walck–Schannon & Hardin comprehensively presents the different modes of cell rearrangement [15].
The remainder of this review will focus on the process of cell intercalation in simple epithelia constituted by apico-basal polarized cells, highlighting our present understanding of how medio-lateral cell neighbour exchange is initiated and how it eventually resolves.
2. Initiating intercalation: establishing cell–cell contacts
Cell intercalation begins with the remodelling of a cell–cell contact, which eventually leads to contact loss (figure 1a). In order to remodel a contact, it is necessary that contact between cells is established so that cells can initially adhere one another. In the absence of cell–cell adhesion (e.g. if cells are packed together but do not form contacts) intercalation cannot be initiated. Cellular adhesion can be mediated, for instance, by adherens junctions, which are known to play a key role in cell–cell coupling and force transmission [16,17]. Adherens junctions, in simple epithelia, are usually located at the cell subapical region and are formed by cadherins (e.g. E-cadherins), which are transmembrane homophilic proteins. While cadherins interact extracellularly with each other, they interact with the actomyosin cytoskeleton intracellularly via the alpha and beta subunits [18–22]. The establishment of cell–cell adherens junctions is thus a necessary condition to initiate junction remodelling.
3. Initiating intercalation: losing cell contact
Once cells have adhered to one another, how can adherens junctions be remodelled to drive contact loss? Interestingly, a contact junction between four intercalating cells usually reduces in size without forming gaps in the tissue (figure 1a(ii) bottom). Junction size reduction is often referred to as junction ‘shrinking’ or ‘shortening’; while these expressions are on one hand simplistic (i.e. do not refer to a precise mechanism driving junction remodelling and can thus be misleading), on the other they are simple, thus appealing to provide an intuitive description of the first phase of cell intercalation (figure 1a, from (i) to (ii)). Apical junction shrinkage initiating cell intercalation has been reported in multiple embryo model systems, such as, as for instance, in chick [23,24], in Xenopus laevis [25], in mice [26] and in Drosophila [27]. In this review, I will mainly refer to the Drosophila model system, where the process of junction shrinking has been best characterized and studied.
(a). Cortical tension anisotropy
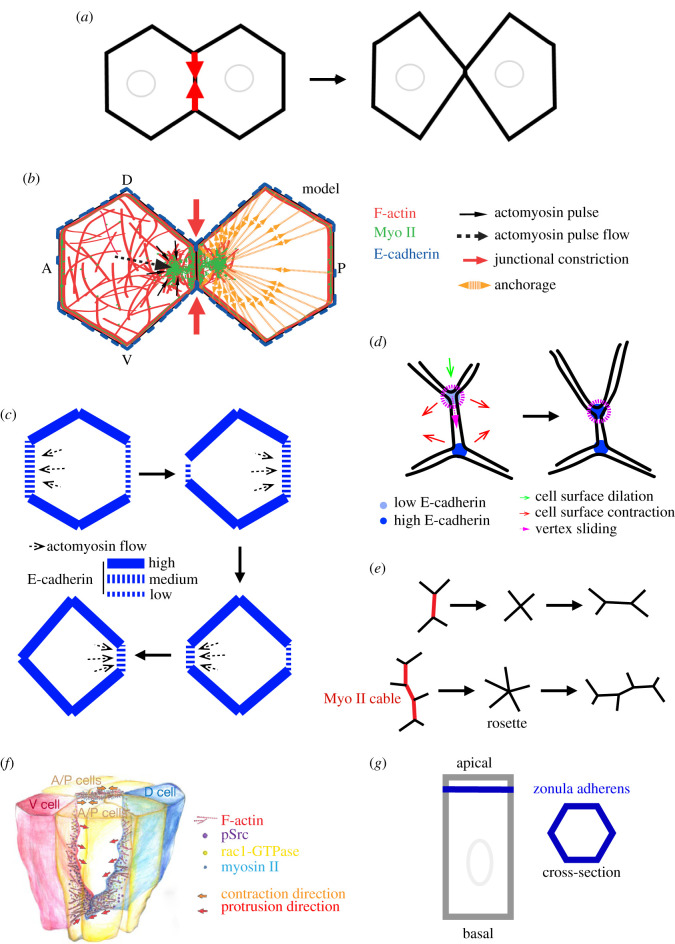
Studies focused in understanding cellular packing and tissue remodelling in the Drosophila model system were the first attempts to shine new light on the process of junction shrinkage [28–30]. In these studies, the authors applied the physical concept of surface tension (line tension) to cell–cell junctions. Cell surface tension is the amount of energy required to increase the cell surface of a unit area. Increasing actomyosin contractility increases surface tension, while increasing E-cadherin-based adhesion lowers surface tension between two cells, eventually driving cell–cell contact reduction and increase, respectively [16]. During germband elongation in the early developing Drosophila embryo, proteins controlling surface tension are distributed in a planar cell polarized fashion: while junctions parallel to the dorsal–ventral (DV) axis of the embryo have higher levels of myosin II and lower levels of E-cadherin (favouring higher surface tension), junctions parallel to the anterior–posterior (AP) axis present lower levels of myosin II and higher levels of E-cadherin (favouring lower surface tension) [31–34]. Tension anisotropy between AP and DV junctions could initiate cell intercalation by driving shrinkage of junctions under higher tension (i.e. parallel to the DV axis). Higher tension along junctions parallel to the DV axis was demonstrated using subcellular laser dissection to selectively disrupt the actomyosin cortical network at different junctions; tension was probed by measuring vertices' (end points of a junction where three cells meet) maximum recoil speed [30]. Cortical tension anisotropy driven by the polarized distribution of actomyosin contractility is the first model to provide new understanding of how cell junctions can be remodelled to initiate cell intercalation (figure 2a).
Figure 2.
Mechanisms driving junction shrinkage. (a) Cortical tension model for junction shrinkage. (b) Medial actomyosin contraction model for junction shrinkage. Adapted with permission from Rauzi et al. [35]. (c) Differential anchorage model for actomyosin pulse flow. (d) Vertex sliding model for junction shrinkage. (e) Representation of a fundamental and a rosette intercalation event. (f) Basal–lateral protrusion model for cell intercalation initiation. Adapted with permission from Sun et al. [36]. (g) Representation of a columnar epithelial cell.
(b). Periodic actomyosin contractions
Periodic actomyosin contractions were reported for the first time in the seminal study by Munro et al. in 2004 in the C. elegans single-cell embryo [37]. These contractions result from the local (few micrometres in diameter) coalescence of the actomyosin network within less than a minute. Since actomyosin periodic contractions are the result of a sudden and local density increase and eventual decrease of filamentous actin (F-actin) and myosin II (Myo II), these contractions are also referred to as ‘pulses’. When F-actin and Myo II are monitored with a fluorescent probe (e.g. eGFP), pulses result from the sudden increase and eventual decrease of signal intensity. Since the seminal work by Munro and colleagues, actomyosin pulses have been reported in numerous studies focused on understanding the underlying mechanisms responsible for cell shape change and tissue morphogenesis during embryo development [35,38–41]. While a large body of in vivo, in vitro and in silico evidence has recently elucidated the nature and the origin of the actomyosin pulses [42–47], here I will focus more specifically on the role of pulses in cell intercalation.
During cell intercalation in the prospective ectoderm of the early–Drosophila embryo, actomyosin pulses form in the apical–medial region of cells, flow towards the junctional cortex and eventually fuse with the junctional actin network. Actomyosin material is thus transferred from the medial to the junctional cell region. By monitoring junction shrinkage during cell intercalation at high temporal resolution, Rauzi et al. [35] showed that junctions shrink in a stepwise fashion: alternating shrinking and stalling phases occur several times before complete contact junction loss. Shrinking phases correlate with pulse formation and flow towards the junctional cortex. By specifically disrupting actomyosin pulses using highly resolved laser dissection, junction shrinkage is hampered. Finally, while medial actomyosin pulsation and flow drive junction shrinkage, junctional actomyosin (partially resulting from the flow and fusion of the medial actomyosin pulses with the junctional actin cortex) stabilizes junction length after shrinkage (figure 2b).
Pulse flow from the medial to the junctional region of the cell is thus a crucial process to drive junction shrinkage. How is medial actomyosin flow oriented? Actin and actomyosin networks can exert traction forces orienting the flow towards regions with higher anchorage [48,49]. Nevertheless, actomyosin pulses flow towards shrinking junctions that have less E-cadherin (i.e. fewer anchoring sites) [32,34]; this is a striking paradox. Interestingly, the levels of E-cadherin at shrinking junctions periodically fluctuate over time scales that are comparable to actomyosin pulse formation time periods [35,49]. Levayer & Lecuit in 2013 tried to solve this paradox, proposing a model in which the medial actomyosin network flows along directions with higher anchorage imbalance and towards junctions with higher levels of E-cadherin (figure 2c). This model only partially solves the paradox since (i) medial actomyosin flow precedes (i.e. cannot be the consequence of) junctional E-cadherin sudden increase [35,49] and reinforcement [50] at shrinking junctions, and also because (ii) medial actomyosin flow follows E-cadherin sudden decrease at the shrinking junctions [35]. Interestingly, recent work by Pinhero and colleagues in 2017 showed that actomyosin flows are triggered by local downregulation of E-cadherin at the cytokinetic furrow during cell division. Flow is proposed to be triggered by local actomyosin network fluidization [51]. Local F-actin fluidization could also take place in a zone close to shrinking junctions following the rapid and periodic decrease of E-cadherin, which would facilitate and eventually direct medial actomyosin flow. Additional work is necessary to further elucidate the mechanisms triggering actomyosin medial flow direction driving junction shrinkage.
(c). Vertex sliding
Vanderleest and colleagues in 2018 proposed a new model for contact junction remodelling based on vertex ‘sliding’ [52]. The authors carefully monitored vertex displacement during cell intercalation in the prospective Drosophila ectoderm. Interestingly, the change of one vertex position at one end of a shrinking junction does not often correlate with the mirrored change in position of the vertex at the other end. In addition, when the position of one vertex shared by two junctions (e.g. a shortening junction parallel to the DV axis and a junction parallel to the AP axis) changes, while one junction shortens (e.g. the junction parallel to the DV axis), the other (e.g. the junction parallel to the AP axis) concomitantly lengthens, such that the overall contact length remains constant (figure 2d). These striking observations suggest that a vertex-specific mechanism could be at play controlling vertex position during contact junction shortening. The authors refer to the change of vertex position as vertex ‘sliding’; this expression, while simplistic, is appealing in that it provides an intuitive description of the process. Vanderleest and colleagues report that vertices slide in a stepwise fashion and that their displacement correlates with fluctuations of E-cadherin at vertices. Low E-cadherin levels correlate with vertex sliding onset, while high E-cadherin levels correlate with vertex stalling. When constant high E-cadherin levels are maintained (e.g. by inhibiting endocytosis), the vertex position persistently stalls and cell intercalation is compromised [34,52]. Levels of E-cadherin thus play a key role in vertex sliding and contact junction remodelling: low E-cadherin levels are necessary to initiate junction shortening (in agreement with previous work [35]), while high E-cadherin levels stabilize contact junction length between intercalating cells. Myo II is enriched at vertices a few seconds prior to E-cadherin enrichment (in agreement with previous studies monitoring junctional E-cadherin fluctuations [35,49]). Increased E-cadherin levels at vertices could be driven by local medial actomyosin contractions focusing E-cadherin proteins tethered to F-actin networks [47,53].
The work of Vanderleest and colleagues strongly supports the idea that vertices are not just geometric entities. Vertices could constitute molecular hubs disassembling junctions at the ‘front’ (shrinking the junctions parallel to the DV axis) and reassembling junctions at the ‘rear’ (lengthening the junctions parallel to the AP axis).
Vanderleest and colleagues also reported that cell surface area fluctuates over time as a consequence of medial actomyosin contractions. By using cross-correlation analysis, the authors show that efficient vertex sliding is achieved when two of the three cells sharing the vertex contract their surface area, while the third cell simultaneously dilates its surface area (figure 2d). The mechanism responsible for polarized vertex sliding (e.g. along the DV axis) remains elusive. One hypothesis is that vertex sliding is facilitated along junctions parallel to the DV axis while it is impaired along junctions parallel to the AP axis that have low and high levels of E-cadherin, respectively [32,34]. A second hypothesis invokes an emerging supracellular mechanism. For instance, synchronized area contraction of anterior and posterior cells, together with the dilation of dorsal and ventral cells of an intercalating quartet, could be more frequent than other possible contraction/dilation combinations. A third hypothesis is that the ‘vertex sliding’ model complements the previously described ‘periodic actomyosin contraction’ and the ‘cortical tension anisotropy’ models. Further work is necessary to better understand how seemingly different models for the same process are compatible and can eventually complement each other.
(d). Rosette formation
Medio-lateral cell intercalation in simple epithelial tissues generally occurs among four cells (i.e. the fundamental intercalating unit). Nevertheless, intercalating units of more than four cells have also been reported and referred to as rosettes (figure 2e) [24,25,54,55]. Rosettes are also driven by actomyosin cortical tension [30,56] and contribute to convergence extension in a way that is equivalent to the additive effects of multiple individual fundamental intercalation units [57]. Rosettes result from the contraction of aligned and juxtaposed junctions enriched in actomyosin, which are referred to as ‘cables’ (figure 2e) [54]. A recent study by Paré and colleagues reported that the ectopic striped expression of Toll receptors can induce actomyosin cable formation in the prospective ectoderm of the Drosophila embryo [58]. It would be interesting in the future to further investigate the origin of cables in intercalating units of more than four cells.
(e). Basal–lateral protrusions
Basal–lateral protrusion is another mechanism bringing two cells in proximity to one another. Basal–lateral protrusion is reported during dorsal midline formation in Caenorhabditis elegans [59–62] and during notochord primordium formation in Ciona intestinalis [63–66]. Cell protrusion directs cell motility similarly to what is reported for non-epithelial cells [67]. A more recent study by Sun and colleagues in 2017 reports basal–lateral cell protrusion formation in tandem with apical junction remodelling [36]. By using two-photon live imaging, the authors monitored intercalating cells in the Drosophila prospective ectoderm at a more basal position of rosettes (figure 2e). Sun and colleagues reported that F-actin-enriched protrusions form in more dorsal and ventral cells of rosettes 15–20 µm from the apical surface. Protrusions are polarized along the DV axis of the embryo and point towards the centre of the rosette (figure 2f). By using one-photon activation optogenetics, the authors showed that basolateral protrusions are Rac1-dependent. The tyrosine kinase Src42A was identified as a factor upstream of Rac1 that specifically affects basal–lateral protrusion. While apical rosette formation is driven by actomyosin cortical tension [30,56], basal rosette formation is mainly driven by Rac1-dependent protrusions. Both apical and basal rosette formation contribute to the extension of the prospective ectoderm. While this study suggests that the formation of apical and basal–lateral rosettes is mutually independent, further work is necessary to confirm this. For example, one could employ strategies to specifically perturb the basal–lateral or the apical machinery while monitoring the apical and basal cell sides, respectively. One such strategy is to implement two-photon activation optogenetics, which has been shown to be an effective technique to modulate protein concentration with spatial, temporal and protein specificity [68–70]. How is basal–lateral cell protrusion polarized? Sun et al. reported that basal rosettes are compromised in eve and Toll-2, 6, 8 mutants. This suggests that the AP gene patterning could control protrusion polarity. What controls the direction of protrusion towards the centre of the rosette? Short-range signalling could control the direction of the protrusive activity similarly to what is observed during radial-cell intercalation in the X. laevis embryo, where cells extend under the control of short-range chemotaxis [71]. Further work is necessary to uncover the guiding signalling cues during basal–lateral rosette formation. This study shows that intercalation taking place in the Drosophila prospective ectoderm shares similarities with other medio-lateral intercalation processes in other model systems that are also mediated by cell protrusive activity [38,60,62,63,72]. The tyrosine kinase Src42A was already known to play a role in cell intercalation and, more specifically, in junction remodelling [73]. Cell–cell contact junctions are indeed also necessary during basal–lateral protrusion-based cell intercalation to mediate traction forces driving net cell displacement [74]. It remains to be conclusively determined if cell contact junctions are established and remodelled at the basal–lateral side of forming rosettes to mediate traction forces. Finally, Sun and colleagues proposed that basal–lateral intercalation mediated by cell protrusion could be a general intercalation mechanism taking place also in fundamental intercalating units (i.e. formed by four cells).
4. Initiating intercalation: gaining a new contact
While cell junction shortening is a process that has been studied extensively, few studies have tackled the question of how a new cell–cell contact subsequently lengthens (figure 1a, from (iii) to (iv)). While junction shortening is a necessary condition for eventual new junction lengthening, Collinet and colleagues reported that junction shortening contributes 30% of the total extension of the intercalating quartet in the prospective Drosophila ectoderm. 70% of the extension then occurs during new junction lengthening [57]. Previous work had hypothesized that new junction lengthening could result from minimizing the energy of the tissue after junction shrinkage [30]. Under this hypothesis, tissue extension is a necessary condition for new junction lengthening. Collinet and colleagues challenged this hypothesis using genetic mutations and physical barriers to constrain the tissue and compromise its extension. Surprisingly, the number of new lengthening junctions in tissue-constrained conditions is equivalent to wild-type conditions. This shows that new junction lengthening (like junction shrinking) is an active cellular process.
What is the cellular mechanism driving new junction lengthening? A study by Yu & Fernandez-Gonzalez, using laser-based induced actomyosin accumulation, showed that medial actomyosin contraction in the anterior and posterior cells of an intercalating quartet is sufficient to drive new junction lengthening [75]. Pulsatile contractions of actomyosin continue to form in intercalating cells also during new junction lengthening. Collinet and colleagues showed that pulses of medial actomyosin correlate with phases of new junction lengthening occurring in a stepwise fashion. By using laser dissection, the authors finally demonstrated that medial actomyosin pulses drive new junction lengthening [57].
Interestingly, during the phase of junction lengthening, low density of E-cadherin is reported; E-cadherin at the new lengthening junction is diluted during the lengthening phase and gradually increases during the stalling phase [57]. This supports the idea that new junctions are gradually established between the dorsal and the ventral cells of the intercalating quartet. Actomyosin is strongly enriched in shrinking junctions but not in lengthening junctions. Bardet and colleagues reported that downregulation of the tumour suppressor PTEN in the developing Drosophila wing blade causes upregulation of Myo II in the newly forming junctions. This prevents new junctions from effectively lengthening and thus compromises cell intercalation [76]. The absence of high levels of actomyosin at the newly forming junctions is thus a necessary condition for new junction lengthening.
During the first phase of cell intercalation (figure 1a, from (i) to (ii)) actomyosin polarized flows play a key role in junction shrinkage. Flow polarization is thought to be driven by junctional E-cadherin polarized distribution [35,49]. During the second phase (figure 1a, from (iii) to (iv)), actomyosin pulses flow towards the newly formed junction, driving junction lengthening. In this second phase, after complete junction shrinkage, E-cadherin junctional distribution is no longer polarized. It is still not clear how medial actomyosin flow is oriented during new junction lengthening. Future work is necessary to test if actomyosin flow polarity is required for new junction lengthening and, if so, how polarity is controlled during this second phase of cell intercalation.
When a junction completely shrinks, the intercalating quartet reaches a critical configuration: this is referred to as the ‘four way’ junction since four cells seem to be in contact in a single punctate zone. This expression is nevertheless misleading. E-cadherin proteins can connect only two cells at a time, therefore dorsal–ventral cell adhesion and anterior-posterior cell adhesion are mutually exclusive within the zonula adherens. Recent work has proposed that the adhesion protein Sidekick, specifically localized at vertices, could play a role in cell intercalation [77,78] and more specifically during new junction formation [79]. This opens new avenues to better elucidate how the adhesive transition is taking place during this critical phase of cell intercalation (figure 1a, from (ii) to (iii)).
5. Resolving intercalation
An epithelial cell quartet (with apical, basal and lateral sides) initiates intercalation by remodelling the contact junctions: a junction shortens and disappears while a new junction forms and lengthens. Contact junctions are typically located in a subapical position called the zonula adherens: a cell contact band a few micrometres wide that encompasses the cell perimeter (figure 2g). As a consequence of junctional remodelling at the zonula adherens, cells change from a prism to a scutoid shape (figure 1b, from 1. to 3.). Scutoids are shapes that result from cells having different apical and basal neighbours. These shapes were initially described in a biological context by Condic and colleagues in 1991 [80] and then theoretically formalized by Gomez-Galvez and colleagues in 2018, who also coined the term ‘scutoid’ [81]. Scutoids have been theoretically predicted as steady state cell shapes formed especially in tubular structures providing mechanical stability. After junctional remodelling, cell neighbour exchange will resolve from apical to basal, completing cell intercalation. Since intercalation is usually not taking place simultaneously along the apical–basal axis and it is initiated at the zonula adherens, the process of intercalation results in a transition of cell shape from prism, to scutoid, to prism again (figure 1b). How do the cells transition from the scutoid to the prism shape (figure 1b, from 3. to 5.)? One possibility is that, while cortical forces initiate cell neighbour exchange at the zonula adherens, protrusions formed all along the lateral side could resolve cell intercalation (seemingly similar to what is reported during rosette formation [36]). Another possibility is that, after junctional remodeling at the zonula adherens, cells ‘accommodate’ apico-basally if this lowers the energy of the system. If cells have different sets of junctions (e.g. adherens junctions, septate junctions, gap junctions, etc.), all junctions along the apical–basal axis need to be remodelled to resolve cell intercalation. Future work is necessary to shine new light on the mechanisms responsible for cell shape remodelling from scutoid to prism resolving cell intercalation. Finally, 3D mathematical models capturing the apical–basal dynamics of resolving T1s are now necessary to help biologist unravel the key principles underlying this fundamental topological transformation.
6. Interplay between intercalation and external forces
Cell intercalation in epithelia is generally driven by cellular active mechanisms generating internal forces. This can be deduced in frog cultured explant showing convergence and extension movements mechanically independent of the rest of the embryo [82] or in sea urchin exogastrulae showing outward archenteron elongation [9]. Direct evidence of cell internal forces driving cell intercalation was reported in the Drosophila embryo (as presented earlier in this review). Nevertheless, during embryo development different tissues can interact, pushing and pulling on each other [40,83–85]. How do external mechanical forces impact on cell intercalation? A first answer to this question is provided by the work of Collinet and colleagues: if external forces are hampered at the boundary of the extending ectoderm tissue in the Drosophila embryo, the number of intercalation events is not reduced but the length and direction of newly extending junctions are altered [57]. This shows that compressive external forces can modulate intercalation shape and dynamics. In the early developing Drosophila embryo, the posterior midgut pulls on one end of the prospective ectoderm. Interestingly more intercalation events occur in the region of the ectoderm closer to the posterior end [58,86]. This suggests that pulling forces also could facilitate cell intercalation.
7. Measuring the contribution of cell intercalation to tissue convergence–extension
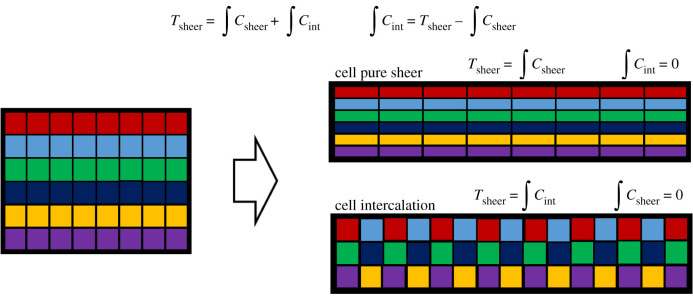
Polarized cell intercalation drives tissue convergence–extension. During convergence–extension, tissues undergo pure shear. Cell intercalation and cell pure shear contribute to tissue pure shear (figure 3). How can the contribution of cell intercalation to tissue pure shear be measured? Cell intercalation events can be measured by tracking and quantifying intercalation events in space and in time [58]. This can be achieved by tracking disappearing and newly forming interfaces or more simply by monitoring cell neighbour variations over time. Nevertheless, intercalation tracking does not provide direct information of the contribution of cell intercalation to tissue pure shear. Butler and colleagues in 2009 implemented a simple and effective method to measure the contribution of cell intercalation to pure shear in the prospective ectoderm of the Drosophila embryo [86]. Since tissue shear results from the sum of the integrated contributions of cell shear and cell intercalation, it is sufficient to measure tissue shear and the integrated contribution of cell shear and to subtract the first from the latter: this results in the integrated contribution of cell intercalation (figure 3). This method is very powerful since it relies on the measurement of only tissue and cell shear, which are computationally less demanding than any topological change measurement.
Figure 3.
Contribution of cell intercalation to tissue pure shear. Tissue pure shear as a result of cell pure shear (top) or cell intercalation (bottom). (Online version in colour.)
8. Conclusion and perspectives
Cell intercalation is implicated in numerous processes encompassing tissue morphogenesis in embryo gastrulation [5,8,9,24,62,63,67], organogenesis [10,11,25–27,85,87], tissue homeostasis [1] and diseases (e.g. cancer cell invasion [4]). Understanding the fundamental mechanisms driving cell intercalation is thus of major importance.
The study of cell intercalation can be undertaken at different spatial and temporal scales. At a cellular resolution and at a time resolution of minutes, cell intercalation can be modelled by a quasi-steady-state distribution of cortical proteins and forces. Surface tension is predicted to increase with higher levels of cortical actomyosin and decrease with higher levels of E-cadherin-based adhesion. While such a model is powerful to eventually predict cellular packing and topology change, it remains a quite simplistic representation of reality. For example, E-cadherin clusters also act as anchoring sites without which actomyosin networks would not be able to generate contractile forces at the cell cortex. Actomyosin contractility and E-cadherin-based adhesion are thus interdependent. To provide a finer understanding of cell intercalation, analyses need to be carried out at a subcellular resolution and at a time resolution of seconds (i.e. the spatio-temporal resolution at which the dynamics of proteins and protein complexes are detectable) to bridge the gap between the cellular and the molecular level. At higher resolutions, pulses of actomyosin become visible. These pulses flow and eventually interact with the junctional cortex. Medial actomyosin pulses, beyond being under the control of upstream signalling factors (e.g. periodic upregulation/downregulation of the Rho pathway [42,43]), depend on the organization of the actomyosin network itself (e.g. F-actin and Myo II turnover rates, cross-linking proteins [45–47,88]). Actomyosin networks are dynamically coupled to the junctional cortex. Medial actomyosin pulses (e.g. via vincuilin) reinforce while cortical actomyosin (e.g. upregulating endocytosis [34]) weakens E-cadherin-based anchoring sites [50]. This results in a dynamic modulation of E-cadherin at the cell cortex [35,49,52]. While medial actomyosin networks asymmetrically coupled to the cortex can generate traction forces orienting actomyosin flows [48,49], actomyosin networks can flow independently of boundary anchoring conditions [47]. Polarized distribution of E-cadherin can modulate actomyosin network properties (e.g. polarized fluidity) that can result in a self-sustained polarized flow [51].
Cell vertices are regions of high E-cadherin density where three or more cells are in proximity. During the first phase of cell intercalation (figure 1a, from (i) to (ii)), vertices appear to shift so that, on one side, one interface reduces in length while, on the other, two interfaces increase in length. Several hypotheses could explain how the process of simultaneous interface shortening and lengthening are coupled. One first hypothesis could be that the interface length change is driven by membrane trafficking (mediated for instance by endo/exocytosis). In this case, equal amounts of membrane need to be added or removed from both cells at a given interface to eventually drive coordinated interface lengthening or shortening, respectively. In addition, since interfaces radiating from the same vertex have equal rate of shortening/lengthening during vertex sliding, membrane trafficking has to be polarized and finely regulated so that membrane removal and addition rates are equal. E-cadherin proteins are modulated at vertices: low levels of E-cadherin correlate with vertex displacement but high levels with vertex stalling [52]. A second hypothesis could thus be that E-cadherin levels are regulated both at vertices and along interfaces to remodel contact junctions. A vertex could thus act as a membrane zipper: spot adherens junctions could be removed from the ‘front’ and new adhesion sites added at the ‘back’ of a sliding vertex. This would result in an apparent and simultaneous shortening and lengthening of interfaces. Photo-bleaching the membrane at the ‘front’ of a sliding vertex while monitoring vertex position using an E-cadherin fluorescent label could be an interesting experiment to validate one of the two hypothesis. For instance, if the photo-bleached membrane portion ends up being at the ‘rear’ of the vertex during vertex sliding, this would support the second hypothesis. Similar experiments would be informative to better decipher the extension of a new contact junction during the second phase of cell intercalation (figure 1a, from (iii) to (iv)). E-cadherin clusters at the cell junctional perimeters could play different roles depending on their specific location. E-cadherin clusters at vertices (together with vertex-specific adhesion complexes) could be key anchoring sites for the medial actomyosin network to exert traction forces for junction remodelling, while E-cadherin polarity at interfaces could direct actomyosin flows and eventual vertex sliding.
In recent years, outstanding work has advanced considerably our understanding of the force-based mechanisms driving cell intercalation. These mechanisms are tuned to provide reversible, irreversible, polarized or randomly oriented cell intercalations that can take place only at the interface between two competing cells, for instance, or throughout a tissue. Different intercalation dynamics and patterns can thus drive tissue morphogenesis, homeostasis or cell competition. We are now at a very exciting time when the knowledge of these fundamental mechanisms can be used to engineer new techniques to synthetically control tissue processes [69,89].
The first key epithelial transformations during early embryo development are elongation and folding. While the first process can separate the future head from the anus, the second can move a great number of cells to the inside of the embryo, where inner organs will form. Intercalation [8] and apical constriction [90] are key cellular processes driving tissue elongation and folding, respectively. Interestingly tissue extension and folding can take place along the same axis (for instance, during archenteron elongation in the sea urchin embryo [9]) or along orthogonal axes (for instance, during neurulation in the X. laevis embryo [82]). In the first case, tissue extension per se contributes to tissue folding. In the second case, extension and folding are orthogonal, thus a priori independent, processes. How can cells drive two simultaneous and independent processes? One possibility is that cells can simultaneously intercalate and apically constrict. If this is the case, how is the cytoskeletal machinery functioning to drive multiple cellular processes? In addition, what are the signalling factors controlling orthogonal tissue shape transformations? This is a new exciting riddle for future studies.
Data accessibility
This article has no additional data.
Competing interests
I declare that I have no competing interests.
Funding
This work was supported by the French government through the UCAJEDI Investments in the Future project managed by the National Research Agency (ANR-15-IDEX-01), the 'Investments for the Future' LABEX SIGNALIFE (ANR-11-LABX-0028-01), the Tramplin ERC programme of the National Research Agency (ANR-16-TERC-0018-01), the ATIP-Avenir programme of the CNRS and the Human Frontier Science Program (CDA00027/2017-C).
References
- 1.Curran S, Strandkvist C, Bathmann J, De Gennes M, Kabla A, Salbreux G, Baum B. 2017. Myosin II controls junction fluctuations to guide epithelial tissue ordering. Dev. Cell 43, 480–492. ( 10.1016/j.devcel.2017.09.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi D, Lopez JH, Schwarz JM, Manning ML. 2014. Energy barriers and cell migration in densely packed tissues. Soft Matter 10, 1885–1890. ( 10.1039/c3sm52893f) [DOI] [PubMed] [Google Scholar]
- 3.Marinari E, Mehonic A, Curran S, Gale J, Duke T, Baum B. 2012. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 484, 542–545. ( 10.1038/nature10984) [DOI] [PubMed] [Google Scholar]
- 4.Levayer R, Hauert B, Moreno E. 2015. Cell mixing induced by myc is required for competitive tissue invasion and destruction. Nature 524, 476–480. ( 10.1038/nature14684) [DOI] [PubMed] [Google Scholar]
- 5.Firmino J, Rocancourt D, Saadaoui M, Moreau C, Gros J. 2016. Cell division drives epithelial cell rearrangements during gastrulation in chick. Dev. Cell 36, 249–261. ( 10.1016/j.devcel.2016.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mongera A, et al. 2018. A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature 561, 401–405. ( 10.1038/s41586-018-0479-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tetley RJ, Staddon MF, Heller D, Hoppe A, Banerjee S, Mao Y. 2019. Tissue fluidity promotes epithelial wound healing. Nat. Phys. 15, 1195–1203. ( 10.1038/s41567-019-0618-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irvine KD, Wieschaus E. 1994. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development 120, 827–841. [DOI] [PubMed] [Google Scholar]
- 9.Hardin JD, Cheng LY. 1986. The mechanisms and mechanics of archenteron elongation during sea urchin gastrulation. Dev. Biol. 115, 490–501. ( 10.1016/0012-1606(86)90269-1) [DOI] [Google Scholar]
- 10.Sanchez-Corrales YE, Blanchard GB, Roper K. 2018. Radially patterned cell behaviours during tube budding from an epithelium. eLife 7, e35717 ( 10.7554/eLife.35717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato K, Hiraiwa T, Maekawa E, Isomura A, Shibata T, Kuranaga E. 2015. Left–right asymmetric cell intercalation drives directional collective cell movement in epithelial morphogenesis. Nat. Commun. 6, 10074 ( 10.1038/ncomms10074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller RE. 1980. The cellular basis of epiboly: an SEM study of deep-cell rearrangement during gastrulation in Xenopus laevis. J. Embryol. Exp. Morphol. 60, 201–234. [PubMed] [Google Scholar]
- 13.Warga RM, Kimmel CB. 1990. Cell movements during epiboly and gastrulation in zebrafish. Development 108, 569–580. [DOI] [PubMed] [Google Scholar]
- 14.McMahon A, Supatto W, Fraser SE, Stathopoulos A. 2008. Dynamic analyses of Drosophila gastrulation provide insights into collective cell migration. Science 322, 1546–1550. ( 10.1126/science.1167094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walck-Shannon E, Hardin J. 2014. Cell intercalation from top to bottom. Nat. Rev. Mol. Cell Biol. 15, 34–48. ( 10.1038/nrm3723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lecuit T, Lenne PF. 2007. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 8, 633–644. ( 10.1038/nrm2222) [DOI] [PubMed] [Google Scholar]
- 17.Heisenberg CP, Bellaiche Y. 2013. Forces in tissue morphogenesis and patterning. Cell 153, 948–962. ( 10.1016/j.cell.2013.05.008) [DOI] [PubMed] [Google Scholar]
- 18.Cavey M, Rauzi M, Lenne P, Lecuit T. 2008. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453, 751–756. ( 10.1038/nature06953) [DOI] [PubMed] [Google Scholar]
- 19.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. 2005. Deconstructing the cadherin-catenin-actin complex. Cell 123, 889–901. ( 10.1016/j.cell.2005.09.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. 2010. Alpha-catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12, 533–542. ( 10.1038/ncb2055) [DOI] [PubMed] [Google Scholar]
- 21.Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. 2014. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science 346, 1254211 ( 10.1126/science.1254211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engl W, Arasi B, Yap LL, Thiery JP, Viasnoff V. 2014. Actin dynamics modulate mechanosensitive immobilization of E-cadherin at adherens junctions. Nat. Cell Biol. 16, 587–594. ( 10.1038/ncb2973) [DOI] [PubMed] [Google Scholar]
- 23.Nishimura T, Honda H, Takeichi M. 2012. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 149, 1084–1097. ( 10.1016/j.cell.2012.04.021) [DOI] [PubMed] [Google Scholar]
- 24.Nishimura T, Takeichi M. 2008. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development 135, 1493–1502. ( 10.1242/dev.019646) [DOI] [PubMed] [Google Scholar]
- 25.Lienkamp SS, Liu K, Karner CM, Carroll TJ, Ronneberger O, Wallingford JB, Walz G. 2012. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat. Genet. 44, 1382–1387. ( 10.1038/ng.2452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. 2009. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat. Genet. 41, 793–799. ( 10.1038/ng.400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warrington SJ, Strutt H, Strutt D. 2013. The Frizzled-dependent planar polarity pathway locally promotes E-cadherin turnover via recruitment of RhoGEF2. Development 140, 1045–1054. ( 10.1242/dev.088724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kafer J, Hayashi T, Maree AFM, Carthew RW, Graner F. 2007. Cell adhesion and cortex contractility determine cell patterning in the Drosophila retina. Proc. Natl Acad. Sci. USA 104, 18 549–18 554. ( 10.1073/pnas.0704235104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farhadifar R, et al. 2007. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095–2104. ( 10.1016/j.cub.2007.11.049) [DOI] [PubMed] [Google Scholar]
- 30.Rauzi M, Verant P, Lecuit T, Lenne P. 2008. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol. 10, 1401–1410. ( 10.1038/ncb1798) [DOI] [PubMed] [Google Scholar]
- 31.Bertet C, Sulak L, Lecuit T. 2004. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667–671. ( 10.1038/nature02590) [DOI] [PubMed] [Google Scholar]
- 32.de Matos Simões S, Blankenship JT, Weitz O, Farrell DL, Masako T, Fernandez-Gonzalez R, Zallen JA.. 2010. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev. Cell 19, 377–388. ( 10.1016/j.devcel.2010.08.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zallen JA, Wieschaus E. 2004. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell 6, 343–355. ( 10.1016/S1534-5807(04)00060-7) [DOI] [PubMed] [Google Scholar]
- 34.Levayer R, Pelissier-Monier A, Lecuit T. 2011. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat. Cell Biol. 13, 529–540. ( 10.1038/ncb2224) [DOI] [PubMed] [Google Scholar]
- 35.Rauzi M, Lenne PF, Lecuit T. 2010. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114. ( 10.1038/nature09566) [DOI] [PubMed] [Google Scholar]
- 36.Sun Z, Amourda C, Shagirov M, Hara Y, Saunders T, Toyama Y. 2017. Basolateral protrusion and apical contraction cooperatively drive Drosophila germ-band extension. Nat. Cell Biol. 19, 375–383. ( 10.1038/ncb3497) [DOI] [PubMed] [Google Scholar]
- 37.Munro E, Nance J, Priess JR. 2004. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424. ( 10.1016/j.devcel.2004.08.001) [DOI] [PubMed] [Google Scholar]
- 38.Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. 2008. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development 135, 2435–2444. ( 10.1242/dev.014704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin AC, Kaschube M, Wieschaus EF. 2009. Pulsed contractions of an actin–myosin network drive apical constriction. Nature 457, 495–499. ( 10.1038/nature07522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solon J, Colombelli J, Brunner D. 2009. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 137, 1331–1342. ( 10.1016/j.cell.2009.03.050) [DOI] [PubMed] [Google Scholar]
- 41.He L, Wang X, Tang HL, Montell DJ. 2010. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat. Cell Biol. 12, 1133–1142. ( 10.1038/ncb2124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mason FM, Xie S, Vasquez CG, Tworoger M, Martin AC. 2016. RhoA GTPase inhibition organizes contraction during epithelial morphogenesis. J. Cell Biol. 214, 603–617. ( 10.1083/jcb.201603077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michaux JB, Robin F, Mcfadden WM, Munro EM. 2018. Excitable RhoA dynamics drive pulsed contractions in the early C. elegans embryo. J. Cell Biol. 217, 4230–4252. ( 10.1083/jcb.201806161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munjal A, Philippe J-M, Munro E, Lecuit T. 2015. A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 524, 351–355. ( 10.1038/nature14603) [DOI] [PubMed] [Google Scholar]
- 45.Senger F, Pitaval A, Ennomani H, Kurzawa L, Blanchoin L. 2019. Spatial integration of mechanical forces by α-actinin establishes actin network symmetry. J. Cell Sci. 132, jcs236604 ( 10.1242/jcs.236604) [DOI] [PubMed] [Google Scholar]
- 46.Belmonte JM, Leptin M, Nedelec F. 2017. A theory that predicts behaviors of disordered cytoskeletal networks. Mol. Syst. Biol. 13, 941 ( 10.15252/msb.20177796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banerjee DS, Munjal A, Lecuit T, Rao M. 2017. Actomyosin pulsation and flows in an active elastomer with turnover and network remodeling. Nat. Commun. 8, 1121 ( 10.1038/s41467-017-01130-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mori M, Monnier N, Daigle N, Bathe M, Ellenberg J, Lénárt P. 2011. Intracellular transport by an anchored homogeneously contracting F-actin meshwork. Curr. Biol. 21, 606–611. ( 10.1016/j.cub.2011.03.002) [DOI] [PubMed] [Google Scholar]
- 49.Levayer R, Lecuit T. 2013. Oscillation and polarity of E-cadherin asymmetries control actomyosin flow patterns during morphogenesis. Dev. Cell 26, 162–175. ( 10.1016/j.devcel.2013.06.020) [DOI] [PubMed] [Google Scholar]
- 50.Kale GR, Yang X, Philippe J-M, Mani M, Lenne P, Lecuit T. 2018. Distinct contributions of tensile and shear stress on E-cadherin levels during morphogenesis. Nat. Commun. 9, 5021 ( 10.1038/s41467-018-07448-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinheiro D, et al. 2017. Transmission of cytokinesis forces via E-cadherin dilution and actomyosin flows. Nature 545, 103–107. ( 10.1038/nature22041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanderleest TE, Smits CM, Xie Y, Jewett CE, Blankenship JT, Loerke D. 2018. Vertex sliding drives intercalation by radial coupling of adhesion and actomyosin networks during Drosophila germband extension. eLife 7, e34586 ( 10.7554/eLife.34586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weng M, Wieschaus E. 2016. Myosin-dependent remodeling of adherens junctions protects junctions from Snail-dependent disassembly. J. Cell Biol. 212, 219–229. ( 10.1083/jcb.201508056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blankenship JT, Backovic ST, Sanny J, Weitz O, Zallen JA. 2006. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev. Cell 11, 459–470. ( 10.1016/j.devcel.2006.09.007) [DOI] [PubMed] [Google Scholar]
- 55.Chacon-Heszele MF, Ren D, Reynolds AB, Chi F, Chen P. 2012. Regulation of cochlear convergent extension by the vertebrate planar cell polarity pathway is dependent on p120-catenin. Development 139, 968–978. ( 10.1242/dev.065326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandez-Gonzalez R, de Matos Simoes S, Röper J-C, Eaton S, Zallen JA. 2009. Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736–743. ( 10.1016/j.devcel.2009.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collinet C, Rauzi M, Lenne P, Lecuit T. 2015. Local and tissue-scale forces drive oriented junction growth during tissue extension. Nat. Cell Biol. 17, 1247–1258. ( 10.1038/ncb3226) [DOI] [PubMed] [Google Scholar]
- 58.Paré AC, Vichas A, Fincher CT, Mirman Z, Farrell DL, Mainieri A, Zallen JA. 2014. A positional Toll receptor code directs convergent extension in Drosophila. Nature 515, 523–527. ( 10.1038/nature13953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sulston JE, Schierenberg E, White JG, Thomson JN. 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64–119. ( 10.1016/0012-1606(83)90201-4) [DOI] [PubMed] [Google Scholar]
- 60.Williams-Masson EM, Heid PJ, Lavin CA, Hardin J. 1998. The cellular mechanism of epithelial rearrangement during morphogenesis of the Caenorhabditis elegans dorsal hypodermis. Dev. Biol. 204, 263–276. ( 10.1006/dbio.1998.9048) [DOI] [PubMed] [Google Scholar]
- 61.Patel FB, et al. 2008. The WAVE/SCAR complex promotes polarized cell movements and actin enrichment in epithelia during C. elegans embryogenesis. Dev. Biol. 324, 297–309. ( 10.1016/j.ydbio.2008.09.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walck-Shannon E, Reiner D, Hardin J. 2015. Polarized Rac-dependent protrusions drive epithelial intercalation in the embryonic epidermis of C. elegans. Development 142, 3549–3560. ( 10.1242/dev.127597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munro EM, Odell GM. 2002. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development 129, 13–24. [DOI] [PubMed] [Google Scholar]
- 64.Keys DN, Levine M, Harland RM, Wallingford JB. 2002. Control of intercalation is cell-autonomous in the notochord of Ciona intestinalis. Dev. Biol. 246, 329–340. ( 10.1006/dbio.2002.0656) [DOI] [PubMed] [Google Scholar]
- 65.Jiang D, Munro EM, Smith WC. 2005. Ascidian prickle regulates both mediolateral and anterior-posterior cell polarity of notochord cells. Curr. Biol. 15, 79–85. ( 10.1016/j.cub.2004.12.041) [DOI] [PubMed] [Google Scholar]
- 66.Oda-Ishii I, Ishii Y, Mikawa T. 2010. Eph regulates dorsoventral asymmetry of the notochord plate and convergent extension-mediated notochord formation. PLoS ONE 5, e13689 ( 10.1371/journal.pone.0013689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shih J, Keller R. 1992. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development 116, 901–914. [DOI] [PubMed] [Google Scholar]
- 68.Guglielmi G, Barry JD, Huber W, De Renzis S. 2015. An optogenetic method to modulate cell contractility during tissue morphogenesis. Dev. Cell 35, 646–660. ( 10.1016/j.devcel.2015.10.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Izquierdo E, Quinkler T, De Renzis S. 2018. Guided morphogenesis through optogenetic activation of Rho signalling during early Drosophila embryogenesis. Nat. Commun. 9, 2366 ( 10.1038/s41467-018-04754-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krueger D, Tardivo P, Nguyen C, De Renzis S. 2018. Downregulation of basal myosin-II is required for cell shape changes and tissue invagination. EMBO J. 37, e100170 ( 10.15252/embj.2018100170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szabo A, Cobo I, Omara S, Mclachlan S, Keller R, Mayor R. 2016. The molecular basis of radial intercalation during tissue spreading in early development. Dev. Cell 37, 213–225. ( 10.1016/j.devcel.2016.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shindo A, Wallingford JB. 2014. PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science 343, 649–652. ( 10.1126/science.1243126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shindo M, Wada H, Kaido M, Tateno M, Aigaki T, Tsuda L, Hayashi S. 2008. Dual function of Src in the maintenance of adherens junctions during tracheal epithelial morphogenesis. Development 135, 1355–1364. ( 10.1242/dev.015982) [DOI] [PubMed] [Google Scholar]
- 74.Shindo A. 2018. Models of convergent extension during morphogenesis. WIREs Dev. Biol. 7, e293 ( 10.1002/wdev.293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu JC, Fernandez-Gonzalez R. 2016. Local mechanical forces promote polarized junctional assembly and axis elongation in Drosophila. eLife 5, e10757 ( 10.7554/eLife.10757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bardet PL, et al. 2013. PTEN controls junction lengthening and stability during cell rearrangement in epithelial tissue. Dev. Cell 25, 534–546. ( 10.1016/j.devcel.2013.04.020) [DOI] [PubMed] [Google Scholar]
- 77.Finegan TM, Hervieux N, Nestor-Bergmann A, Fletcher AG, Blanchard GB, Sanson B. 2019. The tricellular vertex-specific adhesion molecule Sidekick facilitates polarised cell intercalation during Drosophila axis extension. PLoS Biol. 17, e3000522 ( 10.1371/journal.pbio.3000522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Letizia A, He DQ, Astigarraga S, Colombelli J, Hatini V, Llimargas M, Treisman JE. 2019. Sidekick is a key component of tricellular adherens junctions that acts to resolve cell rearrangements. Dev. Cell 50, 313–326. ( 10.1016/j.devcel.2019.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uechi H, Kuranaga E. 2019. The tricellular junction protein Sidekick regulates vertex dynamics to promote bicellular junction extension. Dev. Cell 50, 327–338. ( 10.1016/j.devcel.2019.06.017) [DOI] [PubMed] [Google Scholar]
- 80.Condic ML, Fristrom D, Fristrom JW. 1991. Apical cell shape changes during Drosophila imaginal leg disc elongation: a novel morphogenetic mechanism. Development 111, 23–33. [DOI] [PubMed] [Google Scholar]
- 81.Gomez-Galvez P, et al. 2018. Scutoids are a geometrical solution to three-dimensional packing of epithelia. Nat. Commun. 9, 2960 ( 10.1038/s41467-018-05376-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keller R. 2002. Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298, 1950–1954. ( 10.1126/science.1079478) [DOI] [PubMed] [Google Scholar]
- 83.Behrndt M, Salbreux G, Campinho P, Hauschild R, Oswald F, Roensch J, Grill SW, Heisenberg C-P. 2012. Forces driving epithelial spreading in zebrafish gastrulation. Science 338, 257–260. ( 10.1126/science.1224143) [DOI] [PubMed] [Google Scholar]
- 84.Rauzi M, Krzic U, Saunders TE, Krajnc M, Ziherl P, Hufnagel L, Leptin M. 2015. Embryo-scale tissue mechanics during Drosophila gastrulation movements. Nat. Commun. 6, 8677 ( 10.1038/ncomms9677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aigouy B, et al. 2010. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142, 773–786. ( 10.1016/j.cell.2010.07.042) [DOI] [PubMed] [Google Scholar]
- 86.Butler LC, Blanchard GB, Kabla AJ, Lawrence NJ, Welchman DP, Mahadevan L, Adams RJ, Sanson B. 2009. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat. Cell Biol. 11, 859–864. ( 10.1038/ncb1894) [DOI] [PubMed] [Google Scholar]
- 87.Wang J, et al. 2005. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat. Genet. 37, 980–985. ( 10.1038/ng1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jodoin JN, Coravos JS, Chanet S, Vasquez CG, Tworoger M, Kingston ER, Perkins LA, Perrimon N, Martin AC. 2015. Stable force balance between epithelial cells arises from F-actin turnover. Dev. Cell 35, 685–697. ( 10.1016/j.devcel.2015.11.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baillie-Johnson P, van den Brink SC, Balayo T, Turner DA, Arias AM.. 2015. Generation of aggregates of mouse embryonic stem cells that show symmetry breaking, polarization and emergent collective behaviour in vitro. J. Vis. Exp. 105, 53252 ( 10.3791/53252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leptin M, Grunewald B. 1990. Cell shape changes during gastrulation in Drosophila. Development 110, 73–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.