Abstract
Prenatal effects on telomere length are increasingly recognized as a potential contributor to the developmental origin of health and adult disease. While it is becoming clear that telomere length is influenced by prenatal conditions, the factors affecting telomere dynamics during embryogenesis remain poorly understood. We manipulated both the pace and stability of embryonic development through varying incubation temperature and its stability in Japanese quail. We investigated the impact on telomere dynamics from embryogenesis to adulthood, together with three potential drivers of telomere shortening, growth rate, oxidative damage and prenatal glucocorticoid levels. Telomere length was not affected by our prenatal manipulation for the first 75% of embryogenesis, but was reduced at hatching in groups experiencing faster (i.e. high temperature) or less stable embryonic development. These early life differences in telomere length persisted until adulthood. The effect of developmental instability on telomere length at hatching was potentially mediated by an increased secretion of glucocorticoid hormones during development. Both the pace and the stability of embryo development appear to be key factors determining telomere length and dynamics into adulthood, with fast and less stable development leading to shorter telomeres, with the potential for adverse associated outcomes in terms of reduced longevity.
Keywords: ageing, telomere, embryo, fetal programming, oxidative stress, glucocorticoid
1. Introduction
Scientific evidence from both a biomedical (e.g. [1,2]) and an ecological perspective (e.g. [3,4]) has established that conditions experienced during early life can have profound effects on adult phenotypes and subsequent life histories. For example, accelerated postnatal growth has been shown in a range of species, including humans, to be associated with reduced longevity [5,6] and increased risks of developing numerous age-related pathologies such as cardiovascular disease and type 2 diabetes later in life [2,7]. Telomere shortening (an important hallmark of ageing [8]) has emerged as one of the key candidates linking these early life conditions to later life adverse effects [9–11]. Telomeres are protective complexes consisting of repeats of a non-coding DNA sequence, proteins and associated RNAs situated at the end of eukaryotic chromosomes. Telomeres serve to identify the chromosome ends, thereby maintaining genome stability, and to prevent the loss of DNA from the chromosome ends that occurs during cell division penetrating into the coding sequences [12]. The length of telomeres is dynamic and results from a balance between loss and restoration processes. In addition to the ‘end-replication problem’, oxidative stress leading to DNA damage can increase telomere loss [13]. While telomere restoration/elongation processes also exist, for instance the enzyme telomerase or the alternative lengthening pathway (ALT), in many species their activity in most somatic tissues is reduced in adulthood [14]. Telomeres shorten with age in a broad range of organisms [15,16], with the more pronounced shortening being observed during growth [17,18], presumably because of the intense cellular proliferation and metabolic activity required to sustain growth [11]. Telomere length and/or shortening rate, especially in early life, have been shown to predict subsequent survival/lifespan in a range of species [19–23], thereby leading to the idea that telomere length could act as a biomarker of individual ‘biological state’. The nature of the link between telomere length and mortality risk (i.e. causal versus correlative) is debated [24], but the recent demonstration that experimental increases in telomere length in the laboratory mouse give rise to an increase in lifespan supports a causal link [25].
While the main focus of research on telomeres during early life has so far been the postnatal growth period [11], changes during the prenatal period are increasingly recognized as potentially even more important [9,26–30], giving rise to the ‘fetal programming of telomere biology hypothesis' [10]. While the ‘initial’ telomere length (i.e. defined by [10] as the telomere length at birth) seems to be of prime importance, the information we have about the prenatal factors determining this ‘initial’ length remains very limited [10].
During the postnatal phase, telomere shortening is thought to be accelerated both by fast growth (e.g. [26]) and/or by growing under harsh environmental conditions (e.g. [31]), and the same pattern could exist at the prenatal stage. However, most of the experiments to date that have attempted to explore the effect of the prenatal environment on telomere dynamics have two main limitations. First, measurements of telomere length have either been done at a single time point or using a cross-sectional approach with the first sampling occurring weeks/months after birth (e.g. [26–28]), precluding separation of the direct effects during embryogenesis from indirect effects linked to postnatal compensatory responses. Second, those experimental manipulations of the prenatal environment that have used mammals required the manipulation to be applied to the mother (e.g. [26,28]), leading to potentially confounding effects or compensatory maternal responses (e.g. preferential allocation of resources to the embryos).
A good model system for investigating the direct effects of prenatal conditions on telomere length and dynamics is an oviparous species, where prenatal conditions can be manipulated directly without interaction with the mother. Telomere length should also be evaluated at different life stages (i.e. embryo, birth, during growth and at adulthood) to better understand the impact of prenatal conditions on ‘initial’ telomere length and subsequent telomere shortening rate. In this context, it has been shown that early stage (72 h) embryonic telomere length decreases with ovulation order in captive zebra finches (Taeniopygia guttata) and that this effect appears to be maintained until adulthood [29]. Additionally, by incubating eggs of wild common terns (Sterna hirundo) at two different temperatures to induce differences in embryo growth rate, Vedder et al. [30] found that slow embryonic growth was associated with longer telomeres at hatching. While the use of stable incubation conditions in that study removed the confounding effect of parental behaviour, constant incubation temperatures are unlikely to be realistic for most egg-laying species [32]. Similarly, variations in the transfer of nutrients, oxygen and hormones from the placenta to the embryos could occur during gestation in mammals and alter embryo development [33]. It is therefore also important to examine the extent to which such unstable developmental conditions could influence embryonic telomere dynamics, and to explore the effects not only on ‘initial’ telomere length, but also on postnatal telomere dynamics. Additionally, it is important to identify the underlying mechanisms driving telomere shortening in response to prenatal conditions. While oxidative stress is a first obvious candidate [13], fast growth and glucocorticoid hormones have also been suggested to influence prenatal telomere shortening [27]. Telomeres are known to be especially prone to oxidative damage due to their high guanine content [13], and oxidative stress has been suggested to be a physiological cost of fast growth [34]. Environmental stressors such as suboptimal incubation temperature could potentially increase glucocorticoid hormones secretion. Increased prenatal exposure to glucocorticoids has previously been shown to accelerate telomere shortening [27], which could either be linked to increased oxidative stress levels, or, as more recently suggested, to a cellular metabolic reprogramming [35].
The main aim of this study was to examine the effects of prenatal growth rate and developmental stability on telomere dynamics from embryogenesis to adulthood, and to investigate the potential underlying mechanisms (i.e. changes in oxidative stress, metabolic rate, prenatal glucocorticoid levels). We used the Japanese quail (Coturnix japonica) as a study system for manipulating prenatal growth rate and stability using modulations of incubation temperature, since incubation in this species has been well studied [36], it has a short generation time and is widely used as a model species in physiological, molecular and behavioural studies [37].
2. Material and methods
(a). Experimental design
All procedures were conducted in accordance with European regulations under the Home Office Project Licence 70/8335 granted to PM and the Personal Licence ICB1D39E7 granted to A.S. Japanese quail are precocial birds that can be raised independently from their parents, therefore avoiding any confounding effect linked to parental care. They reach sexual maturity and therefore adulthood very quickly (ca 50–60 days), and are short-lived (2.5–5 years) especially for an avian species of their body size [37]. Japanese quail eggs were obtained from Moonridge Farm (Devon, UK) and delivered within 48 h of collection. Identity of the parents was unfortunately unavailable. However, given that Japanese quail lay a maximum of one egg a day [37] and that all the experimental eggs were collected on the same day, it is very likely that all of the eggs would have originated from different females. In total, 120 eggs were sacrificed as embryos (see below for details) and 164 were incubated until hatching. Eggs were stored 24 h at 14°C upon reception before being incubated.
Eggs were incubated in four artificial incubators (Brinsea Octagon Advance 20 EX) with automatic egg turning and humidity set at 45% until standardized embryonic day 15 (stED15; see below for description of development times). Egg turning was stopped at stED15 and humidity was increased to 65% for hatching purposes. Experimental temperature conditions were chosen, based on the literature [36] and pilot experiments, so as to maximize differences in developmental speed while minimizing the risks of having differences in hatching success between groups (i.e. to avoid the selective disappearance of embryos in some groups). Eggs were incubated at three constant temperatures (electronic supplementary material, figure S1): high (H) = 38.4°C, medium (M) = 37.7°C and low (L) = 37.0°C. Additionally, a fourth group was incubated under ‘unstable’ (U) temperature conditions, with an incubation temperature of 37.7°C but five incubation recesses of 30 min during the day (i.e. mimicking the female leaving the nest to forage; [38]). Incubation recesses were achieved by using an automatic power switch; temperature within the incubator during the recesses was measured using a digital thermometer, and averaged over the five recesses of 1 day (averaged temperature is presented in electronic supplementary material, figure S1, minimum temperature during incubation recess was 29.5°C). While the incubation temperature of this unstable group outside the recess periods was similar to the medium temperature group (37.7°C), its mean daily incubation temperature was 37.0°C, so similar to the low-temperature group. This incubation pattern creates developmental instability by decelerating metabolism during each incubation recess (i.e. 15% decrease in embryo heart rate halfway through the 30 min incubation recess, see electronic supplementary material, S2 and figure S2). While incubation recesses are common in many bird species including Japanese quail [38], incubating birds will minimize the frequency and duration of such recesses if environmental conditions are favourable (e.g. [39]).
Animal husbandry rooms were maintained at 21°C on a 14 L : 10 D cycle throughout the experiment, and hatching was monitored during the 14 h of light starting from stED15 onwards. If hatching occurred overnight, hatching time was considered to be 06.00 the next morning. Body mass was recorded on the day of hatching (D0) and chicks were placed for 24 h in a larger incubator set at 37°C before being placed into their respective enclosure at D1 where an additional heat source was provided until day 15 post-hatching (D15) (Brinsea Comfort brooder 40, 42 W), as well as ad libitum food (Heygates starter crumbs, 22% protein) and water. We used group-specific enclosures of 4.3 m2 to avoid late-hatching chicks (from L and U groups) being out-competed by older chicks (from H and M groups) since hatching occurred asynchronously between groups (see results for details). Chick food was switched to pellets (Heygates quail and partridge pellets, 16% protein) at D15. Chicks were maintained in mixed-sex groups until D25 when they could be sexed morphologically. Females were then kept in groups in the enclosures (enclosure size was adjusted to the number of females) and males were placed in pairs in 0.8 m2 cages to avoid female exhaustion due to male harassment and to limit male–male conflicts. Body mass was recorded for each bird at D0, D1, D3, D5, D10, D15, D20, D30, D45 (by which point birds had reached adult body size), D60 (sexual maturity reached by all birds), D90. Most birds were euthanized at D90–120 for other experimental purposes, but eight birds were kept alive until approximately 1 year of age (D360) to gather data on long-term telomere dynamics.
(b). Sampling procedures
Differences in embryo developmental rate among treatments were initially assessed by measuring embryo mass (after removing yolk and blotting the embryo on absorbent paper) on day 13 of incubation (ED13; eight eggs sacrificed per treatment). For assessment of embryo telomere length, it was important to collect samples at a similar developmental stage between experimental groups since telomere length is likely to be affected by the number of cell divisions that have occurred (and hence by developmental stage). Since our pilot experiments revealed a difference of approximately 24 h in incubation time to hatching between the H and M group, and another 24 h between the M and L/U groups, we chose to euthanize and sample embryos at a standardized developmental stage, being the ED13 of the medium temperature treatment (hereafter referred as stED13). Therefore, we euthanized embryos from the high-temperature group at ED12 and embryos from the unstable and low-temperature groups at ED14 so that all embryos would be approximately at the same developmental stage at the time of sampling. We confirmed the validity of our approach by comparing the body mass of these embryos at stED13, finding no significant differences between groups (H = 4.24 ± 0.13 g, M = 4.26 ± 0.16 g, L = 4.15 ± 0.13 g, U = 4.41 ± 0.19 g; N = 14–16 per group; GLM: F3,56 = 0.5, p = 0.68).
Given that a longitudinal sampling approach is more powerful at revealing changes in telomere length with age than typical cross-sectional sampling, we used red blood cells (RBCs) to measure repeatedly postnatal telomere lengths of the same individuals [16]. We collected blood in embryos at stED13 from the jugular vein using heparinized capillaries following euthanasia by decapitation. Blood was collected at the postnatal stage by puncturing the wing vein with a 26 G needle and using heparinized capillaries. We collected blood at each of D1, D20 and at D60 for telomere length measurement for a subsample of 61 of the 103 birds that hatched (+8 birds at D360), but blood volume was too low to get enough DNA for TRF telomere analysis for 23 of these 61 chicks at D1. Blood was centrifuged 10 min at 3000g and 4°C to separate plasma from RBCs, and was subsequently flash-frozen in liquid nitrogen and stored at −80°C until laboratory analysis. Importantly, telomere length has been shown to be correlated between RBCs and other tissues in birds [40], but a recent study pointed out that it could be life-stage dependent [41]. Therefore, to evaluate the relevance of using RBC telomere length in Japanese quails as a potential indicator of overall telomere length, we compared telomere length in RBCs and in heart samples for a subsample of stED13 embryos (N = 7) and adults (D90; N = 14). Heart samples (10–20 mg) were collected from the tip of the heart following euthanasia (cervical dislocation for adults) and dissection on ice, and were quickly flash frozen in liquid nitrogen and stored at −80°C until analysis.
(c). Measurement of embryo heart rate and plasma corticosterone
We measured embryo heart rate as an indicator of embryo metabolism using a non-invasive infrared methodology on the 60 eggs being sacrificed at stED13 to control for known effects of developmental stage on embryo heart rate [42] (see electronic supplementary material, S2 for details). Corticosterone (CORT) is the main glucocorticoid hormone in birds. Plasma total CORT levels of embryos at stED13 were determined by immunoassay (see electronic supplementary material, S2 for details).
(d). Measurement of DNA damage and telomere length
One of the predominant forms of free radical-induced oxidative lesions in DNA, 8-hydroxydeoxyguanosine (8-OHdG), was quantified in DNA extracted from RBCs as described previously [43]. Telomere length was measured using the in-gel telomeric restriction fragment (TRF) method as described previously [44]. Detailed methodologies are available in electronic supplementary material, S3.
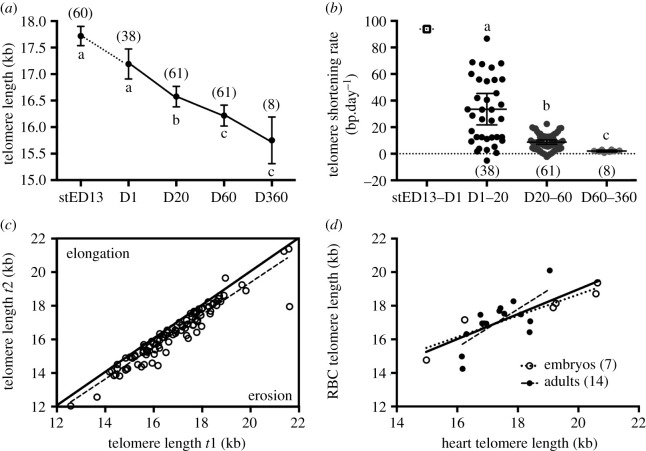
RBC telomere length decreases with age in Japanese quail from embryogenesis to adulthood (figure 1a, GEE: p < 0.001), with the rate of shortening itself decreasing gradually with age (figure 1b, GEE: p < 0.001). Based on the eight individuals sampled twice at adulthood (D60 and D360), the estimated adult telomere shortening rate is −745 ± 109 bp yr−1. Japanese quail exhibit a very high intra-individual repeatability/consistency in telomere length (figure 1c, n = 160, R adjusted for age and between-gel effects = 0.93, 95% CI = [0.90–0.96], p < 0.001). Controlling for the experimental treatment in the analysis only reduced very slightly (R = 0.91, 95% CI = [0.88–0.96], p < 0.001) the within-individual repeatability estimate. Telomere length in RBCs is significantly correlated with telomere length in the heart (figure 1d, Spearman N = 21, σ = 0.80, p < 0.001), a relationship that is found in both embryos (N = 7, σ = 0.96, p < 0.001) and adults (N = 14, σ = 0.64, p = 0.015). Telomere length does not significantly differ between heart and RBC samples (exact Wilcoxon test; overall: N = 21, Z = −1.65, p = 0.10; embryos: N = 7, Z = −1.69, p = 0.11; adults: N = 14, Z = −0.63, p = 0.55). Thus, telomere lengths in RBCs can be used as representative of those in other tissues of the quail.
Figure 1.
Characterizing the telomere biology of Japanese quail. (a) Telomere lengths of Japanese quail red blood cells from embryogenesis to 360 days postnatal. stED13 represents a standardized developmental stage between experimental groups as explained in the main text methods. Data plotted as means ± se; solid lines linking means indicate longitudinal data and the dotted line cross-sectional data. (b) Intra-individual telomere shortening rate until 360 days postnatal. Data plotted as individual data points, with horizontal lines indicating means ± s.e. Cross-sectional average ‘shortening rate’ between stED13 and D1 is presented for illustration purposes. (c) Intra-individual consistency/repeatability of telomere length. Telomere length at the first sampling occasion (t1) is plotted against telomere length at the second sampling occasion (t2) for each consecutive measurement (i.e. D1 versus D20, D20 versus D60 and D60 versus 360) for each bird. Line of equality is presented as a solid line, while the dotted line represents the relationship in our dataset. (d) Correlation between heart and RBC telomere length in Japanese quail embryos (stED13) and adult individuals. Solid line represents the overall correlation while dotted lines represent stage-specific correlations. Letters indicate significant differences according to post-hoc tests, and numbers in brackets represent sample sizes.
(e). Data analysis
Data were analysed using SPSS 24 for all statistical tests, except the within-individual repeatability analysis of telomere lengths (see above) that was conducted using the RptR package in R 3.4.2 [45]. Differences in average hatching success between groups were analysed using a GLMM following a binary distribution. Differences in embryo mass at ED13, embryo heart rate at stED12, incubation time to hatching and plasma CORT were analysed using GLMs with experimental treatment as a fixed factor, and associated post-hoc tests. We used generalized estimating equations (GEEs) following a normal distribution to investigate the effects of Age (i.e. repeated effect), Treatment, Sex and their interactions on body mass dynamics (reduced to the 61 individuals for which we had telomere data, but results are similar when using the full dataset), telomere length dynamics and RBC DNA damage, and the associated post-hoc tests (non-significant interactions were removed from the final models). Sex was not included in final models for telomere length and DNA damage since preliminary analyses revealed no significant effect of Sex either as a main factor or in interaction with other factors. All means are presented ± s.e., and significance level was set at p ≤ 0.05.
3. Results
(a). Developmental and postnatal consequences of incubation temperature and stability
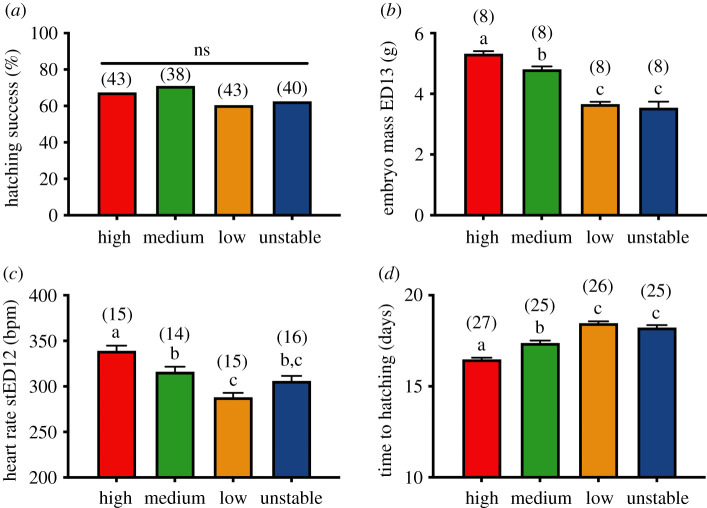
Hatching success did not statistically differ between experimental groups (figure 2a, p = 0.75). Experimental treatments altered embryo growth and developmental rate (figure 2b, GLM: F3,28 = 49.0, p < 0.001), as shown by the increasing embryo mass at ED13 with increasing incubation temperature (H > M > L, all p < 0.044). Embryos from the unstable group were smaller than the ones from high- and medium-temperature treatments (all p < 0.001), but not significantly different from the embryos incubated at low temperature (figure 2b, p = 1.00). Embryo heart rate differed among experimental groups at the same developmental stage (figure 2c, GLM: F3,56 = 15.8, p < 0.001), decreasing from high to low temperature (H > M > L, all p < 0.025). Embryos from the unstable group had a heart rate that was intermediate between those from the low and medium temperature groups (figure 2c, L-U: p = 0.10, M-U: p = 1.0). Incubation time to hatching significantly differed among experimental groups (figure 2d, GLM: F3,99 = 64.1, p < 0.001). Eggs incubated at lower temperatures hatched later (L > M > H, all p < 0.001), and eggs incubated under unstable conditions hatched later than both high and medium temperatures (all p < 0.001), but were not significantly different in hatching time from low temperature ones (p = 0.92, figure 2d). However, despite these prenatal differences in developmental rate, there were no overall effects of incubation conditions on postnatal growth (see electronic supplementary material, S4, table S1 and figure S3 for details on weak age × sex × treatment effects).
Figure 2.
Differences between the four incubation temperature treatment groups in: (a) hatching success, (b) embryo total mass at embryonic day 13 (ED13), (c) embryo heart rate at standardized embryonic day 12 (stED12), and (d) time from the beginning of incubation to hatching. Data plotted as means ± s.e. Details of statistical tests are given in the text, letters indicate significant differences according to post-hoc tests, and numbers in brackets represent sample size. (Online version in colour.)
(b). Effect of prenatal conditions on telomere length and dynamics
The experimental manipulation of prenatal conditions significantly influenced telomere length (GEE: p = 0.034), through an interaction between age and treatment (figure 3, GEE: p = 0.009). Post-hoc tests revealed no significant differences in telomere lengths between groups at the stED13 embryo stage (electronic supplementary material, S5 and figure S4A; all p > 0.45). This lack of difference in telomere length was also true for a subsample of ED13 embryos (i.e. embryos of the same age but different developmental stages, dependent on their incubation temperature regime, see figure 2b; H = 18.72 ± 1.26 kb, M = 18.80 ± 1.18 kb, L = 18.83 ± 0.62 kb, U = 18.41 ± 1.08 kb; N = 3–4 per group; GLM: F3,11 = 0.03, p = 0.99). However, by day 1 post-hatching both high temperature and unstable chicks exhibited shorter telomeres than did medium and low-temperature chicks (electronic supplementary material, S5 and figure S4B; H-M: p = 0.020, H-L: p = 0.020, H-U: p = 0.24, M-L: p = 0.58, M-U: p = 0.003, L-U: p = 0.002). In the middle of the postnatal growth phase (day 20) chicks from the low-temperature group still had significantly longer telomeres than unstable and high-temperature groups (electronic supplementary material, S5 and figure S4C; L-U: p = 0.015, L-H: p = 0.050), while medium temperature chicks exhibited an intermediate telomere length (electronic supplementary material, S5 and figure S4C; all p > 0.20). At adulthood (day 60), the pattern remained the same as at day 20, with birds from the low-temperature group having significantly longer telomeres than unstable and high-temperature groups (electronic supplementary material, S5 and figure S4D; L-U: p = 0.007, L-H: p = 0.022), and medium-temperature birds having an intermediate telomere length (electronic supplementary material, S5 and figure S4D; all p > 0.13).
Figure 3.
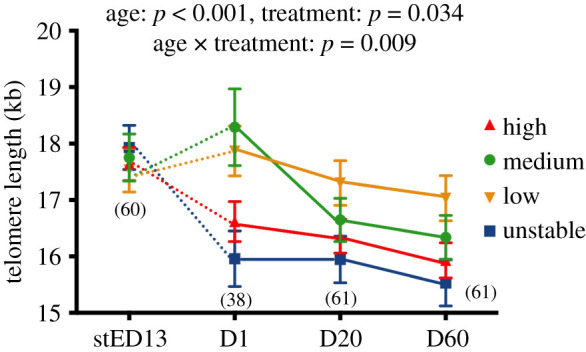
Effects of experimental incubation temperatures on changes in telomere length with age. stED13 represents a standardized developmental stage between experimental groups as explained in the methods; D1 = day after hatching, D20 = chick and D60 = adult. Data plotted as means ± s.e. Details of statistical tests are given in the text, solid lines indicate longitudinal data and dotted lines cross-sectional data, and numbers in brackets represent sample sizes. (Online version in colour.)
The changes in telomere length with age varied significantly between experimental groups (figure 3). In both the high temperature (electronic supplementary material, S5 and figure S4E) and unstable (electronic supplementary material, 5 and figure S4H) groups, telomere length decreased during late embryogenesis (from stED13 to day 1 post-hatching; U: p = 0.001; H: p = 0.020) and from juvenile to adulthood (day 20–60 post-hatching; U: p < 0.001; H: p < 0.001), but not significantly so during the early chick stage (between day 1 and 20; U: p = 0.95; H: p = 0.32). By contrast, telomere length did not significantly change in the late embryo stage in the medium (electronic supplementary material, S5 and figure S4F) and low (electronic supplementary material, S5 and figure S4G) temperature groups (M: p = 0.48; L: p = 0.55), but subsequently decreased both from day 1 to 20 (M: p = 0.001; L: p = 0.002) and from day 20 to 60 (M: p = 0.003; L: p < 0.001).
(c). Effect of prenatal conditions on DNA damage and embryo corticosterone levels
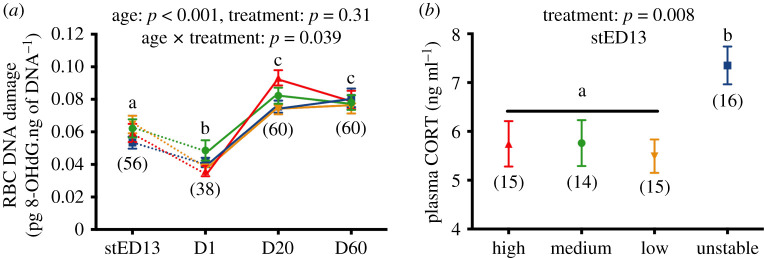
The experimental manipulation of prenatal conditions significantly influenced DNA damage through an interaction between age and treatment (figure 4a, GEE: p = 0.039). Post-hoc tests revealed no significant differences in DNA damage between groups at the stED13 embryo stage (electronic supplementary material6, figure S5A; all p > 0.098), day 1 post-hatching (electronic supplementary material, S6 and figure S5B, all p > 0.055) or day 60 post-hatching (electronic supplementary material, S6 and figure S5D, all p > 0.30). However, at day 20 post-hatching, DNA damage levels were higher in the high-temperature group compared to both low and unstable groups (p < 0.001 and p = 0.002, respectively), while medium temperature chicks exhibited intermediate levels (all p > 0.095, electronic supplementary material, S6 and figure S5C). Age-related variations in DNA damage also slightly differed between experimental groups (electronic supplementary material, S6 and figure S5E–H).
Figure 4.
Effects of experimental incubation temperatures on (a) changes in DNA damage levels with age, (b) plasma corticosterone (CORT) levels in embryos. stED13 represents a standardized developmental stage between experimental groups as explained in the methods; D1 = day after hatching, D20 = chick and D60 = adult. Data plotted as means ± s.e. Details of statistical tests are given in the text, solid lines indicate longitudinal data and dotted lines cross-sectional data, letters indicate significant differences according to post-hoc tests, and numbers in brackets represent sample sizes. (Online version in colour.)
Plasma CORT in embryos significantly differed between experimental groups (GLM: F3,56 = 4.3, p = 0.008), with embryos from the unstable group having higher CORT levels than other groups (figure 4b, U versus H/M/L: all p < 0.045, differences between H/M/L: all p > 0.96).
(d). Stage-dependent correlations between body mass and telomere length
The changing correlations between phenotypic and physiological traits over time are reported in electronic supplementary material, S7 and figure S6. While a clear positive correlation between body mass and telomere length was apparent in embryos (r = 0.55, p < 0.001, N = 60), this relationship tended to be negative just after hatching (r = −0.29, p = 0.08, N = 38) and non-significant later in postnatal development (day 20: r = −0.13, p = 0.32, N = 61; day 60: r = −0.03; p = 0.80, N = 61).
4. Discussion
Our results highlight the importance of both the pace and stability of embryo development in determining not only the ‘initial’ telomere length (i.e. the length at birth), but also how it then changes through to adulthood. Our experimental treatments altered the pace of development and metabolism in the predicted direction without affecting hatching success or postnatal growth rate. Therefore, any effect we observed on telomere lengths is unlikely to be driven by selective mortality or differences in postnatal size/growth pattern between experimental groups. We found no evidence that the impact of prenatal conditions on telomere length was driven by differences in oxidative stress, although increased prenatal exposure to glucocorticoids could be an important factor mediating the impact of developmental instability on telomere length.
(a). Developmental and postnatal consequences of incubation temperature and stability
The incubation temperature affected the pace of development and metabolism in the expected direction, with higher temperatures increasing embryo metabolic rate (estimated here using heart rate) and growth rate and reducing the time to hatching. Unstable incubation conditions produced an overall developmental rate that was relatively similar to that of the low incubation temperature group, suggesting that the average daily temperature is the main driver of average developmental speed since the U and L groups shared the same daily average. Embryo heart rate in the unstable group was intermediate between that of the low- and medium-temperature groups, probably because we conducted our measurements outside incubation recess periods (i.e. at 37.7°C, the same temperature as the medium group). However, the experimental incubation temperature regimes did not affect either the hatching success or size of the hatched chicks, in contrast to a recent study that found a reduced viability and hatching size of Japanese quail eggs incubated at a lower temperature (36.0°C) than our low-temperature treatment (37.0°C) [46].
(b). Prenatal growth rate and stability as determinants of telomere length and dynamics
While neither embryo growth rate nor the stability of incubation conditions influenced telomere length measured in late embryos (at stED13, i.e. 75% of prenatal development), both factors clearly affected perinatal telomere dynamics since embryos growing fast (H group, see [30] for similar results) or under less stable conditions (U group) exhibited shorter telomeres at day 1 post-hatching (ca 5 days later). The fact that telomerase activity declines toward the end of embryo development [47] could be one reason explaining why the effects of our treatments only became apparent by the time of hatching. This hypothesis is supported by the lack of significant difference in telomere length between embryos of the same age (but at different developmental stages (i.e. ED13)), and also by the fact that while the correlation between body mass and telomere length was positive in embryos, it became negative just after hatching. One initial hypothesis to explain the perinatal shortening observed in response to fast growth (i.e. high temperature) or developmental instability (i.e. unstable temperature) was that differences in oxidative stress levels might occur between groups, leading to effects on telomere dynamics [13]. Indeed, both high metabolic rate (i.e. here linked to high incubation temperature) and variations in metabolic rate resembling the hibernation-arousal situation (i.e. here experienced by embryos from the unstable group due to repeated shifts from low to high temperature) have been linked to increased oxidative stress levels [48,49]. However, our results on oxidative damage to DNA (i.e. no significant differences between groups in DNA damage before day 20 post-hatching) do not support this hypothesis. Prenatal exposure to glucocorticoids has been shown to accelerate telomere shortening [27], and therefore variation in prenatal glucocorticoid levels linked to incubation temperature stability could contribute to the observed differences in hatching telomere length, since we found that embryos experiencing unstable incubation conditions had higher plasma glucocorticoid levels and shorter telomeres at hatching. This effect on telomeres could be a consequence of either reduced telomerase activity ([50], but see [51]) or reprogramming of cellular metabolism as recently proposed by the metabolic telomere attrition hypothesis [35]. However, shorter telomeres at hatching in the high-temperature group could not be explained neither by differences in prenatal glucocorticoid exposure, nor by oxidative stress (see above). Instead, they may be related to the increased rate of cellular division likely associated with their fast metabolism and embryonic growth. Since the size at hatching did not differ between groups, it is possible that fast embryonic growth increased the rate of telomere loss per round of cellular division. It is worth noting that captive Japanese quails have been artificially selected over the long-term for early life productivity and not for lifespan. Consequently, there might be little selection for telomere maintenance in this model species. Therefore, it is possible that the effects observed in the present study may be more pronounced than those observed in wild animals under similar conditions (e.g. see [30] for similar results but a weaker effect size in a wild bird species).
The prenatal treatment also affected early postnatal telomere dynamics, with chicks from the high and unstable temperature treatment groups showing no significant shortening from day 1 to day 20 while low and medium temperature ones did. It is possible that compensatory mechanisms (e.g. telomerase activity) were triggered following the initial quick perinatal decline in telomere length observed in these groups, yet without being able to fully compensate for their initially shorter telomeres. Indeed, most of the original differences observed in telomere length at hatching between experimental groups remained relatively unchanged until adulthood. While postnatal telomere shortening rate might be more variable between individuals living in the wild, there is evidence that the rank order of telomere lengths across individuals is retained over time even in natural conditions [52]. Our results therefore support the fetal programming of telomere biology hypothesis [10] and prove experimentally the importance of the prenatal stage in determining telomere length over the life course of individuals [10,53].
5. Conclusion
Since short telomeres have been associated with reduced chances of survival [19,21] and increased risks of ageing-related diseases [53], our study supports the hypothesis that environmental conditions experienced during embryonic life influence future survival prospects and health state through their effects on ‘initial’ telomere length and subsequent postnatal shortening. We show that both the pace and stability of embryonic growth can affect telomere length, and that the perinatal period appears to be a critical period determining telomere length and dynamics up to adulthood. While the exact mechanisms behind this perinatal telomere shortening and its long-term effects on lifespan and disease risk remain to be identified, our model offers important opportunities to identify these mechanisms and test potential intervention strategies to prevent perinatal telomere shortening.
Supplementary Material
Acknowledgements
We are grateful to Mark Haussmann, Winnie Boner and Kate Griffith for help in setting up TRF methodology, and to Franklin Lo, Becky Shaw and Katie Byrne for their help in collecting body mass data. We are particularly grateful to the late Graham Law for his dedication and inspiring approach to ethics and animal welfare, as well as to his team for taking care of animal husbandry. We are grateful to José Noguera and three anonymous reviewers for useful comments on a previous draft. Finally, A.S. is grateful to the crew of the Marion Dufresnes and the French Polar Institute (IPEV) for hosting him from 56 to 21° South while writing the manuscript.
Data accessibility
The dataset used in this manuscript is available at: https://doi.org/10.6084/m9.figshare.9994829.v1 [54].
Authors' contributions
A.S. designed the study, conducted the experimental work, data analysis and wrote the manuscript. P.M. and N.B.M. had input on study design and data analysis, and commented on the manuscript.
Competing interests
We declare having no competing interests.
Funding
The project was funded by a Marie Skłodowska-Curie Postdoctoral Fellowship (grant no. 658085) to A.S., and A.S. was supported by a ‘Turku Collegium for Science and Medicine’ Fellowship at the time of writing.
References
- 1.Barker DJ, Martyn CN. 1991. The maternal and fetal origins of cardiovascular disease. J. Epidemiol. Community Health 46, 8–11. ( 10.1136/jech.46.1.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarry-Adkins JL, Ozanne SE. 2017. Nutrition in early life and age-associated diseases. Ageing Res. Rev. 39, 96–105. ( 10.1016/j.arr.2016.08.003) [DOI] [PubMed] [Google Scholar]
- 3.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. [DOI] [PubMed] [Google Scholar]
- 4.Metcalfe N, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe N, Monaghan P. 2003. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 38, 935–940. ( 10.1016/S0531-5565(03)00159-1) [DOI] [PubMed] [Google Scholar]
- 6.Ozanne SE, Hales CN. 2004. Lifespan: catch-up growth and obesity in male mice. Nature 427, 411–412. ( 10.1038/427411b) [DOI] [PubMed] [Google Scholar]
- 7.Tarry-Adkins JL, Ozanne SE. 2014. The impact of early nutrition on the ageing trajectory. Proc. Nutr. Soc. 73, 289–301. ( 10.1017/S002966511300387X) [DOI] [PubMed] [Google Scholar]
- 8.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153, 1194–1217. ( 10.1016/j.cell.2013.05.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wust S, Wadhwa PD. 2011. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl Acad. Sci. USA 108, E513–E518. ( 10.1073/pnas.1107759108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Entringer S, de Punder K, Buss C, Wadhwa PD. 2018. The fetal programming of telomere biology hypothesis: an update. Phil. Trans. R. Soc. B 373, 20170151 ( 10.1098/rstb.2017.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monaghan P, Ozanne SE. 2018. Somatic growth and telomere dynamics in vertebrates: relationships, mechanisms and consequences. Phil. Trans. R. Soc. B 373, 20160446 ( 10.1098/rstb.2016.0446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Lange T, Lundblad V, Blackburn EH. 2006. Telomeres. New York, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 13.Reichert S, Stier A. 2017. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 13, 20170463 ( 10.1098/rsbl.2017.0463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shay JW, Reddel RR, Wright WE. 2012. Cancer and telomeres—an ALTernative to telomerase. Science 336, 1388–1390. ( 10.1126/science.1222394) [DOI] [PubMed] [Google Scholar]
- 15.Gomes NMV, Shay JW, Wright WE. 2010. Telomere biology in Metazoa. FEBS Lett. 584, 3741–3751. ( 10.1016/j.febslet.2010.07.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stier A, Reichert S, Criscuolo F, Bize P. 2015. Red blood cells open promising avenues for longitudinal studies of ageing in laboratory, non-model and wild animals. Exp. Gerontol. 71, 118–134. ( 10.1016/j.exger.2015.09.001) [DOI] [PubMed] [Google Scholar]
- 17.Benetos A, . et al. . 2013. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 12, 615–621. ( 10.1111/acel.12086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spurgin LG, Bebbington K, Fairfield EA, Hammers M, Komdeur J, Burke T, Dugdale HL, Richardson DS. 2017. Spatio-temporal variation in lifelong telomere dynamics in a long-term ecological study. J. Anim. Ecol. 282, 20142263 ( 10.1111/1365-2656.12741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. 2003. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395. ( 10.1016/S0140-6736(03)12384-7) [DOI] [PubMed] [Google Scholar]
- 20.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, Boonekamp JJ. 2018. The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Phil. Trans. R. Soc. B 373, 20160447 ( 10.1098/rstb.2016.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Chronic infection. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 23.Fairlie J, Holland R, Pilkington JG, Pemberton JM, Harrington L, Nussey DH. 2015. Lifelong leukocyte telomere dynamics and survival in a free-living mammal. Aging Cell 15, 140–148. ( 10.1111/acel.12417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons MJ, . P. 2015. Questioning causal involvement of telomeres in aging. Ageing Res. Rev. 24, 191–196. ( 10.1016/j.arr.2015.08.002) [DOI] [PubMed] [Google Scholar]
- 25.Muñoz-Lorente MA, Cano-Martin AC, Blasco MA. 2019. Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat. Commun. 10, 1–14. ( 10.1038/s41467-019-12664-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarry-Adkins JL, Martin-Gronert MS, Chen JH, Cripps RL, Ozanne SE. 2008. Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. FASEB J. 22, 2037–2044. ( 10.1096/fj.07-099523) [DOI] [PubMed] [Google Scholar]
- 27.Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. 2012. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. R. Soc. B 279, 1447–1456. ( 10.1098/rspb.2011.1913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allison BJ, Tarry-Adkins JL, Ozanne SE, Giussani DA. 2016. Divergence of mechanistic pathways mediating cardiovascular aging and developmental programming of cardiovascular disease. FASEB J. 30, 1968–1975. ( 10.1096/fj.201500057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguera JC, Metcalfe NB, Reichert S, Monaghan P. 2016. Embryonic and postnatal telomere length decrease with ovulation order within clutches. Sci. Rep. 6, 25915 ( 10.1038/srep25915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vedder O, Verhulst S, Zuidersma E, Bouwhuis S. 2018. Embryonic growth rate affects telomere attrition: an experiment in a wild bird. J. Exp. Biol. 221, 181586 ( 10.1242/jeb.181586) [DOI] [PubMed] [Google Scholar]
- 31.Stier A, Delestrade A, Bize P, Zahn S, Criscuolo F, Massemin S. 2016. Investigating how telomere dynamics, growth and life history covary along an elevation gradient in two passerine species. J. Avian Biol. 47, 134–140. ( 10.1111/jav.00714) [DOI] [Google Scholar]
- 32.Deeming DC. 2002. Nests, eggs, and incubation: new ideas about avian reproduction. Oxford Ornithology Series; Oxford, UK: Oxford University Press. [Google Scholar]
- 33.Fowden AL, Forhead AJ, Coan PM, Burton GJ. 2008. The placenta and intrauterine programming. J. Neuroendocrinol. 20, 439–450. ( 10.1111/j.1365-2826.2008.01663.x) [DOI] [PubMed] [Google Scholar]
- 34.Smith SM, Nager RG, Costantini D. 2016. Meta-analysis indicates that oxidative stress is both a constraint on and a cost of growth. Ecol. Evol. 6, 2833–2842. ( 10.1002/ece3.2080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casagrande S, Hau M. 2019. Telomere attrition: metabolic regulation and signalling function? Biol. Lett. 15, 20180885 ( 10.1098/rsbl.2018.0885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romao JM, de Moraes TGV, de Teixeira RSC, Buxade CC, Cardoso WM. 2010. Incubation of Japanese quail eggs at different temperatures: hatchability, hatch weight, hatch time and embryonic mortality. Arch. Vet. Sci. 14, 155–162. ( 10.5380/avs.v14i3.14887) [DOI] [Google Scholar]
- 37.Ottinger MA. 2001. Quail and other short-lived birds. Exp. Gerontol. 36, 859–868. ( 10.1016/S0531-5565(00)00246-1) [DOI] [PubMed] [Google Scholar]
- 38.Orcutt FS Jr, Orcutt AB. 1976. Nesting and parental behavior in domestic common quail. The Auk 93, 135–141. [Google Scholar]
- 39.Lothery CJ, Thompson CF, Lawler ML, Sakaluk SK. 2014. Food supplementation fails to reveal a trade-off between incubation and self-maintenance in female house wrens. PLoS ONE 9, e106260 ( 10.1371/journal.pone.0106260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reichert S, Criscuolo F, Verinaud E, Zahn S, Massemin S. 2013. Telomere length correlations among somatic tissues in adult zebra finches. PLoS ONE 8, e81496 ( 10.1371/journal.pone.0081496.t004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt JE, Heidinger BJ. 2016. Telomere correlations during early life in a long-lived seabird. Exp. Gerontol. 85, 1–18. ( 10.1016/j.exger.2016.09.011) [DOI] [PubMed] [Google Scholar]
- 42.Sheldon EL, McCowan LSC, McDiarmid CS, Griffith SC. 2018. Measuring the embryonic heart rate of wild birds: an opportunity to take the pulse on early development. Auk 135, 71–82. ( 10.1642/AUK-17-111.1) [DOI] [Google Scholar]
- 43.Stier A, Schull Q, Bize P, Lefol E, Haussmann M, Roussel D, Robin J-P, Viblanc VA. 2019. Oxidative stress and mitochondrial responses to stress exposure suggest that king penguins are naturally equipped to resist stress. Sci. Rep. 9, 8545 ( 10.1038/s41598-019-44990-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kärkkäinen T, Bize P, Stier A. 2020. Correlation in telomere lengths between feathers and blood cells in pied flycatchers. J. Avian Biol. 51, jav.02300 ( 10.1111/jav.02300) [DOI] [Google Scholar]
- 45.Stoffel MA, Nakagawa S, Schielzeth H. 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. ( 10.1111/2041-210X.12797) [DOI] [Google Scholar]
- 46.Ben-Ezra N, Burness G. 2017. Constant and cycling incubation temperatures have long-term effects on the morphology and metabolic rate of Japanese quail. Physiol. Biochem. Zool. 90, 91–105. ( 10.5061/dyad.76qt1) [DOI] [PubMed] [Google Scholar]
- 47.Ulaner GA, Hu J-F, Vu TH, Giudice LC, Hoffman AR. 1998. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 58, 4168–4172. [PubMed] [Google Scholar]
- 48.Hermes-Lima M, Zenteno-Savín T. 2002. Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp. Biochem. Physiol. Part C 133, 537–556. ( 10.1016/S1532-0456(02)00080-7) [DOI] [PubMed] [Google Scholar]
- 49.Stier A, Bize P, Habold C, Bouillaud F, Massemin S, Criscuolo F. 2014. Mitochondrial uncoupling prevents cold-induced oxidative stress: a case study using UCP1 knockout mice. J. Exp. Biol. 217, 624–630. ( 10.1242/jeb.092700) [DOI] [PubMed] [Google Scholar]
- 50.Choi J, Fauce SR, Effros RB. 2008. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav. Immun. 22, 600–605. ( 10.1016/j.bbi.2007.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES. 2012. Chronic stress elevates telomerase activity in rats. Biol. Lett. 8, 1063–1066. ( 10.1098/rsbl.2012.0747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bichet C, Bouwhuis S, Bauch C, Verhulst S, Becker PH, Vedder O. 2020. Telomere length is repeatable, shortens with age and reproductive success, and predicts remaining lifespan in a long-lived seabird. Mol. Ecol. 29, 429–441. ( 10.1111/mec.15331) [DOI] [PubMed] [Google Scholar]
- 53.Aviv A, Shay JW. 2018. Reflections on telomere dynamics and ageing-related diseases in humans. Phil. Trans. R. Soc. B 373, 20160436 ( 10.1098/rstb.2016.0436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stier A, Metcalfe N, . B, Monaghan P.. 2020. Data from: Pace and stability of embryonic development affect telomere dynamics: an experimental study in a precocial bird model. Figshare ( 10.6084/m9.figshare.9994829.v1) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Stier A, Metcalfe N, . B, Monaghan P.. 2020. Data from: Pace and stability of embryonic development affect telomere dynamics: an experimental study in a precocial bird model. Figshare ( 10.6084/m9.figshare.9994829.v1) [DOI]
Supplementary Materials
Data Availability Statement
The dataset used in this manuscript is available at: https://doi.org/10.6084/m9.figshare.9994829.v1 [54].