Abstract
In humans and other mammals, defensive responses to danger vary with threat imminence, but it is unknown how those responses affect decisions to help conspecifics. Here, we manipulated threat imminence to investigate the impact of different defensive states on human helping behaviour. Ninety-eight healthy adult participants made trial-by-trial decisions about whether to help a co-participant avoid an aversive shock, at the risk of receiving a shock themselves. Helping decisions were prompted under imminent or distal threat, based on temporal distance to the moment of shock administration to the co-participant. Results showed that, regardless of how likely participants were to also receive a shock, they helped the co-participant more under imminent than distal threat. Reaction times and cardiac changes during the task supported the efficacy of the threat imminence manipulation in eliciting dissociable defensive states, with faster responses and increased heart rate during imminent compared to distal threats. Individual differences in empathic concern were specifically correlated with helping during imminent threats. These results suggest that defensive states driving active escape from immediate danger may also facilitate decisions to help others, potentially by engaging neurocognitive systems implicated in caregiving across mammals.
Keywords: altruism, prosocial, defensive state, freezing, fight-or-flight, fear
1. Introduction
In situations that threaten our health or life, the motivation to protect ourselves may conflict with that to protect others (for example, the desire to avoid injury or death may keep us from jumping on the train tracks to save someone who fell in). Decisions to help others in such situations differ from more common forms of everyday helping, in that they require the integration of two highly salient cues—the danger to ourselves, and the distress of someone in need. In humans, investigation of the neurocognitive bases of helping has mainly focused on the latter (i.e. on how empathy for others' distress triggers motivation to help) [1,2]. However, the role of concurrent defensive responses to danger has been overlooked. Especially, it is unknown how situational aspects like imminence of danger impact subsequent decisions to help (in the example above, whether the train is fast approaching or still far away).
Despite their risk, decisions to help others under threat are observed in humans [3,4], and other primates [5]. A dominant view in psychology is that empathy is the key proximate mechanism driving these decisions, as the embodied representation of another individual's distress triggers situation-specific behaviours to alleviate it [2,6]. In a broader evolutionary framework, the capacity to resonate with others' emotions is argued to have evolved from the mother–offspring bond, and reflects the functioning of a mammalian offspring care system [7]. This system allows for cues of vulnerability and distress to be quickly recognized, and to trigger caregiving behaviours that can sometimes generalize towards non-kin [7,8]. In support of the empathy–care–altruism link, it has been demonstrated that individuals higher on empathic concern, a component of empathy thought to reflect feelings of care and compassion [1,9], are more likely to give up money [10] or take painful electric shocks to reduce the pain of others (particularly ingroup members [11]). Moreover, greater overlap in neural activation between experienced and observed distress has been linked with real-life extraordinary altruistic behaviours, like non-directed organ donations [12,13].
With few exceptions [11,14], the majority of experimental studies on human altruism have assessed actions that benefit others at a variable cost, usually monetary [10,15–17], but in the absence of immediate physical risk. Some studies have investigated altruism in risky contexts specifically, but measured decisions to help avatars in virtual reality environments, rather than real conspecifics under real threat [18]. It has been argued that there are not only obvious ethical and practical challenges in recreating real-life dangerous situations in the laboratory, but the neurocognitive processes involved in less risky altruistic actions are likely to be the same; thus, the experimental study of those lower risk actions may inform about the processes implicated in more extreme forms of altruism [8]. Nevertheless, the absence of immediate physical risk in experimental studies may obscure the contribution of psychological processes other than empathy on helping under threat, most notably that of defensive processes.
Previous research on stress has suggested that defensive states can impact prosocial decisions [19]. Evidence of a facilitating effect of stress on prosociality has been obtained in studies that induced stress through paradigms like the Trier Social Stress Test, and measured subsequent increase in prosocial behaviour in economic exchange games [20–23] and hypothetical moral decisions [24]. The extent to which stress drives prosocial versus antisocial outcomes has also been associated with individual differences in, for instance, physiological responses to the stressor [25] and ability to empathize with the other [26]. Despite these previous indications that defensive responses to acute stress may influence prosocial action, the potential mechanisms behind those effects are unclear. Also, induced stress paradigms are not entirely comparable to real-life threatening scenarios wherein there is immediate physical risk associated with helping.
It has been proposed that, in humans and other mammals, defensive responses triggered by potentially harmful events are part of a defence continuum, whereby graded behavioural, physiological and neural responses are produced as a function of threat imminence. Distal or unpredictable threats trigger risk assessment that allows for slower, flexible and more strategic escape decisions; as threat imminence increases, more fixed and species-specific behaviours are activated, such as freezing or, when immediate avoidance is necessary, fight-or-flight [27–30]. In recent years, neural correlates of these defensive states have been described in humans. Functional imaging studies have shown that distal, retreating or slow-moving threats preferentially engage so-called cognitive fear circuits, which include the ventromedial prefrontal cortex, posterior cingulate, basolateral amygdala and hippocampus [31–33]. Proximal, looming or fast-moving threats instead engage a ‘reactive fear’ network, including the central amygdala, periaqueductal grey, middle cingulate cortex, hypothalamus and anterior insula, which coordinates faster and more reflexive escape decisions [31–34]. The flow of information between these neural circuits enables adaptive switches between different defensive states as a function of dynamic threat properties like spatial proximity, direction or speed of movement [35], which ultimately inform the animal about the imminence of danger.
Although previous research has characterized how different defensive states impact individual escape decisions [33], it remains unknown how those states affect decisions towards others in threatening situations, namely whether to help them or not. In this pre-registered study (https://osf.io/fxmns), we tried to answer this outstanding question using threat imminence as a tool to induce different defensive states. To do so, we developed a novel paradigm in which participants were asked to make trial-by-trial decisions about whether or not to help a co-participant (a confederate) avoid aversive electrical shocks, at the risk of also receiving a shock. Importantly, helping decisions were prompted under distal or imminent threat, based on the moment of shock administration to the co-participant.
Our first question was whether the willingness to help the co-participant avoid the shock was affected by threat imminence. More frequent helping decisions under distal threat would suggest that defensive states associated with the engagement of slower and more flexible decision processes facilitates helping behaviour under threat. Conversely, increased helping under imminent threat would indicate reactive defensive responses to immediate danger promote altruistic helping, in line with previous demonstrations that acute stress fosters prosocial behaviour [19].
Our second question concerned the impact of risk level on the frequency of helping behaviour. A recent study assessing avoidance of slow and fast virtual predators reported shock intensity only impacted escape decisions to slow, but not fast predators [33]. In line with these findings, we predicted the risk level of receiving a shock following a help decision would only impact decisions during distal, but not imminent threats.
Our third and final question concerned the influence of individual differences in empathy on helping decisions under threat. Previous research has supported a link between altruism and empathic concern [10,11,36], a component of empathy that reflects other-focused concern and motivation to alleviate their distress [37]. We thus hypothesized that higher scores on a trait measure of empathic concern would be associated with more frequent helping behaviour. Importantly, we predicted empathic concern would be more strongly associated with helping under imminent threat. Since higher threat imminence favours the activation of rapid and reflexive responses, it presumably hinders the engagement of slower and more taxing processes like cognitive control and emotional regulation [31,33]. Those processes may, in addition to empathic concern, contribute to decisions to help others in threatening contexts [38]; if they are hindered under imminent compared to distal threat, we would expect empathic concern to be the key individual motivator of helping decisions in situations of higher threat imminence.
2. Methods
(a). Participants
Based on power calculations performed on G × Power 3.1.9.2, a sample of 123 individuals was initially pre-registered to ensure 80% power to detect an r of at least 0.25 at α = 0.05 on correlation analyses. However, due to unforeseen logistical limitations (resources and availability of the researcher playing the role of confederate), data collection was interrupted at n = 98. Based on the initial power calculations, this sample size was still sufficient to guarantee 80% power to detect small-medium effects on the pre-registered ANOVA, including main effects of within- (n = 36) and between-subject (n = 98) factors and interaction (n = 52), as well as to detect an r = 0.3 (n = 83) at α = 0.05.
One hundred healthy volunteers were recruited through flyers on- and off-campus, and local online recruitment systems. Two participants were excluded from the study due to doubts about the veracity of the experiment. The remaining 98 participants were randomly assigned to two experimental groups (high and low risk), based on the risk level of helping decisions in the experimental task (more details below). Groups did not significantly differ in age or empathic concern scores (table 1). All participants were screened for a history of psychiatric and neurological diagnoses, brain injuries and substance abuse. Participants provided informed consent prior to the experiment and were compensated with two movie tickets. The study was approved by the regional ethics board in Stockholm, Sweden.
Table 1.
Sample gender, age and empathic concern descriptive statistics (M and s.d.).
| full sample (n = 98) | high-risk group (n = 50) | low-risk group (n = 48) | |
|---|---|---|---|
| gender breakdown | 56 F | 25 F | 31 F |
| age, M (s.d.) | 26.04 (4.53) | 26.53 (4.21) | 25.56 (4.84) |
| empathic concern, M (s.d.) | 14.67 (7.89) | 14.22 (7.90) | 15.14 (7.97) |
(b). Experimental task
In each testing session, a participant and a confederate (henceforth, co-participant) were informed the experiment consisted of two parts, which would be randomly assigned to each one of them by a coin flip. Participant and co-participant were then accompanied to separate testing rooms and did not interact again during the experiment (detailed information about testing procedures and debriefing are available in the electronic supplementary material).
Participants performed a task wherein they made trial-by-trial decisions about whether or not to help the co-participant avoid aversive electrical shocks to the wrist, at the risk of also being shocked (figure 1). Threat imminence was manipulated based on previous work [32,34], by varying the spatial position and movement of a visual cue signalling either threat (a spider, associated with the shock) or safety (a butterfly, not associated with the shock) on a computer screen. In addition, a webcam feed of the co-participant was presented on the screen throughout the task. Unknown to the participant, the video feed was in fact pre-recorded, and edited to select unique clips for each trial of the task (more details about the video stimuli provided in electronic supplementary material).
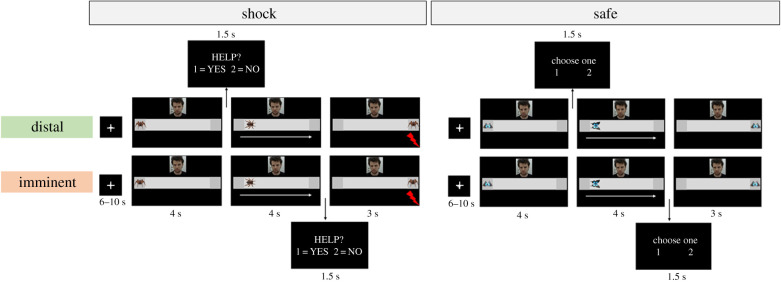
Figure 1.
Schematic of the experimental task. Participants made trial-by-trial decisions about whether or not to help a co-participant avoid an aversive electrical shock, at the risk of receiving a shock themselves. The task included shock (left panel) and safe trials (right panel). Responses were prompted either in the beginning of the trial, when the visual cue was static on the left side (distal), or at the end of the trial, after the visual cue had moved to an endpoint on the right, and thus immediately before shock delivery (imminent). In the schematic, this is represented by the response slides and vertical arrows signalling when responses were prompted. (Online version in colour.)
Participants were informed that, throughout the experiment, they and the co-participant would see the same screen. Each trial started with a static cue on the left side of the screen (4 s), which then moved to the right (4 s). In shock trials, the co-participant would be administered an aversive shock to the wrist when the cue reached the right end of the screen, unless participants decided to help him. To decide whether they wanted to help the co-participant avoid the upcoming shock, participants made forced-choice responses by pressing 1 (Help) or 2 (Don't help) on the keyboard as soon as the response slide was displayed (1.5 s). Responses were prompted sometimes in the beginning of the trial, when the visual cue was static on the left side (distal threat), and other times at the end of the trial, after the visual cue had moved to an endpoint on the right, and thus immediately before shock delivery (imminent threat). Outcomes of participants' decisions were as follows: if they chose not to help, the co-participant would always receive a shock; if they chose to help, there would be a predefined probability of both participant and co-participant receiving a shock (30% in the low-risk group and 70% in the high-risk group). These contingencies were intended to simulate potential outcomes of offering assistance to others in a real-life dangerous situation, wherein helping others may not always be successful, putting both the helper and the helped at risk. Participants were instructed to respond as quickly as possible. Also, to discourage missed responses, they were informed that a shock would be delivered to both participants (with 100% chance) whenever a response was not detected. Shock administration always happened at the end of the trial, and participants were able to see the outcome of their decisions on the screen (i.e. the co-participant receiving or not receiving a shock; 3 s).
Safe trials followed an identical structure, with response slides presented at distal or imminent stages in relation to the end of the trial. However, participants were instructed that no shocks would be given and they should arbitrarily choose to press 1 or 2 when the response slide was displayed. It was made clear to them that their choice would have no consequences for them or the co-participant. The inclusion of responses on safe trials was meant to make them match the shock condition, especially given indication that physiological arousal may increase not only in response to emotional content but also increased task demands [39].
The task included 40 trials split into four 10-trial blocks. Two blocks included distal trials (decisions to help were prompted under distal threat) and two included imminent trials (decisions to help prompted under imminent threat). The order of blocks was randomized across participants. Safe and shock trials (20 of each) were randomized within blocks. The task was programmed and delivered using E-prime 2.0 (Psychology Software Tools, Inc., www.pstnet.com).
(c). Questionnaire measures
After the experimental task, participants completed the Interpersonal Reactivity Index (IRI) [37], a self-report measure of empathic tendencies. It consists of four subscales (Empathic concern, Personal distress, Perspective taking and Fantasy), and each item is rated on a 5-point Likert scale ranging from ‘Does not described me well’ to ‘Describes me very well’. We were specifically interested in the Empathic concern subscale, thought to reflect a tendency to feel compassion or concern for others. Participants were also given a number of post-task questions designed to assess the believability of the experiment (see electronic supplementary material).
(d). Recording and pre-processing of physiological measures
Delivery of electrical stimulation and recording of physiological responses were done using BIOPAC systems (Santa Barbara, CA, USA) and AcqKnowledge 4.1.1. software. Electrical shocks consisted of a 100 ms DC-pulse delivered through two electrodes placed on the participant's right wrist. Shocks were individually calibrated to an intensity level ‘not painful, but very uncomfortable’. Heart rate was collected through two electrodes on the collarbones and one ground electrode placed on the ankle. Heart rate recordings were visually inspected for quality. Trials where the QRS complex was unclear or with movement-related artefacts were removed from analysis. Additionally, participants with more than six unusable trials (30%) were dropped from the analysis. Automated peak detection and stimulus–response algorithms implemented in Acqknowledge were used to identify R-peaks, and to calculate heart rate (in beats per minute, bpm) in the 4 s time window after the onset of distal and imminent events, as well as in the 6 s window after fixation onset, which was used as baseline. Changes in heart rate related to distal and imminent events were baseline-corrected and used in the statistical analysis. Heart rate was also pre-processed and analysed using a model-based approach integrated in the Matlab-based software PsPM (Psycho-Physiological Modelling) v. 4.2.1 [40]. The PsPM approach models evoked heart period (ms) and not heart rate (bpm), and assigns each heart period to the following heartbeat. The pre-processed time-series is then analysed using a GLM approach. These data were used to plot average cardiac changes over time during the task (figure 3c; more details about this method provided in electronic supplementary material).
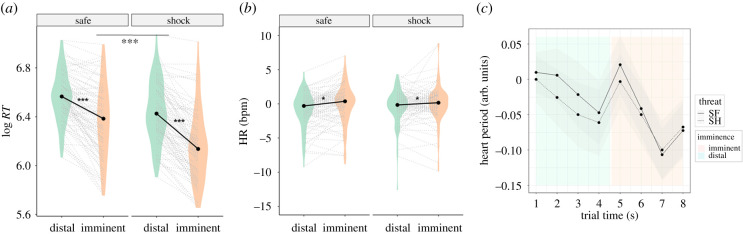
Figure 3.
(a) Violin plot depicting the reaction times (log RT), showing responses were faster during threat than safe trials, and imminent than distal threats; dotted lines represent individual data from both groups (main effects of imminence and threat, ***p < 0.001). (b) Violin plot depicting heart rate (HR) in bpm, showing HR was slower during distal compared to imminent trials; dotted lines represent individual data from both groups (main effect of imminence, *p = 0.015). (c) Heart period over time (note that heart period interpretation is inverse to rate, with lower values indicating increase in heart rate). (Online version in colour.)
(e). Statistical analysis
(i). Pre-registered analyses
Our key dependent variable was helping behaviour. Helping behaviour was operationalized as the percentage of helping responses per individual in each condition. To assess the impact of risk level and threat imminence on helping under threat, helping percentages were analysed in a mixed factor Type III ANOVA with imminence (distal, imminent) as within-subjects factor, and risk level (low-risk group, high-risk group) as between-subject factor. To assess the link between empathic traits and helping, we ran correlations between IRI's empathic concern score and helping percentage in distal and imminent conditions. We additionally ran follow-up correlations (Sidak-corrected for multiple comparisons) within each group.
(ii). Analyses not included in the pre-registration
Additional analyses were carried out to (i) provide confirmatory validation of the effects, (ii) provide more complete information about the data, and (iii) verify the effectiveness of the experimental manipulation.
Specifically, we analysed single trial dichotomous helping responses (help or no help) using a mixed-effects logistic regression. This approach allowed us to account for variation in helping behaviour explained by random sampling of participants and trial number (random effects), in addition to that explained by threat imminence and risk level (fixed effects). Mixed-effects approaches have been suggested to offer several advantages in relation to traditional repeated-measures ANOVAs [41], among which increasing the generalizability of research findings to other individuals and stimuli [42]. Our models predicted the likelihood of helping versus not helping on each trial, as a function of threat imminence, risk level and imminence × risk level interaction (fixed effects), as well as subject and trial number (random effects). Several models using bound optimization by quadratic approximation (bobyqa) were fitted and compared using likelihood ratio tests. We report results from the best fitting model as indexed by Akaike information criterion (AIC), which included fixed effects of threat imminence and risk level, and no threat imminence × risk level interaction (which was not significant).
To provide additional confirmation, we also repeated the aforementioned analyses (ANOVA, correlations and mixed-effects logistic regression) in a subsample excluding participants who had a 100% helping rate in both conditions (distal and imminent) (n = 32, n remaining = 66). The rationale for this decision was that participants who helped in 100% of the trials in both conditions might be at ceiling for reasons not related to the experimental manipulations (e.g. they did not find the shocks aversive enough), which could obscure the effects.
Finally, analyses to ascertain the effectiveness of the threat imminence manipulation were performed on reaction time and heart rate data, which provide important information about the underlying defensive state. Reaction times were expected to be slower during distal relative to imminent trials, following suggestions that defensive responses to lower/moderate threat imminence are characterized by behavioural immobility (i.e. freezing) in preparation of subsequent active avoidance if/when immediate escape becomes necessary (i.e. fight-or-flight) [43]. Heart rate deceleration or bradycardia has been frequently used as a proxy for freezing in humans, whereas subsequent tachycardia typically indexes active avoidance [43–46]. We thus verified whether distal compared to imminent trials were associated with differential heart rate.
Reaction times were log transformed and data from two participants excluded, one because they did not make any responses during safe trials, the other was an outlier (more than 2.5 s.d.s below the mean). Transformed values were then used in a threat (safe, shock) by imminence (distal, imminent) by risk level (high, low) ANOVA. Heart rate in bpm, time-locked to stimulus onset and baseline corrected, was entered in a threat (safe, shock) by imminence (distal, imminent) by risk level (high, low) ANOVA.
The significance threshold was set at α = 0.05, as pre-registered. Although the inclusion of additional statistical tests is known to alter the pre-specified Type I error rate, the added analyses are of great value to provide a more thorough picture of our data. For the sake of transparency, and in line with suggestions that more important information can be derived from treating p-values continuously rather than as thresholds [47], full p-values are reported throughout (for both pre-registered and additional analyses), in order to provide the reader with enough information to assess the value of the effects. Data and code are available on the OSF project page (https://osf.io/nb6cf/).
3. Results
(a). Threat imminence facilitated helping under threat
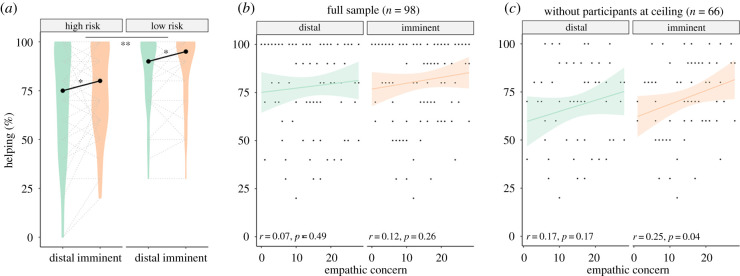
Results showed participants helped more under imminent compared to distal threat (F1,96 = 5.16, p = 0.025), and when the risk of receiving a shock was lower (30%) compared to higher (70%; F1,96 = 9.53, p = 0.003) (table 2 and figure 2). The imminence × risk level interaction was not statistically significant (F1,96 = 0.96, p = 0.33).
Table 2.
Descriptive statistics for helping responses (%).
| distal |
imminent |
|||
|---|---|---|---|---|
| M (s.d.) | Mdn | M (s.d.) | Mdn | |
| high-risk level (n = 50) | 71.2 (28.1) | 75 | 75.4 (22.3) | 80 |
| low-risk level (n = 48) | 85.6 (18.2) | 90 | 87.3 (17.6) | 95 |
Figure 2.
(a) Violin plots depicting helping percentage in each group, showing more frequent helping during imminent relative to distal threats, and in the low relative to high-risk group; dotted lines represent individual data (note: some participants have overlapping lines) (b,c). Scatter plots depicting the correlation between empathic concern and helping percentage in the full sample and subsample without participants at ceiling (*main effect of imminence, p = 0.025; **main effect of risk, p = 0.003). (Online version in colour.)
We validated these results by addressing the same question using a mixed-effects logistic regression on single trial dichotomous responses (help or no help). The results tracked closely with those of the ANOVA, revealing increased probability of helping under imminent threat (β = 0.14, s.e. = 0.04, z = 3.8, p = 0.0001, 95% CI [0.07, 0.21]), and when the risk of shock was lower (β = 1.83, s.e. = 0.71, z = 2.56, p = 0.010, 95% CI [0.43, 3.23]).
To further confirm these effects, we repeated the analyses after removing participants at ceiling (i.e. who helped the co-participant in 100% of both distal and imminent trials; n = 32). In the remaining subsample (n = 66), results were in the same direction, with participants helping more under imminent threat (ANOVA: F1,64 = 4.74, p = 0.033, logistic mixed regression: β = 0.13, s.e. = 0.04, z = 3.79, p = 0.0001, 95% CI [0.07, 0.21]), and when the risk was low (ANOVA: F1,64 = 5.81, p = 0.019; logistic mixed regression: β = 0.65, s.e. = 0.34, z = 1.93, p = 0.053, 95% CI [−0.009, 1.31]). Again, there was no indication of a significant imminence × risk level interaction (F1,64 = 0.48, p = 0.49).
Overall, these results indicate that individuals helped more in the low-risk group. Importantly, independent of the risk involved in the decision, increased threat imminence facilitated helping behaviour.
(b). Empathic concern and helping behaviour under imminent threat
To assess the association between helping behaviour and empathic tendencies, we performed correlations between the empathic concern score from the IRI ([37]) and helping percentage under distal and imminent threat. In the full sample (n = 98), these correlations were not statistically significant (distal: r = 0.07, p = 0.49; imminent: = 0.12, p = 0.26). Akin to previous analyses, we repeated the correlations after excluding the 32 participants at ceiling (final n = 66). This approach was deemed particularly useful here, given correlations are highly affected by extreme values. After removal of participants at ceiling, we observed higher EC was associated with increased helping behaviour during imminent (r = 0.25, p = 0.04), but not distal threats (r = 0.17, p = 0.17).
At an exploratory level, we also performed follow-up correlations (Sidak corrected) between helping responses and empathic concern within the high and low-risk groups, after exclusion of participants at ceiling. Specifically, for the high-risk group, empathic concern was not significantly associated with helping under either distal (r = 0.05, p = 0.75) or imminent threat (r = 0.08, p = 0.65). For the low-risk group, empathic concern was only associated with helping under imminent threat (r = 0.50, p = 0.007; distal threat: r = 0.38, p = 0.04). While exploratory and performed in a smaller subset of participants (38 in the high-risk group and 28 in the low-risk group), these additional analyses suggest the association between helping behaviour and empathic concern may, in addition to threat imminence, depend on risk level.
(c). Effectiveness of the threat imminence manipulation
(i). Increased threat imminence was associated with faster reaction times
To determine whether our threat imminence manipulation induced different defensive states, we analysed reaction times during the task. We found that responses were faster during imminent relative to distal trials (F1,94 = 220.87, p < 0.001), in shock compared to safe trials (F1,94 = 95.61, p < 0.001), and when the risk was low rather than high (F1,94 = 4.98, p = 0.028). We also observed a significant imminence × threat interaction (F1,94 = 15.96, p < 0.001), driven by faster reaction times under imminent versus distal trials in the threat compared to the safe condition (table 3 and figure 3a). There were no significant interactions with risk level (risk level by imminence: F1,94 = 0.17, p = 0.68; risk level by threat: F1,94 = 1.75, p = 0.20; risk level by imminence by threat: F1,94 = 1.95, p = 0.17).
Table 3.
Descriptive statistics for reaction times (ms) and heart rate (bpm).
| distal |
imminent |
|||
|---|---|---|---|---|
| SF | SH | SF | SH | |
| reaction time (ms) | 723 (146) | 646 (158) | 603 (177) | 492 (154) |
| heart rate (bpm) | −1.08 (3.64) | −0.61 (3.18) | −0.28 (3.43) | −0.33 (3.64) |
(ii). Increased threat imminence was associated with heart rate acceleration
To further confirm the effectiveness of the threat imminence manipulation, we compared heart rate during distal and imminent threats. Results showed heart rate was increased in response to imminent compared to distal trials (F1,88 = 6.04, p = 0.015) (table 3 and figure 3b). We also observed a significant imminence × risk level interaction (F1,88 = 5.35, p = 0.023), driven by a greater impact of imminence when the risk was low than high. The effect of threat and remaining interactions was not significant (threat: F1,88 = 0.43, p = 0.52; risk level × threat: F1,88 = 2.16, p = 0.15; imminence × threat: F1,88 = 2.38, p = 0.13; risk × imminence × threat: F1,88 = 0.22, p = 0.64).
Taken together, our reaction time and heart rate effects suggest the threat imminence manipulation successfully induced shifts between defensive states, with distal threats eliciting slower responses and bradycardia, and imminent threats eliciting faster responses and tachycardia.
4. Discussion
This study assessed whether willingness to help others in threatening situations is affected by threat imminence. We showed that, regardless of the risk involved, participants were more likely to help when the threat was imminent rather than distal, suggesting that defensive states elicited in situations of imminent danger may promote altruistic helping behaviour.
Akin to other mammals, humans respond to threatening stimuli gradually as a function of imminence [27,28]. When threat imminence increases, active avoidance is enabled by activation of neural circuits that coordinate fast and species-specific defensive behaviours (e.g. fight-or-flight). Our results suggest that the same way increased imminence triggers active escape from self-directed threats, it also promotes active helping when others are under threat. Human research examining the interplay between helping and defensive responses under real threat is lacking, but some animal studies have addressed the link between defence and caregiving, and its underlying mechanisms. At the neural level, switching from freezing to active avoidance as a function of threat imminence has been linked to oxytocin-mediated exchanges between the basolateral and central amygdala nuclei [48–51]. Notably, in rodents, central amygdala activation by oxytocin has also been shown to trigger maternal and caregiving behaviours in virgin females [52], and to enhance maternal defensive aggression [53,54]. More importantly, in rats, the ability to inhibit freezing in favour of active threat coping behaviours is necessary to allow females to engage in offspring defence [55]. These previous findings in animals demonstrate that neurohormonal circuits activated in situations of imminent danger are not only implicated in active individual defence, but also enable caregiving behaviours like offspring protection. While the discussion about underlying mechanisms remains speculative at this point, one possibility raised by our results is that, in humans, increased threat imminence may also enable defensive states that facilitate care to conspecifics in the form of defensive helping.
In previous animal studies, defensive helping has mainly been observed towards offspring [54,55], although there is evidence of harm aversion and helping of unfamiliar conspecifics in rodents [56,57] and non-human primates [58,59]. It has been suggested that human altruism and, at a more general level, the capacity to care for others' welfare, results from neurohormonal circuitry that evolved in mammals to support offspring care [7]. In highly social species, particularly those with high levels with alloparenting like primates, this circuitry presumably evolved to generalize to others beyond kin, and is involved in manifestations of care for strangers, such as costly helping in humans [8,60]. Our results suggested that individuals scoring higher in empathic concern, which reflects motivation to care for others’ wellbeing, helped a stranger more frequently under imminent threat, further supporting that increased threat imminence may engage processes implicated in caregiving motivation in humans.
Our results are also consistent with studies suggesting acute stress promotes subsequent prosocial behaviour in humans [19,21–24]. Of particular relevance are the findings reported by Tomova et al. [20], in which induced stress was associated with subsequent increased activation in the anterior insula and anterior midcingulate cortex in response to other's pain, regions that have also been shown to be critically implicated in ‘reactive fear’ circuits [33,34].
Our interpretation of the findings is based on the assumption that our threat manipulation successfully elicited different defensive states. Supporting this assumption is the fact that we manipulated threat imminence in a manner consistent with previous studies that demonstrated dissociable neural and behavioural responses in humans to distal versus imminent threats [31,34]. To further verify the manipulation was effective, we analysed response latency and heart rate as indicators of defensive state. Participants displayed faster responses and increased heart rate during imminent compared to distal threats, in line with evidence of behavioural immobility and fear bradycardia during freezing, and active avoidance and tachycardia during fight-or-flight [44,45,61–64].
In addition to hypotheses related to the impact of threat imminence and empathic concern on helping, we tested whether the risk level of helping affected decisions differently as a function of threat imminence. Our results showed participants helped more if the risk of shock was low, but provided no evidence for an interaction between risk and threat imminence, contrary to previous reports that shock level had a greater impact on escape decisions when facing slow versus fast predators [33]. Methodological differences between the two studies, including different outcome variable (help or escape responses), are likely to account for this discrepancy. The most critical difference is that, in our study, risk level was manipulated between subjects, ensuring that within-individual risk level and spatio-temporal distance of threat did not simultaneously contribute to imminence perception. Rather, our risk manipulation allowed us to assess the influence of overall dangerousness of the situation on helping behaviour.
One limitation of our study is the low threat value of the task relative to real threatening situations, which could compromise the generalizability of the findings to real life. Indeed, helping a co-participant avoid aversive shocks in a controlled and safe laboratory experiment does not entail the same risk as many real-life dangerous situations. This aspect may have contributed to the small effect size of imminence on helping, albeit significant and consistent with the size of effects expected in adequately powered psychological experiments like our own [65,66]. Importantly, the lack of evidence for an interaction between risk level and threat imminence across behavioural analyses suggests the effect of imminence on helping might persist in a higher risk context.
5. Conclusion
This study provides new evidence that situations of imminent danger can promote defensive helping in humans, potentially by activating neural circuitry also implicated in caregiving responses. Our study thus supports prior accounts suggesting acute stress or fight-or-flight states may facilitate prosocial and even heroic action in situations of immediate need [19]. Importantly, grounded on a threat imminence framework that allows for a more systematic examination of different defensive states, our results provide insights into the potential neurocognitive mechanisms behind the facilitating effect of threat imminence on helping. Additional research is warranted to replicate this effect, specifically using indices of neural function that can confirm the mechanisms implicated in helping decisions under threat.
Supplementary Material
Acknowledgements
We thank A. Walsh and A. Marsh for helpful comments on the manuscript. We also thank E. Estonius and J. Wall for assistance in pre-processing of physiology data.
Ethics
This study has been approved by the Swedish Ethical Review Authority (Etikprövningsmyndigheten; Dnr: 2019-04335). All research participants provided written consent.
Data accessibility
Data, code and materials are available on Open Science Framework (OSF; https://osf.io/nb6cf/).
Authors' contributions
J.B.V. conceived and designed the study, analysed data and drafted the manuscript. E.E. performed data collection and stimuli development. S.S. analysed data. A.O. provided critical input throughout all stages, including design and manuscript writing. All authors approved the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the Knut and Alice Wallenberg Foundation (KAW 495 2014.0237) and a Consolidator Grant (2018-00877) from the Swedish Research Council (Vetenskapsrådet) to A.O.
References
- 1.Batson CD, Fultz J, Schoenrade PA. 1987. Distress and empathy: two qualitatively distinct vicarious emotions with different motivational consequences. J. Pers. 55, 19–39. ( 10.1111/j.1467-6494.1987.tb00426.x) [DOI] [PubMed] [Google Scholar]
- 2.de Waal FBM, Preston SD. 2017. Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev. Neurosci. 18, 498–509. ( 10.1038/nrn.2017.72) [DOI] [PubMed] [Google Scholar]
- 3.Oliner SP. 2004. Do unto others: extraordinary acts of ordinary people, 312 p Boulder, CO: Basic Books. [Google Scholar]
- 4.Rand DG, Epstein ZG. 2014. Risking your life without a second thought: intuitive decision-making and extreme altruism. PLoS ONE 9, e109687 ( 10.1371/journal.pone.0109687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Waal FBM, Lanting F. 1998. Bonobo: the forgotten ape, 1st edn Berkeley, CA: University of California Press. [Google Scholar]
- 6.Batson CD. 2014. The altruism question: toward a social-psychological answer. New York, NY: Psychology Press. [Google Scholar]
- 7.Preston SD. 2013. The origins of altruism in offspring care. Psychol. Bull. 139, 1305–1341. ( 10.1037/a0031755) [DOI] [PubMed] [Google Scholar]
- 8.Marsh AA. 2019. The caring continuum: evolved hormonal and proximal mechanisms explain prosocial and antisocial extremes. Annu. Rev. Psychol. 4, 347–371. ( 10.1146/annurev-psych-010418-103010) [DOI] [PubMed] [Google Scholar]
- 9.Strauss C, Lever Taylor B, Gu J, Kuyken W, Baer R, Jones F, Cavanagh K. 2016. What is compassion and how can we measure it? A review of definitions and measures. Clin. Psychol. Rev. 47, 15–27. ( 10.1016/j.cpr.2016.05.004) [DOI] [PubMed] [Google Scholar]
- 10.Feldmanhall O, Dalgleish T, Mobbs D. 2013. Alexithymia decreases altruism in real social decisions. Cortex. 49, 899–904. ( 10.1016/j.cortex.2012.10.015) [DOI] [PubMed] [Google Scholar]
- 11.Hein G, Silani G, Preuschoff K, Batson CD, Singer T. 2010. Neural responses to ingroup and outgroup members' suffering predict individual differences in costly helping. Neuron 68, 149–160. ( 10.1016/j.neuron.2010.09.003) [DOI] [PubMed] [Google Scholar]
- 12.Brethel-Haurwitz KM, Cardinale EM, Vekaria KM, Robertson EL, Walitt B, VanMeter JW, Marsh AA. 2018. Extraordinary altruists exhibit enhanced self–other overlap in neural responses to distress. Psychol. Sci. 29, 1631–1641. ( 10.1177/0956797618779590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connell K, Brethel-Haurwitz KM, Rhoads SA, Cardinale EM, Vekaria KM, Robertson EL, Walitt B, VanMeter JW, Marsh AA. 2019. Increased similarity of neural responses to experienced and empathic distress in costly altruism. Sci. Rep. 9, 1–11. ( 10.1038/s41598-018-37186-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockwood PL, Hamonet M, Zhang SH, Ratnavel A, Salmony FU, Husain M, Apps MA. et al. 2017. Prosocial apathy for helping others when effort is required. Nat. Hum. Behav. 1, 0131 ( 10.1038/s41562-017-0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FeldmanHall O, Dalgleish T, Thompson R, Evans D, Schweizer S, Mobbs D. 2012. Differential neural circuitry and self-interest in real vs hypothetical moral decisions. Soc. Cogn. Affect. Neurosci. 7, 743–751. ( 10.1093/scan/nss069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gospic K, Sundberg M, Maeder J, Fransson P, Petrovic P, Isacsson G, Karlström A, Ingvar M. et al. 2014. Altruism costs—the cheap signal from amygdala. Soc. Cogn. Affect. Neurosci. 9, 1325–1332. ( 10.1093/scan/nst118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greening S, Norton L, Virani K, Ty A, Mitchell D, Finger E. 2014. Individual differences in the anterior insula are associated with the likelihood of financially helping versus harming others. Cogn. Affect. Behav. Neurosci. 14, 266–277. ( 10.3758/s13415-013-0213-3) [DOI] [PubMed] [Google Scholar]
- 18.Zanon M, Novembre G, Zangrando N, Chittaro L, Silani G. 2014. Brain activity and prosocial behavior in a simulated life-threatening situation. Neuroimage. 98, 134–146. ( 10.1016/j.neuroimage.2014.04.053) [DOI] [PubMed] [Google Scholar]
- 19.Buchanan TW, Preston SD. 2014. Stress leads to prosocial action in immediate need situations. Front. Behav. Neurosci. 8, 5 ( 10.3389/fnbeh.2014.00005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomova L, Majdandžic J, Hummer A, Windischberger C, Heinrichs M, Lamm C. 2017. Increased neural responses to empathy for pain might explain how acute stress increases prosociality. Soc. Cogn. Affect. Neurosci. 12, 401–408. ( 10.1093/scan/nsw146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Dawans B, Fischbacher U, Kirschbaum C, Fehr E, Heinrichs M. 2012. The social dimension of stress reactivity: acute stress increases prosocial behavior in humans. Psychol. Sci. 23, 651–660. ( 10.1177/0956797611431576) [DOI] [PubMed] [Google Scholar]
- 22.von Dawans B, Ditzen B, Trueg A, Fischbacher U, Heinrichs M. 2019. Effects of acute stress on social behavior in women. Psychoneuroendocrinology 99, 137–144. ( 10.1016/j.psyneuen.2018.08.031) [DOI] [PubMed] [Google Scholar]
- 23.Youssef FF, Bachew R, Bissessar S, Crockett MJ, Faber NS. 2018. Sex differences in the effects of acute stress on behavior in the ultimatum game. Psychoneuroendocrinology 96, 126–131. ( 10.1016/j.psyneuen.2018.06.012) [DOI] [PubMed] [Google Scholar]
- 24.Singer N, Sommer M, Döhnel K, Zänkert S, Wüst S, Kudielka BM. 2017. Acute psychosocial stress and everyday moral decision-making in young healthy men: the impact of cortisol. Horm. Behav. 93, 72–81. ( 10.1016/j.yhbeh.2017.05.002) [DOI] [PubMed] [Google Scholar]
- 25.Starcke K, Polzer C, Wolf OT, Brand M. 2011. Does stress alter everyday moral decision-making? Psychoneuroendocrinology 36, 210–219. ( 10.1016/j.psyneuen.2010.07.010) [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Ma J, Nater UM. 2019. How cortisol reactivity influences prosocial decision-making: the moderating role of sex and empathic concern. Front. Hum. Neurosci. 13, 415 ( 10.3389/fnhum.2019.00415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanchard RJ, Blanchard DC. 1990. Anti-predator defense as models of animal fear and anxiety. In Fear and defence (eds Brain PF, Parmigiani S, Blanchard R, Mainardi D), pp. 89–108. Amsterdam, The Netherlands: Harwood Academic Publishers. [Google Scholar]
- 28.Fanselow MS, Lester L. 1988. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. In Evolution and learning (eds Bolles RC, Beecher MD), pp. 185–212. Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- 29.McNaughton N, Corr PJ. 2004. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 28, 285–305. ( 10.1016/j.neubiorev.2004.03.005) [DOI] [PubMed] [Google Scholar]
- 30.Mobbs D, Headley DB, Ding W, Dayan P. 2020. Space, time, and fear: survival computations along defensive circuits. Trends Cogn. Sci. 24, 228–241. ( 10.1016/j.tics.2019.12.016) [DOI] [PubMed] [Google Scholar]
- 31.Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. 2007. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 317, 1079–1083. ( 10.1126/science.1144298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T. 2010. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc. Natl Acad. Sci.USA 107, 20 582–20 586. ( 10.1073/pnas.1009076107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi S, Hassabis D, Sun J, Guo F, Daw N, Mobbs D. 2018. How cognitive and reactive fear circuits optimize escape decisions in humans. Proc. Natl Acad. Sci. USA 115, 3186–3191. ( 10.1073/pnas.1712314115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. 2009. From threat to fear: the neural organization of defensive fear systems in humans. J. Neurosci. Off. J. Soc. Neurosci. 29, 12 236–12 243. ( 10.1523/JNEUROSCI.2378-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer C, Padmala S, Pessoa L. 2019. Dynamic threat processing. J. Cogn. Neurosci. 31, 522–542. ( 10.1162/jocn_a_01363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batson CD, O'Quin K, Fultz J, Vanderplas M, Isen AM. 1983. Influence of self-reported distress and empathy on egoistic versus altruistic motivation to help. J. Pers. Soc. Psychol. 45, 706–718. ( 10.1037/0022-3514.45.3.706) [DOI] [Google Scholar]
- 37.Davis MH. 1983. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 44, 113–126. ( 10.1037/0022-3514.44.1.113) [DOI] [Google Scholar]
- 38.Hortensius R, de Gelder B. 2018. From empathy to apathy: the bystander effect revisited. Curr. Dir. Psychol. Sci. 27, 249–256. ( 10.1177/0963721417749653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackersie CL, Calderon-Moultrie N. 2016. Autonomic nervous system reactivity during speech repetition tasks: heart rate variability and skin conductance. Ear Hear. 37(Suppl. 1), 118S–125S. ( 10.1097/AUD.0000000000000305) [DOI] [PubMed] [Google Scholar]
- 40.Paulus PC, Castegnetti G, Bach DR. 2016. Modeling event-related heart period responses. Psychophysiology 53, 837–846. ( 10.1111/psyp.12622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gelman A, Hill J. 2006. Data analysis using regression and multilevel/hierarchical models, 1st edn Cambridge, NY: Cambridge University Press. [Google Scholar]
- 42.Yarkoni T.2019. The generalizability crisis. PsyArXiv. See https://osf.io/jqw35 .
- 43.Roelofs K. 2017. Freeze for action: neurobiological mechanisms in animal and human freezing. Phil. Trans. R. Soc. B 372, 20160206 ( 10.1098/rstb.2016.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagenaars MA, Oitzl M, Roelofs K. 2014. Updating freeze: aligning animal and human research. Neurosci. Biobehav. Rev. 47, 165–176. ( 10.1016/j.neubiorev.2014.07.021) [DOI] [PubMed] [Google Scholar]
- 45.Hashemi MM, Gladwin TE, de Valk NM, Zhang W, Kaldewaij R, van Ast V, Koch SB, Klumpers F, Roelofs K. 2019. Neural dynamics of shooting decisions and the switch from freeze to fight. Sci. Rep. 9, 1–10. ( 10.1038/s41598-019-40917-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hermans EJ, Henckens MJAG, Roelofs K, Fernández G. 2013. Fear bradycardia and activation of the human periaqueductal grey. Neuroimage 66, 278–287. ( 10.1016/j.neuroimage.2012.10.063) [DOI] [PubMed] [Google Scholar]
- 47.McShane BB, Gal D, Gelman A, Robert C, Tackett JL. 2019. Abandon statistical significance. Am. Stat. 73(Suppl. 1), 235–245. ( 10.1080/00031305.2018.1527253) [DOI] [Google Scholar]
- 48.Terburg D, et al. 2018. The basolateral amygdala is essential for rapid escape: a human and rodent study. Cell 175, 723–735; e16. ( 10.1016/j.cell.2018.09.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huber D, Veinante P, Stoop R. 2005. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308, 245–248. ( 10.1126/science.1105636) [DOI] [PubMed] [Google Scholar]
- 50.Tovote P, et al. 2016. Midbrain circuits for defensive behaviour. Nature 534, 206–212. ( 10.1038/nature17996) [DOI] [PubMed] [Google Scholar]
- 51.Viviani D, Charlet A, Burg Evd, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. 2011. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333, 104–107. ( 10.1126/science.1201043) [DOI] [PubMed] [Google Scholar]
- 52.Pedersen CA, Ascher JA, Monroe YL, Prange AJ. 1982. Oxytocin induces maternal behavior in virgin female rats. Science 216, 648–650. ( 10.1126/science.7071605) [DOI] [PubMed] [Google Scholar]
- 53.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. 2005. Brain oxytocin correlates with maternal aggression: link to anxiety. J. Neurosci. 25, 6807–6815. ( 10.1523/JNEUROSCI.1342-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bosch OJ. 2013. Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defence. Phil. Trans. R. Soc. B 368, 20130085 ( 10.1098/rstb.2013.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rickenbacher E, Perry RE, Sullivan RM, Moita MA. 2017. Freezing suppression by oxytocin in central amygdala allows alternate defensive behaviours and mother-pup interactions. eLife 6, e24080 ( 10.7554/eLife.24080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartal I B-A, Shan H, Molasky NMR, Murray TM, Williams JZ, Decety J, Mason P. 2016. Anxiolytic treatment impairs helping behavior in rats. Front. Psychol. 7, 850 ( 10.3389/fpsyg.2016.00850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hernandez-Lallement J, Attah AT, Soyman E, Pinhal CM, Gazzola V, Keysers C. 2020. Harm to others acts as a negative reinforcer in rats. Curr. Biol. 30, 949–961. ( 10.1016/j.cub.2020.01.017) [DOI] [PubMed] [Google Scholar]
- 58.Clay Z, Waal Fd. 2013. Bonobos respond to distress in others: consolation across the age spectrum. PLoS ONE 8, e55206 ( 10.1371/journal.pone.0055206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto S, Humle T, Tanaka M. 2012. Chimpanzees' flexible targeted helping based on an understanding of conspecifics’ goals. Proc. Natl Acad. Sci. USA 109, 3588–3592. ( 10.1073/pnas.1108517109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brethel-Haurwitz KM, O'Connell K, Cardinale EM, Stoianova M, Stoycos SA, Lozier LM, VanMeter JW, Marsh AA. 2017. Amygdala-midbrain connectivity indicates a role for the mammalian parental care system in human altruism. Proc. R. Soc. B 284, 20171731 ( 10.1098/rspb.2017.1731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gladwin TE, Hashemi MM, van Ast V, Roelofs K. 2016. Ready and waiting: freezing as active action preparation under threat. Neurosci. Lett. 619, 182–188. ( 10.1016/j.neulet.2016.03.027) [DOI] [PubMed] [Google Scholar]
- 62.Riskind JH, Sagliano L, Trojano L, Conson M. 2016. Dysfunctional freezing responses to approaching stimuli in persons with a looming cognitive style for physical threats. Front. Psychol. 7, 521 ( 10.3389/fpsyg.2016.00521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Löw A, Weymar M, Hamm AO. 2015. When threat is near, get out of here: dynamics of defensive behavior during freezing and active avoidance. Psychol. Sci. 26, 1706–1716. ( 10.1177/0956797615597332) [DOI] [PubMed] [Google Scholar]
- 64.Wendt J, Löw A, Weymar M, Lotze M, Hamm AO. 2017. Active avoidance and attentive freezing in the face of approaching threat. Neuroimage. 158, 196–204. ( 10.1016/j.neuroimage.2017.06.054) [DOI] [PubMed] [Google Scholar]
- 65.Collaboration OS. 2015. Estimating the reproducibility of psychological science. Science 349, aac4716 ( 10.1126/science.aac4716) [DOI] [PubMed] [Google Scholar]
- 66.Schäfer T, Schwarz MA. 2019. The meaningfulness of effect sizes in psychological research: differences between sub-disciplines and the impact of potential biases. Front. Psychol. 10, 813 ( 10.3389/fpsyg.2019.00813) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, code and materials are available on Open Science Framework (OSF; https://osf.io/nb6cf/).