Abstract
Angiosperm pollen grain diameter varies greatly from a few microns to over 100, but the selective forces driving the interspecific variation in pollen size remain unclear. Although both pre- and post-pollination hypotheses have been proposed, empirical evidence remains scarce. Here we propose that visits by pollen-foraging pollinators have selected against large pollen grains. An association between pollinator behaviour and pollen grain size was confirmed by field studies of 80 flowering species in natural communities, showing that pollinators positively collected pollen in those species with relatively smaller pollen grains but rarely did so in species with larger ones. Allowing for the confounding effects of pollinator type, flower size or style length and pollen grain number, we found a significant effect of pollen-foraging behaviour on variation in pollen grain size, particularly in bee-pollinated plants. While these results suggest that many plant species whose pollen is collected or consumed by pollinators produce small pollen grains, it remains unclear whether pollen grain size is directly affected by pollinator foraging habit or indirectly mediated by pollen number trade-offs.
Keywords: pollen grain size evolution, style length, pollinator type, grooming behaviour, size-number trade-off, phylogeny
1. Introduction
Pollination mutualisms are often complicated by the fact that the agents of pollen dispersal are usually attracted to flowers by the prospect of nourishment. Floral visitors and plants consequently have conflicting agendas, especially when the nourishment sought by the floral visitors is pollen [1–3]. Bees have evolved various structural and behavioural adaptations to promote pollen collection, as the development of their larvae relies on the pollen protein [4]. For example, harvested pollen is often packed on the corbiculae of bees where it is not available for stigmatic deposition or ovule fertilization. It remains unclear how plants mitigate pollen loss to bee visitors [5], although in a few species this has been resolved by the evolution of heteranthery, a partitioning in function in anthers between pollinating and feeding [6].
Among angiosperm species, pollen grain volume ranges over almost five orders of magnitude, the diameter ranging from less than 10 μm (e.g. in forget-me-not, Myosotis) to over 100 μm (in cotton or cucumber) [7–10]. Why are pollen grains so large in some species but relatively smaller in most species (ca. 20–40 µm)? Hypotheses to explain pollen grain size variation can be broadly categorized pre- or post-pollination selection. Numerous observations support the post-pollination hypothesis, for example, stigma depth/style length is often positively associated with pollen grain size [11–16]. Here, larger pollen grains may outperform smaller grains on stigmas in a long race because of faster germination or tube growth, resulting in a higher siring success [17–21]. However, correlations between pollen grain size and style length may simply be the result of intrinsic scaling relationships and have nothing to do with variation in fertilization success of different sized grains [22–24]. The null hypothesis of allometric scaling of sexual organs and flower size is referred as the allometry hypothesis here (electronic supplementary material, table S1).
Early workers proposed that pre-pollination foraging economics would also select on pollen grain size. In particular, it was suggested that bees would prefer small (lipid-rich, starchless) pollen grains over large (starchy) grains because larger grains were envisioned as having relatively lower nutritional value [25]. However, this pre-pollination hypothesis has not been supported by subsequent studies considering phylogenetic relatedness and pistil characteristics [14], or analysis of nutrition components [15]. Instead, Harder [14] proposed another pre-pollination hypothesis that the comb-like structures on bee limbs would be more efficient at grooming large pollen grains than small ones. Consequently, one may expect that bee-pollinated plants would evolve smaller pollen grains to escape from grooming. However, Harder [14] found no evidence for associations between pollen grain size and the effectiveness of grooming. Although the idea appears to have been abandoned, it was never fully investigated using modern phylogenetic methods coupled with direct examinations of pollinator grooming behaviour and variation in pollen grain size.
Here, we re-visit pre- and post-pollination hypotheses explaining pollen size variation (electronic supplementary material, table S1). The evolution of pollen grain size may be constrained by the numbers of grains per flower given that there is a size-number trade-off [26,27], reflecting an allocation strategy for male investment [28]. To disentangle confounding effects of the pollen size evolution, we ask whether interspecific pollen size variation is the result of allometric growth of flower size, or post-pollination stigma/stylar interactions (i.e. the stylar interaction hypothesis) or whether variation is associated with pollinator foraging behaviour (the pollinator foraging hypothesis). We propose that pollen grain size should be associated with pollinator behaviour. More specifically, large pollen would be favoured in the species whose pollen is little exploited by pollinators, whereas in species visited by pollen-collecting foragers, large numbers of smaller pollen grains may enhance reproductive success by increasing the chances that some pollen grains are not groomed.
2. Material and methods
(a). Measurement of pollen grain size and number
We collected pollen grains from open flowers of 80 native species from 25 families in a field station of Central China Normal University, Shangri-La Alpine Botanical Garden (SABG, 27°54′ N, 99°38′ E, 3300–3350 m above sea level) in Yunnan Province, southwest China. These pollen grains were made into temporary slides with gelatin. To estimate pollen grain size, equatorial and/or polar diameters of 5–20 grains per species were measured under a light microscope based on pollen shape (electronic supplementary material, figure S1 and table S2). As the sampled pollen was basically spherical or ellipsoidal, the value of the long (polar) axis was used as pollen diameter in the comparisons across species. Pollen grain numbers per flower were collected from our previous studies in SABG, sampling 10 flowers that were nearly opening [29] or 20 flowers per species [30].
(b). Pollen-foraging behaviour
To examine the pollinator foraging hypothesis, we investigated pollinator groups and pollinator feeding pollen behaviour on flowers in natural communities in SABG. Our previous studies over years there indicated that diverse insects acted as effective pollinators including bumblebees, solitary bees, hoverflies, other flies, butterflies, hawkmoths and other moths (see [31–34]). To identify whether pollinators collect pollen, we spent hundreds of hours on clear days observing pollinator foraging activities on 80 flowering species from 25 families (electronic supplementary material, table S2). These species were native, flowering in the wild and open to natural visitors. We observed for at least 20 foraging bouts of each floral visitor or for more than 4 h to record whether the insect bodies contacted anthers/pollen and conspecific stigmas during foraging, and whether the visitors consumed or groomed pollen, particularly into the bees' corbiculae or scopae (electronic supplementary material, figures S2 and S3). As large pollen grains commonly appear in Cucurbitaceae, Geraniaceae, Malvaceae and Liliaceae whose pollen grains are usually exposed to visitors [34] without physical protection from pollen collectors, we examined pollen visibility in these 80 species to test whether large pollen grains are associated with pollen exposure and pollinator feeding behaviour. Pollen in each species was categorized as exposed (anthers and pollen are visible to visitors) or concealed (anthers and pollen are hidden in the corolla tube) (see [34]).
(c). Measurements of flower size and style length
To test the post-pollination stylar interaction hypothesis for the interspecific pollen size variation, we examined the relationship between pollen grain diameter and style length. Previous analysis of pollen grain size and number suggested that the size of sexual organs could be related to flower size [24,26]. To test the allometry hypothesis, we measured flower size. To estimate style length, we measured the distance from the corolla base to the top of the pistil with a digital caliper on 3–30 fresh flowers (34 species) or on photos of herbarium specimens from Chinese Virtual Herbarium (http://www.cvh.ac.cn/) (46 species) using Digimizer software (v. 4.6.0). Meanwhile, the surface area of the corolla of each of the 80 species was measured to estimate flower size with herbarium specimens using Digimizer software [35]. For bowl-shaped flowers, we measured the total area of the corolla. For tubular and bilaterally symmetrical flowers, flower size was calculated as the lateral area multiplied by two. If species had special corolla shapes such as the beak-like upper lips in Pedicularis species, areas of these parts were then added to the total area [31].
(d). Data analysis
To test the three hypotheses for pollen size evolution (electronic supplementary material, table S1), we built a phylogenetic tree of the 80 species from SABG with one outgroup based on internal transcribed spacer (nrITS) and two chloroplast markers (matK, rbcL regions). All gene sequences were downloaded from NCBI (https://www.ncbi.nlm.nih.gov/). GenBank accession numbers are shown in the electronic supplementary material, table S1. The sequences were assembled using Geneious v. 11.0 (Biomatters, Auckland, New Zealand), they were aligned using Mafft v. 7.3.0 [36] and were edited using BioEdit v. 7.2.5 [37]. Aligned matrices of three DNA regions were combined using SequenceMatrix v. 1.8 [38]. Bayesian inference (BI) methods were used for phylogenetic reconstruction. Partitioned BI analyses were performed using MrBayes v. 3.2.6 [39], with DNA substitution models selected for each gene partition by the Bayesian information criterion using jModeltest v. 2.0 [40,41]. Markov chain Monte Carlo analyses were run in MrBayes for 10 million generations for each dataset with each run comprising four incrementally heated chains. The first 25% of the trees were discarded as burn-in. The remaining trees were used to generate a majority-rule consensus tree. Both BI analyses and jModeltest were performed at the CIPRES Science Gateway (http://www.phylo.org).
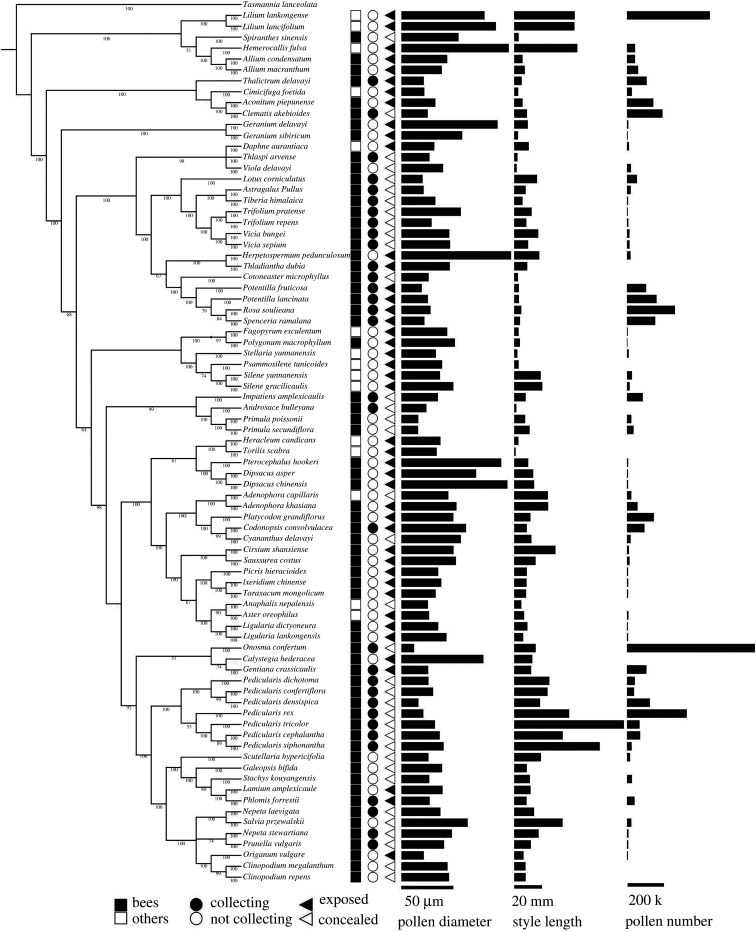
To see whether variation in pollen size is associated with pollen consumption by flower visitors (electronic supplementary material, figure S1, S2 and S3), we mapped pollen diameter, pollinator foraging habits (whether or not pollinators consume/collect pollen), pollen visibility (whether or not pollen is concealed or physically protected from consumption) and pollen number on the phylogenetic tree at the Interactive Tree Of Life (https://itol.embl.de/) (figure 1).
Figure 1.
Reconstruction of the phylogeny of 80 flowering species from 25 families which were studied in Shangri-La, southwest China with pollinator types (bee pollinators, other pollinators), pollen grooming/collecting behaviour (positively collecting, not or rarely grooming/collecting), pollen visibility (exposed, concealed pollen) indicated by closed or open symbols respectively, pollen grain diameter, style length and pollen number (related to bar lengths) mapped onto it.
To examine the association between flower size (visual area of corolla), style length, and pollen number and diameter, we conducted bivariate correlation in SPSS v. 22.0 (IBM Inc., New York, NY, USA). As pollen-related traits usually correlate with flower size, partial correlation analysis with flower size as the control variable was performed to account for the effect of flower size. The phylogenetically independent contrast (PIC) analyses and calculation of Felsenstein's contrasts correlation [42] between flower size, pollen grain size, pollen number and style length were performed in Mesquite v. 2.75 [43] with the phenotypic diversity analysis program (PDAP) package [44].
To examine the effects of pollinator type, grooming behaviour and pollen visibility on pollen grain size and pollen number, we logarithmically transformed data of pollen grain size and number and then conducted generalized linear model (GLM) analysis (normal distribution and an identity function) with pollen size or pollen number as the dependent variable, and pollinator type, grooming behaviour and pollen visibility as the fixed factors. Also, we conducted the same analysis using a phylogenetic linear model by maximum likelihood using Pagel's lambda model [45]. This analysis was performed with the function phylolm of the package phylolm [46] in R v. 3.5.0 [47] separately. The outgroup from the BI tree was pruned before analyses.
To remove the confounding effects of flower size and style length on pollen grain size and number in bee-pollinated species, we further calculated the ratio of pollen diameter to style length, the ratio of pollen diameter to flower size and the ratio of pollen number to flower size (see [48]). Then, we conducted GLM analysis (normal distribution and an identity function) with these ratios as the dependent variable and grooming behaviour as fixed factors. To examine whether exposed species' pollen is less likely to be depleted by pollen collectors than the concealed pollen in bee-pollinated species, GLM analysis (normal distribution and an identity function) was performed with the proportion of species with exposed pollen as dependent variable and pollen grooming behaviour as the fixed factor.
3. Results
(a). Correlations of pollen-related traits
Pollen-related traits including pollen grain size and number, flower size and style length varied greatly among the 80 species for which pollinator foraging behaviour was observed in natural communities (figure 1; electronic supplementary material, table S1). For example, pollen diameter (mean ± s.e. = 42.0 ± 2.4 µm, n = 80; electronic supplementary material, figure S4) varied around 10-fold from the smallest (11.8 µm in Onosma confertum (Boraginaceae)) to the largest (106.1 µm in Herpetospermum pedunculosum (Cucurbitaceae)). Pollen number per flower (mean ± s.e. = 62 991 ± 14 621, n = 64) varied from fewer than 700 in Geranium sibiricum to over 700 000 grains in O. confertum (figure 1). Pollen size was correlated positively with flower size and style length, and negatively with pollen number. These correlations between flower size and pollen-related traits were confirmed based on phylogenetically independent contrasts except for pollen size and number (table 1). However, the partial correlation analysis with flower size as the control variable indicated that only pollen size and pollen number were correlated (r = −0.653, p < 0.001), while there was no significant correlation between pollen size and style length (p = 0.563), or pollen number and style length (p = 0.218). These results suggest an intra-sexual trade-off between pollen size and number that was strongly correlated with flower size, an intrinsic factor, while interspecific variation in allocation to pollen size and number could be driven by extrinsic factors.
Table 1.
The Pearson's correlation and Felsenstein's contrast (left/right) values (upper right) and p-values (lower left) between flower size, style length, pollen number and pollen grain diameter based on bivariate correlation analysis and the phylogenetically independent contrast (PIC) analysis of the 80 wild species in Shangri-La, southwest China. (Significant R values are in italics.)
| flower size | style length | pollen number | pollen size | |
|---|---|---|---|---|
| flower size | 0.583/0.649 | 0.620/0.609 | 0.267/0.302 | |
| style length | <0.001/<0.001 | 0.229/0.358 | 0.267/0.382 | |
| pollen number | <0.001/<0.001 | 0.068/0.004 | -0.384/-0.082 | |
| pollen size | 0.016/0.007 | 0.017/<0.001 | 0.002/0.517 |
(b). Factors affecting pollen size and number
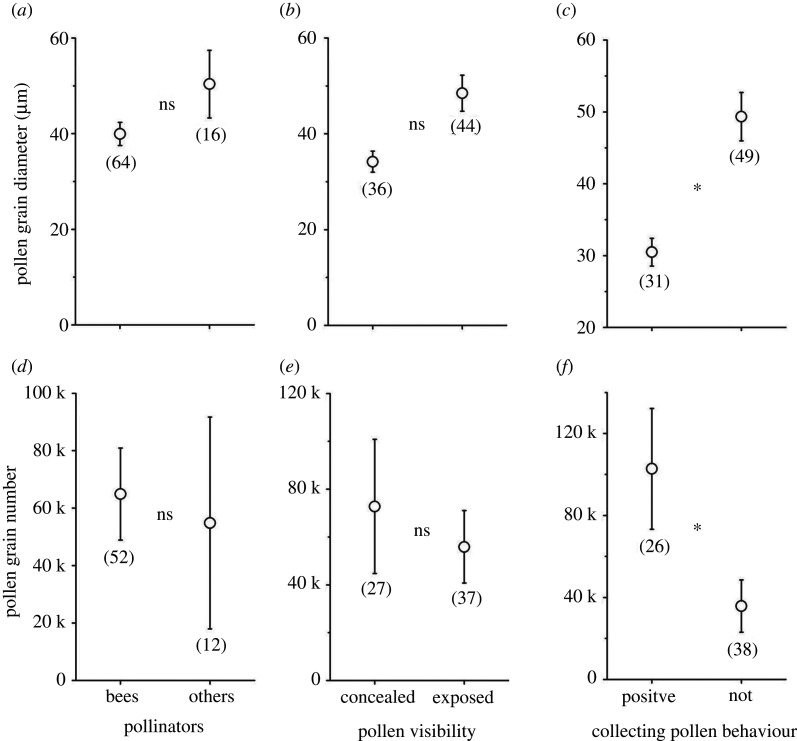
Under a GLM, pollen size or number in species mainly pollinated by bees did not differ significantly from that of species pollinated by other insects (table 2a). However, pollen grains were significantly larger in species with exposed pollen than in those with concealed pollen (table 2a), but the relationship between pollen visibility and pollen size disappeared under phylogenetic analysis (table 2b), perhaps because pollen exposure is a conservative trait within plant families. Under the phylogenetic linear model, effects of either pollinator type or pollen visibility on both pollen grain size and number were not significant, but the presence or absence of pollen-foraging behaviour by pollinators significantly affected both pollen size and number (figure 2 and table 2b).
Table 2.
Comparison of pollen size and number per flower between different pollinator types, different pollinator foraging behaviour (whether or not visitors positively collect pollen) and pollen visibility under (a) generalized linear model analysis, with the coefficient of variation (CV) in pollen grain diameter and number, and (b) phylogenetic linear model analysis. (Italicized values indicate significant differences at p < 0.05.)
| factors | pollen grain diameter (μm) |
pollen number |
|||||
|---|---|---|---|---|---|---|---|
| Wald χ2 | p | CV | Wald χ2 | p | CV | ||
| (a) generalized linear model analysis | |||||||
| pollinator type | bee pollinators | 2.531 | 0.112 | 0.49 | 0.057 | 0.812 | 1.78 |
| other pollinators | 0.60 | 2.33 | |||||
| grooming behaviour | grooming | 31.928 | <0.001 | 0.35 | 10.729 | 0.001 | 1.46 |
| no grooming | 0.48 | 2.20 | |||||
| pollen visibility | exposed pollen | 9.418 | 0.002 | 0.51 | 1.625 | 0.202 | 1.64 |
| concealed pollen | 0.39 | 2.00 | |||||
| dependent variable | factors | estimate | s.e. | t | p | ||
| (b) phylogenetic linear model analysis | |||||||
| pollen size | pollinator type | 7.686 | 5.921 | 1.298 | 0.198 | ||
| grooming behaviour | −19.357 | 5.092 | −3.802 | <0.001 | |||
| pollen visibility | 7.042 | 5.011 | 1.405 | 0.164 | |||
| pollen number | pollinator type | −0.214 | 0.203 | −1.054 | 0.296 | ||
| grooming behaviour | 0.485 | 0.186 | 2.601 | 0.012 | |||
| pollen visibility | −0.076 | 0.178 | −0.424 | 0.673 | |||
Figure 2.
Comparison of pollen grain size (mean ± s.e., n = 80 species) and number (mean ± s.e., n = 64 species) between pollinator types (a,d), pollen visibility (b,e) and pollen-foraging behaviour whether pollinators positively collect pollen or not (c,f), all estimated by phylogenetic linear model analysis (*, p < 0.05; ns, no significant difference). The number of species is shown under each bar.
Our field observations of pollinator foraging behaviours showed that bees did not collect pollen in 33 (51.6%) of the 64 bee-pollinated plant species; bees collected nectar but rarely or never positively gathered pollen into their corbiculae (figure 1; electronic supplementary material, figure S2 and S3). Pollen grains of plant species pollinated by pollen collectors were significantly smaller (figure 2c) and more numerous (figure 2f) than those of species pollinated by insects that did not positively collect pollen from that species.
To isolate the confounding effect of pollinator type on pollen grain size, we analysed the 64 species whose major pollinators were bees, showing that pollen grain diameter was significantly larger (Wald χ2 = 32.981, p < 0.001) in species from which bees did not collect pollen (48.8 ± 3.7 µm, n = 33) than in species from which bees did collect pollen (30.5 ± 1.9 µm, n = 31). Correspondingly, the proportion of species with exposed pollen was significantly higher (Wald χ2 = 12.470, p = 0.001) in plants from which bees did not collect pollen (66.7 ± 8.3%) than in those from which they did positively collect it (25.8 ± 8.0%). Bee-pollinated species had significantly more (Wald χ2 = 11.003, p = 0.001) pollen grains if bees collected their pollen (10 2754 ± 29 441, n = 26) than for species where bees did not collect their pollen (27 013 ± 8137, n = 26). However, pollen number did not significantly differ (Wald χ2 = 1.268, p = 0.260) between species with concealed (78 716 ± 31 421, n = 24) and exposed pollen (53 026 ± 12 988, n = 28).
A positive relationship between pollen grain size and style length (r = 0.313, p = 0.012) among the 64 bee-pollinated species was also observed under the PIC analysis. The ratio of pollen grain diameter to style length in these bee-pollinated species was significantly higher (Wald χ2 = 4.795, p = 0.029) in species in which bees did not positively collect pollen (6.8 ± 1.0) than in those where they did collect it (4.1 ± 0.7), confirming that large pollen was usually not exploited by bees. Similarly, the ratio of pollen diameter to flower size was significantly higher (Wald χ2 = 14.546, p < 0.001) in species without pollen collection (0.98 ± 0.06) than in species with pollen collected by bees (0.73 ± 0.03). However, the ratio of pollen number to flower size did not differ significantly (Wald χ2 = 0.035, p = 0.851) between species with (2.28 ± 0.08) and without (2.26 ± 0.11) pollen collection, indicating that pollen size rather than number was likely to be affected by pollen collection by pollinators.
4. Discussion
The PIC analysis suggested a positive relationship between pollen grain size and style length across species, not inconsistent with the post-pollination hypothesis for the evolution of pollen grain size. These pollen-related trait correlations disappeared in the partial analysis as the control of flower size; however, a trade-off between pollen size and number appeared. To reduce the confounding effects of pollinator type and flower size, our comparison of pollen size/style length ratios in 64 bee-pollinated species showed that pollen size was strongly affected by pollen-feeding habits.
In contrast with a basic assumption in previous analyses that bees are generalized pollen collectors, our direct observations in the field showed that bumblebees foraged for nectar only and avoided collecting pollen on 52% of bee-pollinated species (figure 2b); pollen grains of those species were lodged on the bee bodies but were rarely groomed into the corbiculae. We observed that the two most abundant bumblebee species did not collect pollen from species in Cucurbitaceae, Malvaceae, Geraniaceae and Liliaceae despite the fact that these pollen grains were relatively large (diameter greater than 80 μm). They did however, collect pollen from other species with relatively small grains (figure 1; electronic supplementary material, figures S2 and S3; [3]). Pollen depletion by bees accounts for a high proportion of pollen loss during pollen transfer [49], however many plants appear to have evolved adaptive strategies to avoid pollen overexploitation by collectors [50]. In a few plant groups, for example, cotton and pumpkin flowers [4], anecdotal observations showed that honeybees did not groom and pack pollen into the corbiculae. Instead, pollen was cleaned from their bodies and discarded. It is thought that spines on the pollen grains of cotton (Gossypium) make pollen packing physically difficult [51] and act as a defence against exploitation. Alternatively large pollen grains may be unfavourable to bumblebees [52] if they are starch-rich but protein-poor [25]. To our knowledge, however, physical and chemical defences protecting pollen from bee collection have been little studied [53], but they could account for the lack of harvesting from some of the large-grained species in this study.
The conflict of interest between pollen consumers and plants also appears to have influenced the evolution of other floral rewards. Unpalatable and toxic floral nectar may filter ineffective pollinators and protect nectar from robbers [54,55]. Recent studies have found that pollen usually contained greater quantities of toxic components than nectar [56], while a chemical defence protecting pollen from collection was confirmed in two bumblebee-pollinated Dipsacus species with exposed pollen on unconcealed anthers [53]. Compared to species in which pollen grains were heavily incorporated into the bees' diet, grains that were rejected were observed to be effectively delivered to stigmas, facilitating pollen transfer [53].
Comparative analyses showed positive relations between pollen size and style length and trade-offs between pollen size and number in some but not in other plant lineages (electronic supplementary material, table S3), but the confounding effect of flower size has rarely been considered. Flowers pollinated by large pollinators such as Lepidoptera, bats or birds usually have large pollen and a long style [25,48]; these plants are likely to have relatively larger flowers than bee-pollinated plants. The PIC analysis showed that pollen size and style length are strongly correlated with flower size across the 80 species (table 1), supporting the allometry hypothesis, but the partial correlation analysis excluding the effect of flower size showed pollen size was only correlated with pollen number. If a number of pollen grains are exploited by pollinators as rewards, a partition in allocation to feeding and pollinating would balance the size of pollen grains, as indicated by pollen size and number of trade-offs. Our survey of pollen grain size and pollinator feeding habits indicated that pollen grains are significantly smaller in species whose pollen is collected or consumed. The coefficient of variation (CV) in pollen-concealed species is smaller than that in pollen-exposed ones (table 2). While pollen size (and number) is consistently smaller (and higher) across species in concealed species, exposed species have either large or small (or few or many) pollen grains. This difference in CV may explain why we found no significant variation in pollen size or number between pollen-exposed and pollen-concealed species (figure 2).
Our analyses removing the confounding effects of pollinator types and flower size showed that the effect of pollen-feeding behaviour on variation in pollen grain size (but not pollen number) remained significant in bee-pollinated species, supporting the pollinator foraging hypothesis. Pollen grains in species pollinated by non-pollen-collecting Lepidoptera, bats or birds are relatively large, perhaps as a result of the same relaxed selection by pollen loss to consumers. Interspecific variation in pollen number per flower can be affected by intrinsic factors such as flower size, pollen size and nutritional content, and extrinsic factors including pollen vector, pollen collection intensity and visitation frequency [15,49,57–59]. For example, bat-pollinated flowers usually produce more and larger pollen grains than hummingbird-pollinated species in a cloud forest in Ecuador [60]. An increase of pollen production would be favoured if larger amounts were efficiently transferred, resulting in a more linear male fitness gain curve under a scarcity of pollinator visits and non-discarding-pollen behaviour [59,60], which could explain some species (i.e. Lepidoptera-pollinated Liliaceae species) producing a large number of relatively large pollen grains.
Our study of pollinator foraging behaviours showed that large pollen grains were associated with species where pollen grains were seldom harvested by pollinators, while small pollen grains were associated with species which were heavily exploited by pollen-collecting foragers. Further studies are needed to clarify whether pollen grain size is directly driven by pollinator foraging habit or indirectly mediated by trade-offs between pollen size and number (i.e. selection is actually on pollen grain number). As predicted, our results indicated that large pollen grains would be favoured where pollen collection is weak or absent. However, it remains unclear why the major pollinators (i.e. bumblebees here) reject collecting large pollen grains. A perspective of pollen-pollen consumer competition could open a new avenue for understanding the evolution of flower–pollinator interactions and male reproductive success in flowering plants.
Supplementary Material
Acknowledgements
We thank laboratory members Q. Fang, J. Tang, T. Wu and C. Zhang for help in the field; Z.D. Fang and staff at the Shangri-La field station for providing logistical support, and W.S. Armbruster, S.C.H. Barrett, S.M. Chang, S.A. Corbet, E.M. Friis, L.D. Harder and Y.Z. Xiong, the associate editor, David Inouye and one anonymous reviewer for help with data analysis, discussions, valuable advice and suggestions to improve the study.
Data accessibility
For reviewers' convenience, we provide related raw data in the electronic supplementary material, table S2.
Authors' contributions
S.Q.H. conceived this project, K.H., and Z.C.W. collected data of pollen-related traits; Z.X.T. collected data of pollinator foraging behaviours in co-flowering species in the field; K.H. collected related data from literature and analysed all data with S.Q.H. S.Q.H. and K.H. wrote a draft; all authors commented on the manuscript. K.H. and Z.X.T. contributed equally to this work.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 31730012)..
References
- 1.Westerkamp C. 1997. Flowers and bees are competitors—not partners. Towards a new understanding of complexity in specialized bee flowers. Acta Hortic. 437, 71–74. ( 10.17660/ActaHortic.1997.437.5) [DOI] [Google Scholar]
- 2.Parker AJ, Williams NM, Thomson JD. 2016. Specialist pollinators deplete pollen in the spring ephemeral wildflower Claytonia virginica. Ecol. Evol. 6, 5169–5177. ( 10.1002/ece3.2252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong ZY, Huang SQ. 2018. Safe sites of pollen placement: a conflict of interest between plants and bees? Oecologia 186, 163–171. ( 10.1007/s00442-017-3999-9) [DOI] [PubMed] [Google Scholar]
- 4.Thorp RW. 2000. The collection of pollen by bees. Plant Syst. Evol. 222, 211–223. ( 10.1007/BF00984103) [DOI] [Google Scholar]
- 5.Minnaar C, Anderson B, de Jager ML, Karron JD. 2019. Plant–pollinator interactions along the pathway to paternity. Ann. Bot. 123, 225–245. ( 10.1093/aob/mcy167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallejo-Marín M, Da Silva EM, Sargent RD, Barrett SCH. 2010. Trait correlates and function of heteranthery. New Phytol. 188: 418–425. ( 10.1111/j.1469-8137.2010.03430.x) [DOI] [PubMed] [Google Scholar]
- 7.Wodehouse RP. 1935. Pollen grains. New York, NY: McGraw-Hill. [Google Scholar]
- 8.Muller J. 1979. Form and function in angiosperm pollen. Ann. Mo. Bot. Gard. 66, 593–632. ( 10.2307/2398913) [DOI] [Google Scholar]
- 9.Wang FX, Qian NF, Zhang YL, Yang HQ. 1995. Pollen flora of China Beijing, China: Science Press. [Google Scholar]
- 10.Willmer P. 2011. Pollination and floral ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 11.Cruden RW, Miller-Ward S. 1981. Pollen-ovule ratio, pollen size, and the ratio of stigmatic area to the pollen-bearing area of the pollinator: a hypothesis. Evolution 35, 964–974. ( 10.1111/j.1558-5646.1981.tb04962.x) [DOI] [PubMed] [Google Scholar]
- 12.Baker HG, Baker I. 1982. Starchy and starchless pollen in the Onagraceae. Ann. Mo. Bot. Gard. 69, 748–754. ( 10.2307/2398994) [DOI] [Google Scholar]
- 13.Plitmann U, Levin DA. 1983. Pollen-pistil relationships in the Polemoniaceae. Evolution 37, 957–967. ( 10.1111/j.1558-5646.1983.tb05624.x) [DOI] [PubMed] [Google Scholar]
- 14.Harder LD. 1998. Pollen-size comparisons among animal-pollinated angiosperms with different pollination characteristics. Biol. J. Linn. Soc. 64, 513–525. ( 10.1111/j.1095-8312.1998.tb00347.x) [DOI] [Google Scholar]
- 15.Roulston TH, Cane JH, Buchmann SL. 2000. What governs protein content of pollen: pollinator preferences, pollen–pistil interactions, or phylogeny? Ecol. Monogr. 70, 617–643. ( 10.1890/0012-9615(2000)070[0617:wgpcop]2.0.co;2) [DOI] [Google Scholar]
- 16.Cruden RW. 2009. Pollen grain size, stigma depth, and style length: the relationships revisited. Plant Syst. Evol. 278, 223– 238 ( 10.1007/s00606-008-0142-8) [DOI] [Google Scholar]
- 17.Cruzan MB. 1990. Variation in pollen size, fertilization ability, and postfertilization siring ability in Erythronium grandiflorum. Evolution 44, 843–856. ( 10.1111/j.1558-5646.1990.tb03809.x) [DOI] [PubMed] [Google Scholar]
- 18.Williams EG, Rouse JL. 1990. Relationships of pollen size, pistil length and pollen tube growth rates in Rhododendron and their influence on hybridization. Sex. Plant Reprod. 3, 7–17. ( 10.1007/BF00189946) [DOI] [Google Scholar]
- 19.Endress PK. 1994. Diversity and evolutionary biology of tropical flowers. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 20.Manicacci D, Barrett SCH. 1995. Stamen elongation, pollen size, and siring ability in tristylous Eichhornia paniculata (Pontederiaceae). Am. J. Bot. 82, 1381–1389. ( 10.1002/j.1537-2197.1995.tb12674.x) [DOI] [Google Scholar]
- 21.McCallum B, Chang S-M. 2016. Pollen competition in style: effects of pollen size on siring success in the hermaphroditic common morning glory, Ipomoea purpurea. Am. J. Bot. 103, 460–470. ( 10.3732/ajb.1500211) [DOI] [PubMed] [Google Scholar]
- 22.Lee S. 1978. A factor analysis study of the functional significance of angiosperm pollen. Syst. Bot. 3, 1–19. ( 10.2307/2418528) [DOI] [Google Scholar]
- 23.Sarkissian TS, Harder LD. 2001. Direct and indirect responses to selection on pollen size in Brassica rapa L. J. Evol. Biol. 14, 456–468. ( 10.1046/j.1420-9101.2001.00285.x) [DOI] [Google Scholar]
- 24.Wang XP, Yu WB, Sun SG, Huang SQ. 2016. Pollen size strongly correlates with stigma depth among Pedicularis species. J. Integr. Plant Biol. 58, 818–821. ( 10.1111/jipb.12477) [DOI] [PubMed] [Google Scholar]
- 25.Baker HG, Baker I. 1979. Starch in angiosperm pollen grains and its evolutionary significance. Am. J. Bot. 66, 591–600. ( 10.1002/j.1537-2197.1979.tb06262.x) [DOI] [Google Scholar]
- 26.Vonhof MJ, Harder LD. 1995. Size-number trade-offs and pollen production by papilionaceous legumes. Am. J. Bot. 82, 230–238. ( 10.1002/j.1537-2197.1995.tb11491.x) [DOI] [Google Scholar]
- 27.Yang CF, Guo YH. 2004. Pollen size-number trade-off and pollen-pistil relationships in Pedicularis (Orobanchaceae). Plant Syst. Evol. 247, 177–185. ( 10.1007/s00606-004-0165-8) [DOI] [Google Scholar]
- 28.Geber MA, Charnov EL. 1986. Sex allocation in hermaphrodites with partial overlap in male/female resource inputs. J. Theor. Biol. 118, 33–43. ( 10.1016/S0022-5193(86)80006-6) [DOI] [Google Scholar]
- 29.Gong YB, Huang SQ. 2014. Interspecific variation in pollen-ovule ratio is negatively correlated with pollen transfer efficiency in a natural community. Plant Biol. 16, 843–847. ( 10.1111/plb.12151) [DOI] [PubMed] [Google Scholar]
- 30.Gao J, Xiong YZ, Huang SQ. 2015. Effects of floral sexual investment and dichogamy on floral longevity. J. Plant Ecol. 8, 116–121. ( 10.1093/jpe/rtv011) [DOI] [Google Scholar]
- 31.Gong YB, Huang SQ. 2009. Floral symmetry: pollinator-mediated stabilizing selection on flower size in bilateral species. Proc. R. Soc. B 276, 4013–4020. ( 10.1098/rspb.2009.1254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang Q, Huang SQ. 2012. Relative stability of core groups in pollination networks in a biodiversity hotspot over four years. PLoS ONE 7, e32663 ( 10.1371/journal.pone.0032663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang Q, Huang SQ. 2013. A directed network analysis of heterospecific pollen transfer in a biodiverse community. Ecology 94, 1176–1185. ( 10.1890/12-1634.1) [DOI] [PubMed] [Google Scholar]
- 34.Xiong YZ, Jia LB, Zhang C, Huang SQ. 2019. Color-matching between pollen and corolla: hiding pollen via visual crypsis? New Phytol. 224, 1142–1150. ( 10.1111/nph.16012) [DOI] [PubMed] [Google Scholar]
- 35.Salvarzi E, Choobineh A, Jahangiri M, Keshavarzi S. 2018. Facial anthropometric measurements in Iranian male workers using Digimizer version 4.1. 1.0 image analysis software: a pilot study. Int. J. Occup. Saf. Ergon. 24, 570–576. ( 10.1080/10803548.2018.1433578) [DOI] [PubMed] [Google Scholar]
- 36.Katoh K, Toh H. 2010. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26, 1899–1900. ( 10.1093/bioinformatics/btq224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic acids symp. Ser. 41, pp. 95–98. London, UK: Information Retrieval Ltd., c1979-c2000. [Google Scholar]
- 38.Vaidya G, Lohman DJ, Meier R. 2011. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27, 171–180. ( 10.1111/j.1096-0031.2010.00329.x) [DOI] [PubMed] [Google Scholar]
- 39.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. ( 10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 40.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 ( 10.1038/nmeth.2109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704. ( 10.1080/10635150390235520) [DOI] [PubMed] [Google Scholar]
- 42.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 43.Maddison WP, Maddison DR. 2011. Mesquite: a modular system for evolutionary analysis. Version 2.75 See http://mesquiteproject.org.
- 44.Midford PE, Garland T Jr, Maddison WP. . 2005. PDAP package of Mesquite.
- 45.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884. ( 10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 46.Ho LST, Ane C. 2014. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63, 397–408. ( 10.1093/sysbio/syu005) [DOI] [PubMed] [Google Scholar]
- 47.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org. [Google Scholar]
- 48.Stroo A. 2000. Pollen morphological evolution in bat pollinated plants. Plant Syst. Evol. 222, 225–242. ( 10.1007/BF00984104) [DOI] [Google Scholar]
- 49.Harder LD, Routley MB. 2006. Pollen and ovule fates and reproductive performance by flowering plants. In Ecology and evolution of flowers, (eds Harder LD, Barrett SCH), pp. 61–80. Oxford, UK: Oxford University Press. [Google Scholar]
- 50.Hargreaves AL, Harder LD, Johnson SD. 2009. Consumptive emasculation: the ecological and evolutionary consequences of pollen theft. Biol. Rev. 84, 259–276. ( 10.1111/j.1469-185X.2008.00074.x) [DOI] [PubMed] [Google Scholar]
- 51.Lunau K, Piorek V, Krohn O, Pacini E. 2015. Just spines -- mechanical defense of Malvaceous pollen against collection by corbiculate bees. Apidologie 46, 144–149. ( 10.1007/s13592-014-0310-5) [DOI] [Google Scholar]
- 52.Vaudo AD, Patch HM, Mortensen DA, Tooker JF, Grozinger CM. 2016. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proc. Natl Acad. Sci. USA 113, E4035-E4042. ( 10.1073/pnas.1606101113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang XY, Tang J, Wu T, Wu D, Huang SQ. 2019. Bumblebee rejection of toxic pollen facilitates pollen transfer. Curr. Biol. 29, 1401–1406. ( 10.1016/j.cub.2019.03.023) [DOI] [PubMed] [Google Scholar]
- 54.Johnson SD, Hargreaves AL, Brown M. 2006. Dark, bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology 87, 2709–2716. ( 10.1890/0012-9658(2006)87[2709:DBNFAA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 55.Barlow SE, Wright GA, Ma C, Barberis M, Farrell IW, Marr EC, Brankin A, Pavlik BM, Stevenson PC. 2017. Distasteful nectar deters floral robbery. Curr. Biol. 27, 2552–2558.e3 ( 10.1016/j.cub.2017.07.012) [DOI] [PubMed] [Google Scholar]
- 56.Palmer-Young EC, Farrell IW, Adler LS, Milano NJ, Egan PA, Junker RR, Stevenson PC. 2019. Chemistry of floral rewards: intra- and interspecific variability of nectar and pollen secondary metabolites across taxa. Ecol. Monogr. 89, e01335 ( 10.1002/ecm.1335) [DOI] [PubMed] [Google Scholar]
- 57.Cruden RW. 2000. Pollen grains: why so many? Plant Syst. Evol. 222, 143–165. ( 10.1007/BF00984100) [DOI] [Google Scholar]
- 58.Muchhala N, Brown Z, Armbruster WS, Potts MD. 2010. Competition drives specialization in pollination systems through costs to male fitness. Am. Nat. 176, 732–743. ( 10.1086/657049) [DOI] [PubMed] [Google Scholar]
- 59.Song YP, Huang ZH, Huang SQ. 2019. Pollen aggregation by viscin threads in Rhododendron varies with pollinator. New Phytol. 221, 1150–1159. ( 10.1111/nph.15391) [DOI] [PubMed] [Google Scholar]
- 60.Muchhala N, Thomson JD. 2010. Fur versus feathers: pollen delivery by bats and hummingbirds, and consequences for pollen production. Am. Nat. 175, 717–726. ( 10.1086/652473) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For reviewers' convenience, we provide related raw data in the electronic supplementary material, table S2.