Abstract
Objective
The purpose of this randomized double-blind placebo controlled clinical trial was to evaluate the effects of a commercial spore-based probiotic supplement consisting of 5 different spore forming bacilli (Bacillus indicus HU36, Bacillus subtilis HU58, Bacillus coagulans SC-208, Bacillus licheniformis, and Bacillus clausii SC-109) on reducing the triglyceride levels (TG) in patients with mild to moderate hypertriglyceridemia (HT).
Study design
A randomized, double-blind, placebo-controlled study with eighty participants with non-fasting triglyceride levels greater than 150 mg/dL.
Methods
Eighty participants with non-fasting triglyceride levels greater than 150 mg/dL were randomized to receive oral probiotic supplement consisting of two capsules containing 5 different spore forming bacilli once daily in the morning or a placebo (rice flour). Their non-fasting triglyceride levels were measured again at six weeks and at twelve weeks.
Results
Compared to the placebo group, participants in the probiotic supplement group had significant lowering of their triglyceride levels after 90 days.
Conclusion
Mild to moderately elevated triglyceride levels can be lowered in patients with mild to moderate HT by a probiotic supplement consisting of five different spore forming bacilli.
Introduction
Hypertriglyceridemia (HT) is common and present in about a third of the population.1 HT leads to an increase in atherogenic LDL levels and a lowering in HDL.2 HT may also increase atherogenesis by the release of free fatty acids, the production of fibrinogen, coagulation factors, and proinflammatory cytokines.3 Most epidemiological studies indicate that HT is associated with obesity, diabetes, metabolic syndrome, and coronary artery disease. Publications have clearly demonstrated that HT is strongly linked with atherosclerosis and an increased risk for acute pancreatitis.4 Several clinical studies have revealed that reducing triglycerides lowers the risk for coronary artery disease.3
A 2009 study showed that the prevalence of HT was 54.3% in patients recently diagnoses with diabetes. Patients with type 2 diabetes commonly have HT.4,5. Cardiovascular diseases (CVD) have been linked to triglycerides. Cholesterol and triglycerides are basically insoluble in plasma.6-8 Whereas cardiovascular risk factors have mainly focused on HDL-C, less interest has been applied to triglycerides. Elevated levels of triglycerides are linked with low levels of HDL-C; in other words, an inverse relationship.2 An early report on the link between CVD and triglycerides was by Albrink in 19599. This study revealed that HT was more important than hypercholesterolemia in link between CVD risk factors in men. Data from Framingham indicated that HT was an even greater risk factor for CVD in women.10 A number of other publications have replicated these findings.11-14
Extremely elevated triglycerides (more than 10 mmol/l) indicate increased risk for pancreatitis and lipemia retinalis. It is established in the medical literature that extremely elevated triglycerides not provoked by nutritional factors are more likely to have a monogenic cause. Mildly to moderately elevated triglycerides are often caused by polygenic disorders. Highly elevated triglyceride levels can also cause fatty liver disease, and are associated with kidney disease, and the use of some medications.15
Triglycerides (TG) are mainly produced in the liver and the intestinal tract. Cholesterol and TG are mostly insoluble in plasma and are transported in lipoprotein particles consisting of a main core of cholesterol esters and TG. Chylomicrons contain mainly TG in their core, and chylomicrons and VLDL are the main carriers of TG in plasma and are the two largest classes of lipoproteins. TG enters into the circulatory system within chylomicrons and are transported to cardiac, skeletal muscle, and adipose tissue. There the triglyceride components are hydrolyzed by lipoprotein lipase, thereby allowing the released free fatty acids to be absorbed by these tissues.16,17
HIV and several antiretroviral treatments including combinations such as ritonavir and lopinavir are linked to HT. Autoimmune diseases, including systemic lupus erythematosus, multiple myeloma, and juvenile dermatomyositis, are also associated with HT. There are immunosuppressant medications which raise triglyceride levels, and HT is prevalent in certain renal diseases, such as renal failure and nephrotic syndrome.18-22
Several recent publications have shown that either fasting or non-fasting TG levels are linked to cardiovascular episodes. However, we still generally measure fasting triglycerides, even though most people exist in a postprandial state, with their circulatory system immersed by triglyceride-rich lipoproteins.16,23,24 Prospective studies have shown that when compared with fasting TG levels, non-fasting TG levels may be a better predictor of CV events in the general population. Recent studies have shown that non-fasting triglycerides were associated with increased stroke risk.5,16,17, 23-27
The treatment of HT is essential in view of the new data in a number of studies demonstrating a causal role of triglycerides in cardiovascular risk. Increased effort is needed in reducing elevated triglycerides in patients by non-pharmacological treatment methods. In general, diet, physical activity, and weight reduction are part of HT treatment. Currently, treatment for HT includes Niacin, which is not as effective as fibrates in lowering triglycerides.28,29 Recent randomized clinical trials using extended release niacin or placebo failed to show any benefits of niacin on CVD outcomes.30,31
Clinicians should review the medications patients are taking that could elevate triglycerides, such as estrogens and retinoic acid products.32 Optimum control in patients with diabetes, thyroid disease, and alcohol consumption are added important points as these contribute to HT.
In patients with HT, pharmacological management alone is insufficient to lower triglyceride levels, and should not be the first line of treatment.4 These new data regarding a causal role for triglycerides in increasing cardiovascular risk should prompt us today to redouble our efforts to reduce hypertriglyceridemia in our patients using non-pharmacological approaches. As this randomized, double-blind, placebo-controlled trial demonstrates, probiotics consisting of five spore forming bacilli effectively lowers triglyceride levels.
Probiotics, a term coined by the Russian Nobel laureate Dr. Ilya Metchnikoff over a hundred years ago, is synonymous with beneficial microbes. Probiotics are live microorganisms that can confer health benefits to the host when given in adequate amounts.34 The search for safe and cost-effective therapy and the concern in medicine over the development of antibiotic resistance has prompted the use of probiotics, with its long history of safety. Research has clearly demonstrated that the proper balance of gut inhabitants are essential in determining health, especially in metabolic disorders.35 Lactobacilli and Bifidobacterium spp. have been globally recognized for their potential health benefits. Spore-forming bacilli have the intrinsic ability to produce a large number of secretory proteins, enzymes, vitamins, carotenoids, and antimicrobial compounds. Furthermore, Bacillus spp. are able to endure and survive the hostile environment of the gastrointestinal tract (GIT) and have higher acid and bile salts tolerance.36,37 Bacillus spore formers have probiotic characteristics, and have also demonstrated to have pathogen exclusion, antimicrobial, anti-oxidant, and immunomodulatory effects.38-40
Bacilli are considered as soil organisms though many of them, including B. subtilis, have been found in ileal biopsies and feces in volunteers.41 A study by Hong el al. supported the growing opinion that B. subtilis and other species had the ability to sporulate anaerobically and produce antimicrobials, adapting to life in the human GIT, therefore becoming gut commensals rather than just soil microorganisms.42 Pinchuck published a study showing the anti-Helicobacter Pylori activity of a probiotic B. subtilis strain, which produced a secretion of aninocoumacin A antibiotic, and also showed antagonistic activity against Shigella flexneri and E. faecium.43 A recent study by Ripert demonstrated that the probiotic B. clausii protected cells from the cytotoxic effect of Clostridium difficile and B. cereus toxins.40 A publication by Joseph confirmed the antimicrobial activity of B. subtilis against foot ulcer bacterial pathogens, with the highest recorded against Klebsiella spp.44
Probiotic Bacillus strains ameliorate the dysbiosis and gut inflammation by reestablishing the gut microbiome toward beneficial microbial population and help the intestinal mucosa to recover from injuries due to illness. The beneficial effects on gut metabolism of B. coagulans was shown by the suppressed production of bile acid, improved intestinal permeability, and decreased bactericidal effect of bile acid, supporting the growth of beneficial microbiota in the intestine.45
Probiotic strains of Bacilli are currently being used for their therapeutic effects on several systemic clinical syndromes, including metabolic syndrome. In a study by Ghoneim, the therapeutic effects of B. subtilis showed the reduction of blood glucose and troponin, as well as on total serum cholesterol, LDL, and VLDL47. Another study on diabetic rats showed that B. subtilis promoted HDL cholesterol and delayed the absorption of LDL cholesterol and triglycerides, in effect demonstrating anti-lipemic effects.48 Probiotic B. clausii consumption was shown to have multiple beneficial effects in a study on human subjects using DNA microarray technique. The genes involved in inflammation, immune response, intestinal permeability, cell adhesion, cell growth, cell differentiation, cell signaling, apoptosis, signal transcription, and transduction in intestinal mucosa were modulated by B. Clausii.49
This randomized, double-blind, placebo-controlled trial demonstrates that probiotics consisting of five spore forming bacilli effectively lowered triglyceride levels in participants with mild to moderate HT.
Methods
Inclusion criteria
In good general health as evidenced by medical history and physical examination.
Willingness to comply with all the study procedures.
Male or Female, age >25 and <70 years old.
For females of reproductive potential: use of highly effective contraception.
Triglyceride levels >150 md/dl
Ability and willingness to take oral supplements and adhere to regimen.
Exclusion criteria
Pregnancy or lactation.
Surgical procedure within the last 120 days.
Use of recreational drugs or addictions.
Alcoholism.
Hepatitis
Currently on or having taken statin drugs within the last 90 days.
Currently on cortisol based medications or having taken these in the last 60 days.
On non-steroidal anti-inflammatory drugs.
Taking or having taken an antibiotic drug within the last 30 days.
Currently under the care of a doctor for any type of medical problem deemed unacceptable for participation in this clinical trial.
Randomizing and blinding
All the randomized assignments were concealed in opaque, sealed envelopes. All participants, doctors, researchers, outcome assessors, were blinded to the research protocol and the random assignment.
Participants and Recruitment
All participants were recruited from newspaper advertising and from posted flyers. They were assessed clinically. After evaluation, 80 participants were randomized to either the intervention or the placebo group. All physicians were trained regarding the administration of the intervention and placebo before the study. All participants were subsequently informed about the research and given an information sheet. All participants were required to provide a signed informed consent that contained the aim and nature of this study.
Statistical Analysis
Baseline variables (sex, age and triglyceride level) were summarized using count/percentage for sex and mean/sd for continuous variables. This was done for the entire cohort and also by treatment. Outcome variables (triglyceride level at 6 and 12 weeks) were compared between treatment groups using two-sample t tests and linear regression models that adjust for baseline variables. Possible interactions between treatment and baseline variables were tested under linear regression models. P values less than .05 (two-sided) were considered statistically significant. All analyses were performed in R 3.4.2.
Results
A total of 80 participants were eligible to enter into the study. All were randomized into the intervention and placebo groups. All 80 participant were reevaluated at 6 weeks and 12 weeks, with no adverse effects reported in either the intervention or placebo groups.
Table 1 summarizes the baseline characteristics of the 80 subjects, overall and by treatment. The study subjects were mostly female (80%), with an average age of 49 years and an average triglyceride level of 207 mg/dl. The treated group had a slightly larger proportion of male subjects, a slightly higher average age and a slightly higher average triglyceride level at baseline. However, these differences were not statistically significant.
Table 1.
Summary of baseline characteristics: count (percentage) for discrete variables and mean (sd) for continuous ones
| Variable | Overall | By Treatment | ||
|---|---|---|---|---|
| (n = 92) | Probiotic (n = 46) | Placebo (n = 46) | P Value | |
| male gender | 18 (20%) | 10 (22%) | 8 (17%) | .79 |
| age (year) | 49.4 (10.4%) | 50.1 (10.1%) | 48.6 (10.8%) | .51 |
| triglyceride (mg/dl) | 206.6 (58.4%) | 214.0 (67.9%) | 199.1 (46.5%) | .22 |
Figure 1 shows the trajectory of triglyceride level in each treatment group. In the treated group, triglyceride level declined sharply from baseline to 6 weeks and further to 12 weeks, whereas the control group did not experience a substantial change in triglyceride level. At 6 weeks, a direct (i.e., unadjusted) comparison of the two treatment groups indicates that probiotic treatment decreases the mean triglyceride level by 37.3 (95% CI: 1.4 to 73.3) mg/dl, which is statistically significant (P = .042). In a linear regression analysis that adjusts for sex, age and triglyceride at baseline, the effect of probiotic treatment at 6 weeks becomes larger (51.7 mg/dl; 95% CI: 22.3 to 80.1) and more significant (P = .0009). At 12 weeks, the unadjusted difference in mean triglyceride level between the two treatment groups (placebo – probiotic) increases to 64.9 (95% CI: 33.5 to 96.2) mg/dl, which is highly significant (P < .0001). After adjusting for sex, age and baseline triglyceride in a linear regression model, the effect of probiotic treatment at 12 weeks becomes even larger (77.7 mg/dl; 95% CI: 52.5 to 102.9) and more significant (P < .0001). There are no significant interactions at either time point (6 or 12 weeks) between treatment and the three baseline variables (P > .4), indicating that the treatment effect is consistent across different subgroups defined by sex, age and baseline triglyceride.
Figure 1.
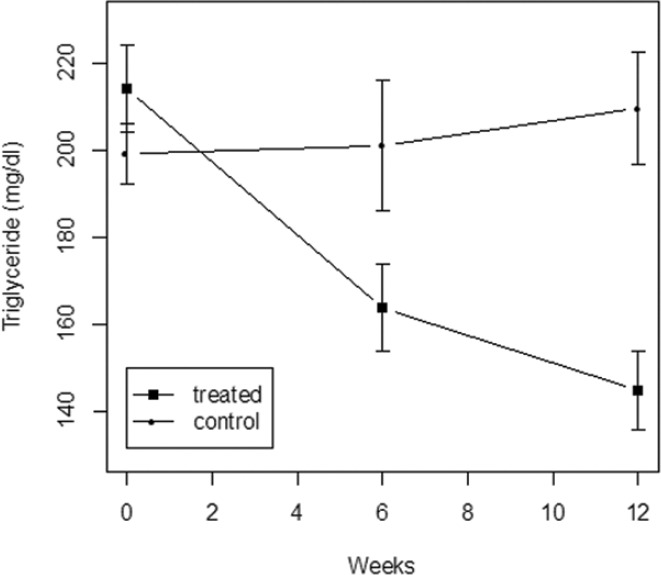
Trajectory of triglyceride level
Discussion
To the best of our knowledge, this study is the first 12 week randomized, double-blind, placebo-controlled trial that has been conducted using spore forming Bacilli probiotic in participants with mild to moderate HT with successful lowering of blood triglyceride levels. The results of this study demonstrated the effectiveness of spore forming Bacilli probiotic in lowering HT in participants and therefore decreasing the risk for CVD events.
Previous studies have demonstrated that the use of 30 day short term spore forming bacilli probiotic altered dietary endotoxemia in human subjects.46 In this study, the improvement in triglyceride levels over 12 weeks is significant in that lowering of CVD risk is of importance due to the epidemiological data linking HT with CVD events.
Conclusion
Fasting elevated triglycerides are a serious marker for arthrogenic potential especially when associated with a low HDL 50. Indeed the triglyceride/HDL ratio has considerable merit in the assessment of cardiovascular risk. Higher ratios >3.5 should be modified. Ideal ratio < 2 should be strived for. This twelve week trial using spore forming bacilli probiotics have remarkable triglyceride lowering. The cardiovascular implications of this study are intriguing especially when we consider the profound impact of lowering triglycerides in the prevention of coronary artery events. For example, in patients with acute coronary syndrome, treated effectively with statins, triglycerides appear to be another important target for subsequent therapy.51,52
Elevated triglyceride–rich lipoproteins must be addressed in attenuating cardiovascular risk. The utilization of probiotics as demonstrated in this analysis should strongly be considered.
Although omega 3s, weight reduction, dietary restriction of carbohydrates, exercise, and statins are important concepts in supporting acceptable triglyceride levels, the initiation of targeted probiotics is yet another simple and easy intervention in attenuating cardiovascular risk.
Limitation
Larger groups of participants in a double-blinded placebo-controlled study would give more data in order to fully determine the effects of spore forming bacilli probiotics on HT. In a similar manner, more data should be obtained with fasting trigclycerides in participants to establish what effects, if any, would spore forming bacilli probiotics have on HT.
References
- 1.Berglund L, Brunzell J, et al. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract Res Clin Endocrinol Metab. 2013;28(3):423-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J. 2014;36(13):774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenenbaum A, Klempfner R, et al. Hypertriglyceridemia: a too long unfairly neglected major cardiovascular risk factor. Cardiovasc Diabetol. 2014;13:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pramono L, Harbuwono D. Managing Hypertriglyceridemia in Daily Practice. Acta Med Indones. 2015. July;47(3):265-71. [PubMed] [Google Scholar]
- 5.Jagla A, Schrezenmeir J. Postprandial triglycerides and endothelial function. Exp Clin Endocrinol Diabetes. 2001;109(4):S533-47. [DOI] [PubMed] [Google Scholar]
- 6.Welty F. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr Cardiol Rep. 2013;15(9):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarwar N, Danesh J, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007;115:450–458. [DOI] [PubMed] [Google Scholar]
- 8.Sarwar N, Sandhu M, et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet 2010;375:1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albrink M, Man E. Serum triglycerides in coronary artery disease. AMA Arch Intern Med. 1959;103:4–8. [DOI] [PubMed] [Google Scholar]
- 10.Castelli W. The triglyceride issue: a view from Framingham. Am Heart J. 1986;112:432–437. [DOI] [PubMed] [Google Scholar]
- 11.LaRosa J. Triglycerides and coronary risk in women and the elderly. Arch Intern Med. 1997;157:961–968. [PubMed] [Google Scholar]
- 12.Robert C, Lanier I. Management of hypertriglyceridemia. Am Fam Physician. 2007;75:1365-71. [PubMed] [Google Scholar]
- 13.Brunzell J. Hypertriglyceridemia. N Eng J Med. 2007;357:1009-17. [DOI] [PubMed] [Google Scholar]
- 14.Berglund L, Brunzell J, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jagla A, Schrezenmeir J. Postprandial triglycerides and endothelial function. Exp Clin Endocrinol Diabetes. 2001;109(4):S533-47. [DOI] [PubMed] [Google Scholar]
- 16.Nordestgaard B, Benn M, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007; 298:299–308. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg I, Eckel R, et al. Triglycerides and heart disease: still a hypothesis? Arterioscler Thromb Vasc Biol. 2011;31(8):1716-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr A, Samaras K, et al. Pathogenesis of hiv-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–1883. [DOI] [PubMed] [Google Scholar]
- 19.Anuurad E, Bremer A, et al. Hiv protease inhibitors and obesity. Current opinion in endocrinology, diabetes, and obesity. 2010;17:478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakeam H, Al-Jedai A, et al. Sirolimus induced dyslipidemia in tacrolimus based vs. Tacrolimus free immunosuppressive regimens in renal transplant recipients. Annals of transplantation : quarterly of the Polish Transplantation Society. 2008;13:46–53. [PubMed] [Google Scholar]
- 21.Kronenberg F. Dyslipidemia and nephrotic syndrome: Recent advances. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2005;15:195–203. [DOI] [PubMed] [Google Scholar]
- 22.Kaysen G. Lipid and lipoprotein metabolism in chronic kidney disease. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2009;19:73–77. [DOI] [PubMed] [Google Scholar]
- 23.Zilversmit D. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clin Chem 1995;41:153–158. [PubMed] [Google Scholar]
- 24.Bansal S, Buring J, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007; 298:309–316. [DOI] [PubMed] [Google Scholar]
- 25.Havel R, Kane J. Introduction: structure and metabolism of plasma lipoproteins. Scriver CR, Beaudet AL, Sly WS, Valle D, The Metabolic and Molecular Bases of Inherited Disease. 7 New York, NY: McGraw-Hill; 1995. pp. 1841–1851. [Google Scholar]
- 26.Marcoux C, Hopkins P, et al. Remnant-like particle cholesterol and triglyceride levels of hypertriglyceridemic patients in the fed and fasted state. J Lipid Res. 2000;41:1428–1436. [PubMed] [Google Scholar]
- 27.Patsch J, Miesenbock G, et al. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb. 1992;12:1336–1345. [DOI] [PubMed] [Google Scholar]
- 28.Gryn S, Hegele R, et al. Novel therapeutics in hypertriglyceridemia. Current Opinion in Lipidology, Issue: Volume 26(6), December 2015, p 484–491. [DOI] [PubMed] [Google Scholar]
- 29.Canner P, Berge K, et al. Fifteen year mortality in Coronary Drug Project patients: long- term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255. [DOI] [PubMed] [Google Scholar]
- 30.Boden W, Probstfield J, et al. Aim-High Investigators: Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 31.Landray M, Haynes R, et al. Hps Thrive Collaborative Group Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014; 371:203–212. [DOI] [PubMed] [Google Scholar]
- 32.Hegele R, Ginsberg H, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2013;2:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elshaghabee F, Rokana N, et al. “Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives” Frontiers in microbiology vol. 8 1490;August 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FAO/WHO (2001). Health and Nutritional Properties of Probiotics in Food Including Powder Milk With Live Lactic Acid Bacteria. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria Rome: Food and Agriculture Organization. [Google Scholar]
- 35.Wang J, Tang H, et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME 2015; J. 9 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bader J, Albin A, et al. Spore-forming bacteria and their utilisation as probiotics. Benef. Microbes. 2012; 3 67–75. [DOI] [PubMed] [Google Scholar]
- 37.Campbell AW. Inflammation and a Solution: Probiotics. Altern Ther Health Med. 2016; 22(5):8-12. [PubMed] [Google Scholar]
- 38.Lefevre M, Racedo S, et al. Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: a randomized, double-blind placebo-controlled study. Immun. Ageing. 2015; 12 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shobharani P, Padmaja R, et al. Diversity in the antibacterial potential of probiotic cultures Bacillus licheniformis MCC2514 and Bacillus licheniformis MCC2512. Res. Microbiol. 2015; 166 546–554. [DOI] [PubMed] [Google Scholar]
- 40.Ripert G, Racedo S, et al. Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by Clostridium difficile and Bacillus cereus toxins. Antimicrob. Agents Chemother. 2016; 60 3445–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fakhry S, Sorrentini I, et al. Characterization of spore forming Bacilli isolated from the human gastrointestinal tract. J. Appl. Microbiol. 2008; 105 2178–2186. [DOI] [PubMed] [Google Scholar]
- 42.Hong H, Khaneja R, et al. Bacillus subtilis isolated from the human gastrointestinal tract. Res. Microbiol 2009. 160 134–143. [DOI] [PubMed] [Google Scholar]
- 43.Pinchuk I, Bressollier P, et al. In vitro Anti-Helicobacter pylori Activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents Chemother. 2001; 45 3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee Y, Yoshitsugu R, et al. Combination of soya pulp and Bacillus coagulans lilac-01 improves intestinal bile acid metabolism without impairing the effects of prebiotics in rats fed a cholic acid-supplemented diet. Br. J. Nutr 2016. 116 603–610. [DOI] [PubMed] [Google Scholar]
- 45.McFarlin B, Henning A, et al. Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. World J Gastrointest Pathophysiol 2017: ; 8(3): 117-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghoneim M, Hassan A, et al. Effect of polysaccharide from Bacillus subtilis sp. on cardiovascular diseases and atherogenic indices in diabetic rats. BMC Complement. Altern. Med 2016; 16:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zouari R, Abdallah-Kolsi R, et al. Assessment of the antidiabetic and antilipidemic properties of Bacillus subtilis SPB1 biosurfactant in alloxan-induced diabetic rats. Pept. Sci. 2015; 104 764–774. [DOI] [PubMed] [Google Scholar]
- 48.Di Caro S, Tao H, et al. Bacillus clausii effect on gene expression pattern in small bowel mucosa using DNA microarray analysis. Eur. J. Gastroenterol. Hepatol. 2005; 17 951–960. [DOI] [PubMed] [Google Scholar]
- 49.Gaziano JM, Hennekens CH, O’Donnell CJ, et al. Fasting triglycerides, high-density lipoprotein and risk of myocardial infarction. Circulation 1997;96:2520-2525. [DOI] [PubMed] [Google Scholar]
- 50.Wan K, Zhao J, Huang H, et al. The association between triglyceride/high-density lipoprotein cholesterol ratio and all-cause mortality in acute coronary syndrome after coronary revascularization. Plos One, April 16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz GG, Abt M, Bao W, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. JACC 2015;65(21):2267-2275. [DOI] [PubMed] [Google Scholar]


