Abstract
Background
The novel coronavirus, named SARS-CoV-2, was first described in December 2019 as a cluster of pneumonia cases in Wuhan, China. It has since been declared a pandemic, with substantial mortality.
Materials and methods
In our case series, we describe the clinical presentation, characteristics, and outcomes of our initial experience of managing 24 critically ill COVID-19 patients at a designated COVID-19 ICU in Western India.
Results
Median age of the patients was 54 years, and 58% were males. All patients presented with moderate to severe acute respiratory distress syndrome (ARDS); however, only 37.5% failed trials of awake proning and required mechanical ventilation. Patients who received mechanical ventilation typically matched the H-phenotype of COVID-19 pneumonia, and 55.5% of these patients were successfully extubated.
Conclusion
The most common reason for ICU admission in our series of 24 patients with severe COVID-19 was hypoxemic respiratory failure, which responded well to conservative measures such as awake proning and oxygen supplementation. Mortality in our case series was 16.7%.
How to cite this article
Shukla U, Chavali S, Mukta P, Mapari A, Vyas A. Initial Experience of Critically Ill Patients with COVID-19 in Western India: A Case Series. Indian J Crit Care Med 2020;24(7):509–513.
Keywords: Acute respiratory distress syndrome, Coronavirus disease-2019, Severe acute respiratory syndrome coronavirus-2
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel pathogen that was first reported from Wuhan, China. It has been shown to cause the disease designated as coronavirus disease 2019 (COVID-19).1 At the time of writing this article, there are more than 3.5 million confirmed cases of COVID-19 worldwide, and mortality associated with this disease has been seen to vary widely across the globe.2 Ever since the first case in India was diagnosed on January 30, 2020, there have been over 80,000 confirmed cases of COVID-19, with over 2,500 deaths. Literature from other countries such as Italy, Spain, France, and the United States has suggested high mortality associated with this disease and overburdening of intensive care units (ICUs). A large number of cases in India have been reported from the state of Maharashtra, which accounts for just over a third of the country's cases. This report from a designated COVID-19 hospital aims to provide a preliminary description of the clinical course and outcomes among patients with severe respiratory distress admitted to ICU with a confirmed diagnosis of COVID-19 in Maharashtra.
Materials and Methods
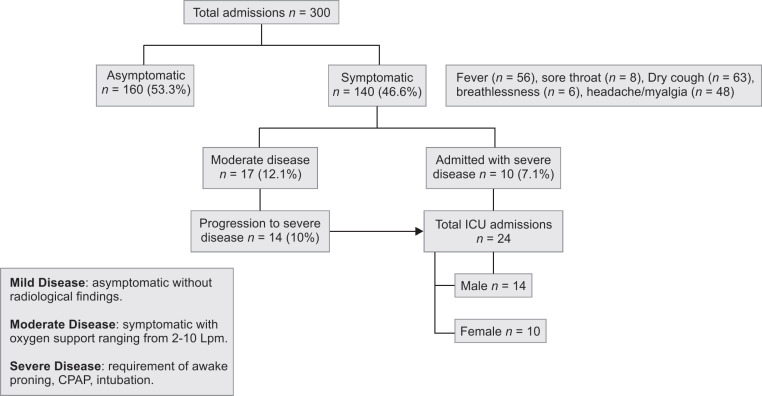
This is a single-center, retrospective, observational experience from a tertiary COVID-19 treatment center in Pune, Maharashtra. The institutional review board approved this study and deidentified patient data were analyzed. Patients with laboratory-confirmed COVID-19 infection, i.e., positive result on real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay of nasopharyngeal swab sample, were admitted to a designated nodal center in the city of Pune, Maharashtra. A total of 300 patients were admitted to the hospital with varying severity of illness between the first week of April and mid-May 2020 (Fig. 1). Of these patients, 140 (46.6%) presented with symptoms such as fever, sore throat, dry cough, breathlessness, headache, or myalgia. Fourteen of these symptomatic patients who were initially admitted with moderate disease without significant respiratory distress deteriorated to severe disease (diagnosed by SpO2 <90% on high-flow oxygen via nonrebreather mask). Ten other patients were referred to our ICU with preexisting severe COVID-19. COVID-19 patients were admitted to ICU when they showed features of moderate-to-severe acute respiratory distress syndrome (ARDS) evidenced by hypoxemia with the PaO2/FiO2 ratio < 200 on arterial blood gas analysis, signs of respiratory distress like tachypnea, and/or accessory muscle usage. A total of 24 ICU admissions were identified, and data pertaining to demographics, clinical signs, blood investigations, and radiological investigations were obtained during the course of their ICU stay. Chest imaging showed bilateral pulmonary opacities not thought to be caused by cardiac failure or volume overload in a majority of patients.
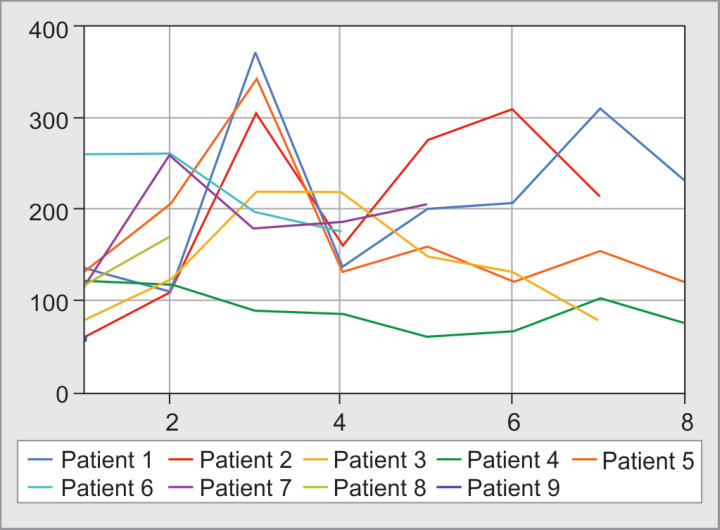
Fig. 1.
P/F ratio for intubated patients (day 1–6). Patients 1, 2, 5, 6, and 7 were extubated. Patient 9 died on day 1 of intubation. Patient 3 died on day 8 and patient 4 died on day 9. Patient 8 died on day 17 of admission
Statistical Analysis
Qualitative variables were described as sample and percentages and quantitative variables as mean (±standard deviation) or median (range). A comparative analysis was performed with a Student's t-test for paired samples. A p value of less than 0.05 was considered statistically significant. Statistical analysis was performed using the Statistical Package for Social Science (SPSS) version 17.0 (SPSS Inc., Chicago, IL).
Results
Out of 300 patients admitted to our hospital with confirmed diagnosis of COVID-19 (Flowchart 1), 24 required admission to the ICU due to falling oxygen saturations despite oxygen therapy. The demographic and baseline clinical characteristics of patients are shown in Table 1. The median age of the patients was 54 years, and 58% were males. Coexisting diseases were seen in nine (37.5%) of the patients, with diabetes mellitus and hypertension being the most commonly encountered chronic medical conditions. All those admitted to ICU had moderate-to-severe ARDS (as per Berlin definition3). These patients had a median PaO2/FiO2 ratio of 119 (range 61–190). All the patients had tachypnea, defined as a respiratory rate of greater than 25 breaths per minute. Chest radiography was performed in all patients on ICU admission, and 21 (87.5%) radiographs were suggestive of bilateral pulmonary infiltrates; 3 (12.5%) radiographs showed unilateral infiltrates, with no pleural effusions visualized. Ten patients were referred directly to ICU after varying duration of illness elsewhere; hence, we could not access those records. Out of the 14 patients who deteriorated while admitted at our center, the average duration from hospital admission to admission in the ICU was 3.7 days. Baseline laboratory investigations are also shown in Table 1, which show a normal median total leukocyte count (TLC), elevated median C-reactive protein (CRP), and a median neutrophil to lymphocyte ratio of 4.7.
Flowchart 1.
Flowchart depicting patients’ journey to ICU. Fever (n = 56), sore throat (n = 8), dry cough (n = 63), breathlessness (n = 6), headache/myalgia (n = 48)
Table 1.
Demographic and clinical characteristics of COVID-19 patients admitted in ICU
| Total (n = 24) | Male (n = 14) | Female (n = 10) | |
|---|---|---|---|
| Age in years | 54 (50–64) | 55 (50–65) | 54 (50–63) |
| Comorbidities | |||
| Diabetes mellitus | 4 (16.7) | 1 (7.14) | 3 (30) |
| Hypertension | 4 (16.7) | 1 (7.14) | 4 (40) |
| Hypothyroid | 2 (8.3) | 0 | 2 (20) |
| Asthma/COPD | 3 (12.5) | 1 (7.14) | 2 (20) |
| Imaging n (%) | |||
| Bilateral infiltrates | 23 (87.5) | 13 (92.8) | 10 (100) |
| Unilateral infiltrate | 1 (12.5) | 1 (7.14) | 0 |
| Baseline PaO2/FiO2 ratio | 119 (72–142) | 99 (74–112) | 139 (80–145) |
| Ventilation | |||
| Intubated | 9 (37.5) | 6 (42.8) | 3 (30) |
| Awake proning | 15 (62.5) | 7 (50) | 8 (80) |
| Duration of mechanical ventilation in days | 6 (3.5–9) | 6 (3.5–8.5) | 7 (6.5–8) |
| Patients extubated | 5 (55.5) | 3 (21.42) | 2 (20) |
| Mortality | 4 (16.7) | 3 (21.42) | 1 (10) |
| Length of ICU stay in days | 9 (7–11) | 7.5 (5–14) | 6 (3–11) |
| Biochemical characteristics at baseline | |||
| C-reactive protein (mg/L) | 112 (85–152) | 112 (77–154) | 116 (42.5–168) |
| Ferritin (ng/mL) | 515 (272–814) | 673 (805–1151) | 285 (222–518) |
| Serum neutrophil/lymphocyte ratio | 4.85 (2.84–7.85) | 5.6 (3.5–8.16) | 3.38 (1.72–5.54) |
| Platelet count (×105/cmm) | 213 (165–345) | 200 (165–313) | 213 (161–357) |
| Total leukocyte count (×103/cmm) | 6.8 (6.1–8.25) | 6.7 (5.1–7.05) | 7.5 (5.3–9.7) |
Values are expressed as n (%) or median (IQR)
All patients were monitored with pulse oximetry, and patients with oxygen saturations of <90% despite high-flow oxygen (>10 L/minute) were encouraged to lie prone. Nine patients (37.5%) required invasive mechanical ventilation. The most common indication for initiation of mechanical ventilation was respiratory distress (rate > 30 minute) along with persistent hypoxemia (no improvement in the P/F ratio despite 4 hours of awake proning) or increasing oxygen demand despite institution of awake proning. For patients who were mechanically ventilated, PaO2/FiO2 ratios prior to intubation were suggestive of moderate-to-severe ARDS. Data pertaining to ventilatory parameters are shown in Table 2. The median FiO2 on day 1 of mechanical ventilation was 0.8 (0.5–1), which subsequently improved to 0.55 (0.5–0.8) on day 3. With regards to ventilation, driving pressure (difference between Pplat and PEEP) on day 1 was 17.5 cm H2O and then decreased to 15 cm H2O by day 5 (Table 2). Immediately following intubation, the median compliance was seen to be 18 mL/cm H2O. The ventilatory parameters in our cohort appear to reflect features of the H-phenotype of COVID-19 pneumonia most frequently.4 All patients who were intubated were positioned prone to treat hypoxemic respiratory failure associated with ARDS.5 Tracheotomy was performed in one patient, on day 9 of mechanical ventilation. Six of the nine patients (66.6%) developed hypotension requiring vasopressor support, with two (22.2%) patients developing secondary infections. Among the patients requiring vasopressor support, three had transient hypotension after intubation, and three had hypotension persisting beyond 12 hours following intubation. All patients received hydroxychloroquine, therapeutic anticoagulation, and systemic glucocorticoid therapy as per local guidelines. The nonintubated patients were asked to lie prone with oxygen support. These were patients who had moderate ARDS, with respiratory rate <30 minute, SpO2 > 85% at all times, and no clinical evidence of respiratory distress.
Table 2.
Ventilatory parameters for intubated patients (n = 9)
| Day 1 | Day 3 | Day 5 | Day 7 | |
|---|---|---|---|---|
| Driving pressure (cm H2O) | 17.5 (15–24) | 20 (12–20) | 15 (12–20) | 15 (11–20) |
| FiO2 | 0.8 (0.5–1) | 0.55 (0.5–0.8) | 0.5 (0.4–0.9) | 0.6 (0.5–0.7) |
| P/F ratio supine | 128.5 (62–198) | 196 (90–306) | 180 (61–270) | 178 (74–280) |
| P/F ratio prone | 162 (93–260) | 263 (90–372) | 190 (62–280) | 208 (103–312) |
| Compliance (mL/cm H2O) | 18 (15.5–30.5) | 18 (15.5–30.5) | 34 (25.5–37.5) | 28 (21–32) |
Note: data expressed as median (IQR)
Outcomes
As of the 2nd week of May, of the 24 patients studied, 4 (16.7%) died, and 20 (83.3%) had been discharged from the ICU. Mortalities included an elderly patient with preexisting hypertension who developed anuric renal failure and multiorgan dysfunction on the 7th day of ICU admission, the second patient died of severe refractory hypoxemia on day 1 of ICU admission, the third patient died of anuric renal failure and multiorgan dysfunction on day 9 of ICU admission, and the fourth mortality was due to secondary sepsis and multiorgan dysfunction on day 17 of ICU admission. The median length of ICU stay was 9 days in survivors. The median duration of mechanical ventilation was 8 days in survivors, with five (55.5%) patients being extubated. Among patients who were proned awake, two required mechanical ventilation after 24 hours of oxygen support. Fifteen patients were managed with awake proning alone and were discharged successfully from the ICU. Median length of ICU stay for nonventilated patients was 8 days. Patients discharged from ICU were discharged from the hospital after two negative swabs as per the guidelines at the time and continue to do well as assessed by telephonic follow-up.
Discussion
This series describes 24 patients with hypoxemic respiratory failure and a confirmed diagnosis of COVID-19 and is the first description of critically ill patients with confirmed COVID-19 in India. The patients in our series presented with symptoms of respiratory distress and signs of moderate-to-severe ARDS similar to previous reports from China and Italy, indicating a similar host response to SARS-CoV-2.6,7 Cough and tachypnea were the most common presenting symptoms; however, only a few patients had fever on admission. Only a third of the patients had preexisting illnesses prior to hospital admission, most often hypertension and diabetes mellitus. Baseline investigations such as TLC and platelet count were found to be within normal range but most of these patients had severe lymphopenia. The neutrophil/lymphocyte ratio (NLR) and other biomarkers of inflammation such as ferritin and CRP were seen to be elevated. These findings are at concurrence with the previous literature, which demonstrated an increased incidence of lymphocytopenia as well as elevated values of NLR on admission in this patient cohort, which are predictive of poor clinical outcomes.8,9
Comparison of patients who required mechanical ventilation with patients who were managed conservatively with awake proning and oxygen supplementation showed no significant differences in terms of age (Table 3). All patients who were mechanically ventilated showed the H-phenotype of COVID-19, which shows high elastance, high lung weight, high lung recruitability, and a high fraction of right-to-left shunt. This phenotype fits the criteria for severe ARDS and is ideally managed as such. Three of our patients got intubated between day 7 and 9 of the start of illness. Baseline investigations for biomarkers of acute inflammation such as CRP, ferritin, and NLR were seen to be comparable between the two groups. Baseline PaO2/FiO2 ratios were significantly different between the two groups, with the median PaO2/FiO2 ratio being lower in the patients who required mechanical ventilation (118 vs 148, p = 0.02). Our findings are in agreement with the previous literature, which suggests that moderate ARDS due to COVID-19 can be managed with measures such as awake proning and high inspired concentrations of oxygen and such patients tend to do well, despite elevated biomarkers of inflammation on ICU admission.10 Deterioration despite the abovementioned measures can be considered an indication for institution of mechanical ventilation in this patient cohort.
Table 3.
Comparison of characteristics between ventilated and nonventilated patients
| Ventilated | Nonventilated | p value | |
|---|---|---|---|
| Age in years | 59.37 ± 13.56 | 57.37 ± 14.62 | 0.74 |
| P/F ratio | 118 (77–134) | 148 (123–238) | 0.02* |
| Baseline investigations | |||
| CRP | 127.78 (50.5–151.5) | 89.67 (11.7–119.1) | 0.34 |
| Ferritin | 339.4 (239.1–790.7) | 279.4 (168.6–1056.9) | 0.38 |
| NLR | 13 (2.89–72) | 3.87 (2.3–6.2) | 0.29 |
| Platelet count | 127.8 (50–161) | 208 (157–275) | 0.22 |
Data expressed as mean ± SD or median (IQR); Student's t test
Patients who needed ventilation had significantly lower P/F ratio
The mortality rate of 16.7% in our series of patients admitted to the ICU is lower than that reported in China as well as hospitals in the United States.9–11 Patients who received mechanical ventilation had high requirements of inspired fraction of oxygen, with higher than normal driving pressures (median, 17 cm H2O) immediately following institution of ventilation. They had low compliance, lower P/F ratios, and needed moderate PEEP to help improve oxygenation (Table 2). Proning helped in all patients except one, where the lung injury did not improve even after seven cycles of proning. Of the nine patients who required mechanical ventilation, five were successfully extubated. The earliest extubation was performed on day 5 of mechanical ventilation, and patients who were successfully extubated tended to show improvements in PaO2/FiO2 ratios by the 3rd day of ventilation (Fig. 1). This suggests that in patients who do not show marked improvement in PaO2/FiO2 ratios by 72 hours, the disease course is likely to be prolonged. Awake proning was seen to lead to marked improvements in the PaO2/FiO2 ratios of patients with hypoxemia despite oxygen therapy, and likely led to lower rates of intubation. This finding is in agreement with previous reports, and awake proning is likely the simplest, most resource-effective method of improving oxygenation in this patient cohort.12,13
The limitation of this study is the small sample of patients from a single center. However, this study provides initial experience regarding management of these patients in India and emphasizes the importance of early screening of patients with COVID-19 to identify those who are critically ill. Critical care management of such patients and early institution of measures such as awake proning and timely initiation of mechanical ventilation may help reduce the mortality and morbidity associated with this condition.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323(8):709–710. doi: 10.1001/jama.2020.1097. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, china: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? http://link.springer.com/ Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. Available from 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. DOI: [DOI] [PubMed] [Google Scholar]
- 6.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335–1335. doi: 10.1001/jama.2020.4344. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Yang A-P, Liu J, Tao W, Li H. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7152924/ Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. DOI: Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. COVID-19 in critically Ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED's experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375–378. doi: 10.1111/acem.13994. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. doi: 10.1186/s13613-020-00650-2. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]