Abstract
Policy Points.
Concerns have been raised about risk selection in the Medicare Shared Savings Program (MSSP). Specifically, turnover in accountable care organization (ACO) physicians and patient panels has led to concerns that ACOs may be earning shared‐savings bonuses by selecting lower‐risk patients or providers with lower‐risk panels.
We find no evidence that changes in ACO patient populations explain savings estimates from previous evaluations through 2015. We also find no evidence that ACOs systematically manipulated provider composition or billing to earn bonuses.
The modest savings and lack of risk selection in the original MSSP design suggest opportunities to build on early progress.
Recent program changes provide ACOs with more opportunity to select providers with lower‐risk patients. Understanding the effect of these changes will be important for guiding future payment policy.
Context
The Medicare Shared Savings Program (MSSP) establishes incentives for participating accountable care organizations (ACOs) to lower spending for their attributed fee‐for‐service Medicare patients. Turnover in ACO physicians and patient panels has raised concerns that ACOs may be earning shared‐savings bonuses by selecting lower‐risk patients or providers with lower‐risk panels.
Methods
We conducted three sets of analyses of Medicare claims data. First, we estimated overall MSSP savings through 2015 using a difference‐in‐differences approach and methods that eliminated selection bias from ACO program exit or changes in the practices or physicians included in ACO contracts. We then checked for residual risk selection at the patient level. Second, we reestimated savings with methods that address undetected risk selection but could introduce bias from other sources. These included patient fixed effects, baseline or prospective assignment, and area‐level MSSP exposure to hold patient populations constant. Third, we tested for changes in provider composition or provider billing that may have contributed to bonuses, even if they were eliminated as sources of bias in the evaluation analyses.
Findings
MSSP participation was associated with modest and increasing annual gross savings in the 2012‐2013 entry cohorts of ACOs that reached $139 to $302 per patient by 2015. Savings in the 2014 entry cohort were small and not statistically significant. Robustness checks revealed no evidence of residual risk selection. Alternative methods to address risk selection produced results that were substantively consistent with our primary analysis but varied somewhat and were more sensitive to adjustment for patient characteristics, suggesting the introduction of bias from within‐patient changes in time‐varying characteristics. We found no evidence of ACO manipulation of provider composition or billing to inflate savings. Finally, larger savings for physician group ACOs were robust to consideration of differential changes in organizational structure among non‐ACO providers (eg, from consolidation).
Conclusions
Participation in the original MSSP program was associated with modest savings and not with favorable risk selection. These findings suggest an opportunity to build on early progress. Understanding the effect of new opportunities and incentives for risk selection in the revamped MSSP will be important for guiding future program reforms.
Keywords: Medicare, accountable care organizations, selection bias, quasi‐experimental studies
In the voluntary medicare shared savings program (mssp), participating accountable care organizations (ACOs) have incentives to reduce total Medicare spending for their attributed patient populations. Specifically, an ACO is eligible for a shared‐savings bonus if total per‐beneficiary Medicare spending is sufficiently below its spending target, or benchmark, and if its performance on a set of quality measures meets minimum standards. In track 1 of the MSSP—the track in which almost all ACOs (99%) participated for the first four years of the program 1 —the shared‐savings bonus was 50% of the difference between an ACO's spending and its benchmark, less a smallpercentage for submaximal quality scores. The benchmark in a given performance year was based on an ACO's average historical spending before MSSP entry for analogously attributed patients, updated to the performance year based on average national Medicare spending growth.
Existing evidence indicates that participation in the MSSP produced modest reductions in Medicare spending for ACO patients, 2 , 3 , 4 , 5 , 6 even after accounting for bonus payments. However, churn in the patients attributed to ACOs and in the providers included in ACO contracts has raised concerns that some of the savings might be an artifact of risk selection—that is, the result of ACOs encouraging high‐cost patients to switch to a non‐ACO provider or excluding clinicians with high‐cost patients from ACO contracts. ACOs do have some incentives to engage in favorable risk selection, but the incentives were limited by several features of the MSSP's original design. Moreover, risk selection can be assessed and addressed by evaluation methods, even if it artificially inflates savings that are calculated by the MSSP by comparing ACO spending with benchmarks. Thus, evidence of risk selection does not imply that estimates of savings from evaluations are biased; if there is favorable risk selection, net savings can still be determined by estimating gross savings using methods that address risk selection and subtracting the bonus payments (which include any unearned gains from risk selection). 7 In the following pages, we describe program features that shape incentives for risk selection in the MSSP before turning to the empirical contributions of this paper.
Incentives for Risk Selection in the MSSP
TIN Level
The MSSP defines ACOs as collections of taxpayer identification numbers (TINs) identifying practices and Centers for Medicare and Medicaid Services (CMS) Certification Numbers (CCNs) identifying certain safety‐net facilities. (To simplify, we refer to both TINs and CCNs as TINs.) An ACO's yearly attributed population includes all patients who receive more qualifying services from that ACO's TINs than any other ACO or non‐ACO TIN. The attribution process assigns patients based on care received from primary care physicians (PCPs; defined by four specialties: internal medicine, family medicine, general practice, or geriatric medicine) as long as patients receive at least one qualifying service from a PCP. Since 88% of patients with a qualifying service have at least one visit with a PCP, attribution is largely based on the PCPs from which patients receive care. Patients without a qualifying service with a PCP are assigned based on receipt of the same services from non‐PCP clinicians. Each year, ACOs in the MSSP can change the TINs in its contract but, unlike ACOs in the Pioneer model, cannot select among clinicians within TINs (ie, all clinicians billing under an included TIN are in the contract).
Until 2017, the MSSP accounted for changes in TIN inclusion each year by adjusting benchmarks to reflect the baseline spending of the revised set of TINs. Thus, ACOs did not have clear incentives to favor lower‐spending TINs because the reduced spending would be offset by reduced benchmarks. Excluding higher‐spending TINs might improve performance on utilization‐based quality measures such as readmission rates, thereby increasing their shared‐savings rate (the percentage of savings they could keep if they qualified for a bonus), but this would not artificially inflate the gross savings. Because the variance of medical spending is greater when spending is higher, an ACO with downside risk for spending in excess of benchmarks (ie, in a two‐sided contract) might avoid TINs with higher spending to minimize the probability of a large loss from random fluctuations in spending. Through 2015, however, almost all ACOs did not assume downside risk, and those that did had less risk for losses than prospect for savings.
Consequently, TIN‐level selection incentives were minimal in the original MSSP and may actually have favored inclusion of higher‐spending TINs for two reasons. First, greater variability in spending at higher levels may present opportunities in one‐sided contracts for larger bonuses (due to random fluctuations). Second, ACOs with higher spending might generate savings more easily, because the costs of lowering wasteful spending are likely lower when there is more wasteful spending to cut (ie, more fat to trim). Indeed, ACOs with higher baseline spending have reduced spending more than other ACOs, on average. 4
In 2017, the MSSP began to blend ACO benchmarks with average regional spending after three years of participation. The implementation of this regional blending will be accelerated by the recent overhaul of the MSSP, “Pathways for Success,” which also requires ACOs to assume more downside risk sooner after MSSP entry. 8 These changes create new incentives for ACOs in the MSSP to select TINs with spending below their regional average, 9 but these incentives were not in place during the period examined by MSSP evaluations to date.
Clinician Level
Given a set of TINs and the attendant benchmark, an MSSP ACO has incentives to exclude clinicians with spending in excess of that predicted by the hierarchical condition categories (HCC) model used to adjust for case mix. 10 To selectively exclude such physicians and their patients, an ACO would have to identify them and arrange for them to bill under a different TIN that is not included in the ACO's contract (because patient attribution is determined by the billing TINs, not clinicians). An ACO also could terminate clinicians’ employment or move them to a different practice, though these behaviors seem implausible and might provoke legal action. The ability to alter the attributed patient population by changing the TIN under which clinicians bill does create some opportunities for risk selection, and there is some anecdotal evidence of ACOs exploiting this mechanism. 11
In particular, one strategy relates to the inclusion of encounters in postacute or long‐term nursing facilities among the qualifying services used by CMS to assign patients to ACOs. Consider an internist or geriatrician who sees patients in the office and on rounds with patients in a skilled nursing facility (SNF), billing both types of services under the same TIN. After entry into the MSSP, the physician's organization could arrange for the SNF encounters to be billed under a separate excluded TIN, thereby causing patients who become acutely ill and receive more postacute facility care than outpatient primary care to be assigned away from the ACO to the excluded TIN in a performance year but not in the baseline period used to calculate a benchmark. The resulting spending reduction would not be corrected by a benchmark reduction, since the ACO did not change its constituent TINs. Patient encounters in SNFs were included in the MSSP attribution rules from 2012 to 2016 and subsequently dropped in 2017.
To the extent that clinician‐level selection results in lower risk‐adjusted spending by favoring clinicians with patients who are lower risk, it should manifest as a change in case mix in the attributed population, assuming that unobservable patient factors not included in the risk adjustment are correlated with the observable factors that are included. It is possible that ACOs could select clinicians based on their efficiency (care patterns), independent of patient risk. This may not manifest as a change in case mix and therefore may not be testable. It is unlikely, however, that ACOs possess the data and analytic capabilities to isolate physicians’ efficiency from the case mix of their patients.
Selecting clinicians based on their efficiency has ambiguous normative implications. While selecting clinicians based on their patients’ risk may be seen as wasteful gaming, selecting clinicians based on their efficiency could foster competition among PCPs to be more efficient as ACO programs expand and exert pressure on PCPs to participate. The associated spending reductions might offset any bonus payments to ACOs engaging in such selection.
Patient Level
To mitigate ACO incentives to increase savings artificially through risk selection or upcoding, the MSSP uses an HCC‐based risk adjustment model to account for changes in case mix. Among continuously served (as opposed to new) patients, the original MSSP rules only applied downward adjustments to ACO benchmarks (ie, if HCC risk scores decreased) to discourage gaming strategies to boost benchmarks by coding more diagnoses. Thus, ACOs had incentives to avoid patients with spending in excess of what the HCC model predicts, to attract patients with below‐predicted spending, and also to avoid continuously assigned patients with risk scores rising faster (due to illness) than the average rates in the broader populations used to update ACO benchmarks. Unlike Medicare Advantage (MA) plans, however, ACOs have no control over benefit or network design and therefore may have fewer means to select favorable risks. In the absence of the provider‐level selection strategies just described or billing manipulation described later in the paper, an ACO would somehow have to induce high‐cost patients in its attributed population to leave the ACO—for example, by dropping them from the practice, successfully referring them to a different PCP, or otherwise limiting their access to the ACO (eg, by capping appointments for high‐risk patients).
A more plausible mechanism for risk selection at the patient level would be for an ACO to bill for high‐risk patients under a TIN not included in the ACO's contract. In the overall Medicare population, the HCC model underpredicts spending in a given year for patients with high spending in the prior year. 12 Thus, ACOs could use data on baseline spending to identify patients whose spending in a performance year is expected to be underpredicted, on average, and arrange for their office visits to be billed under a different TIN. For several reasons, however, the gains from such a strategy may be smaller than they seem. First, the MSSP truncates spending per beneficiary at the 99th percentile, which lessens the underprediction problem. 12 Second, to gain from this strategy ACOs must alter assignment for high‐cost patients who would otherwise remain attributed to an ACO. Patients with the highest baseline spending, however, are least likely to remain assigned to the same ACO. 13 Some will become unassignable because they no longer use qualifying services (eg, patients at the end of life), while others suffering health shocks (eg, a new cancer diagnosis) may shift their care to different providers. Churn is naturally higher among high‐risk patients who develop needs for additional care. Third, ACOs receive lagged claims. This weakens predictions of performance‐year spending based on past spending because the correlation in spending between two periods decays as more time elapses between periods. Fourth, manipulating billing for different patients at different times may require advanced information systems that many ACOs lack. These caveats notwithstanding, the opportunity to select favorable risks via billing manipulation is a weakness in the current MSSP design.
In addition to supply‐side selection efforts by ACOs, high‐risk patients may exhibit stronger or weaker demand for care in ACOs. Many ACOs target high‐risk patients for enhanced care management, and prior research has found that ACO efforts have been associated with improved overall care ratings among high‐risk patients. 14 Thus, the tailored care ACOs offer may attract, rather than repel, high‐risk patients.
Attracting low‐risk patients may be more feasible than avoiding high‐risk patients. For example, ACOs could reach out to healthy patients without qualifying services and schedule visits for them (eg, annual wellness visits [AWVs]), thereby increasing the number of attributed patients with below‐predicted spending. The proportion of Medicare beneficiaries without a qualifying service is modest, however, and includes a subgroup of high‐risk patients (eg, decedents, hospice enrollees, and those using only emergency and inpatient care). Thus, the strategy would apply to an even smaller share of beneficiaries. Moreover, targeting of such efforts is likely to be highly imperfect because ACOs cannot know ex ante which patients would not use care by the end of a year. Imperfect targeting would contribute to higher spending due to the additional office visits and related services for patients whose attribution is not altered. Evidence to date suggests that clinicians may be motivated to provide AWVs to a broad patient population. Medicare patients with the lowest annual spending are not more likely to receive AWVs than those with higher spending, 15 , 16 and AWVs have largely substituted for office visits as opposed to lowering the proportion of patients with no office visits. Nevertheless, this potential selection strategy merits examination.
Contributions of This Paper
In this paper, we use evaluation methods to estimate savings in the MSSP that address selection bias, gauge the potential for residual selection, and test for risk selection that may have contributed to shared‐savings bonuses but not to bias in our evaluation. First, we report new estimates of overall savings through 2015 from a difference‐in‐differences analysis that addresses bias from provider‐level selection using an intention‐to‐treat approach. 5 Specifically, we hold ACOs’ providers constant over time. These estimates reflect the combined results of earlier work that compared savings between physician‐group and hospital‐based ACOs. As in those stratified analyses, 5 we find no evidence of patient risk selection and estimate overall gross savings in excess of bonus payments. We also consider the implications of differential changes in organizational structure among non‐ACO providers (eg, from different exposure to provider consolidation) and demonstrate that our finding of larger savings among physician group ACOs is robust to this consideration.
Second, we implement alternative approaches to eliminate residual risk selection that may have gone undetected by tests of observable patient characteristics in our evaluation (summarized in Table 1). These include use of patient fixed effects in difference‐in‐differences models, an intention‐to‐treat analysis holding patients’ baseline assignments to providers (prior to the start of MSSP incentives) constant, prospective assignments based on utilization two years prior to each study year, and an area‐level analysis defining MSSP exposure based on program penetration in patients’ hospital referral region (HRR). We provide empirical evidence that these approaches—while ensuring no bias from selection of patients with fixed characteristics predictive of lower spending—introduce other sources of bias. Nevertheless, these approaches produce results that are generally consistent with our main findings. We also consider an alternative patient attribution approach (using data on referring PCPs) to address potential selection bias from ACO efforts to use AWVs to boost attribution of healthy patients who might otherwise be unattributed. This approach increases savings estimates, providing evidence against successful use of AWVs to select lower‐risk patients.
Table 1.
Approaches to Assess and Address Residual Risk Selection in Evaluation
| Analytic Strategy | How Strategy Intends to Address Risk Selection | Potential Problems With Strategy |
|---|---|---|
| Patient fixed effects | Eliminate differential changes in fixed characteristics of patients attributed to ACOs vs. non‐ACO providers by comparing within‐patient changes in spending | Limiting to a continuous cohort introduces large within‐patient health declines that could differ between ACO and non‐ACO providers in absence of MSSP if:
|
| Intention‐to‐treat with fixed baseline assignments | Eliminate differential changes in patient attribution to ACOs vs. non‐ACO providers by holding baseline assignments constant over the study period | In absence of MSSP, baseline attribution to ACOs associated with subsequent differential changes in spending as patients switch providers if:
|
| Area‐level analysis | Eliminate re‐sorting of patients to ACO vs. non‐ACO providers after MSSP entry by basing exposure on area‐level MSSP penetration instead of patient‐level attribution to an ACO |
Allows bias from selective program participation in low‐spending‐growth areas Does not control for market‐level determinants of spending growth, including differential changes in population health Ecological fallacy (eg, if most effective ACOs are in low‐growth areas) |
| Attribution based on referring PCP | Augment attribution using the referring PCP for services used by unattributed patients to mitigate bias from ACO use of annual wellness visits or other strategies to attract low‐cost patients who would not otherwise be attributed |
Narrowly addresses only 1 potential selection behavior 13% of beneficiaries remain unassigned |
Abbreviations: ACO, accountable care organizations; MSSP, Medicare Shared Savings Program; PCP, primary care physician.
Third, we test for risk selection that may have been successfully eliminated in our evaluation but would have contributed to bonus payments (Table 2). We do so by allowing the provider composition of ACOs to change over time, as it did, and repeating our evaluation analysis. We also analyze patterns of patient and physician exit from ACOs over three performance years. We find that ACOs did not systematically favor providers with lower‐risk patients or lower spending as they changed their provider composition. We also demonstrate that analyses of patient or physician exit can be misleading without considering the counterfactual—churn in the absence of MSSP incentives. Last, we test for ACO manipulation of the TINs used by physicians for billing to achieve a lower‐cost attributed population during the performance period. We find no evidence of this behavior.
Table 2.
Approaches to Assess Risk Selection Contributing to Bonuses but Removed in Evaluation
| Selection Strategy | Analytic Approach to Assess Extent of Selection | Interpretation |
|---|---|---|
| Reconfiguration of ACOs to favor lower‐cost providers | Compare savings estimates from main approach holding ACO TINs or clinician NPIs constant vs. approach allowing changes in provider composition after first performance year | Greater savings estimated when allowing provider turnover would suggest selective inclusion of lower‐cost providers. Since ACO benchmarks adjust for changes in TINs, only increases in savings from within‐TIN changes in clinician composition could contribute to bonuses and constitute risk selection. |
| Gaming of CMS attribution algorithm via manipulation of TINs used for billing |
1. Compare savings with and without adjustment for patient characteristics when employing the CMS attribution algorithm 2. Using CMS attribution algorithm, compare savings when holding ACO composition fixed as sets of TINs vs. NPIs |
1. Attenuation of savings by patient covariate adjustment in analysis using the CMS attribution rules but not in our main approach (using only PCP office visits for attribution) would suggest risk selection enabled by the CMS rules that was removed in our evaluation but could have contributed to bonuses. 2. If ACOs strategically changed the TINs used for billing by member clinicians to induce attribution of lower‐cost patients, savings estimates should be attenuated by holding ACOs constant as sets of NPIs. |
| Exclude high‐risk patients or physicians with high‐risk patients | Compare differences in exit from ACO‐attributed populations between higher‐ vs. lower‐risk patients (or differences in exit from ACO TINs between PCPs with higher‐ vs. lower‐risk patients) with analogous differences in patient or PCP exit among non‐ACO TINs | Disproportionate exit of higher‐risk patients or PCPs with higher‐risk patients that is greater for ACOs than non‐ACO providers would suggest potential risk selection (a necessary but not sufficient observation). |
Abbreviations: ACO, accountable care organizations; CMS, Centers for Medicare and Medicaid Services; NPI, national provider identifier; PCP, primary care physician; TIN, tax identification number.
Finally, we discuss the implications of our findings for ACO policy in Medicare. Although we do not find evidence of risk selection in the early years of the MSSP, ACOs do have incentives to engage in favorable risk selection, and the opportunities to do so have grown. We discuss program reforms that could strengthen incentives for providers to participate in the MSSP and lower spending while mitigating incentives for risk selection.
Data and Methods
Evaluation of the MSSP Through 2015
Using Medicare claims for 20% random annual samples of fee‐for‐service Medicare beneficiaries from 2009 to 2015, we conducted a difference‐in‐differences analysis comparing beneficiaries attributed to providers that entered the MSSP in 2012, 2013, or 2014, with local beneficiaries attributed to nonparticipating providers (control group), before and after program entry by participating providers. In each year, we attributed beneficiaries to the ACO or non‐ACO TIN that accounted for the plurality of allowed charges for their office visits with PCPs (Current Procedural Terminology [CPT] codes 99201–15, 99241–45, G0402, and G0438‐39 in carrier claims and corresponding revenue center codes in outpatient claims for specific safety‐net settings). 17 In the pre‐entry period, attribution to an ACO means attribution to a group of providers who subsequently enter the MSSP.
We limited the qualifying services used for assignment to office visits with PCPs to achieve greater comparability between the ACO and control group. Use of all qualifying services in the CMS assignment rules, which include evaluation and management services from outpatient specialists and physicians in nursing facilities, introduces systematic differences between ACO‐attributed patients and the control group. 5 This occurs because many ACOs do not provide specialty care or postacute or long‐term care in nursing facilities. 4 , 18 , 19 Consequently, beneficiaries using nursing facility care or only specialty care would be disproportionately assigned to the control group. When comparison groups in a difference‐in‐differences analysis systematically differ, a stronger common shocks assumption is required; in this context, drivers of spending growth other than the MSSP would be more likely to differentially affect the attributed populations of ACO vs. non‐ACO providers if the populations differ.
Our modifications to beneficiary assignment also minimized bias from gaming strategies that involve changes in the TINs used for billing (described earlier). For example, a patient who requires more postacute care than primary care would be assigned by the CMS algorithm to the TIN billing for the postacute care but in our analysis would remain assigned to the ACO or non‐ACO TIN providing the primary care.
As expected from the dominant role of primary care in the CMS attribution algorithm, our assignments and CMS assignments overlapped substantially. For example, 89% of beneficiaries we attributed in 2013 to ACOs entering the MSSP in 2012‐2013 were found in the 2013 MSSP Beneficiary‐level attribution file; 20 of those, the assigned ACO matched in more than 99% of cases. Of beneficiaries in the 2013 MSSP Beneficiary‐level attribution file, we attributed 84% to ACOs; of those, the assigned ACO matched in 96% of cases.
After assigning beneficiaries to providers, we fit the following linear regression model:
where Y is the annual Medicare spending for beneficiary i in year t attributed to ACO k or a non‐ACO TIN and residing in HRR h; ACO is a vector of indicators for each ACO with the non‐ACO control group as the omitted reference group; HRR_Year is a vector of indicators for each HRR‐year combination; ACOcohort_Post is a vector of indicators of attribution to a specific entry cohort of ACOs (2012, 2013, or 2014 cohort) in a specific postentry year; Covariates is a vector of the patient characteristics listed in Table 4; and β1‐β4 are vectors of coefficients corresponding to each term.
Table 4.
Differential Changes From the Pre‐entry Period to Each Performance Year in the Characteristics of Patients Served by ACOs, as Compared With the Control Group, by Entry Cohort of ACOs a
| Differential Change From Pre‐entry Period to Each Performance Year for ACOs vs. Control Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2012 Entry Cohort | 2013 Entry Cohort | 2014 Entry Cohort | |||||||
| Patient Characteristic | Unadjusted Pre‐entry Sample Mean,b $/Patient | 2013 | 2014 | 2015 | 2013 | 2014 | 2015 | 2014 | 2015 |
| Age (year) | 72.2 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 |
| Female sex (%) | 58.5 | −0.2 | −0.3 | −0.2 | −0.1 | −0.2 | −0.2 | 0.0 | −0.1 |
| Race or ethnic group c (%) | |||||||||
| Non‐Hispanic white | 83.5 | 0.0 | −0.1 | −0.2 | 0.0 | −0.1 | −0.2 | 0.0 | 0.1 |
| Non‐Hispanic black | 8.5 | −0.1 | 0.0 | 0.2 | 0.0 | 0.0 | 0.2 | −0.1 | −0.2 |
| Hispanic | 4.7 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Other | 3.2 | 0.0 | 0.0 | −0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Medicaid recipient (%) | 15.3 | −0.2 | −0.2 | −0.2 | −0.1 | −0.3 | −0.1 | −0.1 | 0.1 |
| Disability was original reason for Medicare eligibility (%) | 21.9 | −0.1 | −0.1 | 0.0 | −0.3 | −0.5 | −0.3 | −0.2 | −0.3 |
| End‐stage renal disease (%) | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| CCW conditions,d no. | |||||||||
| Through prior year | 5.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Through 3 years priore | 4.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| History of hip fracture (%) | 2.9 | −0.1 | −0.1 | −0.1 | 0.0 | 0.0 | 0.0 | 0.0 | −0.1 |
| History of myocardial infarction (%) | 4.2 | 0.0 | 0.0 | 0.0 | −0.1 | −0.1 | 0.0 | 0.0 | 0.0 |
| HCC risk scoref | |||||||||
| Based on claims in prior year | 1.23 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 |
| Based on claims 3 years priore | 1.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| ZCTA‐level characteristic | |||||||||
| % below federal policy level | 9.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| % with high school diploma | 75.4 | −0.1 | −0.2 | −0.2 | 0.0 | 0.0 | −0.1 | 0.0 | 0.0 |
| % with college degree | 19.4 | −0.2 | −0.2 | −0.2 | 0.0 | 0.0 | 0.0 | −0.1 | 0.0 |
Means and percentages were adjusted for geography to reflect comparisons within hospital referral regions. ZCTA denotes ZIP Code tabulation area.
In the analyses, the pre‐entry period differed for each entry cohort, but for the purpose of describing the study sample in this table, years 2009–2011 were used to calculate a single mean for each characteristic.
Race or ethnic group was determined from Medicare Master Beneficiary Summary Files.
Chronic conditions from the Chronic Conditions Data Warehouse (CCW) included 27 conditions: acute myocardial infarction, Alzheimer's disease, Alzheimer's disease and related disorders or senile dementia, anemia, asthma, atrial fibrillation, benign prostatic hyperplasia, chronic kidney disease, chronic obstructive pulmonary disease, depression, diabetes, heart failure, hip or pelvic fracture, hyperlipidemia, hypertension, hypothyroidism, ischemic heart disease, osteoporosis, rheumatoid arthritis or osteoarthritis, stroke or transient ischemic attack, breast cancer, colorectal cancer, endometrial cancer, lung cancer, prostate cancer, cataracts, and glaucoma. Analytic models included indicators for all 27 conditions and indicators for the presence of multiple conditions ranging from 2 to 9 or more conditions. Counts of conditions included all conditions except cataracts and glaucoma.
For analyses of CCW condition indicators and hierarchical condition categories (HCC) scores derived from earlier claims, we limited the sample to a subgroup of beneficiaries who were also continuously enrolled in fee‐for‐service Medicare 3 years prior to the study year. The purpose of this was to assess the extent to which any differential changes may have been due to differential changes in coding practices in response to MSSP incentives.
HCC risk scores are derived from demographic and diagnostic data in Medicare enrollment and claims files, with higher scores indicating higher predicted spending in the subsequent year. For each beneficiary in each study year, we assessed the HCC score based on enrollment and claims data in the prior year, two years prior, and three years prior, in each case requiring continuous enrollment in fee‐for‐service Medicare in the study year and the year of claims used to calculate HCC scores.
The ACO fixed effects adjust for pre‐entry differences between ACOs and the control group and for any changes in the distribution of ACO‐attributed beneficiaries across ACOs. The HRR‐year fixed effects adjust for geographic differences between ACOs and the control group and for regional changes in spending for the control group. Thus, the estimated effect of MSSP participation (β3) is the difference between spending for ACO‐attributed patients in a postentry year and spending that would be expected for ACO patients if the change from the pre‐entry period to that year was equal to the change observed for patients in the same HRR served by non‐ACO providers (the differential change in spending for ACO patients, or gross savings). We used a robust variance estimator, specifying clusters as ACOs (for ACO‐attributed beneficiaries) or HRRs (for the control group). Specifying HRRs as the clusters for all beneficiaries yielded similar results. Additional details of the methods, including exclusion of patients attributed to Pioneer ACOs, have been published elsewhere. 5
To eliminate bias from selective dropout of ACOs by 2015, our analysis followed an intention‐to‐treat approach in which we retained all ACOs through 2015 regardless of participation status. To eliminate bias arising from changes in the sets of TINs or physicians composing ACOs, we held constant from 2009 to 2015 the definition of ACOs as collections of TINs or physician National Provider Identifiers (NPIs), in the latter case modifying attribution to assign patients to a group of ACO NPIs or a non‐ACO NPI. We held the PCPs in each ACO constant to eliminate bias from changes in ACO PCP composition instead of using fixed effects for beneficiaries’ assigned PCP, because PCP fixed effects could introduce bias if, for example, ACOs shift patients to more cost‐effective clinicians (eg, by prioritizing ACO patients for scheduling with their established PCPs). We did not wish to remove the effects of such strategic shifting from our evaluation of savings. As previously reported, 5 including PCP fixed effects did not alter our conclusions.
We conducted additional analyses to assess potential violations of the identifying assumption of our difference‐in‐differences analysis: that the ACO control group difference would have remained constant in the absence of MSSP participation. We estimated differential changes in patient characteristics and compared savings estimates with and without adjustment for fixed and time‐varying patient covariates. For time‐varying covariates potentially affected by the MSSP (eg, HCC scores via upcoding), we checked the sensitivity of results to adjusting for values derived from data several years prior to a given study year, as opposed to the year before. In addition to regression adjustments, we also implemented a propensity‐score weighting technique to balance covariates between ACOs and the control group in each year. 5 , 21
We estimated differences in pre‐entry trends between ACOs and the control group and conducted falsification tests treating pre‐entry years as hypothetical entry years. We also conducted falsification tests treating both non‐ACO TINs that were large enough after the start of the MSSP to participate (an expected 5,000‐plus assigned beneficiaries in the full Medicare population) and the 2015 MSSP entrants (which we did not analyze in the main analyses) as hypothetical entrants in various years. In addition to testing whether large provider organizations or groups that eventually joined the MSSP had slower spending growth when not participating, these falsification tests also explored whether our intention‐to‐treat approach, which categorized TINs by their ACO status at the outset of MSSP entry and held the treatment group of TINs constant, might produce differential reductions in spending in the absence of the MSSP.
Finally, we considered potential bias from differential changes in the share of patients served by higher‐ or lower‐spending organizations, as might result from differential exposure to mergers, acquisitions, and organizational expansion. In particular, our finding of greater savings in physician group ACOs could be partially explained by differential spending increases expected from hospital acquisition of physician practices and associated price increases (Medicare reimburses care in hospital‐owned settings at higher rates than in the independent office setting). In previous work, we conducted falsification tests treating non‐ACO physician groups as hypothetical ACOs and concluded that the differential exposure of the control group to hospital‐physician consolidation (and expansion of hospital outpatient departments) contributed minimally to overall estimated savings for physician group ACOs.
In this paper, we also consider the addition of non‐ACO organizational fixed effects to our models as an alternative strategy to address this concern by basing estimation on within‐organization changes in spending. Specifically, we included fixed effects for the TIN or CCN to which non‐ACO beneficiaries were assigned, in addition to ACO fixed effects for ACO‐assigned beneficiaries. These additional controls would adjust for differential price increases in the control group resulting from expansion of hospital outpatient departments or hospital acquisition of physician practices (assuming acquired practices bill under the acquiring hospitals’ outpatient TINs). Controlling for TIN/CCN fixed effects, however, could also introduce bias by selectively excluding in pre‐entry or postentry years certain groups of clinicians and patients whose TINs or CCNs are not consistently present throughout the study period. For example, observations for patients of retiring solo practitioners would be excluded before but not after the retirements cause patients to re‐sort to other practices. Likewise, if practices acquired by other organizations bill under the acquiring organization's TIN and have persistently distinct practice patterns, comparisons of within‐TIN changes in spending between ACOs and non‐ACO providers could be confounded by differential changes in TIN membership and the fixed practice patterns of member clinicians. In that case, a model without provider fixed effects would be less prone to bias than one with provider fixed effects. Thus, estimates controlling for provider fixed effects are hard to interpret.
Moreover, attempts to adjust for differential provider consolidation among ACO and non‐ACO providers may bias savings estimates if the effects of consolidation in one group spill over onto spending in the other group. If, for example, primary care practice ACOs save in part by steering their patients away from high‐priced hospital‐owned specialty clinics and imaging facilities, an unadjusted spending trend reflecting the expansion of hospital‐based health systems in the control group may better approximate counterfactual ACO spending in the absence of steerage efforts.
Approaches to Assess and Address Residual Risk Selection
Patient Fixed Effects
Conceptual considerations
One approach to ensure that differential changes in population composition do not contribute to savings estimated by difference‐in‐differences analysis is to use patient fixed effects in the model to control for all characteristics of patients that are fixed. Replacing ACO fixed effects with patient fixed effects in the model just described produces a difference‐in‐differences estimate based on within‐patient changes. Specifically, for a given performance year, the estimate becomes the mean within‐patient difference between spending for a patient attributed to an ACO in the performance year and spending in years when the patient is not attributed to an ACO in a performance year, minus the concurrent within‐patient spending difference for patients not attributed to an ACO in the performance year.
There are two major drawbacks to this approach. First, basing the estimation on within‐patient changes converts the analysis from one of annual cross‐sectional samples, each representative of the fee‐for‐service Medicare population, to a longitudinal cohort of patients who were alive, enrolled in fee‐for‐service Medicare, and eligible for attribution in both the pre‐ and postentry periods. As illustrated in Figure 1, the spending trends over the study period for these two samples differ dramatically. Adjusted annual Medicare spending of the serial cross‐sectional samples analyzed in our main evaluation approach increased by $374 per patient (4.2%) from 2009 to 2015, demonstrating that, despite substantial turnover in the sample over time, spending growth reflected modest secular trends. In contrast, adjusted spending increased by $1,740 per patient (26.4%) for a longitudinal cohort of continuously enrolled and attributable patients that would serve as the basis for estimation of savings in a model with patient fixed effects. The spending increase is most rapid at the end of the study period. This reflects the fact that patients must remain alive from the preperiod to 2015 to contribute to estimation of 2015 savings in a model with patient fixed effects, but they may then die or enter a long‐term care facility, for example, and no longer be alive or attributable on the basis of outpatient primary care in 2016. Thus, the cohort becomes more acutely ill (in ways not accounted for by the adjustments) as they near the end of the cohort inclusion period, unlike patients in consistently defined annual cross‐sectional samples. Figure 1 demonstrates how the rapid increase in spending occurs near the end of the inclusion period, no matter when that inclusion period ends.
Figure 1.
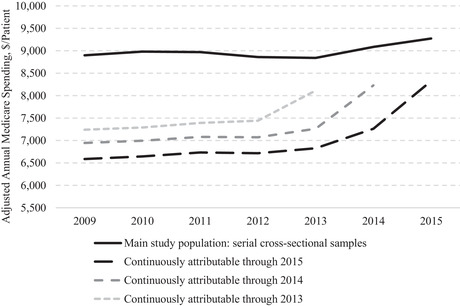
Adjusted Annual Per‐Patient Medicare Spending From 2009 to 2015 for Serial Cross‐Sectional Samples vs. Longitudinal Cohorts of Continuously Attributable Patientsa
aDecedents excluded throughout study period in all groups. Continuously attributable cohorts are subgroups of the main study population who have at least one office visit with a primary care physician in each year to support attribution.
Thus, an analysis with patient fixed effects requires the strong assumption that within‐patient spending changes would be the same for ACO and non‐ACO patients in the absence of the MSSP. This implies both similar health declines and similar treatment of patients with declining health by ACO and non‐ACO providers, yet the rapid acceleration in spending for the longitudinal cohorts in Figure 1 is likely to vary across different patient populations and providers. In contrast, an analysis of serial cross‐sectional samples allows patients to die or experience health declines consistently across time. The analysis can therefore net out differences in health care needs or treatment patterns between severely ill ACO and non‐ACO patients (because severely ill patients are consistently present in the pre‐ and postperiod).
Second, because patient attribution to ACOs is time‐varying, the difference‐in‐differences estimator in a model with patient fixed effects reflects not only pre‐ to postperiod changes in spending associated with a patient's provider entering the MSSP but also changes in spending associated with changes in patient attribution from a non‐ACO to ACO provider, or vice versa, during the postperiod. If patients are assigned to ACOs vs. non‐ACO providers based on their time‐varying health care needs, the estimates from a model with patient fixed effects would be biased because differences in spending caused by endogenous assignment to ACO or non‐ACO providers would not be differenced out, as they would be in a model without patient fixed effects. This second source of bias may interact with the first (eg, if sorting based on time‐varying needs is prominent among patients experiencing severe health declines).
To ameliorate the bias due to shifts in attribution between ACO and non‐ACO providers in the postperiod, ACO attribution in the postperiod could be treated as an absorbing state (turned on indefinitely after the first postperiod year of ACO attribution). However, this would not remove bias from endogenous sorting into ACOs in an initial postperiod year and would tend to bias estimates away from savings because attribution of high‐risk patients is less stable (as described later in the paper); thus, treating ACO attribution as an absorbing state would selectively retain high‐risk patients in the ACO group selectively in the postperiod.
Recognizing these conceptual concerns, results from a model with patient fixed effects must be interpreted with caution. Although patient fixed effects eliminate bias from differential compositional changes in the fixed characteristics of patients exposed to the MSSP, their deployment can exacerbate bias from differential changes in time‐varying characteristics within patients, effectively reversing the bias corrections achieved by a difference‐in‐differences comparison of serial cross‐sectional samples that are stably different (at the population level) in their fixed and time‐varying characteristics.
Empirical Analysis
To understand the impact of using patient fixed effects, first we limited our study sample to a longitudinal cohort of continuously attributable patients that supports estimation of a difference‐in‐differences from within‐patient changes and reestimated our main difference‐in‐differences model. The resulting estimate might differ from our main estimates for several reasons, including the concerns described earlier and the much lower mean spending for this cohort (Figure 1). Second, we substituted patient fixed effects for the ACO fixed effects in the model to isolate the incremental effect of holding the patient constant. Third, to gauge selection bias introduced by this approach, we compared estimates with and without adjustment for patients’ time‐varying characteristics.
Holding Baseline Assignments Fixed and Use of Time‐Varying Prospective Assignment
Conceptual Considerations
Another approach to eliminating bias from risk selection is to hold patients’ attribution to providers at baseline constant. This type of intention‐to‐treat approach was implemented by the Medicare Payment Advisory Commission, for example. 22 In addition to removing the contribution of differential changes in patient attribution from the difference‐in‐differences estimate (by disallowing changes in attribution), this approach also does not require utilization of qualifying services to categorize patients into ACO and non‐ACO groups after the initial year. This latter advantage may address bias from differential changes in the attributed patient population caused by provision of qualifying services (such as AWVs). More generally, ACO effects on primary care use and patient attribution are endogenous, though in prior work we found no evidence of differential changes in ACO provision of PCP office visits that would substantiate this concern. 5
Like the use of patient fixed effects, however, use of baseline patient assignments can introduce other sources of bias. If ACO and non‐ACO providers differ in their reimbursement rates or practice patterns in the absence of MSSP exposure, or if patient attribution to ACO vs. non‐ACO providers (in the absence of MSSP exposure) is influenced by their time‐varying health needs, we should expect spending differences between groups of patients defined by their baseline assignments to change over time, even if the MSSP has no effect on spending. In the framework of an instrumental variable analysis, the exclusion restriction is unlikely to hold when using baseline assignment as an instrument for MSSP exposure in the postperiod. That is, baseline assignment to ACO vs. non‐ACO providers likely predicts changes in spending that are not solely reflective of greater exposure to the MSSP.
The bias arises because a constraint is applied asymmetrically in time. It is therefore similar to the problem noted earlier of requiring a cohort to be alive and continuously enrolled for some period and also to the problem of regression to the mean when matching on time‐varying variables. 23 , 24 For example, outpatient Medicare spending for patients of independent physician groups is likely to be lower than for other patients, on average, because they are likely to receive less outpatient care at more generously reimbursed hospital‐owned facilities. Consequently, spending for patients initially attributed to independent primary care groups is likely to increase over time relative to a local control group served by a mix of PCPs in independent and hospital‐based practices. As patients switch practices, the proportion of patients attributed to hospital‐based practices can only increase among those initially attributed to independent groups, whereas switching would be bidirectional in the control group, leading to a smaller net shift to hospital‐based practices. Thus, an evaluation holding baseline assignments constant would tend to underestimate savings by independent physician group ACOs, all else equal. More generally, use of baseline assignments could bias overall MSSP savings estimates if the mix of independent and hospital‐based practices participating in the MSSP differs from the surrounding delivery system.
Similarly, practice patterns might differ systematically between ACOs and non‐ACO providers. The substantial patient churn in provider patient populations 13 , 25 , 26 could therefore introduce bias in an evaluation using time‐invariant baseline assignments to define comparison groups that would not be present in an evaluation using time‐varying assignments.
In addition, changes in health care needs may cause changes in attribution of patients to ACO or non‐ACO providers, whether because of true change in providers or the attribution algorithm. If patients are disproportionately assigned to ACOs when they become ill and to non‐ACO providers when they are healthy, or vice versa, one would expect differences in spending between patients initially assigned to ACOs and non‐ACO providers to converge as their health status reverts to the population mean. Use of the CMS attribution algorithm could exacerbate this source of bias. Its inclusion of services in postacute facilities would cause acutely ill patients to be disproportionately assigned away from ACOs at baseline, 27 inducing a subsequent differential increase in spending for patients assigned to ACOs at baseline as the control group's acute care needs subside and the ACO group's needs emerge.
We do not attempt to assess or address these sources of potential bias introduced by using baseline assignments. Rather, we note that the bias is difficult to predict and could be substantial, interpret savings estimates produced by this approach with caution, and conduct falsification tests to determine whether this approach might estimate an erroneous differential change in spending in the absence of MSSP participation.
Finally, in an alternative approach, we prospectively assign beneficiaries to providers in study year t based on utilization in year t‐2. For example, we based assignments in study year 2009 on 2007 claims and assignments in study year 2015 on 2013 claims. By consistently applying this alternative assignment approach to each study year, we address the limitations of the baseline assignment approach noted earlier while still eliminating any growth in savings estimates over the postentry period that might be due to patient risk selection. The effect of any differential re‐sorting of ACO and non‐ACO patients in 2014 or 2015 is eliminated because 2013 is the last year used for attribution. Like the baseline assignment approach, this approach also eliminates the effect of differential changes in ACO or non‐ACO organizational structure over postentry years on assignment to the ACO or control group (ie, the effect of changes in the practices or physician composition represented by ACO or non‐ACO TINs).
Empirical Analysis
First, we assigned patients to ACOs or non‐ACO TINs in 2009 based on office visits with PCPs. We then fit the model described in the previous section, limiting the sample to beneficiaries with a 2009 assignment, replacing the time‐varying indicators for the ACO or cohort to which a patient is assigned with time‐invariant 2009 assignments. We dropped the 2009 data from our analysis to minimize bias from regression to the mean that would arise because we require a qualifying service in 2009 but not after that.
Assuming absence of the biases described earlier, the differential change in spending for patients assigned to ACOs at baseline estimated by this reduced form model is interpretable as attributable to the MSSP. Because only 66.6% of patients assigned to an ACO in 2009 were assigned to an ACO in 2015 (among those eligible for assignment in both years), we inflate the differential change estimate to recover the MSSP effect as if all patients assigned at baseline to ACOs and none assigned to non‐ACO providers were exposed to the MSSP in the performance years. To do so, we estimated the difference in the probability of being assigned to an ACO in a performance year between patients assigned to ACOs and non‐ACO providers at baseline, among those with an assignment in 2009 and the performance year. We use the inverse of this difference, which averaged roughly two for performance year 2015, as the inflation factor. We use this approximation in lieu of a formal two‐state estimation procedure to avoid limiting the analysis to a cohort of continuously attributable patients, which would negate one of the advantages of holding the baseline assignment constant and require a stronger common shocks assumption (as described earlier). In falsification tests, we applied the same estimation procedure in hypothetical entry years to large non‐ACO TINs and ACOs that entered the MSSP in 2015.
When employing assignments based on claims in year t‐2, we limit our main study sample to those who were continuously enrolled in fee‐for‐service Medicare in year t‐2. We then substitute these prospective assignments for the retrospective assignments (based on claims in year t) and estimate our main model. We then inflate estimates by the inverse of the difference in the probability of being assigned to an ACO in year t between patients assigned to ACOs and non‐ACO providers in year t‐2.
Area‐Level Analysis
Conceptual Considerations
Another approach to eliminate bias from strategic selection of lower‐risk patients by ACOs is to compare spending changes between areas with higher vs. lower exposure to the MSSP. Basing exposure on an area‐level measure of MSSP penetration (an ecologic instrument) rather than patient‐level attribution to an ACO ensures that systematic re‐sorting of lower‐risk patients to ACOs after program entry would not contribute to savings estimates, assuming that the mechanisms for risk selection do not change patients’ location of residence. This approach also captures spillover effects of ACO efforts to lower spending on patients served by, but not attributed to, ACOs, as well as any spillover effects on practice patterns of other providers.
This strategy, too, is not without its disadvantages. First, the counterfactual (spending in the absence of MSSP participation) is no longer based on local trends in spending for an unexposed group but rather based on average national spending growth in HRRs with no (or less) MSSP participation. Greater MSSP participation in low‐growth regions (selection relative to benchmarks based on national spending growth) would therefore contribute to savings in an area‐level analysis but not in our main analysis. As described later, we take an intention‐to‐treat approach to remove bias from selective ACO continuation or expansion in areas determined by ACOs to be low‐growth ex post (eg, based on their bonuses), but this does not remove bias from selective entry based on ex ante knowledge of spending growth. Because spending growth is challenging to predict—for example, regional growth in one period does not predict growth in the next28,29—we would not expect bias from selective entry but cannot exclude this possibility.
Second, because few HRRs had no MSSP penetration, and no HRRs had 100% penetration, an area‐level analysis requires strong parametric assumptions about the relationship between MSSP penetration and spending growth and extrapolation to estimate an effect of 100% vs. 0% participation that is analogous to effects estimated by our main evaluation approach. Third, like any area‐level analysis, inferences about lower‐level units are subject to ecological fallacy. For example, ACOs that most effectively reduce spending could be in low‐penetration areas. Fourth, differences in fixed or time‐varying characteristics of the Medicare fee‐for‐service population between areas may be less stable than differences between providers within areas, on average. For example, growing MSSP penetration may be correlated with faster or slower growth in regional Medicare Advantage enrollment, potentially causing differential changes in the study population that would be minimized in a within‐area analysis. Finally, and perhaps most important, an area‐level analysis does not hold constant market‐level changes in unobserved determinants of spending growth, and spending growth is known to vary widely across regions.
Empirical Analysis
For each performance year, we calculated MSSP penetration in each HRR as the proportion of attribution‐eligible beneficiaries attributed to an ACO in a given program year, using our main method of attribution and an intention‐to‐treat approach that holds constant ACO definitions as the sets of TINs included at the outset of program participation and retains exiting ACOs as continuing in the program. MSSP penetration in 2014, for example, is the proportion of beneficiaries in an HRR attributed in 2014 to an ACO in the 2012, 2013, or 2014 entry cohorts. We then fit the following model for Medicare spending (Y) for beneficiary i in year t and HRR h:
where HRR and Year are vectors of HRR and year fixed effects, respectively, and ACOPenetration × ProgramYr is an interaction between ACO penetration and indicators of each program year from 2012 to 2015, allowing the effect of ACO penetration to differ in each program year as more ACOs enter and continuing ACOs gain experience. (The interaction creates four variables equal to the MSSP penetration in HRR h in program year t when Year is program year t, and zero otherwise.) To gauge whether this area‐level approach was more or less immune to bias from changes in population characteristics than our main within‐area approach, we compared the differential changes in patient characteristics estimated in our main approach with analogous differential changes associated with 100% increases in area‐level MSSP participation.
Attribution Based on Referring PCPs
Conceptual Considerations
While attributing patients based on PCP office visits only minimizes some forms of bias, it leaves an average of 23% of beneficiaries unassigned in each year. To reduce this and to address potential selection bias from ACO efforts to boost attribution of low‐cost patients without altering patients’ actual PCP, we modified the attribution procedure to use information about the referring PCP for other services. Thus, in a year in which a patient sees a specialist or has an imaging procedure or laboratory test but does not have an office visit with a PCP, we can attribute the patient to the PCP listed as the referring physician for those other services. This approach should reduce bias from a differential increase in the assigned share of low‐risk ACO patients after MSSP entry, whether because of strategic AWVs or other ACO efforts to enhance primary care access (though we did not find evidence of this in prior work). 5
Empirical Analysis
We used Medicare Carrier claims to determine the most common NPI with a PCP specialty appearing in the referring NPI field of a beneficiary's claims. For a given year, we then attributed the beneficiary to an ACO if that NPI was listed in the ACO's participant list in the first year of MSSP participation. We implemented these alternate assignments if the patient had no office visits with a PCP and reestimated savings using our main evaluation approach. Doing so increased the proportion of beneficiaries with an assignment in a given year from 77% to 87%, on average. Among beneficiaries for whom assignments could be made using either approach, 88.8% were assigned to the same ACO or to the control group in both cases, indicating that the most common referring PCP is usually the PCP providing the most office visits.
Assessing Risk Selection Potentially Contributing to Bonuses but Removed in Evaluation
Reconfiguration of ACOs to Favor Lower‐Cost Primary Care Providers
To assess the extent to which ACOs reconfigured their provider composition over performance years to favor primary care practices or PCPs with lower per‐patient spending, we modified our difference‐in‐differences analysis to allow the sets of TINs or PCPs (NPIs) constituting each ACO to change over the performance years per the annual MSSP Provider‐level ACO participation files. 30 The changes in ACO PCPs reflected both changes in TINs and changes in the PCPs billing under the included TINs. Because the CMS participation files are available only for ACOs participating in the MSSP, we limited this analysis to ACOs participating through 2015 to eliminate effects of ACO dropout.
We then compared savings estimates when holding the set of TINs or NPIs constant, as in our main approach, with estimates when allowing them to change. Greater savings produced by the compositional changes would be a necessary but not sufficient condition for concluding that ACOs favored providers with lower spending as they evolved. Such a finding would not be sufficient because it might be expected from attenuation bias in our intention‐to‐treat analysis, which treated TINs or PCPs no longer exposed to ACO incentives as still part of an ACO. In addition, ACOs may have successfully identified providers who were more responsive to MSSP incentives, as opposed to providers with lower baseline spending.
Moreover, greater spending reductions produced by changes in ACO TIN inclusion would be negated by benchmark adjustments in the MSSP's calculation of shared savings, as noted earlier. Thus, compositional changes favoring lower‐cost providers would only contribute to bonus payments if the changes in PCP composition of ACOs produced greater spending reductions than the changes in TIN composition of ACOs.
Gaming of CMS Attribution Algorithm via Manipulation of TINs Used for Billing
As noted previously, our modifications to the attribution rules would act to minimize bias from ACO manipulation of the TINs used for billing to shift the attributed population toward lower‐cost patients. To assess the potential for this selection strategy, among others, we assessed the effect of patient covariate adjustment on savings estimates when employing the original CMS attribution algorithm, which included additional qualifying services (CPT codes 99304–99310, 99315–99316, 99318, 99324–99328, 99334–99337, 99339–99340, 99341–99345, 99347–99350) and an additional step to attribute beneficiaries with no services from PCPs on the basis of services from non‐PCPs (specialists, nurse practitioners, and physician assistants). 17 Specifically, we implemented the CMS algorithm to attribute beneficiaries to providers and repeated our evaluation analyses, holding constant the sets of TINs composing ACOs over the study period. We compared gross savings estimates with vs. without adjustment for observable patient characteristics. Substantial attenuation of savings estimates by patient covariate adjustment in analyses using the CMS attribution algorithm but not in our main approach (using only office visits with PCPs for attribution) would suggest risk selection that was removed in our evaluation but may have contributed to bonus payments. This assumes that risk selection is based on observables or that unobservable factors used to select are correlated with the observables.
Employing the CMS attribution algorithm, we also compared savings estimates from evaluation analyses holding constant the composition of ACOs as fixed sets of TINs vs. fixed sets of clinician NPIs (the NPIs billing primarily under TINs included in ACOs in their first year of participation). If ACOs strategically changed the TINs used for billing by member clinicians to cause selective attribution of lower‐cost patients (eg, by shifting billing for nursing facility services to excluded TINs), then savings estimates should be attenuated by holding ACOs constant as sets of NPIs. For example, if ACOs shifted billing for nursing facility services, but not office visits by the same clinician, to an excluded TIN, or if ACOs shifted billing by clinicians with high‐cost patients to an excluded TIN, the billing changes would increase savings when ACOs are defined as sets of TINs but not when they are defined as sets of NPIs. In the latter case, patients would remain assigned to an ACO even if their assigned clinician changed the TIN used to bill for all or some of their services.
Patient and Physician Exit From ACOs
We also examined whether higher‐risk patients or PCPs with higher‐risk patients were more likely to exit from ACOs. We categorized beneficiaries attributed to ACOs in 2013 or 2014 (year t) into deciles based on their concurrent HCC score (ie, using diagnoses from year t). We then compared the proportion who were no longer attributed to the same ACO in the subsequent year (t+1) across deciles. We used the MSSP Beneficiary‐level attribution files to determine actual beneficiary assignments in years t and t+1 and limited the sample to beneficiaries who were attributed to ACOs that remained in the MSSP in 2015, so that patient exit could be interpreted as the patient, the patient's physician, or the physician's practice leaving an ACO, rather than an ACO leaving the MSSP. We additionally limited the sample to beneficiaries continuously eligible for attribution from 2013 to 2015 so that exit did not reflect lack of a qualifying service.
In an alternate analysis, we used our attribution approach (based on office visits with PCPs) and held ACO composition of TINs constant (using ACO composition upon MSSP entry) so that patient exit could be interpreted as the patient or the patient's PCP leaving a set of ACO TINs (the more relevant quantity, since ACO benchmarks adjust for TIN inclusion). In each version, we calculated the difference in HCC scores between leavers and stayers and fit a model of HCC scores as a function of ACO fixed effects and an indicator of leaving to estimate the mean within‐ACO difference in HCC scores between leavers and stayers, thereby controlling for any relationship between organizational case mix and patient churn.
Prior research demonstrates that attribution in the MSSP is less stable over time for higher‐risk patients because attribution is based on utilization. 13 , 22 , 27 Higher‐risk patients use more qualifying services provided by more TINs (Appendix Table 1) and have a higher risk of health declines that may prompt a change in provider. Hence, they should be more likely to have changes in attribution due to changes in health care needs that cause them to favor different established providers in different years or switch to new providers. Differential exit from ACOs of high‐risk patients is therefore not necessarily the consequence of risk selection. Moreover, it may not lead to a differential change in the average risk of ACO‐attributed patients relative to non‐ACO patients, because the risk of continuously assigned patients changes over time and new patients enter the ACO‐assigned population.
To characterize the relationship between assignment churn and patient risk in the absence of MSSP incentives, we conducted a falsification test in which we applied the preceding analyses of patient exit to large nonparticipating TINs (those meeting the MSSP eligibility criterion of 5,000‐plus beneficiaries). For consistency with the analysis of exit determined from the MSSP Beneficiary‐level attribution file, we used the CMS attribution algorithm. This comparison remained inconsistent, however, because changes in TIN inclusion contributed to patient exit from ACOs and we could not simulate such compositional changes among non‐ACO providers. To achieve a more consistent comparison, we employed our attribution approach in an alternate version that held ACO or non‐ACO composition constant over time.
We conducted an analogous analysis at the PCP level to characterize the relationship between the average health risk of a PCP's patients and the probability of PCP exit from the ACO. Specifically, we modified our attribution method to attribute beneficiaries to a PCP NPI, rather than to an ACO or non‐ACO TIN, based on qualifying office visits. We focused on PCPs actively billing for visits from 2012 to 2015 so that exit from an ACO or non‐ACO TIN by 2015 would reflect a switch to a different practice or different TIN for billing purposes, as opposed to exit from the workforce. We also limited the analysis to PCPs with at least 20 attributed patients per year (accounting for 85.7% of patient‐years) to reduce sampling error in estimation of PCPs’ average patient risk and to avoid giving undue weight to exiting PCPs with very few patients.
Using 2012–2013 data, we estimated the average HCC risk score of each PCP's attributed patients by fitting a linear regression model of patients’ HCC scores as a function of PCP fixed effects and an indicator for year. We categorized PCPs into deciles based on their patients’ mean HCC score. We determined the primary TIN under which PCPs billed in 2013 from the Medicare Data on Provider Practice and Specialty file. 31 Among PCPs billing under TINs included by the 2012 or 2013 entry cohorts of ACOs upon program entry (per the MSSP Provider‐level research identifiable file), we then determined the proportion of PCPs in each decile who were no longer billing under any of those TINs in 2015. Similarly, among PCPs billing under large non‐ACO TINs in 2012 or 2013, we determined the proportion in each decile no longer billing under any of those TINs by 2015.
Because ACOs and large non‐ACO TINs differ, our falsification analyses could not reliably establish a counterfactual (the extent to which higher‐risk patients, or PCPs with higher‐risk patients, would exit ACOs in the absence of MSSP incentives). Nevertheless, a relationship between patient risk and patient or PCP exit that is similar for ACOs and non‐ACO TINs would reject an interpretation of a strong relationship for ACOs as prima facie evidence of strategic risk selection—including manipulation of TINs used for billing—in response to MSSP incentives.
Results
Main Evaluation of the MSSP Through 2015
Table 3 summarizes the overall results of our main evaluation approach. In the pre‐entry period, ACO spending levels and trends did not differ from those for local controls. Estimates of annual gross savings grew over performance years to $302 per patient by 2015 in the 2012 entry cohort and $139 per patient in the 2013 cohort. Overall gross savings did not grow in the 2014 cohort over two performance years and were not significant in 2015. Aggregating these gross savings across all ACO‐attributed patients from 2013 to 2015, multiplying by five to correct for the 20% sampling, and subtracting bonus payments yielded a total programwide estimate of net savings to Medicare from 2013 to 2015 of $358 million. 5
Table 3.
Estimated Gross Savings by MSSP Entry Cohort and Performance Year
| Estimated Gross Savings (Adjusted Differential Change in Spending From Pre‐entry Period to Performance Year for ACOs vs. Control Group), b $/Patient (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Entry Cohort | Unadjusted Pre‐entry Sample Mean, a $/Patient | Adjusted Pre‐entry Difference in Annual Spending Level Between ACOs and Control Group, $/Patient (95% CI) | Adjusted Pre‐entry Difference in Annual Spending Trend Between ACOs and Control Group, $/Patient (95% CI) | 2013 | 2014 | 2015 |
| 2012 (n = 114 ACOs) | 9,649 | 139 (−79, 357) | −3 (−58, 53) | −129 (−261, 2) | −291 (−425, −157) | −302 (−437, −166) |
| 2013 (n = 106 ACOs) | 9,649 | 31 (−84, 146) | −5 (−39, 29) | −15 (−112, 82) | −114 (−214, −14) | −139 (−243, −35) |
| 2014 (n = 115 ACOs) | 9,649 | 33 (−90, 155) | 8 (−18, 34) | — | −72 (−150, 7) | −36 (−122, 50) |
Abbreviations: ACO, accountable care organization; CI, confidence interval; MSSP, Medicare Shared Savings Program.
In the analyses, the pre‐entry period differed for each entry cohort, but for the purpose of describing the study sample in this table, years 2009–2011 were used to calculate a single mean for each characteristic.
A negative differential change in spending indicates savings.
Differential changes from the pre‐entry period to 2015 in ACO‐attributed patients’ sociodemographic and clinical characteristics, relative to local control patients attributed to non‐ACO providers, were consistently minimal (Table 4). These findings included minimal differential changes in patients’ history of hip fracture or acute myocardial infarction, conditions that have been used as exogenous markers of health risk (though could be affected by efforts to improve quality). 32 Not only were all differential changes in observable patient characteristics small, but also there is no suggestion in the Table 4 estimates of consistently greater imbalance in entry cohorts with greater savings or of growing imbalance within cohorts as savings grew.
Estimates were nearly identical for the 2012 and 2013 cohorts with and without adjustment for patient covariates and with and without propensity‐score weighting (Figure 2). Holding ACO definitions constant as sets of PCPs instead of TINs increased gross savings slightly in the 2012 cohort and appreciably in the 2013 cohort. Thus, we can reject changes in the PCPs billing under ACO TINs as contributing to the main estimates of savings. Falsification tests of pre‐entry years for ACOs and hypothetical entry years for 439 large non‐ACO TINs revealed no evidence of differential reductions in spending in the absence of MSSP participation (Appendix Figure 1 and Appendix Table 2).
Figure 2.
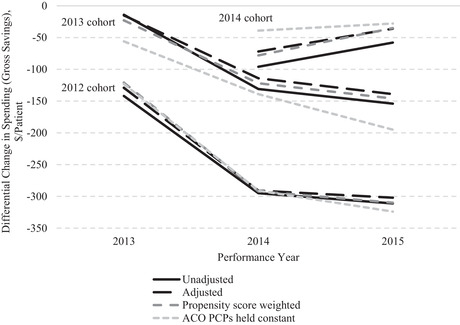
Effects of Adjusting Patient Characteristics and Holding PCP Composition of ACOs Constant on Savings Estimates in Main Analysis
Abbreviations: ACO, accountable care organization; PCP, primary care physician.
Finally, adding non‐ACO TIN/CCN fixed effects to models had modest and inconsistent effects on savings estimates for physician group ACOs (Appendix Table 3). Because these estimates are challenging to interpret for reasons previously discussed, and because adjusted estimates indicated consistently greater savings than unadjusted estimates (suggesting residual bias toward the null introduced by the fixed effects), we favor our previously reported approach of conducting falsification tests for non‐ACO physician groups as a way to gauge the sensitivity of our results to differential shifts in non‐ACO patients across organizations or differential changes in non‐ACO organizational structure relative to ACOs. Replacing ACO fixed effects with ACO entry cohort indicators also did not substantively affect estimates.
Approaches to Assess and Address Residual Risk Selection
Patient Fixed Effects
After limiting the study population to a longitudinal cohort of continuously enrolled beneficiaries who were attributable to an ACO or non‐ACO provider in at least 1 pre‐MSSP year and in 2015, gross savings estimates were attenuated and less precise (Table 5), as expected from the substantially lower spending for this cohort (Figure 1) and its smaller size (35% of beneficiaries and 55% of beneficiary‐year observations in the full study population). Within this cohort, replacing ACO fixed effects with patient fixed effects increased savings by $1 per patient in the 2012 entry cohort, decreased savings by $33 per patient in the 2013 cohort, and increased savings by $52 per patient in the 2014 cohort (Table 5), providing no consistent evidence that turnover in ACO‐attributed populations differentially favored patients with fixed characteristics predictive of lower spending. In models with patient fixed effects, estimates of gross savings adjusted for time‐varying patient factors were consistently greater (larger savings) than unadjusted estimates (Table 5), suggesting that restricting to a cohort of continuously attributable patients and implementing patient fixed effects introduced differential changes in time‐varying characteristics that biased savings toward zero and were not present in our main analysis.
Table 5.
Impact of Patient Fixed Effects on Estimated Savings
| Estimated Gross Savings in 2015 a (Differential Change in Spending From Pre‐entry Period to 2015 for ACOs vs. Control Group), $/Patient (95% CI) | ||||
|---|---|---|---|---|
| Continuously Attributable Sample, c Patient Fixed Effects Added to Model | ||||
| Entry Cohort | Primary Sample and Approach, No Patient Fixed Effects in Model, Adjusted b | Continuously Attributable Sample, c No Patient Fixed Effects in Model, Adjusted b | Adjusted b | Unadjusted |
| 2012 | −302 (−437, −166) | −203 (−299, −107) | −204 (−281, −126) | −161 (−240, −83) |
| 2013 | −139 (−243, −35) | −109 (−225, 8) | −76 (−154, 1) | −72 (−150, 6) |
| 2014 | −36 (−122, 50) | −18 (−134, 99) | −70 (−144, 4) | −43 (−118, 32) |
Abbreviations: ACO, accountable care organizations; CI, confidence interval; TIN, tax identification number.
A negative differential change in spending indicates savings.
Adjusted for patient characteristics.
Sample limited to beneficiaries continuously enrolled in fee‐for‐service Medicare from 2009 to 2015 who were attributable to an ACO or non‐ACO TIN in 2015 and at least 1 year from 2009 to 2011 (and thus could contribute to estimation of a differential change in a model with patient fixed effects). We further excluded decedents, long‐term nursing home residents, and beneficiaries enrolled in hospice so that the large increase in spending in 2015 displayed in Figure 1 would not be even larger (ie, to limit potential bias from allowing these health declines in at the end of the study period but not earlier).
Holding Baseline Assignments Fixed and Use of Time‐Varying Prospective Assignments
Estimates of gross savings from analyses holding patients’ baseline assignments fixed across the study period were generally similar to estimates from our main approach (Figure 3). Falsification tests applying the same approach to large non‐ACO TINs or the 2015 entry cohort of MSSP ACOs as hypothetical entrants in 2013 yielded significant differential spending increases in hypothetical performance years, despite preperiod spending differences from the control group that were similar to those for ACOs (Appendix Table 4). Although the results of these falsification tests suggest that we may have underestimated savings in our main intention‐to‐treat approach (eg, from attenuation bias due to retaining ACOs and ACO TINs no longer participating), they suggest more generally that an approach using baseline assignments may introduce bias of unknown magnitude and direction.
Figure 3.
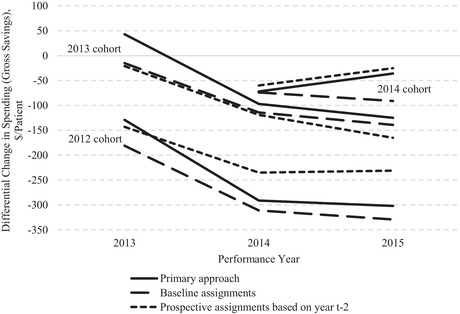
Comparison of Savings Estimates From Primary Approach vs. Holding Baseline Assignments Constant vs. Using Prospective Assignments From Two Years Before Each Study Yeara
aEstimates from analyses holding baseline assignments fixed over study period were inflated to correct for attenuation bias as described in the methods section. Similarly, estimates from analyses using prospective assignments based on claims two years before each study year were inflated to correct for attenuation bias introduced by prospective assignment. Of beneficiaries prospectively assigned to an ACO in a cohort in year t‐2, an average of 81% remained assigned to an ACO in year t; thus, inflation factors averaged 1.23 across cohorts.
Estimated gross savings when using prospective assignments based on service use in year t‐2 also were generally similar to our primary approach (Figure 3). The use of prospective assignments attenuated savings estimates somewhat in the 2012 entry cohort and led to somewhat larger savings estimates in the 2013 cohort. Comparison of estimates adjusted and unadjusted for patient covariates suggested that the use of prospective assignment introduced some bias toward the null from adverse selection into ACOs, as the adjustments increased savings estimates in the 2012–2013 cohorts by $36 to $155 per beneficiary (ie, spending reductions were more negative with adjustments).
Area‐Level Analysis
Adjusted gross savings associated with a 100% increase in MSSP penetration in the area‐level analysis were larger than gross savings estimated in our main approach and grew from $299 per patient in 2013 to $463 per patient in 2015. Although the larger savings may be indicative of spillovers, we also found that populations in HRRs with greater growth in MSSP participation became differentially lower risk relative to HRRs with lower growth in participation (Table 6). Unlike in our main analysis, adjusted gross savings in 2015 ($463 per patient) was substantially smaller than unadjusted gross savings ($788 per patient) because of the growing imbalance in patient characteristics described in Table 6.
Table 6.
Comparison of Balance on Patient Characteristics in Within‐Area Patient‐Level Analysis vs. Area‐Level Analysis
| Differential Change From Pre‐entry Period to 2015 Associated With MSSP Exposure | ||
|---|---|---|
| Patient Characteristic | Primary Approach (Exposure = Patient Attribution to MSSP ACO in 2015) a | Area‐Level Approach (Exposure = 100% MSSP Penetration in HRR in 2015) |
| Age (year) | 0.1 | 0.1 |
| Female sex (%) | −0.2 | −0.6 |
| Race or ethnic group b (%) | ||
| Non‐Hispanic white | −0.1 | −1.5 |
| Non‐Hispanic black | 0.0 | 0.0 |
| Hispanic | 0.0 | 0.1 |
| Other | 0.0 | 1.3 |
| Medicaid recipient (%) | −0.1 | 0.4 |
| Disability was original reason for Medicare eligibility (%) | −0.2 | −0.7 |
| End‐stage renal disease (%) | 0.0 | −0.2 |
| CCW conditions, c no. | ||
| Through prior year | 0.0 | −0.1 |
| Through 3 years prior d | 0.0 | 0.0 |
| HCC risk score e | ||
| Based on claims in prior year | 0.01 | −0.04 |
| Based on claims 3 years prior d | 0.00 | −0.03 |
| ZCTA‐level characteristic | ||
| % Below federal policy level | 0.0 | 0.0 |
| % With high school diploma | −0.1 | 0.0 |
| % With college degree | −0.1 | 0.2 |
Estimates from Table 2, pooled across 2012–2014 entry cohorts
ZCTA denotes ZIP Code tabulation area.
Race or ethnic group was determined from Medicare Master Beneficiary Summary Files.
Chronic conditions from the Chronic Conditions Data Warehouse (CCW) included 27 conditions: acute myocardial infarction, Alzheimer's disease, Alzheimer's disease and related disorders or senile dementia, anemia, asthma, atrial fibrillation, benign prostatic hyperplasia, chronic kidney disease, chronic obstructive pulmonary disease, depression, diabetes, heart failure, hip or pelvic fracture, hyperlipidemia, hypertension, hypothyroidism, ischemic heart disease, osteoporosis, rheumatoid arthritis or osteoarthritis, stroke or transient ischemic attack, breast cancer, colorectal cancer, endometrial cancer, lung cancer, prostate cancer, cataracts, and glaucoma. Analytic models included indicators for all 27 conditions and indicators for the presence of multiple conditions ranging from 2 to 9 or more conditions. Counts of conditions included all conditions except cataracts and glaucoma.
For analyses of CCW condition indicators and hierarchical condition categories (HCC) scores derived from earlier claims, we limited the sample to a subgroup of beneficiaries who were also continuously enrolled in fee‐for‐service Medicare 3 years prior to the study year. The purpose of this was to assess the extent to which any differential changes may have been due to differential changes in coding practices in response to MSSP incentives.
HCC risk scores are derived from demographic and diagnostic data in Medicare enrollment and claims files, with higher scores indicating higher predicted spending in the subsequent year. For each beneficiary in each study year, we assessed the HCC score based on enrollment and claims data in the prior year, 2 years prior, and 3 years prior, in each case requiring continuous enrollment in fee‐for‐service Medicare in the study year and the year of claims used to calculate HCC scores.
Attribution Based on Referring PCPs
Attribution based on the dominant referring PCP when beneficiaries had no PCP office visits to support attribution increased gross savings estimates by $50 to $112 per patient (Appendix Table 5). In previous work, we also found that modifying attribution to include office visits with nurse practitioners and physician assistants (who conduct AWVs in many practices) did not substantively change estimates. 5
Assessing Risk Selection Potentially Contributing to Bonuses but Removed in Evaluation
Reconfiguration of ACOs to Favor Lower‐Cost Primary Care Providers
Allowing the TINs composing ACOs to change after the first performance year did not appreciably affect adjusted gross savings in the 2012 entry cohort, decreased savings in the 2013 entry cohort, and increased savings in the 2014 cohort (Table 7). The changes in adjusted savings due to compositional changes in the 2013 and 2014 cohorts were at least partly mediated by shifts to providers with sicker (2013 cohort) or healthier (2014 cohort) patients, as opposed to providers with different levels of efficiency, based on comparisons of adjusted and unadjusted estimates when allowing ACO TIN composition to change (Table 7).
Table 7.
Impact on Estimated Savings of Allowing Provider Composition of ACOs to Change Over Time a
| Estimated Gross Savings in 2015 a | ||||||
|---|---|---|---|---|---|---|
| (Differential Change in Spending From Pre‐entry Period to 2015 for ACOs vs. Control Group), $/Patient (95% CI) | ||||||
| ACOs Defined as Sets of TINs | ACOs Defined as Sets of PCPs | |||||
| Entry Cohort | Composition Fixed Over Time, Adjusted b | Composition Allowed to Change, Adjusted b | Composition Allowed to Change, Unadjusted | Composition Fixed Over Time, Adjusted b | Composition Allowed to Change, Adjusted b | Composition Allowed to Change, Unadjusted |
| 2012 | −286 (−431, −142) | −296 (−455, −136) | −307 (−563, −50) | −303 (−408, −199) | −257 (−367, −146) | −243 (−385, −102) |
| 2013 | −115 (−223, −7) | −62 (−185, 61) | 44 (−143, 230) | −167 (−274, −60) | −146 (−251, −41) | −120 (−263, 23) |
| 2014 | −33 (−122, 56) | −116 (−308, 76) | −215 (−456, 26) | −52 (−143, 39) | −12 (−205, 182) | −107 (−334, 120) |
Abbreviations: ACO, accountable care organization; CI, confidence interval; MSSP, Medicare Shared Savings Program.
Limited to ACOs still participating in 2015 for which time‐varying ACO definitions are available in the MSSP Provider‐level research identifiable file.
Adjusted for patient characteristics.
Compared with changes produced by allowing the composition of TINs to change, allowing ACOs’ composition of PCPs to change caused lesser changes in adjusted savings (Table 7), suggesting no systematic selection of lower‐cost PCPs within ACO TINs. These findings provide no consistent evidence of favorable risk selection mediated by changes in ACO provider inclusion to increase bonuses, but they do support our main intention‐to‐treat approach to eliminate bias from changes in ACO provider composition.
Gaming of CMS Attribution Algorithm via Manipulation of TINs Used for Billing
In analyses employing the CMS attribution algorithm and holding ACO TIN composition constant over performance years, adjustment for patient covariates had modest and inconsistent effects, providing no consistent evidence of patient‐level risk selection within ACO TINs via gaming of the attribution algorithm. Adjusted gross savings in 2015 was 94% of unadjusted gross savings in the 2012 cohort, 133% of unadjusted gross savings in the 2013 cohort, and 74% of the (smaller) unadjusted gross savings in the 2014 cohort (Appendix Table 6).
Use of the CMS attribution algorithm caused trends in the pre‐entry period to differ between ACOs and the control group in a direction that would exaggerate savings estimates if the trend difference continued over performance years. The trend difference was due to the inclusion of visits in nursing facilities, which were largely dropped from the attribution algorithm in a 2017 rule change, 33 and not due to the inclusion of outpatient visits with specialists, which increased differences in preperiod levels but not trends (Appendix Table 7). These findings support our a priori decision to base attribution only on PCP office visits in our main approach and suggest that savings estimated with use of the CMS attribution algorithm may be biased.
In analyses using the CMS attribution algorithm, adjusted gross savings were substantively similar when treating ACOs as fixed groups of initially participating TINs or as fixed groups of clinicians billing under those TINs in the ACOs’ first year of participation (Appendix Table 6). Savings were somewhat smaller when defining ACOs as fixed groups of clinicians, but so were differences in preperiod trends (data not shown), suggesting that redefining ACOs as groups of clinicians attenuated estimates by correcting for bias related to preexisting trends as opposed to bias from gaming the TINs used for billing. (Such gaming should have manifested as larger and more consistent effects of adjustment for patient characteristics on savings estimates in Appendix Table 6.) Taken together with the robustness of our primary results to adjustment for patient characteristics and redefining ACOs as fixed sets of PCPs (nearly identical estimates as shown in Figure 2), the results of these analyses allow us to reject various gaming strategies at the clinician or patient level—that is, excluding billing by clinicians with sicker patients or shifting billing for high‐cost patients or postacute visits to excluded TINs—as contributing more than minimally to savings bonuses.
Patient and Physician Exit From ACOs
Among patients attributed to an ACO in 2013 or 2014 per the CMS Beneficiary‐level attribution file, the proportion no longer attributed to the same ACO in the subsequent year per the attribution file was higher among patients with higher HCC scores—for example, 22.4% in the highest decile of HCC scores vs. 15.3% in the second to lowest decile (Figure 4A). As has been previously described, 13 the exit rate was also higher among patients in the lowest decile of risk scores, consistent with their low use of qualifying services providing a less reliable basis for assessing a patient's regular source of care. We observed a nearly identical pattern when using the CMS attribution algorithm to assign patients to large non‐ACO TINs, with exit rates rising from 15.2% among patients in the second‐lowest decile of HCC scores to 22.3% in the highest decile (Figure 4A). These results are consistent with the lack of differential changes detected in our analysis of measurable patient characteristics (Table 4), which quantify the net effect of nonrandom patient exit and entry and suggest churn is similar in both ACO and non‐ACO groups.
Figure 4.
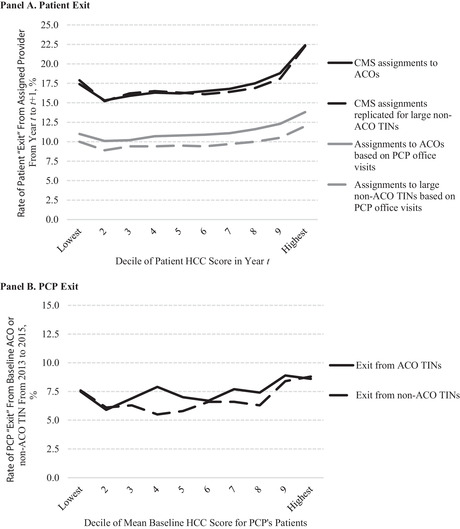
Patient and PCP Exit From ACOs and Large Non‐ACO TINs by Decile of Patient Risk
Abbreviations: ACO, accountable care organization; CI, confidence interval; CMS, Centers for Medicare and Medicaid Services; HCC, hierarchical condition categories; PCP, primary care physician; TIN, tax identification number.
Results were similar when attribution was based on office visits with PCPs; patient exit rates were higher for patients with higher HCC scores in both ACOs and large non‐ACO TINs (Figure 4A). Within ACOs, the mean difference in HCC scores between patients exiting and staying was nearly identical for ACOs (0.095; p < 0.001) and large non‐ACO TINs (0.096; p < 0.001). Thus, in the absence of MSSP incentives, higher‐risk patients had less stable assignments over time, as might be expected because they receive more services from more physicians in PCP specialties (Appendix Table 1). When attribution was based on office visits with PCPs only, exit rates were substantially lower (Figure 4A), as expected by the exclusion of other qualifying services in the CMS attribution algorithm (eg, postacute visits) that directly reflect changes in patients’ health care needs and thus introduce instability in attribution. 27
In analogous analyses at the PCP level, PCPs with higher‐risk patients also had higher rates of exit from both ACO TINs and large non‐ACO TINs by 2015 (Figure 4B). The mean difference in PCPs’ mean patient HCC score between exiting and staying PCPs was 0.029 (p = 0.009) for ACOs and 0.026 (p = 0.005) for non‐ACO TINs. These differences were reduced to 0.019 (p = 0.09) and 0.018 (p = 0.05), respectively, after adjustment for ACO or TIN fixed effects to estimate within‐ACO or within‐TIN differences, suggesting that physician turnover is higher in organizations serving higher‐risk patients.
We cannot explain the residual differences in patient risk between exiting and staying PCPs. One possibility is that physicians who have been at a practice longer may be more likely to switch practices and also may have sicker patients due to aging of their patient panel. Regardless of the explanation, our analysis demonstrates that PCP turnover is greater for PCPs with higher‐risk patients in the absence of MSSP incentives. Thus, greater exit from ACOs by physicians with higher‐risk patients should not be interpreted as evidence of risk selection by ACOs. 34
Discussion
Through 2015, we estimate that the MSSP lowered Medicare spending modestly for ACO patients. We implemented several measures to minimize selection bias and found no evidence that residual selection drove the estimated savings. The larger savings and greater growth in savings reported for physician group ACOs were similarly robust in previous work. 5 Based on the results of falsification tests and analyses controlling for both ACO and non‐ACO practice effects, we can also exclude differential increases in the share of non‐ACO patients served by higher‐spending organizations or in reimbursement rates for non‐ACO providers (eg, from hospital‐physician consolidation) as likely explanations for the larger savings among physician group ACOs.
In addition, we detected no evidence of risk selection that may have contributed to ACO bonuses but was eliminated by our evaluation approach. Specifically, we did not find evidence that ACOs consistently manipulated their composition of providers to favor practices or physicians with lower‐risk patients (or more efficient practice patterns). The lack of detectable risk selection is consistent with ACOs’ limited incentives to favor practices (TINs) with low spending under the benchmarking rules during the first phase of the MSSP (through 2016) and a constrained ability to select specific physicians or patients within practices for inclusion or exclusion.
As the basis for benchmarks increasingly transitions to ACOs’ regional spending average (or a regional‐national blend under Pathways), as opposed to their own historical spending, ACOs have new opportunities, and thus incentives, to favor practices with risk‐adjusted spending below the regional (or national) average. Identifying practices with lower predicted spending levels is easier than identifying those with slower predicted spending growth, because spending levels are strongly correlated over time, whereas growth rates are not. 28 , 29 Wide variation in risk‐adjusted spending levels among providers within regions 35 , 36 suggests opportunities for ACOs in the revamped MSSP, Pathways for Success, to earn bonuses by selectively including or excluding practices. Such practice‐level selection may be easier than physician‐ or patient‐level selection because it requires only a change in ACO participant lists and because spending can be more reliably profiled at the practice level than at the physician or patient level. Already there is evidence of selective participation in the MSSP at the ACO level in the wake of recent regionalization of benchmarks; ACOs with high spending for their region have disproportionately exited, while entrants disproportionately have low spending for their region. 9 , 37
In the short run under Pathways, selective inclusion of low‐spending practices by ACOs (or selective participation of low‐spending ACOs) will be costly to the Medicare program, effectively increasing subsidies to providers that have lower‐risk patients or are already more efficient. 9 In the long run, selectively attracting more efficient practices could conceivably enhance social welfare if demand for efficiency in the MSSP applies sufficient pressure on other providers to become more efficient—and thus attractive to ACOs for referrals or contract inclusion—as the program expands. Such gains, however, would require the prospect of financial gains from efficiencies to be more attractive than the fee‐for‐service status quo; in turn, this requires strong incentives for a broad swath of providers to participate and save in the MSSP.
As new incentives under Pathways play out, evaluations that judge ACO spending against valid counterfactuals, rather than benchmarks, will continue to be important to quantify savings from changes in care delivery. Unbiased estimates, however, may be increasingly elusive as new payment models proliferate and expand. In addition, patient‐level risk selection within ACO practices could emerge after the initial years of the MSSP, further compromising quasi‐experimental attempts to quantify program impact.
Improving the MSSP
For the MSSP to be successful, a primary goal must be to strengthen incentives for ACOs to lower spending without discouraging participation, particularly by inefficient providers with the greatest potential to generate savings. Incentives for ACOs to lower spending have been weak to date, primarily because benchmarks have been rebased after each three‐year contract period to reflect ACOs’ most recent spending. Rebasing both limits ACOs’ time to recoup the fixed costs of initiatives to improve care efficiency and diminishes the marginal incentives to save. 38 An ACO that lowers spending in one contract period is disadvantaged in the subsequent contract period with a lower benchmark. If the ACO then allows spending to return to its prior level, it incurs a penalty in a two‐sided contract; or, if it incurs the additional costs of keeping spending lower, it does not get rewarded for doing so. In a one‐sided contract without downside risk, an ACO that increases spending in one contract period is not penalized for doing so and is rewarded in the subsequent period with a higher benchmark; by temporarily increasing spending, it can earn a future bonus without exerting further effort as its spending falls to its prior level.
To strengthen incentives to lower spending, the link between benchmarks and prior savings must be severed. The MSSP has weakened this link by basing benchmarks in part on average regional spending. However, the ACO‐specific historical spending component of benchmarks continues to be rebased every contract period, and the initial 10%‐25% weight given to the regional component appears to have already discouraged participation by providers with high spending. We have recommended eliminating rebasing of the historical component, a slower convergence of benchmarks for ACOs with higher or lower initial spending, limited downside risk so that such convergence does not ensure short‐term losses for ACOs with initially high spending and discourage their participation, and higher shared‐savings rates for ACOs with lower risk‐adjusted spending for whom the costs of lowering spending are presumably greater because they have less waste to cut. 39
The major constraint on MSSP policy is the voluntary nature of the program; policy to date has underestimated the participation consequences of benchmark changes and downside risk. We believe a voluntary program that continues to offer weak incentives for providers with high spending to participate and save, and substantial subsidies for providers with low spending, will unravel into a costly and ineffective program. To be successful, a voluntary program may require a longer transition period in which benchmarks are based entirely on ACOs’ baseline spending (ie, no regional component) and updated annually without rebasing (eg, at a fixed percentage or at a blend of national and regional spending growth). If strengthening incentives to participate and save among providers with high spending successfully fosters convergence in spending between providers, a less voluntary model with greater downside risk and more aggressive benchmark convergence could be considered.
Program success will also require measures to minimize incentives for risk selection, which are generally exacerbated by measures to strengthen incentives for ACOs to lower spending. For example, rebasing weakens not only incentives to save but also incentives to risk select, as it periodically negates the gains achieved via either mechanism. There is ample room for improvement in the method of risk adjustment. For example, the ACO Consumer Assessment of Healthcare Providers and Systems survey offers additional information about patients’ health and social risk that can be used to enhance prediction and limit risk score inflation from coding practices. More advanced methods of prediction such as machine learning also could be used. However, the predictive accuracy of risk adjustment is unlikely to become sufficient to eliminate selection incentives. Residual selection due to hard‐to‐measure risk factors will contribute to spending above benchmarks and influence ACO decisions about participation and configuration. Moreover, better prediction can be counterproductive. For example, adding patients’ baseline spending to the HCC model substantially improves predictive accuracy 12 but would greatly weaken ACO incentives to reduce spending, since lowering spending would in turn lower benchmarks.
In light of the inherent limitations of risk adjustment, further measures will be necessary. We believe that the most critical and effective step to address risk selection in the MSSP is to further limit ACOs’ risk for extreme spending. This could be accomplished, for example, by truncating per‐beneficiary spending at the 97th or 95th percentile. Doing so may confer additional benefits by refocusing organizational attention on systemic changes in care delivery, as opposed to the high‐risk case management strategies that have been featured in ACO efforts despite questionable conceptual and empirical bases. 40 , 41 , 42 In addition, benchmarks can be adjusted to achieve normative goals, such as assuring fair payments for safety‐net providers.
Finally, complementary steps could minimize opportunities for ACOs to game the MSSP by shifting billing for specific patients, or by specific clinicians, to excluded TINs. Ideally, ACOs could be redefined as collections of organizations, including all owned practices, rather than collections of billing entities. CMS possesses data, such as the Provider Enrollment, Chain, and Ownership System (PECOS), that can be used to identify all TINs used by practices that are owned or operated by the same entity; CMS also could require ACO member organizations to submit such data. ACOs could then be required to include the TINs for all practices owned or operated by its constituent organizations and the data on ownership used for audit purposes. If an organization split out certain patients or physicians into a newly created TIN, for example, redefining ACOs in this manner would ensure that the TIN is included in the ACO's contract. Similarly, an organization would not be able to include in its ACO contract only its practices that compare favorably against a regional or national benchmark.
Alternatively, CMS could redefine ACOs as collections of NPIs billing under a set of TINs and base benchmarks on baseline spending billed by the same NPIs. This might be a sensible modification to limit billing‐related gaming strategies over a short term within a contract period but would do nothing to address selective inclusion of certain TINs by an organization to exploit a regional benchmark. If implemented over a longer period, however, the basis for determining the historical component of an ACO's benchmark could grow tenuous and noisy. Due to practice and workforce turnover, only a modest proportion of an ACO's NPIs may have a history of actively billing in the same market during a baseline period 5‐10 years earlier. Limited representation during a baseline period would introduce increasing random error into benchmarks over time, presenting increasing uncertainty to ACOs about whether spending reductions will be rewarded and thus weakening incentives to save.
For these reasons, we favor redefining ACOs as collections of organizations, with each organization defined as all TINs used by practices the organization owns or operates at a given time. This would allow physician and practice composition of organizations to change from baseline to performance year. Empirical analysis could help guide rules for defining ACOs, but ultimately these considerations become less consequential with stronger measures in place to address risk selection.
As long as incentives to save are strengthened and incentives for risk selection mitigated, we are less concerned about incentives for organizations to selectively include more efficient practices or physicians. Increased demand for more efficient providers could be desirable over the long run, and related offsets to savings in the short run would be tolerable if the savings are greater.
Implications for Research Methods
Our analyses also have implications for the application of quasi‐experimental research methods. First, our findings exemplify the importance of establishing a plausible counterfactual when attempting to draw causal conclusions. Our estimates of early savings are greater than those generated by comparisons with ACO benchmarks, which systematically underestimated ACO savings in the first phase of the program and more recently have overestimated savings. 37 , 43 In addition, our tests of risk selection reveal that analysis of patient or physician exit from ACOs can be misleading without considering exit under a counterfactual scenario in which providers do not face MSSP incentives. 34 When comparing against churn among non‐ACO providers, we found no evidence of risk selection at the clinician or patient level; turnover is higher for higher‐risk patients and their clinicians, regardless of MSSP incentives.
Second, our analyses illustrate common trade‐offs between approaches to address different sources of bias; strategies that ensure elimination of one source of bias can exacerbate bias from other sources. In particular, our findings demonstrate that analytic steps to eliminate bias from changes in fixed characteristics of patients due to changes in ACO population composition can exacerbate bias from within‐patient changes in time‐varying characteristics. Although we found no suggestion of residual selection in robustness checks of our main approach—which allowed patient turnover within ACOs—the same checks suggested introduction of bias by use of patient fixed effects, baseline assignments, or area‐level comparisons to hold patients constant. The estimates produced by these approaches were generally consistent with those from our main approach but less robust in sensitivity or falsification analyses. Thus, an overemphasized conceptual concern about one source of potential bias (in this case, selection on unobserved fixed traits of patients) can lead to a suboptimal approach, if not erroneous conclusions. Our findings suggest that assessing assumptions with observable information can help guide choice of an approach. Since identifying assumptions in quasi‐experimental studies cannot be tested directly (counterfactuals cannot be observed), one must ultimately rely on tests of observable quantities to gauge the extent of residual bias. By that standard, we have most confidence in the estimates produced by our primary approach and would advise caution in interpreting estimates produced by the alternative approaches.
Limitations
Because providers were not randomized to the MSSP, our analysis was subject to forms of selection bias other than risk selection conditional on participation. One concern is that providers opted into the MSSP based on anticipated changes in spending growth. Spending trends in the pre‐entry period, however, were nearly identical for ACOs and local control groups, on average, suggesting that ACOs did not select into the MSSP based on established trajectories. Although we could not rule out selective entry as contributing to gross savings estimates, we do not find it plausible that ACOs could accurately predict their future risk‐adjusted spending growth relative to their region. We further note that ACOs had incentives to enter if their anticipated spending growth was slower than national spending growth (the basis for updating benchmarks), not local spending growth (the basis for counterfactuals in our evaluation). Thus, even if ACOs managed to selectively enter in a way that resulted in bonus payments, that would not necessarily bias our estimates of gross savings, and the unearned bonuses would be accounted for in our calculation of net savings. However, because regional spending growth in one period is not predictive of regional spending growth in the next, and because regional spending levels are not correlated with regional spending growth, ACOs’ basis for predicting their region's growth relative to the nation is limited.
Clearly, ACOs were likely to have greater capacity to respond to MSSP incentives than nonparticipating providers. But in the absence of selection on future changes in spending growth, nonequivalence between ACO and non‐ACO providers would compromise only the external validity (generalizability), not the internal validity, of our findings. As long as the differential reductions in spending we estimated were due to provider responses to new incentives, they would be valid estimates of the causal effects of MSSP participation on participants. We would not expect ACOs to slow fee‐for‐service spending, counter to their financial self‐interest, in the absence of an incentive to do so.
Another concern is that other time‐varying determinants of spending growth affected ACOs and non‐ACO providers differently because they are different. However, spending levels were similar for ACOs and non‐ACO providers, and differential changes in spending for ACO patients in pre‐entry years and for patients of large non‐ACO providers were small. Thus, violations of the common shocks assumption would have had to coincide with the staggered entry of ACOs into the MSSP and grow with longer participation. 5
Nevertheless, in the absence of randomization to the MSSP, we cannot entirely exclude the possibility of selection bias contributing to our estimates of gross savings. The source of bias, however, would have to evade detection by the many robustness checks we conducted. We also cannot reject the possibility of some gaming behavior undetected by our many tests. The costs of such gaming, however, would be reflected in bonus payments. Thus, our estimates of net savings would still be valid as long as our approach generated unbiased estimates of gross savings.
Conclusion
Through its first three full years of operation, we found that participation in the MSSP was associated with modest savings and not with favorable risk selection. These findings suggest an opportunity to build on early progress. Understanding the effect of stronger selection incentives on savings in the revamped MSSP will be important to guide future program reforms.
Funding/Support: This research was supported by grants from the National Institute on Aging of the National Institutes of Health (P01AG032952) and Arnold Ventures. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Arnold Ventures.
Conflict of Interest Disclosures: All authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. McWilliams reports serving as an unpaid member of the board of directors for the Institute for Accountable Care. No other conflicts reported.
Acknowledgments: The authors thank Pasha Hamed, MA, for statistical programming support.
Supporting information
Appendix Table 1. Number of Qualifying Services Received and Number of TINs “Competing” for Assignment, by Decile of Per‐beneficiary Spending
Appendix Table 2. Falsification Tests of 439 Non‐ACO TINs Large Enough to Participate in the MSSP, and of 2015 MSSP Entrants Prior to Entry
Appendix Table 3. Gross Savings Estimates by ACO Type With and Without Adjustment for Non‐ACO TIN/CCN Fixed Effects in Addition to ACO Fixed Effects
Appendix Table 4. Application of Baseline Assignment Approach in Falsification Tests Treating Large Non‐ACO TINs or 2015 MSSP Entrants as Hypothetical Entrants in 2013
Appendix Table 5. Attribution Based on Referring PCP When Beneficiary Has No Office Visits With a PCP
Appendix Table 6. Tests of ACO Gaming of CMS Attribution Algorithm via Manipulation of TINs Used for Billing
Appendix Table 7. Comparison of Preperiod Differences in Spending Levels and Trends by Different Methods of Patient Attribution
Appendix Figure 1. Falsification Tests Estimating Differential Changes in Spending for ACOs Hypothetically Entering in Pre‐entry Period Years
References
- 1. Centers for Medicare and Medicaid Services . Fast Facts: All Medicare Shared Savings Program ACOs and Pioneer ACOs. Washington, DC: Centers for Medicare and Medicaid Services; 2015. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/PioneersMSSPCombinedFastFacts.pdf. Accessed March 20, 2020. [Google Scholar]
- 2. Colla CH, Lewis VA, Kao LS, O'Malley AJ, Chang CH, Fisher ES. Association between Medicare accountable care organization implementation and spending among clinically vulnerable beneficiaries. JAMA Intern Med. 2016;176:1167‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McWilliams JM. Changes in Medicare Shared Savings Program savings from 2013 to 2014. JAMA. 2016;316:1711‐1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in Medicare. N Engl J Med. 2016;374:2357‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McWilliams JM, Hatfield LA, Landon BE, Hamed P, Chernew ME. Medicare spending after 3 years of the Medicare Shared Savings Program. N Engl J Med. 2018;379:1139‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trombley MJ, Fout B, Brodsky S, McWilliams JM, Nyweide DJ, Morefield B. Early effects of an accountable care organization model for underserved areas. N Engl J Med. 2019;381:543‐551. [DOI] [PubMed] [Google Scholar]
- 7. McWilliams JM, Zaslavsky AM, Landon BE, Chernew ME. Spending reductions in the Medicare Shared Savings Program: selection or savings? The Incidental Economist June 17, 2019. https://theincidentaleconomist.com/wordpress/mssp-selection-savings/. Accessed March 20, 2020.
- 8. Medicare Program ; Medicare Shared Savings Program; accountable care organizations—pathways to success and extreme and uncontrollable circumstances policies for performance year 2017. Final rules. Fed Regist. 2018;83(249):67816 https://www.govinfo.gov/content/pkg/FR-2018-12-31/pdf/2018-27981.pdf. Accessed March 20, 2020. [PubMed] [Google Scholar]
- 9. McWilliams JM, Landon BE, Rathi VK, Chernew ME. Getting more savings from ACOs—can the pace be pushed? N Engl J Med. 2019;380:2190‐2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Medicare program ; Medicare Shared Savings Program: accountable care organizations. Final rule. Fed Regist. 2011;76(212):67802 http://www.gpo.gov/fdsys/pkg/FR-2011-11-02/pdf/2011-27461.pdf. Accessed March 20, 2020. [PubMed] [Google Scholar]
- 11. Friedberg MW, Chen PG, Simmons M, et al. Effects of Health Care Payment Models on Physician Practice in the United States. Follow‐up Study. Santa Monica, CA: Rand Corporation; 2018. https://www.rand.org/pubs/research_reports/RR2667.html. Accessed March 20, 2020. [PMC free article] [PubMed] [Google Scholar]
- 12. Medicare Payment Advisory Commission . Chapter 2: Improving risk adjustment in the Medicare program. In: Report to the Congress: Medicare and the Health Care Delivery System Washington, DC: MedPac; 2014. http://www.medpac.gov/docs/default-source/reports/jun14_ch02.pdf. Accessed March 20, 2020. [Google Scholar]
- 13. McWilliams JM, Chernew ME, Dalton JB, Landon BE. Outpatient care patterns and organizational accountability in Medicare. JAMA Intern Med. 2014;174:938‐945. [DOI] [PubMed] [Google Scholar]
- 14. McWilliams JM, Landon BE, Chernew ME, Zaslavsky AM. Changes in patients' experiences in Medicare accountable care organizations. N Engl J Med. 2014;371:1715‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ganguli I, Souza J, McWilliams JM, Mehrotra A. Trends in use of the US Medicare annual wellness visit, 2011–2014. JAMA. 2017;317:2233‐2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ganguli I, Souza J, McWilliams JM, Mehrotra A. Practices caring for the underserved are less likely to adopt Medicare's annual wellness visit. Health Aff (Millwood). 2018;37:283‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Medicare and Medicaid Services . Medicare Shared Savings Program: shared savings and losses and assignment methodology; specifications. Washington, DC: Centers for Medicare and Medicaid Services; 2014. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/Shared-Savings-Losses-Assignment-Spec.pdf. Accessed March 20, 2020. [Google Scholar]
- 18. Colla CH, Lewis VA, Bergquist SL, Shortell SM. Accountability across the continuum: the participation of postacute care providers in accountable care organizations. Health Serv Res. 2016;51:1595‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of Pioneer accountable care organizations. N Engl J Med. 2015;372:1927‐1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shared Savings Program Accountable Care Organizations Beneficiary‐level RIF . Research Data Assistance Center (ResDAC) website. https://www.resdac.org/cms-data/files/ssp-aco-beneficiary-level-rif. Accessed March 20, 2020.
- 21. Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. Journal of the American Statistical Association. 2018;113:390‐400. [Google Scholar]
- 22. Medicare Payment Advisory Commission . Chapter 6: Assessing the Medicare Shared Savings Program's effects on spending. In: Report to Congress: Medicare and the Health Care Delivery System Washington, DC: MedPac; 2019. http://www.medpac.gov/docs/default-source/reports/jun19_ch6_medpac_reporttocongress_sec.pdf?sfvrsn=0. Accessed March 20, 2020. [Google Scholar]
- 23. Daw JR, Hatfield LA. Matching and regression to the mean in difference‐in‐differences analysis. Health Serv Res. 2018;53:4138‐4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chabe‐Ferret S. Should we combine difference in differences with conditioning on pre‐treatment outcomes? Toulouse School of Economics working paper number 17‐824. https://www.tse-fr.eu/publications/should-we-combine-difference-differences-conditioning-pre-treatment-outcomes. Published June 2017. Accessed March 20, 2020.
- 25. Pham HH, Schrag D, O'Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356:1130‐1139. [DOI] [PubMed] [Google Scholar]
- 26. Lewis VA, McClurg AB, Smith J, Fisher ES, Bynum JP. Attributing patients to accountable care organizations: performance year approach aligns stakeholders' interests. Health Aff (Millwood). 2013;32:587‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McWilliams JM, Chernew ME, Zaslavsky AM, Landon BE. Post‐acute care and ACOs: who will be accountable? Health Serv Res. 2013;48:1526‐1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McWilliams JM, Song Z. Implications for ACOs of variations in spending growth. N Engl J Med. 2012;366:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chernew M, Decicca P, Town R. Managed care and medical expenditures of Medicare beneficiaries. J Health Econ. 2008;27:1451‐1461. [DOI] [PubMed] [Google Scholar]
- 30. Shared Savings Program Accountable Care Organizations Provider‐level RIF . Research Data Assistance Center (ResDAC) website. https://www.resdac.org/cms-data/files/ssp-aco-provider-level-rif. Accessed March 20, 2020.
- 31. Medicare Data on Provider Practice and Specialty (MD‐PPAS) . Research Data Assistance Center (ResDAC) website. https://www.resdac.org/cms-data/files/md-ppas. Accessed March 20, 2020.
- 32. Colla CH, Wennberg DE, Meara E, et al. Spending differences associated with the Medicare Physician Group Practice Demonstration. JAMA. 2012;308:1015‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sawhney TG, Fitch K, Gusland C. The exclusion of some nursing facility visits from MSSP assignment has potential unintended consequences. Milliman Issue Brief. https://milliman-cdn.azureedge.net/-/media/products/aco-insight/pdfs/exclusion-nursing-facility-visits-unintended-consequences.ashx. Published April 2018. Accessed April 24, 2020.
- 34. Markovitz AA, Hollingsworth JM, Ayanian JZ, et al. Risk adjustment in Medicare ACO program deters coding increases but may lead ACOs to drop high‐risk beneficiaries. Health Aff (Millwood). 2019;38:253‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rose S, Zaslavsky AM, McWilliams JM. Variation in accountable care organization spending and sensitivity to risk adjustment: implications for benchmarking. Health Aff (Millwood). 2016;35:440‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwartz AL, Zaslavsky AM, Landon BE, Chernew ME, McWilliams JM. Low‐value service use in provider organizations. Health Serv Res. 2018;53:87‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chernew ME, de Loera‐Brust A, Rathi V, McWilliams JM. MSSP participation following recent rule changes: what does it tell us? Health Affairs Blog. November 22, 2019. https://www.healthaffairs.org/do/10.1377/hblog20191120.903566/full/. Accessed March 20, 2020. [Google Scholar]
- 38. Douven R, McGuire TG, McWilliams JM. Avoiding unintended incentives in ACO payment models. Health Aff (Millwood). 2015;34:143‐149. [DOI] [PubMed] [Google Scholar]
- 39. McWilliams JM, Chernew ME, Landon BE. Comment on: [CMS–1701–P] Medicare Program; Medicare Shared Savings Program; Accountable Care Organizations—Pathways to Success; proposed rule. 2018. https://hmrlab.hcp.med.harvard.edu/files/hmrtest2/files/september_18_2018_comment_letter_re_cms-1701-p_medicare_program_mssp.pdf. Accessed March 20, 2020.
- 40. McWilliams JM, Schwartz AL. Focusing on high‐cost patients: the key to addressing high costs? N Engl J Med. 2017;376:807‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glied S. How policymakers can foster organizational innovation in health care. Health Affairs Blog. July 15, 2016. https://www.healthaffairs.org/do/10.1377/hblog20160715.055867/full/. Accessed March 20, 2020. [Google Scholar]
- 42. McWilliams JM, Chernew ME, Landon BE. Medicare ACO program savings not tied to preventable hospitalizations or concentrated among high‐risk patients. Health Aff (Millwood). 2017;36:2085‐2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chernew ME, Barbey C, McWilliams JM. Savings reported by CMS do not measure true ACO savings. Health Affairs Blog. June 19, 2017. https://www.healthaffairs.org/do/10.1377/hblog20170619.060649/full/. Accessed March 20, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Table 1. Number of Qualifying Services Received and Number of TINs “Competing” for Assignment, by Decile of Per‐beneficiary Spending
Appendix Table 2. Falsification Tests of 439 Non‐ACO TINs Large Enough to Participate in the MSSP, and of 2015 MSSP Entrants Prior to Entry
Appendix Table 3. Gross Savings Estimates by ACO Type With and Without Adjustment for Non‐ACO TIN/CCN Fixed Effects in Addition to ACO Fixed Effects
Appendix Table 4. Application of Baseline Assignment Approach in Falsification Tests Treating Large Non‐ACO TINs or 2015 MSSP Entrants as Hypothetical Entrants in 2013
Appendix Table 5. Attribution Based on Referring PCP When Beneficiary Has No Office Visits With a PCP
Appendix Table 6. Tests of ACO Gaming of CMS Attribution Algorithm via Manipulation of TINs Used for Billing
Appendix Table 7. Comparison of Preperiod Differences in Spending Levels and Trends by Different Methods of Patient Attribution
Appendix Figure 1. Falsification Tests Estimating Differential Changes in Spending for ACOs Hypothetically Entering in Pre‐entry Period Years


