Abstract
Recent adjustments to the histological diagnosis and the introduction of molecular classification are providing renewed support for the paradigm that antibody-mediated rejection (ABMR) is an important clinical problem for which there is an urgent need for better therapies. Acute ABMR is observed when the graft is exposed to rapid increases high-titer donor-specific antibodies (DSA) that are most often generated as anamnestic responses in sensitized recipients or de novo responses in non-sensitized patients that are non-adherent. Chronic ABMR is associated with slower increases in DSA, which may be high or low titer and transient or persistent. These DSA elicit cycles of injury and repair that manifest as multilamination of the peritubular capillary basement membrane or arteriopathy manifesting as intimal fibrosis. Mitigating the problem of AMBR requires the anamnestic and de novo DSA responses to be prevented, and established DSA responses to be reversed. To this end, a better understanding the immunobiology of DSA production is necessary, and also the development of assays capable of detecting early humoral immune responses. How recent advances in understanding the immunobiology of B cells, and areas requiring further investigation that might lead to new therapies or better diagnosis are discussed in this review.
Introduction
Under current standard of care, the median graft survival for first kidney allografts from deceased donors in the United States from 2008–2015 (https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/) is 93.2% at 1-year post-transplantation, but declines to 74.4 % at 5-years post-transplant. Comparable graft survival rates are reported for heart and liver transplantation, while the survival rates for lung and small bowel transplants are considerably lower. Chronic antibody-mediated rejection (ABMR) has emerged as a leading cause of graft loss for kidney transplants, although other mechanisms including T cell-mediated rejection (TCMR), infections and immunosuppression-related drug toxicity have also been implicated (1–3). Chronic ABMR appears to be less responsive to current immunosuppression compared to TCMR, clinical trials of ABMR have been inconclusive at best, and there is currently no accepted standard of treatment (4). These observations have led to the current paradigm that ABMR is an important cause for graft loss, and therapies capable of reversing chronic ABMR are critically necessary (4). As a result, there is strong interest in understanding the immunobiology of B cell activation leading to memory B cell and plasma cells (PC) differentiation, and in delineating the biological differences between acute primary and recall B cell responses compared to chronic donor-specific antibody (DSA) production. The anticipation is that such insights will lead to more effective therapeutic strategies. Here recent advances in the basic science of B cell biology that leads to antibody production are reviewed, and how these mechanistic insights might lead to new treatments for reversing acute and chronic ABMR are discussed.
Donor-specific humoral responses in naïve recipients (Fig 1)
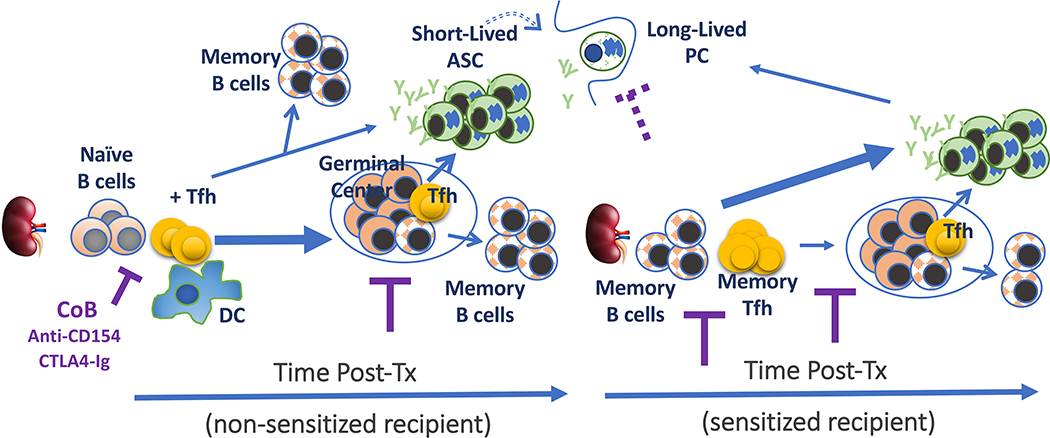
Fig 1.
Elaboration of donor-specific B cell responses in non-sensitized and sensitized recipients. Thickness of arrowed lines represent relative differentiation fate. ASC: antibody secreting cell; COB: co-stimulation blockade; DC: dendritic cell; PC: plasma cell; Tfh: T follicular helper cell.
Experimental and clinical data support the paradigm that B cell responses elicited by allografts are predominantly T cell-dependent, since the absence of T cells or co-stimulation blockade results in the inhibition of DSA production (5, 6). Upon initial exposure to alloantigen, the primary B cell response undergo on an orchestrated series of interactions that result in the generation of memory B cells and antibody-secreting cells (ASC) that comprise short-lived plasmablasts and long-lived PC. The initiation of a B cell response requires antigen to enter secondary lymphoid organs (SLOs) such as the spleen and draining lymph nodes, where they are captured by specialized subcapsular macrophages that then transfer intact antigens to follicular dendritic cell (FDCs) embedded in the B cell follicle. The non-phagocytic FDCs retain and display intact antigens that are usually coated with antibody and/or complement, for extended periods of time (weeks) to follicular B cells. In the case of allogeneic transplantation, recipient donor-specific B cells encountering FDC-bound opsonized antigen, receive activation signals through the B cell receptor (BCR), complement receptors (namely CR2), and other receptors that modulate BCR signaling such as FcγRII, CD22 and CD72 (reviewed in (7)). As a consequence of these signals, the activated B cell down-regulates the chemokine receptor, CXCR5, and upregulate CCR7 and EBI2, which guide B cells to exit the follicle and migrate to the T-B interface to receive T cell help from T follicular helper (Tfh) cells. To engage in cognate T-B interactions, the recipient B cell has to internalize the BCR-bound alloantigen, process and present allo-peptides on MHC Class II to recipient CD4+ T cells with indirect allo-specificity.
At the same time, donor antigen is picked up by recipient dendritic cells (DCs) migrating through the transplanted organ, by DCs encountering donor antigen draining into the SLOs, or by the transfer from donor DCs through exosomes, trogocytosis or similar mechanisms (8–10). Donor antigens are then processed by the DCs and presented to alloreactive CD4+ T cells, after which a subset differentiates into Tfh cells that move to the T-B interface. The interaction between Tfh and B cells can last from minutes and up to an hour, and is mediated by the T cell receptor (TCR) and Class II on B cells, integrins such as LFA1 on T cells and ICAM1 and ICAM2 on B cells, and costimulatory molecules such as CD28 and CD154 on T cells and CD80/86 and CD40 on B cells (7, 11–13). B cells that receive T cell help differentiate into extrafollicular memory B cells or ASCs, or enter into a GC reaction. Within the GC, B cells undergo multiple rounds of somatic hypermutation and class-switch recombination, ultimately emerging as high-affinity, class-switched memory B cells and long-lived PCs.
The GC is anatomically divided into the light and dark zones. Within the light zone, high-affinity B cells presenting the most abundant antigen:MHC Class II complexes outcompete low-affinity B cells, for interactions with limiting numbers of CXCR5+PD1hiICOS+ Tfh cells (14). The ICOS:ICOSL interaction and homotypic interactions with SLAM family molecules (SLAM, CD84 and Ly108) mediate Tfh:B cell interaction. By recruiting the phosphatase, SHP1, SLAM molecules function similarly to PD1 in restraining GC B cell responses (15). In contrast, the SLAM-associated protein, SAP, is critical for productive Tfh:B cell interactions, by functioning as an adaptor that recruits the tyrosine kinase, Fyn (16), and modulating the recruitment of the SHP1 by SLAM (17, 18). Additional critical components of Tfh help for GC B cells are the costimulatory molecules, CD154:CD40 and CD28:CD80/CD86, and cytokines, IL-4 and IL-21. Indeed, we and others have reported that the blockade of CD40:CD154 or CD28:B7 co-stimulation not only prevents the induction of DSA responses but also reverses established GC and DSA responses in both mice and humans (19–24). The Tfh cytokines, IL-4 and IL-21 play non-redundant roles, and the inhibition of both IL-4 and IL-21 production results in severe defects in B cell responses (25).
After receiving help from Tfh cells, B cells exit the light zone and enter the dark zone where they undergo cytosine deaminase (AID)-mediated hypermutation and cell division. A consequence of BCR somatic hypermutation is non-productive BCR mutations that result in B cells undergoing apoptosis (26). Surviving B cells with productive BCRs migrate back into the light zone to undergo another round of Tfh-mediated selection (27). After repeated rounds of entry and exit of the dark zone, the B cells emerge from the GC as memory B cells early in the GC response, or as long-lived PCs later in the GC response.
Immunobiology of Memory B cells
Memory B cells are generated in two distinct waves: extrafollicular pre-GC memory B cells are generated early upon antigen exposure, while GC-dependent memory B cells with limited somatic hypermutations are released at the initial phase of the GC response (28). Extrafollicular memory B cells tend to be IgM, whereas the post-GC B cells have undergone class-switch recombination and express BCRs with the new isotypes (29, 30). There is significant heterogeneity in the ratios of IgM and class-switched memory B cells generated during a primary immune response, with some types of infections and immunizations generating up to 50% of memory IgM B cells (31–34). Currently, the factors determining the ratio of IgM and IgG memory B cells are not known, what is the ratio of IgM versus IgG memory B cells elicited by alloantigen, and whether the mode of allosensitization affects this ratio.
IgM versus IgG memory B cells have distinct functional capabilities, which are dictated in part, by their BCRs. The intracellular tails of IgM and IgD BCRs have only a three amino acid whereas the IgG, IgE and IgA isotype BCRs have significantly longer intracellular tails with distinct baseline as well as antigen-driven signaling abilities. Overall, IgG and IgE BCRs provide stronger signals compared to IgM and IgD BCRs, suggesting a more vigorous response to antigen and propensity to differentiate into PCs (35–37). Conversely, memory B cells with IgM BCR survive longer than those with IgG BCR, consistent with the notion that these B cells are epigenetically poised to respond to a range of antigens that may be more similar to the naïve B cell repertoire than the PC repertoire (29). Using mutant mice that allow the effects of class-switching and somatic hypermutation to be independently controlled, Gitlin et al. (38), reported that the level of somatic hypermutation of the BCR determines the longevity of memory B cells. More recently, Pape et al. (39) suggested that affinity of the germline-encoded BCR determined the long-term stability of the switched Ig memory B cells. The issue of longevity of memory B cells has important implications to protective immunity following vaccinations, and to highly sensitized patients waiting for compatible organ transplantation. Thus, preclinical studies assessing the magnitude of alloreactive memory B cell frequency and their longevity in response to different modes of allosensitization are critically needed to guide the development of optimized desensitization protocols for the highly sensitized individuals.
If memory B cells are released from the GC significantly earlier in the immune response compared to long-lived PCs secreting high-affinity class-switch antibodies, then treatments that successfully reverse DSA responses may prevent the generation of long-lived PCs but not memory B cells. As a result, such allosensitized recipients may lack circulating DSA but harbor memory B cells that are able to mediate an anamnestic DSA response. Since sensitization in the clinic is detected by the presence of DSA, and there is currently no clinical assay for quantifying memory donor-specific B or T cells, some DSA-negative patients might incorrectly be classified as non-sensitized. Thus, the ability to quantify donor-specific memory B cells in the clinical setting is critically needed, and a number of assays are currently being developed (reviewed (40)). Notably, the ability to quantify the frequency of all circulating memory B cells on the basis of the expression of CD27 and other markers, in pre- and post-transplantation patients, represents a feasible approach but it is suboptimal, and an assay that quantifies the frequency of donor-specific memory B cells g optimally identify patients with the potential of mounting a recall humoral response.
Immunobiology of Plasma cells
Antibody-secreting cells can be divided into those that are short-lived and transiently detectable in the blood post-immunization, and those that are long-lived PCs in the bone marrow, SLO, organs such as the lung, intestine and chronically inflamed tissues (41). Halliley et al. (42) identified four PC subsets in the human bone marrow (BM), based on the loss of expression of the B cell marker CD19 and acquisition of the PC markers, CD38 and CD138. The CD19−CD38hiCD138+ subset was shown to be morphologically distinct, differentially expressing PC-associated genes, and exclusively harboring PCs specific for viral (mumps and measles) antigens to which the subjects had not been exposed for more than 40 years. Collectively their data suggested that the CD19−CD38hiCD138+ cells were the long-lived PCs, whereas the CD19+CD38hiCD138− subset with higher expression of Ki-67, HLA-DR and FcγRIIb, and lower expression of CD28 was considered to be the more recently generated PCs. In addition, they confirmed the preferential expression of CD28 on the long-lived PC subsets, which may explain the gradual disappearance of pre-existing anti-HLA IgG in belatacept-treated recipients (43, 44). In contrast to these observations, Groves et al. (45) more recently reported on the presence of both CD38highCD27+ CD19-positive and CD19-negative ASCs in the bone marrow and spleen, with CD19-positive ASCs present at higher frequencies than CD19-negative ASCs. Furthermore, both CD19-positive and CD19-negative ASCs secreted antibodies specific for childhood (polio) as well as recent (influenza) vaccines, thus suggesting a more heterogenous population of ASCs responsible for serological memory. Resolving the phenotype of long-lived PCs in the bone marrow, SLO, tissues and blood is critical if we are to identify novel therapies that specifically target the PC subsets that are responsible for the circulating DSA in highly sensitized patients waiting for a compatible organ transplant.
Garimalla et al. (46) reported that three CD19-positive and two CD19-negative ASC subsets appear in the peripheral blood at 7 days post-tetanus vaccination. The CD19-positive subsets represented 95% of the blood ASC, and made up a slightly smaller percentage of ASC in the bone marrow. Analysis of the VH repertoire demonstrated strong oligoclonality with extensive interconnectivity among the 5 ASC subsets and with switched memory B cells, but each subset had distinct transcriptomes with respect to cell cycle, hypoxia, TNFα and unfolded protein responses. The ability to detect ASCs generated early in response to vaccination suggest new possibilities for assessing de novo sensitization. Finally, both CD19-positive and CD19-negative ASC lack expression of CD20, thus treatment with anti-CD20 (Rituxan or Rituximab) should have no effect on ASC and the antibodies they produce.
Studies comparing the human B cell, plasma cell and circulating antibody repertoire following boost with tetanus vaccination suggested that the anti-tetanus serum IgG repertoire represented only a small fraction (<5%) of peripheral blood ASC response, and an even smaller fraction of anti-tetanus memory B cell repertoire (47). While the basis for the selection for PCs responsible for long-lived circulating antibody is currently not understood, their observations may provide an explanation into why some patients that are DSA negative mount a strong recall humoral response upon allogeneic transplantation. These observations also underscore the need for a memory donor-specific B cell assay to more accurately identify pre-sensitized transplant recipients lacking circulating DSA.
Recall humoral responses
The notion of a recall response has its roots in infection-induced protection to reinfection dating as far back as 400 BC, and constitutes the basis for vaccination (reviewed (48)). While serological memory is mediated by long-lived PCs that maintain constant levels of antibodies in the serum and body fluids in an antigen-independent fashion, recall antibody responses upon antigen re-exposure is the result of memory B cells rapidly differentiating into ASCs. Experimental data suggest that the type of memory B cell and their BCR affinity determines their differentiation fate: in the presence of circulating antibodies, only B cells that express high-affinity BCR (often class-switched) can compete with circulating antibodies to bind to antigen and enter into a recall antibody response (29). In the absence of circulating antibodies, lower affinity BCRs can also bind to antigen and become activated. Another factor determining the subset of memory B cells responds during the recall is the lapsed time since the sensitizing event, because different memory B cell subsets have different life spans - memory B cells derived from, and expressing, lower-affinity IgM receptors persist longer than those derived from or expressing higher-affinity IgG receptors having shorter half-lives. Finally, some memory B cells, most likely those with higher-affinity, class-switched BCRs differentiate directly into antibody-secreting cells, whereas others enter a GC response to emerge as antibody-secreting cells producing high-affinity, class-switched antibodies (49–51). We previously reported that exposure of sensitized mice to the same sensitizing alloantigen results in the rapid differentiation into memory B cell response with minimal GC responses (21). Nevertheless, it is possible that exposure to allogeneic allografts that have similar but non-identical HLA eplets, might stimulate memory B cells to enter into GC B cell response. These different differentiation fates of memory B cells dictates the therapeutic window for targeting GC responses versus the need to target ASC/PC during an anamnestic response.
Eyer et al. (52) developed new approaches to analyze at a single-cell level to gain insights into the quality of ASC generated after secondary and tertiary tetanus immunization in mice. They confirmed that the increase in the numbers of splenic ASCs was earlier, and of a larger magnitude and higher affinity compared to bone marrow ASC, consistent with ASCs generation in the spleen and a subset migrating to the bone marrow. A novel finding was that antigen re-challenge induced an overall increase in secretion rate by ASC. Notably there was an enormous heterogeneity in the rates of antibody secretion by each ASC that spanned 3 logs. and secretion rates was not correlated with affinity of the secreted antibody. The basis for this heterogeneity is not known and it remains to be determined whether this level of ASC heterogeneity is observed following allogeneic transplantation, where the quantity of alloantigen is orders of magnitude higher and the “danger” signals triggering immune responses are different from those elicited by Freund’s adjuvant used in those studies. Insights into the signals that determine secretion rates of ASCs might lead to the identification of new therapeutic targets.
Acute antibody-mediated rejection (Fig 2A)
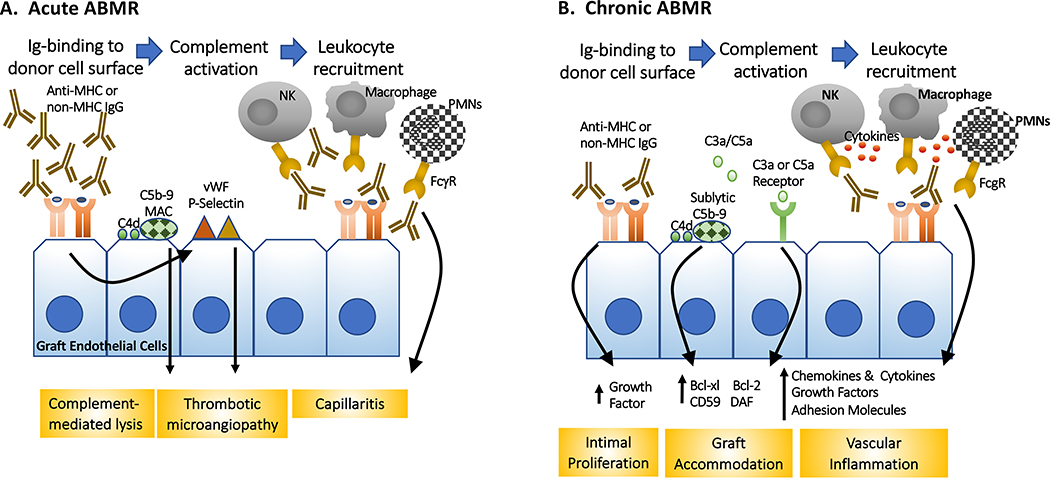
Fig 2.
Mechanisms of acute and chronic AMBR. (A) Acute ABMR is triggered by a rapid exposure of the donor graft to high titer DSA, resulting in lytic complement activation, thrombosis and leukocyte recruitment. (B) In contrast, chronic ABMR is mediated the exposure of the donor endothelial cells to gradually increasing DSA that result in more modest cell injury and inflammation, thereby allowing for the induction of graft accommodation and repair mechanisms that can become independent of DSA binding to graft endothelial cells. FcγR: Fc-gamma receptors; MAC: membrane attack complex; PMNs: polymorphonuclear neutrophils; vWF: von Willebrand factor
The experimental and clinical evidence that a rapid exposure of an allograft to high levels of circulating DSA induces acute ABMR, which in clinical kidney transplantation is characterized by (i) the presence of circulating DSA directed against HLA or other antigens; (ii) biopsy evidence of antibody interactions with vascular endothelium, e.g. c4d deposition; (iii) histological evidence of acute microvasculature inflammation and injury, such as capillary dilatation, cytoplasmic swelling of endothelial cells and the presence of intracapillary monocytes, macrophages, natural killer (NK) cells, T cells, neutrophils and/or eosinophils. Macrovascular lesions of arteritis and monocytic and lymphocytic inflammation of macrovessels are increasingly recognized as part of AMR pathology (1), and more recently, gene-expression profiling (53, 54) has been introduced to provide greater diagnostic precision.
Investigations into the mechanisms of ABMR have primarily focused on the effects of DSA-binding to the allograft endothelial cells, mediating local inflammation and antibody-mediated rejection (55, 56). The consequence to graft endothelial cells and smooth muscle cells depends on the specificity and the isotype of the DSA (57). Studies by Reed and colleagues demonstrated that anti-Class I IgG induces MHC clustering, promotes focal adhesion kinase-dependent proliferation and the migration of smooth muscle cells, which collectively may contribute to the neointimal changes seen in chronic rejection (58). Anti-MHC Class I antibodies have also been reported to induce early endothelial activation, increase adhesion molecule expression, and signal the synthesis of chemokines and cytokines. Recent studies suggest that anti-DQ, alone or in combination with anti-DR IgG, stimulated pro-inflammatory IL-6 production by endothelial cells, and compromise the induction of FoxP3+ regulatory T cells (59). Despite these recent findings, the mechanistic basis for the clinical observations that anti-Class II antibodies (especially anti-DQ) tend to be more pathogenic and resistant to therapeutic intervention remains incompletely understood (reviewed (55)).
Current DSA quantification have focused on HLA, however approximately 10–23% of recipients are pre-sensitized to non-HLA antigens (60, 61) and 22% form non-HLA antibodies after transplant (62). Non-MHC antibodies directed at endothelial and smooth muscle cells, which may include those that are autoreactive or polyreactive, have been implicated in ABMR. One of the best characterized of these antibodies is specific for angiotensin II type 1 receptor, which can signal to induce hypertension and vasculopathy in renal and cardiac transplantation (63). More recently, Pineda S et al. (64) used an integrative computational approach to identify 95 non-MHC variants residing in 72 unique genes that appear functionally relevant to ABMR of kidney allografts. These genes were enriched for active transmembrane transporter activity and immune response-activating cell-surface receptor signaling pathway. The importance of these antibodies in predicting and mediating rejection, either independently or in conjunction with anti-HLA antibody quantification, requires further validation.
A consequence of class-switch recombination in post-GC ASCs is the production of antibodies that have different functional abilities, depending on their ability to trigger complement and bind to Fc-receptors. Human IgG1 and IgG3 strongly mediate antibody-dependent cellular cytotoxicity (ADCC), and complement dependent cytotoxicity, whereas IgG2 and IgG4 generally induce more subtle responses, and Fc glycosylation may further alter function (65, 66). Consistent with the possibility that IgG subclasses have different function in vivo, some studies have reported a correlation between ability DSA to activate complement and worse allograft outcome (67, 68). However, others have countered that the efficiency of ex vivo complement activation is determined more by the titer of the DSA than the IgG subclass (69), suggesting that functional differences due to isotype may be overwhelmed by high titer DSA. The importance of recruitment of FcR-expressing cells such as NK cells to the elaboration of ABMR has been demonstrated experimentally, and confirmed as part of the gene signature of ABMR (70, 71). Finally, while the focus of DSA immunobiology has been on the consequences of their binding to graft endothelial or smooth muscle cells, IgG binding to soluble antigens or cells can generate opsonins that promote the activation of APCs and the T cell responses they elicit (72), suggesting the need for a broader view of the role of DSA in allograft rejection.
Chronic ABMR (Fig 2B)
Over the past decade, there has been increasing acceptance that the late loss of allograft is primarily mediated by antibodies through processes collectively referred to as chronic ABMR (2). Chronic ABMR in renal allografts is diagnosed histologically by the presence of glomerulopathy, multilamination of the peritubular capillary basement membrane or arteriopathy that manifests as intimal fibrosis (1), and develops in patients with pre-formed DSA or those that develop de novo DSA as a result of inadequate immunosuppression or early TCMR (73). In the former, it is reasonable to hypothesize that pre-formed DSA is the product of long-lived PCs, whereas in the latter, inadequate immunosuppression or previous TCMR might allow for a development of acute T and B cell responses that when unchecked, results in the generation of long-lived PCs responsible for sustained circulating DSA levels. What is less understood mechanistically, and more difficult to study in experimental models, is the immunobiology of DSA responses when immunosuppression is inadequate. It is possible that this results in blunted Tfh responses (74), which may impose a more stringent selection for high affinity B cells and PCs. Conversely, calcineurin inhibitors may blunt BCR signaling (75), and thus favoring the generation of memory B cells over PCs. As a result, the net effect of immunosuppression on the memory B cell and PC generation cannot be accurately predicted, and whether those responses evolve over time by acquiring resistance to standard immunosuppression requires further investigation.
Allografts exhibiting chronic ABMR pathology may be observed in the absence of DSA or current/recent antibody interaction with vascular endothelium (e.g. C4d), while histological evidence of antibody binding to graft tissue may not predict graft dysfunction (76, 77). Various explanations have been suggested, including limitations of DSA quantification that is largely limited to HLA-reactive IgG, unequal pathogenicity of different IgG isotypes with different Fc glycosylation patterns, differential expression of target antigens by the allograft, and the possibility that the slow appearance of low titer DSA may induce protective and the induction of repair or resistance mechanisms that collectively result in graft accommodation. Several in vitro studies have implicated a number of mechanisms mediating graft accommodation, including the upregulation of protective genes such as complement regulatory proteins, hemeoxygenase 1, cAMP-dependent protein kinase A and PI3K/AKT, and anti-apoptotic genes Bcl-2 and Bcl-xL, as well as increased endothelial anti-oxidant responses (55, 78, 79). Whether different organ types exhibit uniquely characteristic defensive strategies remain to be investigation, and whether sustained injury might elicit repair mechanisms that eventually become dysregulated over time and independent of the initial DSA-mediated injury.
Consistent with the notion that de novo DSA develops as a result of inadequate immunosuppression or non-adherence, recent reports suggest that the incidence of pure chronic ABMR is relatively rare while mixed T cell and antibody rejection is more frequently encountered (73, 80). When both donor-specific T and B cell responses develop, only DSA is assessed clinically and its presence may simply indicate an overall more mature immune response, which may be more difficult to reverse by immunosuppression and where the allograft had incurred more damage and were unable to fully recover their function (81). Indeed, post-transplant DSA associated with TCMR has a worse prognosis than either DSA or TCMR alone (82–85). Thus, DSA associated with mixed rejection would require more aggressive immunosuppression to rapidly reverse both T, B and plasma cell responses, whereas DSA associated with pure ABMR may require immunosuppression targeting T:B interactions and PCs. In all cases, novel approaches to repair allograft function would be beneficial to reverse the effects of immune damage.
Regulation of DSA production and regulatory B cells
Humoral responses ultimately have to be halted, so new antibody responses can develop and a broad repertoire of circulating protective antibodies may be maintained. Follicular regulatory T cells (Tfr) have been identified as a subset of FoxP3+ regulatory T cell (Treg) with a T follicular (CXCR5+PD-1+) phenotype with a specialized role in regulating T-dependent B cell responses (86, 87). While early reports suggested that these cells functioned to inhibit autoreactive B cells that may inadvertently be activated during responses to foreign antigens or pathogens, subsequent reports suggested that Tfr also functions to inhibit responses to foreign antigens or pathogens (88). Recent studies by Clement et al. (89), using mice in which Tfrs were inducibly deleted, reported that Tfrs controlled IgG and IgE responses to vaccines, allergens and autoantigens, and that they did so by restraining early pre-GC B cell responses. They also showed that the Tfr suppressive program was controlled by the transcription factors FoxP3, that FoxP3 modified the Tfh program into a Tfr program, while the chromatin-modifying enzyme EZH2 was essential for maintaining the Tfr cell transcriptional program and optimal suppressive function (90). As Tregs are being tested for tempering alloreactivity in transplant recipients, it will be interesting to test whether such cells can differentiate into Tfrs that have utility in controlling chronic humoral responses.
A second subset of cells that might function to inhibit B cell responses are the regulatory B cells (Bregs). Bregs were originally described as B cells that had the ability to downregulate inflammation in numerous pathological processes, including autoimmune diseases, transplant rejection, anti-tumor responses, and infections (reviewed in (91, 92). Several phenotypes have been reported, including those that have a transitional 2 (T3) phenotype (CD19+CD1dhiCD21hiCD23hiCD24hi), CD5+ CD1d+ (B10), Tim-1+, or marginal zone (MZ) B cells, plasmablasts, and PCs. In humans, transitional B cells CD24hiCD38hi, CD19+CD24hiCD27+ B10 cells, CD27interCD38+ plasmablasts, Tim-1+ Bregs, and CD25+CD71+B regulatory 1 cells have been described to exhibit regulatory function. These observations raise the possibility that Bregs may not actually be a unique developmental lineage like FoxP3+ regulatory T cells (Tregs), but a conglomeration of B cells at different stages of development responding to environmental cues to acquire the ability to secrete anti-inflammatory cytokines, such as IL-10, TGF-β, and IL-35. These Bregs may directly inhibit T effector, including Tfh, responses, or indirectly by inducing Tregs and/or Tfr. Mohib et al. (93) recently reported that IL-10 tagged Bregs preferentially, over non-Bregs, engaged T cells at the T:B border in an antigen-preferred manner, providing support direct Breg suppression of T cells, including those that may be destined to become Tfh. Finally whether Bregs after encountering T cells differentiate into ASC or memory B cells was not investigated.
There is considerable literature implicating a role for Bregs in clinical transplantation. The first frequently cited study was the report by Clatworthy et al. (94) that the depletion of B cells with anti-CD20 resulted in a higher incidence of acute rejection (5 of 6 patients) in kidney transplant recipients compared to those treated with anti-CD25 induction (0 of 7 patients). However, an alternative explanation for those observations might be that the depletion of B cells in the context of steroid-free immunosuppression, resulted in inflammation that promoted alloreactivity. A second series of frequently cited studies is the identification of an enriched molecular signature of B cells in tolerant compared to stable immunosuppressed recipients (95–97). However, the B cell signature was lost when tolerant patients were compared to healthy controls, raising the possibility that reduced B cell frequency was due to immunosuppression in stable transplant recipients. Indeed, subsequent studies examining the impact of pharmacological immunosuppression, revealed an impact of steroids, azathioprine as well as calcineurin-inhibitors on circulating B cells including transitional B cells (98) (99). Thus, the B cell tolerance signature, and the role for Bregs in the maintenance of clinical transplantation tolerance has to be reexamined. Finally, that Bregs might regulate T cell responses was suggested by a third series of observations that patients with decreased ratios of transitional B cells (TrB; CD19+CD24hiCD38hi) producing IL-10 versus TNF-α was associated with patients with graft dysfunction and rejection (82). Subsequently, Cherukuri et al. (100) reported that a simpler approach of quantifying reduced T1/T2 ratios was able to predict subsequent kidney allograft deterioration. Those observations are consistent with the ability of B cells, by virtue of the production of IL-10 or other immune-modulatory cytokines, to modulate T cell and subsequently, B cell responses. Whether this correlation reflects causation is unclear, and whether the patients with reduced T1/T2 ratios have increased propensity to develop DSA was not reported. Finally, it remains to be determined whether T1/T2 ratios can be manipulated by the immunosuppression type, or if they are determined by baseline characteristics of the donor and/or recipient.
Conclusions
This overview of the immunobiology of the B cell responses leading to antibody production focuses on recent insights into the unexpected heterogeneity of memory B cell and PC responses, and how they might be leveraged into identifying new therapeutic targets. For instance, that memory B cells may exist either as long-lived minimally mutated IgM or shorter-lived mutated IgG memory underscores the necessity and challenges of quantifying such cells in sensitized patients waiting transplantation or post-transplant recipients. Likewise, there is considerable heterogeneity in the ASC response, which encompasses both the CD19-positive and CD19-negative subsets originating from SLOs, that are detectable in peripheral blood and subsequently in the bone marrow. These early ASCs have vastly different rates of antibody secretion, but the signals that control antibody-secretion rates is not understood, nor how early ASC subsets access the long-lived niches in the bone marrow and ultimately penetrate into the circulating antibody repertoire is also not known. It is clear that such insights will be invaluable in identifying therapeutic interventions for preventing, mitigating, or even reversing serological memory. The variable pathogenicity of DSA is also incompletely understood. For example, clinical data indicate that antibodies specific for MHC Class II are more resistant to reversal and are more pathogenic, but there remains no compelling mechanistic explanation to explain these differences. It is also unclear whether allografts under standard immunosuppression can preferentially elicit Tfh and humoral responses, or if effector T cell responses that mediate cellular rejection are invariably elicited. In addition, the clinical importance of chronic ABMR as a mediator of late graft loss has been challenged recently, and the possibility that other immune mechanisms may elicit the same histological features as chronic ABMR should be explored (101). Finally, other functions of B cells as antigen presenting cells and cytokine producers have been reported, and their contribution to allograft rejection in the clinic remains incompletely defined. These unknowns provide abundant research opportunities for basic scientists, who and when working in concert with clinicians, may identify new ways to not only control or reverse established DSA responses but meet the challenge of “one transplant for life”.
Acknowledgment
This work was supported in part by grants (R01AI142747, P01AI097113) from the National Institute of Allergy, Immunology and Infectious Diseases, National Institutes of Health.
Abbreviations
- ABMR
antibody-mediated rejection
- AID
cytosine deaminase
- ASC
antibody secreting cells
- BCR
B cell receptor
- Bregs
regulatory B cells
- DCs
dendritic cells
- DSA
donor-specific antibodies
- FDC
follicular dendritic cell
- MZ
marginal zone
- PC
plasma cells
- Tregs
regulatory T cells
- SLO
secondary lymphoid organs
- TCMR
T cell-mediated rejection
- TCR
T cell receptor
- Tfh
T follicular helper
- TrB
transitional B cells
Footnotes
Disclosure
The author of this manuscript has conflicts of interest to disclose as described by the American Journal of Transplantation. This review is part of a supplement funded by CSL Behring.
References
- 1.Loupy A, Lefaucheur C. Antibody-Mediated Rejection of Solid-Organ Allografts. N Engl J Med. 2018;379(12):1150–60. [DOI] [PubMed] [Google Scholar]
- 2.Halloran PF, Chang J, Famulski K, Hidalgo LG, Salazar ID, Merino Lopez M, et al. Disappearance of T Cell-Mediated Rejection Despite Continued Antibody-Mediated Rejection in Late Kidney Transplant Recipients. J Am Soc Nephrol. 2015;26(7):1711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halloran PF, Reeve JP, Pereira AB, Hidalgo LG, Famulski KS. Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: new insights from the Genome Canada studies of kidney transplant biopsies. Kidney Int. 2014;85(2):258–64. [DOI] [PubMed] [Google Scholar]
- 4.Bohmig GA, Eskandary F, Doberer K, Halloran PF. The therapeutic challenge of late antibody-mediated kidney allograft rejection. Transpl Int. 2019;32(8):775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Ma L, Yin D, Shen J, Chong AS. Long-term control of alloreactive B cell responses by the suppression of T cell help. J Immunol. 2008;180(9):6077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabant M, Gorbacheva V, Fan R, Yu H, Valujskikh A. CD40-independent help by memory CD4 T cells induces pathogenic alloantibody but does not lead to long-lasting humoral immunity. Am J Transplant. 2013;13(11):2831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cyster JG, Allen CDC. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell. 2019;177(3):524–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest. 2016;126(8):2805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, Paster JT, LeGuern C, Germana S, et al. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol. 2016;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morelli AE, Bracamonte-Baran W, Burlingham WJ. Donor-derived exosomes: the trick behind the semidirect pathway of allorecognition. Curr Opin Organ Transplant. 2017;22(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong S, Zhang Z, Liu H, Tian M, Zhu X, Zhang Z, et al. B Cells Are the Dominant Antigen-Presenting Cells that Activate Naive CD4(+) T Cells upon Immunization with a Virus-Derived Nanoparticle Antigen. Immunity. 2018;49(4):695–708 e4. [DOI] [PubMed] [Google Scholar]
- 12.Qi H, Chen X, Chu C, Liu D, Ma W, Wang Y, et al. Tfh cell differentiation and their function in promoting B-cell responses. Adv Exp Med Biol. 2014;841:153–80. [DOI] [PubMed] [Google Scholar]
- 13.Hu J, Havenar-Daughton C, Crotty S. Modulation of SAP dependent T:B cell interactions as a strategy to improve vaccination. Curr Opin Virol. 2013;3(3):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwickert TA, Victora GD, Fooksman DR, Kamphorst AO, Mugnier MR, Gitlin AD, et al. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J Exp Med. 2011;208(6):1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity. 2018;49(2):264–74 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Detre C, Keszei M, Romero X, Tsokos GC, Terhorst C. SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions. Semin Immunopathol. 2010;32(2):157–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. [DOI] [PubMed] [Google Scholar]
- 18.Crotty S T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Chen J, Young JS, Wang Q, Yin D, Sciammas R, et al. Tracing Donor-MHC Class II Reactive B cells in Mouse Cardiac Transplantation: Delayed CTLA4-Ig Treatment Prevents Memory Alloreactive B-Cell Generation. Transplantation. 2016;100(8):1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Yin H, Xu J, Wang Q, Edelblum KL, Sciammas R, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am J Transplant. 2013;13(9):2280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Wang Q, Yin D, Vu V, Sciammas R, Chong AS. Cutting Edge: CTLA-4Ig Inhibits Memory B Cell Responses and Promotes Allograft Survival in Sensitized Recipients. J Immunol. 2015;195(9):4069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young JS, Chen J, Miller ML, Vu V, Tian C, Moon JJ, et al. Delayed Cytotoxic T Lymphocyte-Associated Protein 4-Immunoglobulin Treatment Reverses Ongoing Alloantibody Responses and Rescues Allografts From Acute Rejection. Am J Transplant. 2016;16(8):2312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bray RA, Tarsitani C, Gebel HM, Lee JH. Clinical cytometry and progress in HLA antibody detection. Methods Cell Biol. 2011;103:285–310. [DOI] [PubMed] [Google Scholar]
- 24.Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L, et al. De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: Post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez DG, Cote CM, Patel JR, Smith CB, Zhang Y, Nickerson KM, et al. Nonredundant Roles of IL-21 and IL-4 in the Phased Initiation of Germinal Center B Cells and Subsequent Self-Renewal Transitions. J Immunol. 2018;201(12):3569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer CT, Gazumyan A, Kara EE, Gitlin AD, Golijanin J, Viant C, et al. The microanatomic segregation of selection by apoptosis in the germinal center. Science. 2017;358(6360). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509(7502):637–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity. 2016;44(1):116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331(6021):1203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012;209(3):597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20(1):67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol. 2010;185(6):3117–25. [DOI] [PubMed] [Google Scholar]
- 33.Shinnakasu R, Inoue T, Kometani K, Moriyama S, Adachi Y, Nakayama M, et al. Regulated selection of germinal-center cells into the memory B cell compartment. Nat Immunol. 2016;17(7):861–9. [DOI] [PubMed] [Google Scholar]
- 34.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol. 2010;185(12):7146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, Robinson MJ, Chen X, Smith GA, Taunton J, Liu W, et al. Regulation of B cell fate by chronic activity of the IgE B cell receptor. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wienands J, Engels N. The Memory Function of the B Cell Antigen Receptor. Curr Top Microbiol Immunol. 2016;393:107–21. [DOI] [PubMed] [Google Scholar]
- 37.Haniuda K, Fukao S, Kodama T, Hasegawa H, Kitamura D. Autonomous membrane IgE signaling prevents IgE-memory formation. Nat Immunol. 2016;17(9):1109–17. [DOI] [PubMed] [Google Scholar]
- 38.Gitlin AD, von Boehmer L, Gazumyan A, Shulman Z, Oliveira TY, Nussenzweig MC. Independent Roles of Switching and Hypermutation in the Development and Persistence of B Lymphocyte Memory. Immunity. 2016;44(4):769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pape KA, Maul RW, Dileepan T, Paustian AS, Gearhart PJ, Jenkins MK. Naive B Cells with High-Avidity Germline-Encoded Antigen Receptors Produce Persistent IgM(+) and Transient IgG(+) Memory B Cells. Immunity. 2018;48(6):1135–43 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bestard O, Cravedi P. Monitoring alloimmune response in kidney transplantation. J Nephrol. 2017;30(2):187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15(3):160–71. [DOI] [PubMed] [Google Scholar]
- 42.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, et al. Long-Lived Plasma Cells Are Contained within the CD19(−)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity. 2015;43(1):132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons RF, Zahid A, Bumb S, Decker H, Sullivan HC, Eun-Hyung Lee F, et al. The impact of belatacept on third-party HLA alloantibodies in highly sensitized kidney transplant recipients. Am J Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L, et al. Posttransplant reduction in preexisting donor-specific antibody levels after belatacept- versus cyclosporine-based immunosuppression: Post hoc analyses of BENEFIT and BENEFIT-EXT. Am J Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groves CJ, Carrell J, Grady R, Rajan B, Morehouse CA, Halpin R, et al. CD19-positive antibody-secreting cells provide immune memory. Blood Adv. 2018;2(22):3163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garimalla S, Nguyen DC, Halliley JL, Tipton C, Rosenberg AF, Fucile CF, et al. Differential transcriptome and development of human peripheral plasma cell subsets. JCI Insight. 2019;4(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavinder JJ, Wine Y, Giesecke C, Ippolito GC, Horton AP, Lungu OI, et al. Identification and characterization of the constituent human serum antibodies elicited by vaccination. Proc Natl Acad Sci U S A. 2014;111(6):2259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33(4):451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McHeyzer-Williams LJ, Dufaud C, McHeyzer-Williams MG. Do Memory B Cells Form Secondary Germinal Centers? Impact of Antibody Class and Quality of Memory T-Cell Help at Recall. Cold Spring Harb Perspect Biol. 2018;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pape KA, Jenkins MK. Do Memory B Cells Form Secondary Germinal Centers? It Depends. Cold Spring Harb Perspect Biol. 2018;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shlomchik MJ. Do Memory B Cells Form Secondary Germinal Centers? Yes and No. Cold Spring Harb Perspect Biol. 2018;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eyer K, Doineau RCL, Castrillon CE, Briseno-Roa L, Menrath V, Mottet G, et al. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring. Nat Biotechnol. 2017;35(10):977–82. [DOI] [PubMed] [Google Scholar]
- 53.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–83. [DOI] [PubMed] [Google Scholar]
- 54.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18(2):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valenzuela NM, McNamara JT, Reed EF. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant. 2014;19(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baldwin WM 3rd, Valujskikh A, Fairchild RL. Mechanisms of antibody-mediated acute and chronic rejection of kidney allografts. Curr Opin Organ Transplant. 2016;21(1):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valenzuela NM, Hickey MJ, Reed EF. Antibody Subclass Repertoire and Graft Outcome Following Solid Organ Transplantation. Front Immunol. 2016;7:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X, Valenzuela NM, Reed EF. HLA class I antibody-mediated endothelial and smooth muscle cell activation. Curr Opin Organ Transplant. 2012;17(4):446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cross AR, Lion J, Poussin K, Assayag M, Taupin JL, Glotz D, et al. HLA-DQ alloantibodies directly activate the endothelium and compromise differentiation of FoxP3(high) regulatory T lymphocytes. Kidney Int. 2019;96(3):689–98. [DOI] [PubMed] [Google Scholar]
- 60.Jackson AM, Lucas DP, Melancon JK, Desai NM. Clinical relevance and IgG subclass determination of non-HLA antibodies identified using endothelial cell precursors isolated from donor blood. Transplantation. 2011;92(1):54–60. [DOI] [PubMed] [Google Scholar]
- 61.Qin Z, Lavingia B, Zou Y, Stastny P. Antibodies against nucleolin in recipients of organ transplants. Transplantation. 2011;92(7):829–35. [DOI] [PubMed] [Google Scholar]
- 62.Sigdel TK, Li L, Tran TQ, Khatri P, Naesens M, Sansanwal P, et al. Non-HLA antibodies to immunogenic epitopes predict the evolution of chronic renal allograft injury. J Am Soc Nephrol. 2012;23(4):750–63. [DOI] [PubMed] [Google Scholar]
- 63.Dragun D, Catar R, Philippe A. Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity. Kidney Int. 2016;90(2):280–8. [DOI] [PubMed] [Google Scholar]
- 64.Pineda S, Sigdel TK, Chen J, Jackson AM, Sirota M, Sarwal MM. Novel Non-Histocompatibility Antigen Mismatched Variants Improve the Ability to Predict Antibody-Mediated Rejection Risk in Kidney Transplant. Front Immunol. 2017;8:1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Taeye SW, Rispens T, Vidarsson G. The Ligands for Human IgG and Their Effector Functions. Antibodies (Basel). 2019;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang TT, Ravetch JV. Functional diversification of IgGs through Fc glycosylation. J Clin Invest. 2019;129(9):3492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369(13):1215–26. [DOI] [PubMed] [Google Scholar]
- 68.Viglietti D, Loupy A, Vernerey D, Bentlejewski C, Gosset C, Aubert O, et al. Value of Donor-Specific Anti-HLA Antibody Monitoring and Characterization for Risk Stratification of Kidney Allograft Loss. J Am Soc Nephrol. 2017;28(2):702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tambur AR, Wiebe C. HLA Diagnostics: Evaluating DSA Strength by Titration. Transplantation. 2018;102(1S Suppl 1):S23–S30. [DOI] [PubMed] [Google Scholar]
- 70.Kohei N, Tanaka T, Tanabe K, Masumori N, Dvorina N, Valujskikh A, et al. Natural killer cells play a critical role in mediating inflammation and graft failure during antibody-mediated rejection of kidney allografts. Kidney Int. 2016;89(6):1293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parkes MD, Halloran PF, Hidalgo LG. Evidence for CD16a-Mediated NK Cell Stimulation in Antibody-Mediated Kidney Transplant Rejection. Transplantation. 2017;101(4):e102–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol. 2011;186(1):214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiebe C, Gibson IW, Blydt-Hansen TD, Pochinco D, Birk PE, Ho J, et al. Rates and Determinants of Progression to Graft Failure in Kidney Allograft Recipients With De Novo Donor-Specific Antibody. Am J Transplant. 2015;15(11):2921–30. [DOI] [PubMed] [Google Scholar]
- 74.Wallin EF, Hill DL, Linterman MA, Wood KJ. The Calcineurin Inhibitor Tacrolimus Specifically Suppresses Human T Follicular Helper Cells. Front Immunol. 2018;9:1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kosowicz JG, Lee J, Peiffer B, Guo Z, Chen J, Liao G, et al. Drug Modulators of B Cell Signaling Pathways and Epstein-Barr Virus Lytic Activation. J Virol. 2017;91(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Q, Hickey M, Drogalis-Kim D, Zheng Y, Gjertson D, Cadeiras M, et al. Understanding the Correlation Between DSA, Complement Activation, and Antibody-Mediated Rejection in Heart Transplant Recipients. Transplantation. 2018;102(10):e431–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chong AS, Rothstein DM, Safa K, Riella LV. Outstanding questions in transplantation: B cells, alloantibodies, and humoral rejection. Am J Transplant. 2019;19(8):2155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Song S, Zhong S, Xiang Y, Li JH, Guo H, Wang WY, et al. Complement inhibition enables renal allograft accommodation and long-term engraftment in presensitized nonhuman primates. Am J Transplant. 2011;11(10):2057–66. [DOI] [PubMed] [Google Scholar]
- 79.Salama AD, Delikouras A, Pusey CD, Cook HT, Bhangal G, Lechler RI, et al. Transplant accommodation in highly sensitized patients: a potential role for Bcl-xL and alloantibody. Am J Transplant. 2001;1(3):260–9. [DOI] [PubMed] [Google Scholar]
- 80.Cherukuri A, Mehta R, Sharma A, Sood P, Zeevi A, Tevar AD, et al. Post-transplant donor specific antibody is associated with poor kidney transplant outcomes only when combined with both T-cell-mediated rejection and non-adherence. Kidney Int. 2019;96(1):202–13. [DOI] [PubMed] [Google Scholar]
- 81.Bouatou Y, Viglietti D, Pievani D, Louis K, Duong Van Huyen JP, Rabant M, et al. Response to treatment and long-term outcomes in kidney transplant recipients with acute T cell-mediated rejection. Am J Transplant. 2019;19(7):1972–88. [DOI] [PubMed] [Google Scholar]
- 82.Cherukuri A, Rothstein DM, Clark B, Carter CR, Davison A, Hernandez-Fuentes M, et al. Immunologic human renal allograft injury associates with an altered IL-10/TNF-alpha expression ratio in regulatory B cells. J Am Soc Nephrol. 2014;25(7):1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cooper JE, Gralla J, Cagle L, Goldberg R, Chan L, Wiseman AC. Inferior kidney allograft outcomes in patients with de novo donor-specific antibodies are due to acute rejection episodes. Transplantation. 2011;91(10):1103–9. [DOI] [PubMed] [Google Scholar]
- 84.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12(5):1157–67. [DOI] [PubMed] [Google Scholar]
- 85.Devos JM, Gaber AO, Teeter LD, Graviss EA, Patel SJ, Land GA, et al. Intermediate-term graft loss after renal transplantation is associated with both donor-specific antibody and acute rejection. Transplantation. 2014;97(5):534–40. [DOI] [PubMed] [Google Scholar]
- 86.Ramiscal RR, Vinuesa CG. T-cell subsets in the germinal center. Immunol Rev. 2013;252(1):146–55. [DOI] [PubMed] [Google Scholar]
- 87.Sage PT, Sharpe AH. T follicular regulatory cells. Immunol Rev. 2016;271(1):246–59. [DOI] [PubMed] [Google Scholar]
- 88.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clement RL, Daccache J, Mohammed MT, Diallo A, Blazar BR, Kuchroo VK, et al. Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat Immunol. 2019;20(10):1360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hou S, Clement RL, Diallo A, Blazar BR, Rudensky AY, Sharpe AH, et al. FoxP3 and Ezh2 regulate Tfr cell suppressive function and transcriptional program. J Exp Med. 2019;216(3):605–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stolp J, Turka LA, Wood KJ. B cells with immune-regulating function in transplantation. Nat Rev Nephrol. 2014;10(7):389–97. [DOI] [PubMed] [Google Scholar]
- 92.Alhabbab RY, Nova-Lamperti E, Aravena O, Burton HM, Lechler RI, Dorling A, et al. Regulatory B cells: Development, phenotypes, functions, and role in transplantation. Immunol Rev. 2019. [DOI] [PubMed] [Google Scholar]
- 93.Mohib K, Cherukuri A, Zhou Y, Ding Q, Watkins SC, Rothstein DM. Antigen-dependent interactions between regulatory B cells and T cells at the T:B border inhibit subsequent T cell interactions with DCs. Am J Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clatworthy MR, Watson CJ, Plotnek G, Bardsley V, Chaudhry AN, Bradley JA, et al. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med. 2009;360(25):2683–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1848–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Le Texier L, Thebault P, Lavault A, Usal C, Merieau E, Quillard T, et al. Long-term allograft tolerance is characterized by the accumulation of B cells exhibiting an inhibited profile. Am J Transplant. 2011;11(3):429–38. [DOI] [PubMed] [Google Scholar]
- 98.Rebollo-Mesa I, Nova-Lamperti E, Mobillo P, Runglall M, Christakoudi S, Norris S, et al. Biomarkers of Tolerance in Kidney Transplantation: Are We Predicting Tolerance or Response to Immunosuppressive Treatment? Am J Transplant. 2016;16(12):3443–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tebbe B, Wilde B, Ye Z, Wang J, Wang X, Jian F, et al. Renal Transplant Recipients Treated with Calcineurin-Inhibitors Lack Circulating Immature Transitional CD19+CD24hiCD38hi Regulatory B-Lymphocytes. PLoS One. 2016;11(4):e0153170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cherukuri A, Salama AD, Carter CR, Landsittel D, Arumugakani G, Clark B, et al. Reduced human transitional B cell T1/T2 ratio is associated with subsequent deterioration in renal allograft function. Kidney Int. 2017;91(1):183–95. [DOI] [PubMed] [Google Scholar]
- 101.Lakkis FG, Chalasani G, Hariharan S. Antibody-Mediated Rejection of Solid-Organ Allografts. N Engl J Med. 2018;379(26):2580. [DOI] [PubMed] [Google Scholar]