Abstract
Introduction
Women and healthcare providers lack adequate information on medication safety during pregnancy. While resources describing fetal risk are available, information is provided in multiple locations, often with subjective assessments of available data. We developed a list of medications of greatest concern during pregnancy to help healthcare providers counsel reproductive-aged and pregnant women.
Methods
Prescription drug labels submitted to the U.S. Food and Drug Administration with information in the Teratogen Information System (TERIS) and/or Drugs in Pregnancy and Lactation by Briggs & Freeman were included (N = 1,186 medications; 766 from three data sources, 420 from two). We used two supervised learning methods (‘support vector machine’ and ‘sentiment analysis’) to create prediction models based on narrative descriptions of fetal risk. Two models were created per data source. Our final list included medications categorized as ‘high’ risk in at least four of six models (if three data sources) or three of four models (if two data sources).
Results
We classified 80 prescription medications as being of greatest concern during pregnancy; over half were antineoplastic agents (n = 24), angiotensin converting enzyme inhibitors (n = 10), angiotensin II receptor antagonists (n = 8), and anticonvulsants (n = 7).
Discussion
This evidence-based list could be a useful tool for healthcare providers counseling reproductive-aged and pregnant women about medication use during pregnancy. However, providers and patients may find it helpful to weigh the risks and benefits of any pharmacologic treatment for both pregnant women and the fetus when managing medical conditions before and during pregnancy.
Keywords: Pregnancy, Medication, Teratogen, Birth defects, Congenital malformations, Teratology
Introduction
Nine out of 10 women in the United States take a medication during pregnancy and 50–70% take a prescription medication (Mitchell et al. 2011; Palmsten et al. 2015). Yet, there is limited, and often inconsistent, information both internationally and in the U.S. about the risks of many medications to the developing fetus, such as birth defects or pregnancy loss (Adam et al. 2011; Noh et al. 2018; Peters et al. 2013). Pregnant women have typically been excluded from clinical trials of medications due to ethical concerns. One assessment found only 10% of medications approved by the U.S. Food and Drug Administration (FDA) between 1980 and 2010 were deemed to have sufficient information to determine fetal risk (Adam et al. 2011). This scarcity of information leaves women and healthcare providers to face difficult decisions about pharmacologic treatment during pregnancy (Hameen-Anttila et al. 2014; Palosse-Cantaloube et al. 2014; Peters et al. 2013). Decisions on medication use and choice of specific medication require careful consideration of the potential risks and benefits for both the woman and the fetus.
To counsel reproductive-aged and pregnant women about medication use, many healthcare providers have relied heavily on the FDA pregnancy categories included in drug product labeling introduced in 1979, which weigh the scientific evidence of risk compared to benefit of use during pregnancy (Lynch et al. 2017; Morgan et al. 2010). However, these categorical ratings (letter designations of A, B, C, D and X) had several limitations; they often did not take into account the most recent available human studies, were frequently misinterpreted as a grading system, and were often used in lieu of the narrative summary provided in the product labeling (Chambers et al. 2008). In 2015, FDA implemented the Pregnancy and Lactation Labeling Rule (PLLR) requiring manufacturers to remove the pregnancy letter category and to revise the content and format of drug product labeling for drugs approved on or after June 30, 2001. In addition, for drugs approved before June 30, 2001, the PLLR requires manufacturers to remove the pregnancy letter category from labeling by June 30, 2018 (Dinatale et al. 2017; U.S. Food and Drug Administration).
Healthcare providers also consult other sources that provide a narrative summary of the scientific literature on the safety of medications during pregnancy (Morgan et al. 2010). These sources include fee-based subscription databases such as REPROTOX™ (www.REPROTOX.org) and TERIS (the Teratogen Information System, https://depts.washington.edu/terisdb/) and textbooks such as Drugs in Pregnancy and Lactation (Briggs and Freeman 2014) and the Catalog of Teratogenic Agents (Shepard and Lemire 2010). While these resources provide information about risks associated with specific prenatal medication exposures, subscription fees may limit accessibility by healthcare providers. Variability also exists in risk ratings between sources due to the subjective nature of assessments (Thorpe et al. 2013). Furthermore, while some lists of teratogenic medications exist, they are often outdated, rely solely on one data source, or are based on the categorical ratings from data sources rather than accounting for information in the narrative summaries (Eltonsy et al. 2016; Webster and Freeman 2003).
Overall, healthcare providers and women do not have easily accessible, high-quality information about the safety of medication use during pregnancy. The goal of this analysis was to use supervised learning methods to systematically evaluate qualitative descriptions of fetal risk and combine information across multiple data sources to develop a list of prescription medications of greatest concern based on currently available information. This list could be used by healthcare providers to facilitate discussions with pregnant women and reproductive-aged women about possible risks associated with specific prenatal medication exposures.
Methods
Data Sources
We used three data sources: manufacturers’ human prescription drug labels submitted to the FDA (“drug labels”), the TERIS database, and the 10th edition of Drugs in Pregnancy and Lactation by Briggs & Freeman. Each provides its own categorization of fetal risk and a narrative summary of fetal risk associated with medication use based on animal and human toxicology data, case reports, case series, and epidemiologic studies (when available). Categorical risk ratings allowed us to develop supervised learning models that could identify statistical patterns in the text used to describe risk. We did not use other data sources that did not include categorical risk ratings.
To obtain a comprehensive list of prescription medications as a basis for our analysis, we downloaded XML files for all prescription drug labels from the National Library of Medicine’s DailyMed website (https://dailymed.nlm.nih.gov/dailymed/) on January 30, 2017. These files contain the most recent human prescription drug labels submitted by manufacturers to the FDA for consideration. DailyMed does not currently provide a list of all FDA-approved human prescription drug labels. We abstracted the FDA pregnancy letter category, narrative summary from the “Pregnancy” section, and “Warnings” content from drug labels.
TERIS, a subscription database, provides a review and interpretation of published literature. We used the TERIS “Summary”, “Comment”, and “Notes” fields and the “magnitude of teratogenic risk to child born after exposure during gestation” rating. TERIS’ teratogenic risk ratings may be “undetermined” or may range from “none” to “high” as decided by the TERIS board. Data from TERIS were provided in a Microsoft Access database by TERIS authors on November 28, 2016.
The 10th edition of Drugs in Pregnancy and Lactation by Briggs and Freeman (2014) (Briggs and Freeman, 2014) was provided as a PDF file by the publisher, with permission from the authors. We abstracted the “pregnancy recommendation” category, which ranged from “compatible” to “contraindicated” and the narrative “fetal risk summary” for each medication.
Categorizing Medications According to Fetal Risk
Using existing data elements as a guide, supervised learning methods (SLM) can group and categorize text information into data suitable for quantitative analyses. We used two SLMs: support vector machine (SVM) with a linear kernel and sentiment analysis. Both used the narrative summaries describing fetal risk of medication use during pregnancy to create prediction models (Cortes and Vapnik 1995; Joachims 1998). To prepare descriptions for analysis, we removed excess whitespace, numbers, symbols, and common English words, and made all letters lowercase. We developed two prediction models (one SVM and one sentiment) per data source, with the goal of identifying “high” risk (vs. “not high” risk) medications to be included on our list. (In preliminary analyses we attempted to use the same model across all three data sources, but the initial model fit was poor, likely because of the different terminology and narrative style used in each data source.) To train the models, we first dichotomized each data source’s fetal risk/pregnancy categories into “high” and “not high” fetal risk (Table 1). Risk categories with insufficient information to determine a risk (i.e., TERIS ‘undetermined’; FDA pregnancy category C or missing; Briggs & Freeman categories indicating no human data and no animal data) were excluded from this training process, but included in the SLM models. (Models were able to use similarities in the narrative summaries between those summaries with and without author ratings to predict risk.) During training, if a data source’s risk category mentioned multiple risk categories (due to risk varying by dose, gestational timing, or type of adverse outcome), we selected the highest risk category. For example, Briggs & Freeman’s pregnancy recommendation for vitamin A says “compatible/contraindicated (doses above U.S. RDA)” during pregnancy, which we categorized as “contraindicated.”
Table 1.
Number of medications by risk category and data source
| Number of Medications by Fetal Risk Category, N (%)a | |||||
|---|---|---|---|---|---|
| TERIS Fetal Risk Ratingb | Briggs & Freeman Pregnancy Recommendationb | FDA Pregnancy Categoryc | |||
| “High Risk” Category | |||||
| High | 17 (1%) | Contraindicated | 141 (12%) | X | 104 (5%) |
| Moderate | 45 (3%) | Human data suggest risk | 152 (13%) | D | 210 (10%) |
| Smalld | 20 (1%) | No or limited human data, animal data suggest high risk | 7 (1%) | ||
| No human or animal data but potential toxicity | 2 (< 1%) | ||||
| Subtotal | 82 (5%) | 302 (26%) | 314 (15%) | ||
| “Not High Risk” Category | |||||
| Minimal | 41 (2%) | Compatible or maternal benefit > fetal risk | 268 (23%) | A | 11 (1%) |
| None | 58 (3%) | Human data suggest low risk | 54 (5%) | B | 369 (17%) |
| Unlikely | 211 (12%) | No or limited human data, animal data suggest risk | 87 (8%) | ||
| No or limited human data, animal data suggest moderate risk | 73 (6%) | ||||
| No or limited human data, animal data suggest low risk | 174 (15%) | ||||
| No or limited human data, probably compatible | 120 (11%) | ||||
| Subtotal | 310 (18%) | 776 (68%) | 380 (18%) | ||
| “Undetermined” Category | |||||
| Undetermined | 1311 (77%) | No human data, no animal data | 63 (6%) | C | 1026 (49%) |
| N/Ac | 386 (18%) | ||||
| Subtotal | 1311 (77%) | 63 (6%) | 1412 (67%) | ||
| Total | 1703 | 1141 | 2106 | ||
The total number of medications in each data source presented in this table is before any de-duplication and matching across data sources
If an author provided multiple categories of fetal risk for a specific medication, the medication was assigned to the highest fetal risk category mentioned. For example, if the fetal risk was “minimal to small”, the medication was assigned to the “small” TERIS fetal risk category
In 2015, FDA implemented the Pregnancy and Lactation Labeling Rule (PLLR) requiring manufacturers to remove the pregnancy letter category and to revise the content and format of drug product labeling for drugs approved on or after June 30, 2001. In addition, for drugs approved before June 30, 2011, the PLLR requires manufacturers to remove the pregnancy letter category from labeling by June 30, 2018 (Dinatale et al. 2017; Miranda-Filho Dde et al. 2016)
According to Adam et al. (2011), even ‘small’ risks might impact decisions about exposed pregnancies (Adam et al., 2011)
SVM text classification uses features of the narrative summaries to develop probabilistic binary linear classifications based on existing risk categorizations (Cortes and Vapnik 1995; Joachims 1998). We used a stratified five-fold method to train SVM models to identify patterns of words and phrases in the narrative summaries associated with “high” risk medications. We used uni-, bi-, and trigrams (one-, two-, and three-word phrases) in a bag-of-words model and used the most frequent 4000 term(s), which were transformed into binary indicators for model training. Data were first split into five equal groups (each representing 20% of the data). For each group, the model trained on the remaining 80% of the data to predict risks for the other 20%. This process occurred five times, so that each medication had a predicted risk (either ‘high’ or ‘not high’). We set the threshold of prediction to achieve at the least an 80% sensitivity. We applied an SVM model built using TERIS to Briggs & Freeman data; however, these models performed poorly, indicating that terminology and language structure are not consistent across data sources. During model testing, we realized that drawing a different 80% training sample could result in slight changes in the SVM model results. To reduce this variability, we ran the SVM model 500 times for each data source and used a conservative approach by only considering a medication “high” risk in the SVM model if all 500 models categorized the medication as “high” risk.
Sentiment analysis uses occurrences of key words from a custom dictionary to predict the opinion of subjective information (Cortes and Vapnik 1995; Joachims 1998). For this approach, we created custom sentiment dictionaries for each data source by identifying words that appeared in at least 80% of “high risk” medications and used these dictionaries to search each data source’s narrative summaries. Across all medications, we set a threshold of two (for TERIS), four (for Briggs & Freeman), and six (for FDA) sentiment words because these cut-offs captured at least 50% of the medications classified as “high” in the dichotomous risk categories. Any medication with the number of sentiment words at or above the cutoff was classified in the sentiment analysis as having a “high” risk.
Development of a List of Medications of Greatest Concern during Pregnancy
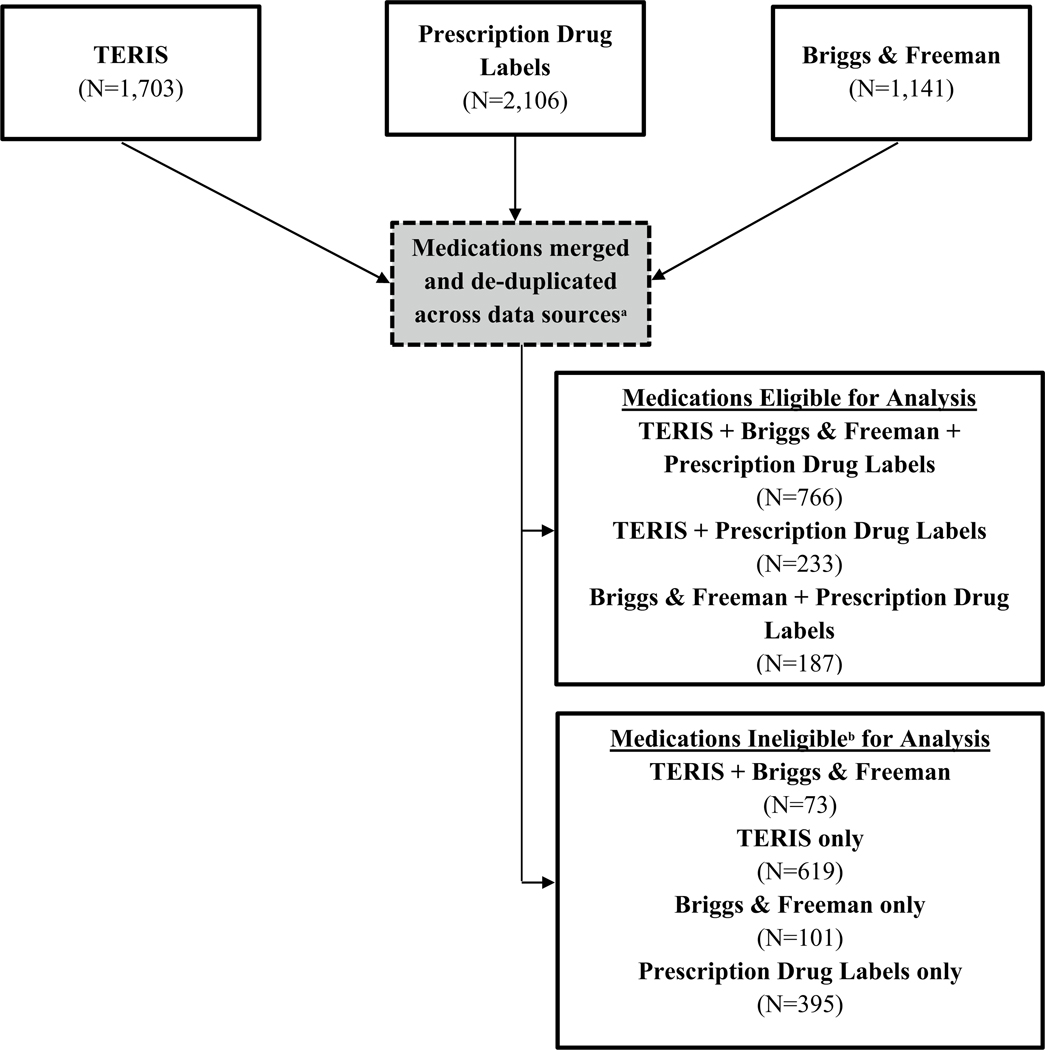
We combined data across all three sources to create our list of medications of greatest concern. However, each data source catalogued medications differently. In the drug product label data, multiple records for a medication could exist (e.g., separate records for each route of administration or inclusion of a salt [e.g., paroxetine and paroxetine hydrochloride]). TERIS and Briggs & Freeman often grouped medications by component or, occasionally, by medication class. TERIS and Briggs & Freeman also included “synonyms” of the medication (i.e., all medications within a class or brand names of medications with the same component). Using indexed medication names and synonyms, we matched medications across the data sources and grouped medications into three categories: (1) medications in all three data sources, (2) medications in two of the three data sources, and (3) medications only in one data source. Because of its complexity, we manually verified the matching process. Medications may have only been included in TERIS or Briggs & Freeman, but not in the drug label data, because they were not prescription medications. If more than one medication from a data source matched to only one medication from another data source, we de-duplicated the data by selecting the highest SLM model risk ratings.
All single-component medications with a prescription drug label for which there was also information in TERIS and/or Briggs & Freeman databases were considered in our analysis (Fig. 1). This approach allowed us to compare medication narrative summaries across at least two data sources. For medications with information in the drug label data and in one other data source (four total SLM models, two from each data source), at least three of the SLM models had to categorize the medication as “high” risk for a medication to be included in our final list of medications. For medications with information in all three data sources, at least four of the six available models had to categorize the medication as “high” risk. We grouped the medications in our final list based on medication class to improve the clinical utility of our list.
Fig. 1.
Matching of medications across data sources. aEach data source classified medications differently. TERIS and Briggs & Freeman often grouped medications by component or, occasionally, by medication class. In the drug label data, multiple records for a medication could exist. For instance, a medication could have separate records for each route of administration or the inclusion of a salt. In addition to the indexed medication name, TERIS and Briggs & Freeman also included “synonyms” of the medication (i.e., all medications within a class if the class was used as the indexing name or brand names of medications that had the same component medication). Using the primary medication name and the synonyms provided by that data source, we matched the medications across the three data sources. bTo be eligible for inclusion in the final list, a medication needed to be in the drug label data and another data source
We used base Python, Python’s Scikit-Learn Library Version 0.19.1, and Natural Language Toolkit Version 3.3, standard open-source libraries for text processing and machine learning models, including SVM. We conducted all other analyses in SAS v.9.3 (Cary, NC). As this analysis relied upon preexisting databases summarizing the safety of medication use in pregnancy and was not research involving human subjects, no statements regarding the 1964 Declaration of Helsinki and its later amendments, patient consent, or Institutional Review Board review were necessary.
Results
Before de-duplication of medications across data sources, 2,106 medications were available in the drug label data, 1,703 in TERIS, and 1,141 in Briggs & Freeman. After dichotomizing each data source’s risk categories, 15% (n = 314) of medications in the drug label data, 5% (n = 82) of medications in TERIS, and 26% (n = 302) of medications in Briggs & Freeman were classified as “high” risk for the purpose of training the models (Table 1; prescription medications classified as “high” for model training purposes are listed in the Supplemental Table). Notably, 77% (n = 1,311) of TERIS medications had a rating of “undetermined” fetal risk, 49% (n = 1,026) of drug labels were pregnancy category C (insufficient human data, animal data indicates some risk), and 6% (n = 63) of Briggs & Freeman medications had limited or no human data and no animal data available to classify fetal risk.
Using each data source’s dichotomized risk categories as the ‘gold standard’, the specificity of SVM and sentiment models were high, ranging from 96 to 99%. However, the sensitivity of the SVM and sentiment models ranged from 51 to 99% (Table 2). If the sources deemed medications to have insufficient data to determine risk, SVM models more often categorized them as ‘high’ risk than sentiment models (e.g., of 1412 medications with ‘undetermined’ risk in drug label data, SVM models coded 327 [23%] as high risk while sentiment models coded 30 [2%] as high risk).
Table 2.
Characteristics of supervised learning models compared to dichotomized risk categories, by data source
| Supervised Learning Models | Dichotomized Risk Categorya | Model Characteristicsb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| High | Not High | Subtotal | Undeterminedc | Totalc | |||||
| Briggs & Freeman (N = 1,141 medications) | |||||||||
| SVMd | |||||||||
| High | 155 | 30 | 185 | 15 | 200 | SEN | 51% | NPV | 84% |
| Not high | 147 | 746 | 893 | 48 | 941 | SPEC | 96% | Accur | 84% |
| Total | 302 | 776 | 1078 | 63 | 1141 | PPV | 84% | ||
| Sentiment | |||||||||
| High | 173 | 24 | 197 | 1 | 198 | SEN | 57% | NPV | 85% |
| Not high | 129 | 752 | 881 | 62 | 943 | SPEC | 97% | Accur | 86% |
| Total | 302 | 776 | 1078 | 63 | 1141 | PPV | 88% | ||
| TERIS (N = 1,703 medications) | |||||||||
| SVMd | |||||||||
| High | 47 | 2 | 49 | 29 | 78 | SEN | 57% | NPV | 90% |
| Not high | 35 | 308 | 343 | 1282 | 1625 | SPEC | 99% | Accur | 91% |
| Total | 82 | 310 | 392 | 1311 | 1703 | PPV | 96% | ||
| Sentiment | |||||||||
| High | 51 | 7 | 58 | 6 | 64 | SEN | 62% | NPV | 91% |
| Not high | 31 | 303 | 334 | 1305 | 1639 | SPEC | 98% | Accur | 90% |
| Total | 82 | 310 | 392 | 1311 | 1703 | PPV | 88% | ||
| Drug Labels (N = 2,106 medications) | |||||||||
| SVMd | |||||||||
| High | 310 | 13 | 323 | 327 | 650 | SEN | 99% | NPV | 99% |
| Not high | 4 | 367 | 371 | 1085 | 1456 | SPEC | 97% | Accur | 98% |
| Total | 314 | 380 | 694 | 1412 | 2106 | PPV | 96% | ||
| Sentiment | |||||||||
| High | 188 | 8 | 196 | 30 | 226 | SEN | 60% | NPV | 75% |
| Not high | 126 | 372 | 498 | 1382 | 1880 | SPEC | 98% | Accur | 81% |
| Total | 314 | 380 | 694 | 1412 | 2106 | PPV | 96% | ||
Accur. Accuracy, NPV Negative Predictive Value, PPV Positive Predictive Value, SEN Sensitivity, SPEC Specificity, SVM Subject Vector Machine model
See Table 1 for details
Calculated using dichotomized risk categories of ‘high’ and ‘not high’ as the gold standard. Medications that the sources categorized as undetermined risk (see Table 1) were not included in models’ characteristic calculations
Provided for informational purposes; these data were not used to calculate the model characteristics
Categorized as ‘High’ if all 500/500 SVM models categorized a medication as high else a medication was categorized as ‘Not High’. See Methods for more details
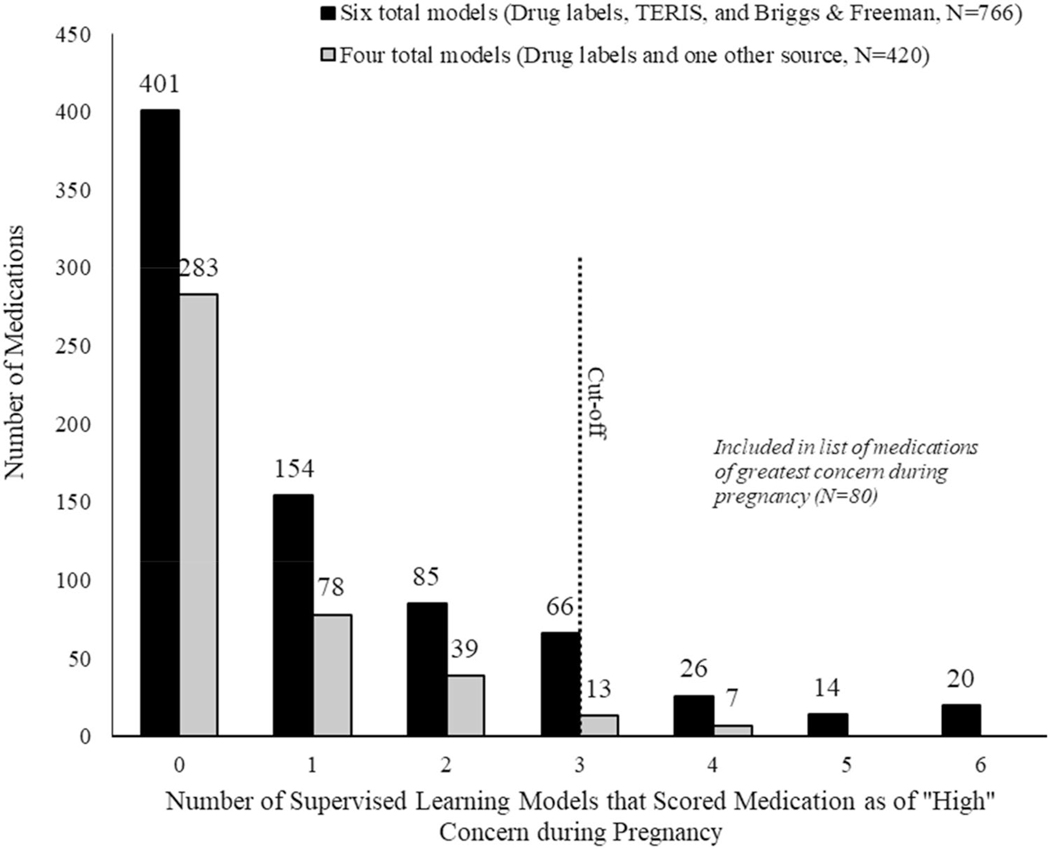
After matching across data sources and de-duplicating the matching results, 1186 medications were available for final analysis (Fig. 1). Of those, over one-third of these medications were categorized by at least one SLM model (n = 502, 42%) as being “high” risk (Fig. 2). However, only 80 medications were categorized as “high” risk in at least three (if four total models) or four (if six total models) models and included in our list of medications of greatest concern (Fig. 2, Table 3). Most (n = 60, 75%) medications in our final list had information in all three data sources. Antineoplastic agents (n = 24), angiotensin converting enzyme inhibitors (n = 10), angiotensin II receptor antagonists (n = 8), and anticonvulsants (n = 7) accounted for over half (61%) of the medications included in our final list.
Fig. 2.
Number of medications that supervised learning models scored as “high” risk during pregnancy, by number of models available (N = 1186)
Table 3.
Lista of prescription medications of greatest concern during pregnancy (N = 80), grouped by medication class (in bold)
| Medications of Greatest Concern during Pregnancy (N = 80) | ||
|---|---|---|
| ACE Inhibitor (N = 10) | Antineoplastic Agent (N = 24) | Ergot Alkaloid (N = 1) |
| Benazepril | Busulfan | Ergotamine |
| Captopril | Carboplatin | Estrogen (N = 2) |
| Enalapril | Chlorambucil | Estradiol |
| Fosinopril | Cisplatin | Estrogens, conjugated |
| Lisinopril | Cyclophosphamide | HMG-COA Reductase |
| Moexipril | Cytarabine | Inhibitor (N = 3) |
| Perindopril | Dactinomycin | Fluvastatin |
| Quinapril | Doxorubicin | Lovastatin |
| Ramipril | Epirubicin | Simvastatin |
| Trandolapril | Etoposide | Immunosupressant Agent (N = 1) |
| Androgen (N = 1) | Fluorouracil | Mycophenolate |
| Testosterone | Gemcitabine | NSAID (N = 4) |
| Angiotensin II Receptor Antagonist (N = 8) | Imatinib | Indomethacin |
| Azilsartan | Letrozole | Meloxicam |
| Candesartan | Mechlorethamine | Nabumetone |
| Eprosartan | Melphalan | Oxaprozin |
| Irbesartan | Mercaptopurine | Opioid (N = 1) |
| Losartan | Methotrexate | Tramadol |
| Olmesartan | Procarbazine | Progestin (N = 2) |
| Telmisartan | Tamoxifen | Hydroxyprogesterone |
| Valsartan | Teniposide | Medroxyprogesterone |
| Anticoagulant (N = 1) | Thalidomide | Prostaglandin (N = 1) |
| Warfarin | Thioguanine | Misoprostol |
| Anticonvulsant (N = 7) | Thiotepa | Retinoid (N = 2) |
| Carbamazepine | Antirheumatic Agent (N = 3) | Acitretin |
| Phenobarbital | Azathioprine | Isotretinoin |
| Phenytoin | Leflunomide | Selective Serotonin Reuptake Inhibitor (N = 2) |
| Primidone | Penicillamine | Paroxetine |
| Topiramate | Antithyroid Agent (N = 1) | Sertraline |
| Trimethadione/Paramethadione | Methimazole | Tetracycline (N = 1) |
| Valproic Acid | Antiviral (N = 1) | Tetracycline |
| Antifungal (N = 1) | Ribavirin | Vitamin (N = 1) |
| Fluconazole | Benzodiazepine (N = 2) | Vitamin A |
| Alprazolam | ||
| Diazepam | ||
This list should not be the sole resource healthcare providers consult and readers should obtain additional information on the relevant dose, timing of administration, and risks associated with any of these medications. Healthcare providers and patients may find it helpful to weigh the specific benefits and risks of pharmacologic treatment to manage medical conditions before and during pregnancy and not simply counsel against use of any medication included in our list
ACE Angiotensin converting enzyme, HMG-COA 3-hydroxy-3-methylglutaryl-coenzym, NSAID Nonsteroidal anti-inflammatory
Discussion
We applied supervised learning methods to existing data sources characterizing medication safety during pregnancy to develop a list of medications of greatest concern during pregnancy. While all use of medication during pregnancy requires a careful assessment of risk versus benefit, using these methods and data sources we identified 80 medications that warrant particular consideration before use by pregnant women due to associations with birth defects, pregnancy loss, or other adverse fetal effects. Antineoplastic agents appeared most frequently in our list, consistent with published reports describing fetal risks associated with many antineoplastic medications (National Toxicology Program). Several additional known teratogens, such as thalidomide, isotretinoin, warfarin, and some anticonvulsant medications were included in our final list (Harden et al. 2009; Lammer et al. 1985; Lenz 1988; Vitale et al. 1999). However, other medications included in our final list are less commonly agreed upon within the scientific literature and in clinical practice. These medications include some selective serotonin reuptake inhibitors (SSRIs) and some non-steroidal anti-inflammatory drugs (Huybrechts et al. 2014; Reefhuis et al. 2015; Rolnik et al. 2017).
Our list can serve as a tool to inform clinical decision-making by identifying some medications of greatest concern, but should not be the sole resource healthcare providers consult. Healthcare providers and patients may find it helpful to weigh the specific benefits and risks of pharmacologic treatment to manage medical conditions before and during pregnancy and not simply counsel against use of any medication included in our list. For example, though the antithyroid medication methimazole is on our list and has been associated with birth defects, use of antithyroid agents by pregnant women with hyperthyroidism not only prevent maternal complications but also prevent fetal hyperthyroidism, which has been associated with intrauterine growth restriction, hydrops fetalis, and fetal death (Alexander et al. 2017; De Groot et al. 2012). Similarly, untreated maternal depression has been associated with poor infant outcomes, and thus some pregnant women, in consultation with their healthcare providers, may decide to continue treatment with specific SSRIs during pregnancy (Ornoy and Koren 2017). Dose is also an important consideration for some medications in our final list; many prenatal vitamins include low doses of vitamin A but at higher doses it can cause birth defects (Duerbeck and Dowling 2012). Lastly, timing of administration is critical as well. Hydroxyprogesterone early in pregnancy has been associated with hypospadias (Carmichael et al. 2005), while use later has been used to reduce preterm births (Meis et al. 2003). Inclusion of a medication on this list does not mean that all pregnant women who take the medication will have adverse outcomes. Conversely, the absence of a specific medication on this list does not imply safety either, but could indicate a lack of reliable information or be the result of incorrect classification by the SLM models. While this list is meant to be a tool for healthcare providers, any pregnant women or women planning a pregnancy who are taking medications on this list should consult a healthcare provider before starting or discontinuing any medications.
Our approach has several limitations. First, we did not consider all medications or teratogenic exposures (e.g., environmental or occupational). For example, only 59% (999/1703) of TERIS entries matched to a drug label and were included in our final analysis; the remaining tended to be chemical compounds or medications either discontinued or not approved in the U.S. We did not include multi-component medication drug product labels, over-the-counter medications or alternative treatments, such as herbals and supplements, which are commonly used during pregnancy and may have risk associated with use (Broussard et al. 2010; Kennedy et al. 2016; Thorpe et al. 2013). Second, we were unable to account for other clinical considerations such as medication dose, gestational timing of exposure, duration of exposure, concomitant exposures, type of adverse outcome, or gradations in the level of risk associated with medications. While this information was often captured in narrative summaries, each data source described these factors differently making it difficult to abstract these details. Third, a different list of medications may result from other analytic approaches and data sources. We could only apply SLM models to data sources with both narrative summaries and risk categories, limiting the number of sources we were able to use. Discontinuation of FDA’s pregnancy letter categories on manufacturers’ drug labeling will require different analytic approaches, but might also allow for the incorporation of other data sources, such as REPROTOX™ and Shepard’s Catalog of Teratogenic Agents. Furthermore, new methods will continue to be developed as the field of natural language processing grows, and different methodological approaches may yield new findings. Fourth, we found that each data source has its own narrative style to describe risk, making it challenging to apply a single model to all sources of information. This variability may be due to differences in the specific purpose of these data sources in terms of focus of review (e.g., teratogenicity, fetal effects alone, or maternal and fetal effects) and in describing risk alone or comparing risk and benefit. Fifth, this list does not offer “safer” alternative medications, although the development of such a list could be a clinically useful companion tool. Sixth, we were limited by the paucity of fetal risk information for most medications provided in our data sources; few medications have sufficient human data to determine fetal risk (Adam et al. 2011). We also only had access to prescription medication labeling submitted for FDA approval, which may have differed from the final approved labeling. Therefore, our list of medications reflects only the current knowledge of risk and safety during pregnancy, which is limited, and does not include newly released medications or medications not yet evaluated by TERIS and/or Briggs & Freeman. This list will need to be updated as new information on associations between medications and pregnancy outcomes becomes available and new medications are put on the market. More high-quality studies and new methods to evaluate teratogenic risk are urgently needed.
This analysis demonstrates a useful method to distill large amounts of text data across multiple sources into a single list and incorporates high-quality information that may not be widely accessible because of barriers such as subscription costs. Before prescribing a medication to pregnant women or women who might become pregnant in the near future, providers should consult the most up-to-date information to best weigh the risks and benefits of all medical treatment.
Supplementary Material
Significance.
Nine out of ten women in the United States take a medication during pregnancy, yet there is limited, and often inconsistent, information about the risks of many medications to the developing fetus.
We applied supervised learning methods to three existing U.S. data sources characterizing medication safety during pregnancy. While all medication use during pregnancy requires a careful assessment of risk versus benefit, using these methods and data sources we identified 80 medications that warrant particular consideration before use by pregnant women due to associations with birth defects, pregnancy loss, or other adverse fetal effects.
Acknowledgements
We thank Jan Friedman and Janine Polifka for sharing the TERIS database and Gerald Briggs for agreeing to share an electronic copy of his textbook with us for our analysis. We also thank Leyla Sahin and Melissa Tassinari from the U.S. Food and Drug Administration for insightful feedback on earlier versions of this manuscript. We also acknowledge Sara Khan, Angel Jose, Jannat Saini, Merika Starr, and Emmy Tran for their support on this analysis.
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
Disclaimer The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10995-020-02942-2) contains supplementary material, which is available to authorized users.
References
- Adam MP, Polifka JE, & Friedman JM (2011). Evolving knowledge of the teratogenicity of medications in human pregnancy. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 157C(3), 175–182. 10.1002/ajmg.c.30313. [DOI] [PubMed] [Google Scholar]
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. (2017). 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid, 27(3), 315–389. 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- Briggs G, & Freeman R (2014). Drugs in pregnancy and lactation: A reference guide to fetal and neonatal risk (10th ed.). Philadelphia, PA: Wolters Kluwer. [Google Scholar]
- Broussard CS, Louik C, Honein MA, Mitchell AA, & The National Birth Defects Prevention Study. (2010). Herbal use before and during pregnancy. American Journal of Obstetrics Gynecology, 202(5), 443 10.1016/j.ajog.2009.10.865. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, & Lammer EJ (2005). Maternal progestin intake and risk of hypospadias. Archives of Pediatrics and Adolescent Medicine, 159(10), 957–962. 10.1001/archpedi.159.10.957. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Polifka JE, & Friedman JM (2008). Drug safety in pregnant women and their babies: Ignorance not bliss. Clinical Pharmacology and Therapeutics, 83(1), 181–183. 10.1038/sj.clpt.6100448. [DOI] [PubMed] [Google Scholar]
- Cortes C, & Vapnik V (1995). Support-vector networks. Machine Learning, 20, 273–297. [Google Scholar]
- De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. (2012). Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism, 97(8), 2543–2565. 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- Dinatale M, Sahin L, Johnson T, Howard T, & Yao L (2017). Medication use during pregnancy and lactation: Introducing the Pregnancy and Lactation Labeling Rule Pediatric Allergy. Immunology, and Pulmonology, 30(2), 132–134. [Google Scholar]
- Duerbeck NB, & Dowling DD (2012). Vitamin A: Too much of a good thing? Obstetrical & Gynecological Survey, 67(2), 122–128. 10.1097/OGX.0b013e318244c52d. [DOI] [PubMed] [Google Scholar]
- Eltonsy S, Martin B, Ferreira E, & Blais L (2016). Systematic procedure for the classification of proven and potential teratogens for use in research. Birth Defects Research Part A: Clinical and Molecular Teratology, 106(4), 285–297. 10.1002/bdra.23491. [DOI] [PubMed] [Google Scholar]
- Hameen-Anttila K, Nordeng H, Kokki E, Jyrkka J, Lupattelli A, Vainio K, et al. (2014). Multiple information sources and consequences of conflicting information about medicine use during pregnancy: A multinational Internet-based survey. Journal of Medical Internet Research, 16(2), e60. 10.2196/jmir.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden CL, Hopp J, Ting TY, Pennell PB, French JA, Hauser WA, et al. (2009). Practice parameter update: management issues for women with epilepsy–focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology, 73(2), 126–132. 10.1212/WNL.0b013e3181a6b2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts KF, Palmsten K, Avorn J, Cohen LS, Holmes LB, Franklin JM, et al. (2014). Antidepressant use in pregnancy and the risk of cardiac defects. New England Journal of Medicine, 370(25), 2397–2407. 10.1056/NEJMoa1312828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachims T (1998). Text categorization with Support Vector Machines: Learning with many relevant features. In Nédellec C & R C (Eds.), Machine Learning: ECML-98: 10th European Conference on Machine Learning Chemnitz, Germany, April 21–23, 1998 Proceedings: Springer, Berlin. [Google Scholar]
- Kennedy DA, Lupattelli A, Koren G, & Nordeng H (2016). Safety classification of herbal medicines used in pregnancy in a multinational study. BMC Complementary and Alternative Medicine, 16, 102 10.1186/s12906-016-1079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, et al. (1985). Retinoic acid embryopathy. New England Journal of Medicine, 313(14), 837–841. 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- Lenz W (1988). A short history of thalidomide embryopathy. Teratology, 38(3), 203–215. 10.1002/tera.1420380303. [DOI] [PubMed] [Google Scholar]
- Lynch MM, Amoozegar JB, McClure EM, Squiers LB, Broussard CS, Lind JN, et al. (2017). Improving safe use of medications during pregnancy: The roles of patients, physicians, and pharmacists. Qualitative Health Research, 27(13), 2071–2080. 10.1177/1049732317732027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. (2003). Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. New England Journal of Medicine, 348(24), 2379–2385. 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- Miranda-Filho Dde B, Martelli CM, Ximenes RA, Araujo TV, Rocha MA, Ramos RC, et al. (2016). Initial description of the presumed congenital Zika Syndrome. American Journal of Public Health, 106(4), 598–600. 10.2105/AJPH.2016.303115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez-Diaz S, et al. (2011). Medication use during pregnancy, with partic***ular focus on prescription drugs: 1976–2008. American Journal of Obstetrics and Gynecology, 205(151), 1–8. 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, Cragan JD, Goldenberg RL, Rasmussen SA, & Schulkin J (2010). Obstetrician-gynaecologist knowledge of and access to information about the risks of medication use during pregnancy. Journal of Maternal-Fetal and Neonatal Medicine, 23(10), 1143–1150. 10.3109/14767051003653252. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. Retrieved July 17, 2017, from https://ntp.niehs.nih.gov/ntp/ohat/cancer_chemo_preg/chemopregnancy_monofinal_508.pdf
- Noh Y, Yoon D, Song I, Jeong HE, Bae JH, & Shin JY (2018). Discrepancies in the evidence and recommendation levels of pregnancy information in prescription drug labeling in the United States, United Kingdom, Japan, and Korea. Journal of Womens Health (Larchmt). 10.1089/jwh.2017.6792. [DOI] [PubMed] [Google Scholar]
- Ornoy A, & Koren G (2017). Selective Serotonin Reuptake Inhibitors during pregnancy: Do we have now more definite answers related to prenatal exposure? Birth Defects Research, 109(12), 898–908. 10.1002/bdr2.1078. [DOI] [PubMed] [Google Scholar]
- Palmsten K, Hernandez-Diaz S, Chambers CD, Mogun H, Lai S, Gilmer TP, et al. (2015). The most commonly dispensed prescription medications among pregnant women enrolled in the U.S. Medicaid program. Obstetrics & Gynecology, 126(3), 465–473. 10.1097/AOG.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palosse-Cantaloube L, Lacroix I, Rousseau V, Bagheri H, Montastruc JL, & Damase-Michel C (2014). Analysis of chats on French internet forums about drugs and pregnancy. Pharmacoepidemiology and Drug Safety, 23(12), 1330–1333. 10.1002/pds.3709. [DOI] [PubMed] [Google Scholar]
- Peters SL, Lind JN, Humphrey JR, Friedman JM, Honein MA, Tassinari MS, et al. (2013). Safe lists for medications in pregnancy: Inadequate evidence base and inconsistent guidance from Web-based information, 2011. Pharmacoepidemiology and Drug Safety, 22(3), 324–328. 10.1002/pds.3410. [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Devine O, Friedman JM, Louik C, Honein MA, & the National Birth Defects Prevention Study. (2015). Specific SSRIs and birth defects: Bayesian analysis to interpret new data in the context of previous reports. BMJ, 351, h3190. 10.1136/bmj.h3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. (2017). Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. New England Journal of Medicine, 377(7), 613–622. 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- Shepard T, & Lemire R (2010). Catalog of teratogenic agents (13th ed.). Baltimore, MA: Johns Hopkins University Press. [Google Scholar]
- Thorpe PG, Gilboa SM, Hernandez-Diaz S, Lind J, Cragan JD, Briggs G, et al. (2013). Medications in the first trimester of pregnancy: Most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiology and Drug Safety, 22(9), 1013–1018. 10.1002/pds.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. (Pregnancy and Lactation Labeling (Drugs) Final Rule. Retrieved June 15, 2017, from https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093307.htm.
- Vitale N, De Feo M, De Santo LS, Pollice A, Tedesco N, & Cotrufo M (1999). Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. Journal of the American College of Cardiology, 33(6), 1637–1641. [DOI] [PubMed] [Google Scholar]
- Webster WS, & Freeman JA (2003). Prescription drugs and pregnancy. Expert Opinion on Pharmacotherapy, 4(6), 949–961. 10.1517/14656566.4.6.949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.