Abstract
Cross-sectionally measured N-terminal pro B-type natriuretic peptide (NT-proBNP) is related to incident dementia. However, data linking changes in NT-proBNP to risk of future dementia are lacking. We aimed to examine the association of change in NT-proBNP over 3.2 years with incident dementia.
We included 4,563 participants in Multi-Ethnic Study of Atherosclerosis (MESA prospective cohort) who were free of cardiovascular disease at enrollment, had NT-proBNP level measured at MESA exams 1 (baseline, 2000 to 2002) and 3 (2004 to 2005) and had no diagnosis of dementia prior to exam 3. The association of change in NT-proBNP level between MESA exams 1 through 3 and all-cause hospitalized dementia (by ICD codes) after MESA exam 3 (2004–2005) through 2015 was assessed using competing-risks Cox proportional hazard regression analysis.
During 45,522 person-years of follow-up, 223 dementia cases were documented. Increase in log-NT-proBNP from MESA exams 1 through 3 was positively associated with incidence of dementia (multivariable HR 1.28, 95% CI 1.001–1.64, p=0.049). An increase of at least 25% in NT-proBNP level from MESA exam 1 through 3 was associated with a 55% (p=0.02) increase in the risk of dementia in multivariable analysis. Addition of temporal NT-proBNP change to a model including risk factors and baseline NT-proBNP improved the prediction of dementia (Harrell’s C statistic from 0.85 to 0.87, P=0.049).
Increase in NT-proBNP is independently associated with future all-cause hospitalized dementia and offers a moderately better predictive performance for risk of dementia compared with risk factors and baseline NT-proBNP.(ClinicalTrials.govIdentifier: NCT00005487)
Keywords: Dementia, Cognition disorders, N-terminal pro-BNP, cohort study, cardiovascular disease
Graphical Abstract
Introduction
Dementia is a major public health problem worldwide and a leading cause of mortality and disability; it is often rated “worse than death” by patients1, 2. Early alterations in brain structure have been shown to precede clinical dementia by 5 to 10 years, but a significant proportion of individuals at risk of dementia are not identified until very late in life and when they clinically become symptomtic3, 4.
Brain alterations leading to dementia are the byproduct of genetic susceptibility combined with multiple risk factor exposures (including CVD risk factors) involving several organ systems in the human body5, 6. Several studies have shown associations between dementia and prior cardiovascular diseases (CVD), in particular atrial fibrillation and heart failure7, 8; but the mechanisms for these associations remain incompletely understood.
N-terminal pro–B-type natriuretic peptide (NT-proBNP) is released in response to myocardial wall stress9 and has been linked to a wide range of adverse cardiovascular outcomes10–15, as well as subclinical myocardial dysfunction16. NT-proBNP has also been associated with subclinical brain damage, cognitive dysfunction and incident dementia17–21; it likely reflects intermediate processes linking CVD to cerebral damage and dysfunction, thus having potential as a surrogate marker for early dementia detection and risk stratification.18–20
Levels of NT-proBNP change substantially over time in response to physiological and pathological alterations22–25. We hypothesized that tracking changes in NT-proBNP level over time may have significant implication for risk stratification and prediction of adverse outcomes over and above the cross-sectional NT-proBNP measurement. Previous studies have linked long-term changes in NT-proBNP level with adverse cardiovascular outcomes in patients with congestive heart failure26, 27, coronary artery disease28 as well as those free of CVD29. However, the implications of changes in NT-proBNP to risk of future dementia have not been explored.
In the present study, we aimed to investigate the relationship between change in NT-proBNP over time with future all-cause hospitalized dementia and measures of cognitive function in a large multiethnic population of men and women without clinical CVD at enrollment in the Multi-Ethnic Study of Atherosclerosis (MESA).
Participants and Methods
The data, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. However, the analytic methods are described in detail in this article.
The design of MESA has been previously described30. Briefly, a total of 6814 men and women aged 45 to 84 years were enrolled between July 2000 and August 2002 from 6 US communities representing 4 racial/ethnic groups (white, black, Hispanic, and Chinese-American). Individuals with any prior clinical diagnosis of CVD at enrollment were excluded. The study protocol was approved by the institutional review board of each MESA field center, and all participants gave informed consent.
Plasma NT-proBNP Measurements
NT-proBNP was measured in 5,597 MESA participants at enrollment (MESA exam 1) between 2000 to 2002. Of these, 4,586 participants underwent repeat NT-proBNP measurements in the follow-up MESA exam 3, performed between 2004–2005.
Blood samples were stored at −70° C and were thawed prior to testing (maximum of 3 freeze-thaw cycles). NT-proBNP was measured using the Elecsys 2010 system (Roche Diagnostics, Indianapolis, IN). All analyses were performed at a core laboratory (Veteran’s Affairs San Diego Healthcare System, La Jolla,CA). Previous studies have indicated that measurements of NT-proBNP using this assay are not significantly altered after 5 freeze-thaw cycles31. Intra- and inter-assay coefficients of variation at various concentrations of NT-proBNP were 1.3% and 4.8%, respectively32. The analytical measurement range for NT-proBNP was 5 to 35,000 pg/mL.
Follow-Up and Endpoints
In MESA, telephone interviews are conducted every 9 to 12 months with each participant to identify interim hospitalizations, outpatient cardiovascular and other diagnoses, and death. Medical records were obtained for those reporting a hospitalization. Probable dementia including Alzheimer’s disease, vascular dementia, and Pick’s disease were classified from the coded hospitalization discharge diagnoses using the International Statistical Classification of Diseases Medical Diagnosis Codes, Ninth Revision. The following diagnosis codes were used to identify dementia: International Classification of Diseases—Ninth Revision (ICD-9): 290, 294, 331.0, 331.1, 331.2, 331.82, 331.83, 331.9, 438.0, and 780.93; ICD-10: F00, F01, F03, F04, G30, G31 (excluding G31.2), I69.91, and R41. In addition, a physician blinded to the ICD-9 codes ascertained potential cases of dementia by reviewing the medical records of each participant, looking for statements that indicated or contradicted the diagnosis of dementia. The use of hospitalization discharge ICD codes for the diagnosis of dementia has been validated and used in prior publications from MESA33–35. In this study, we only included dementia events that occurred after MESA exam 3 through 2015.
The diagnoses of interim stroke, heart failure, and coronary heart disease were adjudicated by 2 independent physicians of the MESA adjudication committee. Full details of the MESA follow-up methods, investigators, and institutions are available at the MESA website (http://www.mesa-nhlbi.org).
Measures of cognitive function were obtained during MESA exam 5 performed between 2010 and 2012, and included the the Cognitive Abilities Screening Instrument (CASI) score, as well as forward and backward Digit Span35.
Statistical Analysis
Data were presented as mean ± SD, median (interquartile range) or number (%), as appropriate. Baseline characteristics were compared between subjects with and without dementia using χ2 tests for categorical variables and 2-sided t tests or the Wilcoxon signed rank test for continuous variables. Variables with non-normal distribution were transformed to logarithmic (NT-proBNP and coronary artery calcium score) or cubic-transfomred values (CASI score) for regression analysis.
We used multivariable competing-risks Cox proportional hazard regression models to investigate the association of temporal change in NT-proBNP levels between MESA exams 1 and 3 and risk of future all-cause hospitalized dementia after MESA exam 336. Change in NT-proBNP levels was included in regression analysis both as continuous but also as a dichotomous variable based on greater than 25% increase in NT-proBNP level since baseline (MESA exam 1). The 25% threshold for change in NT-proBNP levels was chosen based on previous reports on intra-individual variability of NT-proBNP indicating that >25% change is deemed clinically significant and unlikely due to measurement error37, 38. We also performed a secondary analysis to assess the risk of future dementia in four groups of participants based on 75th percentile of baseline NT-ptoBNP level and 25% or more increase in NT-proBNP level over time. Death prior to the diagnosis of dementia was considered as a competing event because it hindered the detection of dementia. We used Harrell’s C-statistic to assess the models’ performance for predicting dementia and to test whether addition of NT-proBNP and its temporal change improved the discriminative performance of models comprising traditional risk factors. The multivariable models included:
Model 1 was adjusted for demographics including age, sex and race/ethnicity.
Model 2 included variables in model 1 in addition to ApoE lipoprotein isoforms and traditional cardiovascular disease risk factors and socioeconomic status at MESA exam 3, including systolic blood pressure, use of antihypertensives, resting heart rate, high-density lipoprotein, total cholesterol, antihyperlipidemics, body mass index, glomerular filtration rate (GFR), diabetes mellitus, income, education level, health insurance status, cigarette smoking and study field center at MESA exam 3. This model was also adjusted for coronary artery calcium score measured in the entire cohort at MESA exam 1, but not at MESA exam 3.
Model 3 was adjusted for all variables in model 2 and also interim cardiovascular events prior to incidence of dementia, including coronary events, heart failure, and clinical stroke as time-varying covariates.
Model 4 was adjusted for all variables in model 3 and left ventricular (LV) end diastolic volume (LVEDV), LV ejection fraction (LVEF), and LV mass (LVM) by magnetic resonance imaging (MRI) obtained at baseline (MESA exam 1) but not at MESA exam 3.
Associations of NT-proBNP levels obtained at MESA exams 1 and 3 as well as its change, with measures of cognitive function at MESA exam 5 were tested using multivariable linear regression analysis adjusted for demographics and traditional risk factors obtained at MESA exams 1 or 3 as well as interim CVD events.
We also used regression analysis to identify the correlates of change in NT-proBNP levels from MESA exam 1. Multivariable models for these analyses were constructed using the variables associated with change in NT-proBNP levels reaching p value <0.2.
Two-tailed P values <0.05 were used for significance testing. All statistical analyses were performed using Stata 14.0 (StataCorp LP; College Station, TX).
Results
Of the 4,586 MESA participants with NT-proBNP measurements at both MESA exams 1 and 3, a total of 4,563 individuals without dementia prior to MESA exam 3 were included in the analysis (Supplemental Figure S1). During 45,522 person-years of follow-up, 223 all-cause hospitalized dementia cases were ascertained. The mean age of the cohort at MESA exam 3 was 65.0 ± 10.01 and 2,219 (48.6%) were men. Baseline characteristics of the participants at MESA exam 3 are described in Table 1. The median NT-proBNP concentration at MESA exams 1 and 3 were 52.3 pg/mL (IQR 23.6 – 107.0) and 65.7 pg/mL (IQR 32.6 – 132.1), respectively. NT-proBNP levels increased by a median of 11.7 pg/mL (IQR −93, 43.9) over a mean of 3.2 ± 0.3 years. Participants with all-cause hospitalized dementia were older and more frequently white, having lower income and lower education level. They were less likely to be without health insurance and had higher systolic and lower diastolic blood pressure, higher use of antihypertensive medications, worse kidney function, slightly lower body mass index, lower total cholesterol and higher use of lipid lowering therapy. Furthermore, participants who developed dementia had higher levels of NT-proBNP (in pg/mL) at MESA exams 1 and 3 as well as higher temporal increase in NT-proBNP levels between the two exams (Table 1).
Table 1.
Clinical characteristics of study participants at MESA exam 3 in those who did and did not develop dementia during follow-up
| Characteristics | No Dementia (N=4,340) | Dementia (N= 223) | P-value |
|---|---|---|---|
| Demographics | |||
| Age, Mean ± SD | 64.3 ± 9.7 | 76.3 ± 7.6 | <0.001 |
| Men, n (%) | 2,097 (48.3) | 122 (54.7) | 0.06 |
| Race/Ethnicity, n (%) | <0.001 | ||
| White | 1,780 (41.0) | 119 (53.4) | |
| Chinese | 580 (13.4) | 13 (5.8) | |
| Black | 1,013 (23.3) | 48 (21.5) | |
| Hispanic | 967 (22.3) | 43 (19.3) | |
| Income, US$ | <0.001 | ||
| < 20,000 | 931 (22.2) | 78 (39.0) | |
| 20,000 – 49,999 | 1,452 (34.6) | 67 (33.5) | |
| ≥ 50,000 | 1,811 (43.2) | 55 (27.5) | |
| Education, n (%) | 0.04 | ||
| High school or less | 1442 (33.3) | 92 (41.3) | |
| College or undergraduate degree | 2058 (47.5) | 97 (43.5) | |
| Graduate degree | 832 (19.21) | 34 (15.2) | |
| Health insurance, n (%) | <0.001 | ||
| Yes | 4056 (93.6) | 220 (99.1) | |
| No | 277 (6.4) | 2 (0.9) | |
| Site, n(%) | 0.03 | ||
| Wake Forest University | 605(13.9) | 39(17.5) | |
| Columbia university | 598(13.8) | 34(15.2) | |
| Johns Hopkins University | 646(14.9) | 31(13.9) | |
| University of Minnesota | 702(16.2) | 49(22.0) | |
| Northwestern university | 809(18.6) | 36(16.1) | |
| University of California, Los Angles | 980(22.6) | 34(15.2) | |
| Cardiovascular risk factors | |||
| Systolic blood pressure, mm Hg; mean ± SD | 122.6 ± 20.2 | 131.2 ± 23.1 | <0.001 |
| Diastolic blood pressure, mm Hg; mean ± SD | 69.7 ± 9.9 | 68.3 ± 10.3 | 0.03 |
| Estimated glomerular filtration rate, mL/min; mean ± SD | 79.3 ± 17.6 | 71.6 ± 19.2 | <0.001 |
| Hypertension medication use, n (%) | 1,898 (44.4) | 141 (65.0) | <0.001 |
| Heart rate, mean ± SD | 64.9 ± 10.2 | 65.6 ± 12.4 | 0.31 |
| Body mass index, kg/m2; mean ± SD | 28.3 ± 5.5 | 27.4 ± 4.8 | 0.02 |
| HDL cholesterol, mg/dL; mean ± SD | 51.5 ± 15.05 | 51.9 ± 14.8 | 0.75 |
| Total cholesterol, mg/dL; mean ± SD | 188.2 ± 36.5 | 182.9 ± 35.2 | 0.04 |
| Antihyperlipidemic use, n (%) | 1,181 (27.6) | 74 (34.1) | 0.02 |
| Diabetes mellitus, n (%) | 642 (14.8) | 33 (14.8) | 0.99 |
| Smoking status, n (%) | 0.18 | ||
| Never | 1,973 (45.8) | 90 (40.7) | |
| Former | 1,904 (44.2) | 112 (50.7) | |
| Current | 433 (10.0) | 19 (8.6) | |
| ApoE isoforms, n(%) | 0.59 | ||
| e2/e2 | 34(0.8) | 1(0.5) | |
| e2/e3 | 492(11.7) | 25(11.6) | |
| e2/e4 | 95(2.3) | 5(2.3) | |
| e3/e3 | 2557(60.9) | 120(55.8) | |
| e3/e4 | 928(22.1) | 58(27.0) | |
| e4/e4 | 94(2.2) | 6(2.8) | |
| NT-proBNP at MESA 1, pg/mLMedian (IQR) | 51.0 (22.9, 102) | 117.3 (52.0, 213) | <0.001 |
| NT-proBNP at MESA 3, pg/mLMedian (IQR) | 63.7(31.3, 125.3) | 146.7(69.0, 317.4) | <0.001 |
| Change in NT-proBNP, pg/mL Median (IQR) | 11.0 (−9.3, 41.3) | 31.2 (−6.8, 102.1) | <0.001 |
NT-proBNP levels and dementia
Log NT-proBNP level at either MESA exams 1 (HR 1.9, p<0.001) or 3 (HR 1.95, p<0.001) was significantly associated with future risk of all-cause hospitalized dementia (Supplemental Table S1). These associations remained significant after adjustment for demographics, socioeconomic status, CVD risk factors, ApoE lipoprotein isoforms and interim CVD events (HR 1.23, 95% CI 1.03–1.45 and 1.25, 95% CI 1.03–1.53 at MESA exams 1 and 3, respectively, Supplemental Table S1).
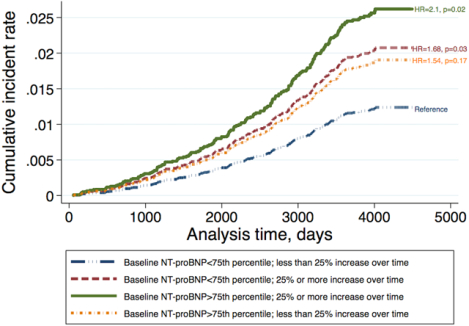
Increase in log-NT-proBNP levels from MESA 1 through MESA 3 exams was associated with significant increase in the risk of future all-cause hospitalized dementia (HR 1.21, 95% CI 1.04–1.42, Table 2). This association remained significant after adjustment for demographics (HR 1.24, 95% CI 1.03–1.49, model 1), traditional risk factors (HR 1.25, 95% CI 1.02–1.52, model 2), interim CVD events (HR 1.25, 95% CI 1.03–1.53, model 3) and MRI derived LVEDV, LVEF and LVM (HR 1.28, 95% CI 1.001–1.64, model 4, Table 2). Participants with greater than 25% increase in NT-proBNP level had 38% to 55% higher risk of dementia in multivariable analysis (Table 2 and Figure 1). On the other hand, any decrease in NT-proBNP levels from MESA 1 to MESA 3 exams was associated with 26–30% reduced risk of future dementia. (Table 2). In a separate analysis (supplemental figure S2), subjects with lower baseline NT-proBNP and smaller increase in its level over time had the lowest risk for future all-cause hospitalized dementia versus those with both high baseline NT-proBNP and greater than 25% increase in its level, in whom the risk of future dementia was the greatest. Participants with either greater baseline NT-proBNP level or greater than 25% increase in its level over time (but not both of these criteria) had moderate risk of future all-cause hospitalized dementia. Interestingly, the risk of future future all-cause hospitalized dementia in subjects with only greater than 25% increase over time (and low baseline NT proBNP) was greater than those with only high baseline NT-proBNP level (and lower than 25% increase in NT-proBNP level).
Table 2.
Hazard ratio of association between change in log-NT-ProBNP level (pg/mL) and risk of future all-cause hospitalized dementia
| Hazard ratio (95% CI) | P-Value | |
|---|---|---|
| Change in log-NT-proBNP | ||
| Unadjusted | 1.21(1.04–1.42) | 0.013 |
| Model 1 | 1.24(1.03–1.49) | 0.024 |
| Model 2 | 1.25(1.02–1.52) | 0.029 |
| Model 3 | 1.25(1.03–1.53) | 0.026 |
| Model 4 | 1.28(1.001–1.64) | 0.049 |
| Increase of ≥ 25% in NT-proBNP | ||
| Unadjusted | 1.45(1.11–1.89) | 0.007 |
| Model 1 | 1.38(1.05–1.81) | 0.019 |
| Model 2 | 1.48(1.1–2.0) | 0.011 |
| Model 3 | 1.49(1.1–2.01) | 0.010 |
| Model 4 | 1.55(1.10–2.25) | 0.023 |
| Any decrease in NT-proBNP | ||
| Unadjusted | 0.73(0.54–0.97) | 0.032 |
| Model 1 | 0.74(0.56–0.99) | 0.041 |
| Model 2 | 0.70(0.50–0.97) | 0.03 |
| Model 3 | 0.70(0.50–0.97) | 0.03 |
| Model 4 | 0.71(0.47–1.1) | 0.101 |
Model 1 was adjusted for age, sex, race/ethnicity, and Log-NT-proBNP at MESA exam 1.
Model 2 included all variables in model 1 in addition to ApoE lipoprotein isoforms and traditional cardiovascular disease risk factors and socioeconomic status at MESA exam 3, including systolic blood pressure, use of antihypertensives, resting heart rate, high-density lipoprotein, total cholesterol, antihyperlipidemics, body mass index, glomerular filtration rate, diabetes mellitus, income, education level, health insurance status, cigarette smoking and study field center at MESA exam 3 and coronary artery calcium score at MESA exam 1.
Model 3 was adjusted for all variables in model 2 and interim cardiovascular events, including coronary events, heart failure, and clinical stroke as time-varying covariates.
Model 4 was adjusted for all variables in model 3 and left ventricular end diastolic volume, left ventricular ejection fraction, and left ventricular mass at MESA exam 1.
Figure 1.
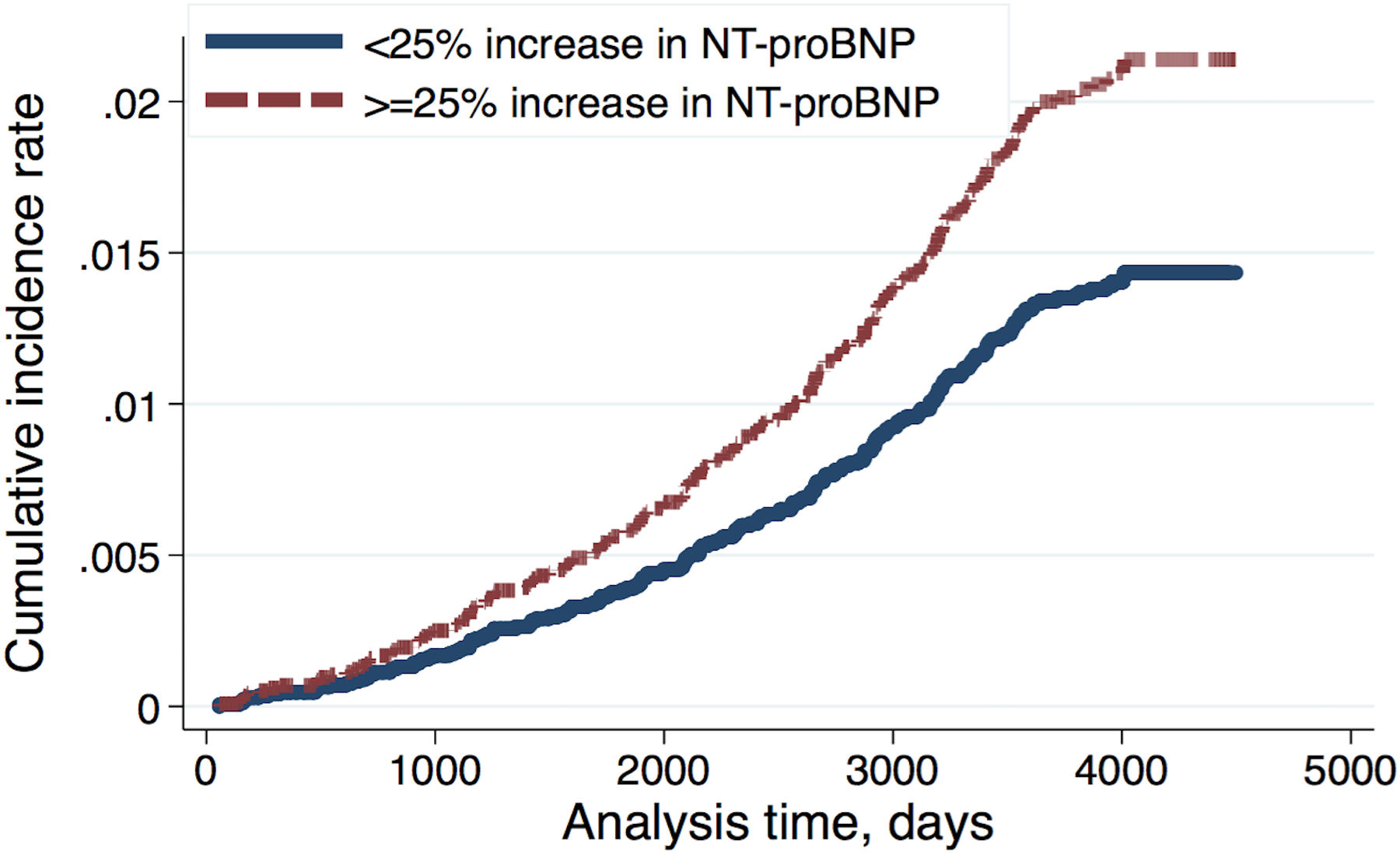
Cumulative incidence rate for future all-cause hospitalized dementia in participants with and without 25% or greater change in NT-proBNP since MESA exam 1 adjusted for baseline demographics (age, sex, race/ethnicity), ApoE lipoprotein isoforms and traditional cardiovascular disease risk factors and socioeconomic status at MESA exam 3, including systolic blood pressure, use of antihypertensives, resting heart rate, high-density lipoprotein, total cholesterol, antihyperlipidemics, body mass index, glomerular filtration rate, diabetes mellitus, income, education level, health insurance status, cigarette smoking and study field center at MESA exam 3 and coronary artery calcium score at MESA exam 1 and interim cardiovascular events, including coronary events, heart failure, and clinical stroke as time-varying covariates.
Risk discrimination
The Harrell’s C statistic for a multivariable model of demographics, traditional risk factors and interim CVD events for prediction of future all-cause hospitalized dementia was 0.85 (95% CI 0.83 – 0.88, Table 3). Addition of NT-proBNP at MESA exam 1 did not improve the discriminative power of the multivariable model (Table 3). Importantly however, the inclusion of change in NT-proBNP significantly increased the Harrell’s C statistics to 0.87 (95% CI 0.84–0.89, p=0.049, Table 3) for dementia prediction over and above baseline NT-proBNP levels.
Table 3.
Risk prediction metrics for the baseline and change in log-NT-proBNP (pg/mL) for predicting future all-cause hospitalized dementia
| Model | Harrell’s C-Statistic (95% CI) | P-Value |
|---|---|---|
| Multivariable model | 0.85(0.83–0.88) | Ref |
| Multivariable model + Baseline log-NT-proBNP | 0.85(0.83–0.88) | 0.26 |
| Multivariable model + Baseline and change in log-NT-proBNP | 0.87(0.84–0.89) | 0.049 |
Multivariable model adjusted for baseline demographics (age, sex, race/ethnicity), ApoE lipoprotein isoforms and traditional cardiovascular disease risk factors and socioeconomic status at MESA exam 3, including systolic blood pressure, use of antihypertensives, resting heart rate, high-density lipoprotein, total cholesterol, antihyperlipidemics, body mass index, glomerular filtration rate, diabetes mellitus, income, education level, health insurance status, cigarette smoking and study field center at MESA exam 3 and coronary artery calcium score at MESA exam 1 and interim cardiovascular events, including coronary events, heart failure, and clinical stroke as time-varying covariates.
NT-proBNP and cognitive function
As shown in Table 4, log NT-proBNP level at MESA exam 1 but not at MESA exam 3 was associated with a lower cubic-transfomed CASI score (β = −7808.98, p=0.02) and backward digit span (β = −0.12, p=0.003) at MESA exam 5. There was no significant association between change in log NT-proBNP level (exam 1 to 3) and exam 5 cognitive function indices.
Table 4.
Association of log-NT-proBNP at MESA exams 1 and 3 and its change with measures of cognitive function at MESA exam 5, β (p value)
| NT-proBNP | MESA exam 1 | MESA exam 3 | Change in log-NT-proBNP |
|---|---|---|---|
| Cubic transformed CASI | −7808.98 (0.009) | −4475.95(0.15) | 3317.71(0.33) |
| Digit span, forward | −0.06(0.2) | −0.07(0.16) | −0.008(0.88) |
| Digit span, backward | −0.12(0.003) | −0.06(0.18) | −0.06(0.18) |
CASI: Cognitive Abilities Screening Instrument. Mean CASI and cubic-transformed CASI for the cohort is 87.1 (11.2) and 689374.6(189370.9).
Regression coefficients adjusted for baseline demographics (age, sex, race/ethnicity), ApoE lipoprotein isoforms and traditional cardiovascular disease risk factors and socioeconomic status at MESA exam 3, including systolic blood pressure, use of antihypertensives, resting heart rate, high-density lipoprotein, total cholesterol, antihyperlipidemics, body mass index, glomerular filtration rate, diabetes mellitus, income, education level, health insurance status, cigarette smoking and study field center at MESA exam 3 and coronary artery calcium score at MESA exam 1 and interim cardiovascular events, including coronary events, heart failure, and clinical stroke as time-varying covariates.
Correlates of changes in log-NT-proBNP
Supplemental Table S2 illustrates the MESA exam 1 correlates of change in log-NT-proBNP levels. Older age and non-black race/ethnicity were associated with greater increase in NT-proBNP levels. Moreover, lower GFR (β = −0.003, p<0.001), hypertension medication use (β = 0.06, p=0.02), diabetes mellitus (β = 0.14, p<0.001), current smoking (β = 0.09, p=0.01), and high coronary artery calcium score (β = 0.01, p=0.01) were correlated with increase in log-NT-proBNP levels. Log-NT-proBNP level at exam 1 was inversely associated with its increase over time (−0.36, p<0.001).
Discussion
In this study, we showed that: a) A 3-year increase in NT-proBNP level is associated with increased risk of future all-cause hospitalized dementia over and above cross-sectionally measured NT-proBNP level at baseline. This association was independent of demographics, CVD risk factors, interim CVD events and left ventricular volume and remodeling indices measured by MRI. On the other hand, any decrease in NT-proBNP level over time was associated with reduced risk of dementia; b) Increase in NT-proBNP levels between the baseline and MESA 3 follow-up exams moderately improved discriminative performance of demographics, CVD risk factors and baseline NT-proBNP levels for predicting future all-cause hospitalized dementia; c) Baseline NT-proBNP level but not its change over time was associated with impaired cognitive function later in life; d) Impaired kidney function, hypertension, diabetes mellitus, smoking, and coronary artery calcification were among the modifiable or treatable correlates of NT-proBNP increase, and thus represent potential targets for dementia prevention. Greater NT-proBNP at MESA 1 exam was associated with smaller increase in its level through MESA exam 3. This is likely attributable to sicker individuals with already high NT-proBNP at MESA 1 exam and little room for increase in NT-proBNP level. The other possible explanation is that patients with lower NT-proBNP at baseline are more likely to be obese and have dyslipidemia and insulin resistance39 and thus are at higher risk of developing diabetes, and cardiovascular disease as well as higher likelihood for greater change in NT-proBNP levels during follow-up.
A few prior studies have demonstrated that NT-proBNP is independently associated with cognitive dysfunction and incident dementia18–21. In two large population-based studies, one in 6,040 participants from the Rotterdam study, and the other in 7,114 subjects from the National FINRISK study, high NT-proBNP was associated with 27% and 32% increase in the risk of dementia, respectively19, 20. Our results are in line with those two studies indicating the association of cross-sectionally measured NT-proBNP with future all-cause hospitalized dementia. However, this study, for the first time, demonstrates the prognostic role of increase in NT-proBNP over time as a predictor of future dementia. In recent years, the prognostic importance of changes in NT-proBNP has been investigated in different clinical settings. In MESA, an increase in NT-proBNP levels has been associated with higher risk of incident coronary heart disease, stroke and death29, 40. A reduction in NT-proBNP level over time was shown to correlate with better outcomes among patients with congestive heart failure with both reduced27 and preserved ejection fraction26. The present study extends our body of knowledge by further emphasizing the prognostic significance of serial NT-proBNP measurements in relation not only to established CVD but also to cerebral morbidity and outcomes. Our findings may have significant implication for early detection and secondary prevention of dementia and suggest that disrupting the pathologic process leading to continuous rise in NT-proBNP may help prevent dementia in older adults.
There are several hypotheses to explain the link between NT-proBNP and its change with dementia. The first and more intuitive possibility is that individuals with elevated NT-proBNP levels are more likely to have both clinically expressed and silent cerebrovascular events; however, change in NT-proBNP remained associated with dementia even after adjusting for CVD risk factors and interim CVD, including clinically manifested cerebrovascular events (stroke)20. Secondly, we propose that the longitudinal increase in NT-proBNP as an index of enhanced myocardial stress, reflects mechanisms leading to progressive subclinical cardiac dysfunction that could entail concomitant myocardial and cerebral microvascular disease41, 42, adverse response to neuroendocrine hormonal overstimulation43 and hypoxia leading to progressive cardiomyocyte and neuronal death16, 44–46. In this regard, overt heart failure, unassociated with clinical cerebrovascular events, has been shown to relate to increased incident dementia7. Previous studies have shown an association between NT-proBNP and hypoperfusion of the myocardium and chronic state of hypoxia41, loss of cardiomyocytes and interstitial fibrosis16. NT-proBNP has been also associated with retinal microvascular damage42. A similar process may explain the association of NT-proBNP and dementia, given that hypoperfusion and microvascular endothelial dysfuction has been linked to structural and functional changes in the brain as well as incident dementia44–46. The fact that increasing NT-proBNP levels remained significantly associated with incident dementia despite adjustment for CVD risk factors, may indicate that brain alterations in patients with subclinical CVD accompanied by high NT-proBNP begin early in the course of progressive cardiac dysfunction. This suggests that early and aggressive control of risk factors associated with increased NT-proBNP levels (such as hypertension, diabetes mellitus, smoking, kidney dysfunction and atherosclerosis as documented in this and other studies) could significantly alter progressive cerebral dysfunction and dementia in older adults.
In the present study, the measures of cognitive dysfunction at MESA exam 5 were only associated with NT-proBNP levels measured at the baseline MESA exam (exam 1) but not with either its change over time or NT-proBNP levels measured at the 3 year follow up examination (exam 3). One possible explanation for this finding is a selection bias arising from people who died before the 10 year follow up exam (MESA exam 5), or did not return to clinic for MESA exam 5 due to dementia, subclinical cognitive dysfunction or other co-morbidities. Further studies are required to assess the relationship of longitudinal changes in cognitive function and changes in NT-proBNP level over time.
The major strengths of our study were the serial measurements of NT-proBNP during a 3-year interval in a large community-based cohort with detailed clinical phenotyping and long follow-up time. The major limitation of our study was the ascertainment of incident dementia cases from hospitalization records using ICD-9 codes. This method of diagnosing dementia cases likely underestimates the incidence of disease, since it only identifies participants who were hospitalized, and in whom dementia was included in their discharge diagnoses. However, this method of identifying dementia events has been recently validated in MESA and has shown to have adequate positive predictive value for diagnosing clinically established dementia33. Also, the biological variability of NT-proBNP due to seasonal changes or circadian rhythm was not taken into account in our analysis; however we expect that these factors have randomly affected the patients with or without dementia and therefore, may have only diluted the effect size but could not change the direction of the observed associations.
In conclusion, in a multi-ethnic large cohort, we demonstrate that an increase in NT-proBNP level over time is significantly associated with risk of future all-cause hospitalized dementia above and beyond clinical risk factors and cross sectionally measured NT-proBNP level. Disruption of the processes leading to increased NT-proBNP by controlling treatable or modifiable cardiovascular risk factors may help prevent dementia in older adults.
Supplementary Material
Perspectives.
This is the first study to demonstrate that increase in NT-proBNP is independently associated with future all-cause hospitalized dementia and offers a moderately better predictive performance for risk of dementia compared with risk factors and baseline NT-proBNP. The greatest risk of dementia was observed in subjects with high baseline NT-proBNP and increase in its level over time. The mechanisms for association of increase in NT-proBNP and dementia is not completely understood and further studies are required to elaborate the mechanisms for this association. However, fidnings of this study may help prevention of dementia by controlling the risk factors that are responsible for increase in NT-proBNP.
Novelty and Significance.
What is new?
NT-ptoBNP, a serum biomarker, is historically related to heart failure.
But it is unknown whether increase in NT-proBNP over time can predict dementia in future.
What is relevant?
This study showed that increase in NT-proBNP is associated with future dementia earlier than diagnosis of clinical dementia or heart failure
Multiple risk factors including hypertension are responsible for increase in NT-proBNP and subsequently dementia
Summary:
Increase in NT-proBNP is independently associated with future dementia. Controlling risk factors may help prevention of dementia.
Acknolegments
We thank the other investigators, the staff, and the participants of MESA (Multi-Ethnic Study of Atherosclerosis) for their valuable contributions. A full list of participating MESA investigators and in- stitutions can be found at http://www.mesa-nhlbi.org.
Source of funding
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
This research was supported by contracts N01-HC-95159, N01- HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01- HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01- HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources (NCRR).
Footnotes
Conflict of interest
None
Contributor Information
Mohammad R Ostovaneh, Division of Cardiology, Johns Hopkins University, Baltimore, MD; Department of Medicine, Penn State College of Medicine, Hershey, PA.
Kasra Moazzami, Division of Cardiology, Johns Hopkins University, Baltimore, MD; Department of Cardiology, Emory University, Atlanta, GA.
Kihei Yoneyama, Division of Cardiology, Johns Hopkins University, Baltimore, MD; St. Marianna University School of Medicine, Kawasaki, Japan.
Bharath A Venkatesh, Division of Cardiology, Johns Hopkins University, Baltimore, MD.
Susan R. Heckbert, Department of Epidemiology, University of Washington, Seattle, WA.
Colin O. Wu, Office of Biostatistics Research, National Heart, Lung and Blood Institute, Bethesda, MD.
Steven Shea, Departments of Medicine and Epidemiology, Columbia University, New York, NY.
Wendy S. Post, Division of Cardiology, Johns Hopkins University, Baltimore, MD.
Annette L. Fitzpatrick, Departments of Family Medicine, Epidemiology and Global Health, University of Washington, Seattle, WA.
Gregory L Burke, Division of Public Health Sciences, Wake Forest University, Winston-Salem, NC.
Hossein Bahrami, Division of Cardiovascular Medicine, University of Southern California, Los Angles, CA.
Otto A. Sanchez, Central Medical Clinic, Saint Paul, MN.
Lori B. Daniels, Department of Medicine, Division of Cardiovascular Medicine, University of California, San Diego, CA.
Erin D. Michos, Division of Cardiology, Johns Hopkins University, Baltimore, MD.
David A Bluemke, Department of Radiology, University of Wisconsin School of Medicine and Public Health, Madison, WI.
João A.C. Lima, Division of Cardiology, Johns Hopkins University, Baltimore, MD.
References
- 1.Todd S, Barr S, Roberts M and Passmore AP. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry. 2013;28:1109–24. [DOI] [PubMed] [Google Scholar]
- 2.Patrick DL, Pearlman RA, Starks HE, Cain KC, Cole WG and Uhlmann RF JAoIM. Validation of preferences for life-sustaining treatment: implications for advance care planning. 1997;127:509–517. [DOI] [PubMed] [Google Scholar]
- 3.Kaye JA, Swihart T, Howieson D, Dame A, Moore MM, Karnos T, Camicioli R, Ball M, Oken B and Sexton G. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–304. [DOI] [PubMed] [Google Scholar]
- 4.Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC and Rossor MN. Imaging of onset and progression of Alzheimer’s disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–5. [DOI] [PubMed] [Google Scholar]
- 5.De Luca V, Spalletta G, Souza RP, Graff A, Bastos-Rodrigues L and Bicalho MA CJN. Definition of Late Onset Alzheimer’s Disease and Anticipation Effect of Genome-Wide Significant Risk Variants: Pilot Study of the APOE e4 Allele. 2019;77:8–12. [DOI] [PubMed] [Google Scholar]
- 6.Forette F, Seux M-L, Staessen JA, Thijs L, Birkenhäger WH, Babarskiene M-R, Babeanu S, Bossini A, Gil-Extremera B and Girerd X JTL. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. 1998;352:1347–1351. [DOI] [PubMed] [Google Scholar]
- 7.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J and Fratiglioni L JAoim. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. 2006;166:1003–1008. [DOI] [PubMed] [Google Scholar]
- 8.Kwok CS, Loke YK, Hale R, Potter JF and Myint PK. Atrial fibrillation and incidence of dementia. A systematic review and meta-analysis. 2011;76:914–922. [DOI] [PubMed] [Google Scholar]
- 9.Daniels LB and Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–68. [DOI] [PubMed] [Google Scholar]
- 10.Oremus M, Don-Wauchope A, McKelvie R, Santaguida PL, Hill S, Balion C, Booth R, Brown JA, Ali U, Bustamam A, Sohel N and Raina P. BNP and NT-proBNP as prognostic markers in persons with chronic stable heart failure. Heart Fail Rev. 2014;19:471–505. [DOI] [PubMed] [Google Scholar]
- 11.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB and Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA and Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–63. [DOI] [PubMed] [Google Scholar]
- 13.Wang AY, Lam CW, Yu CM, Wang M, Chan IH, Zhang Y, Lui SF and Sanderson JE. N-terminal pro-brain natriuretic peptide: an independent risk predictor of cardiovascular congestion, mortality, and adverse cardiovascular outcomes in chronic peritoneal dialysis patients. J Am Soc Nephrol. 2007;18:321–30. [DOI] [PubMed] [Google Scholar]
- 14.Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, Ibsen H, Torp-Pedersen C and Hildebrandt PR. N-terminal pro-brain natriuretic peptide, but not high sensitivity C-reactive protein, improves cardiovascular risk prediction in the general population. Eur Heart J. 2007;28:1374–81. [DOI] [PubMed] [Google Scholar]
- 15.Rutten JH, Mattace-Raso FU, Steyerberg EW, Lindemans J, Hofman A, Wieberdink RG, Breteler MM, Witteman JC and van den Meiracker AH. Amino-terminal pro-B-type natriuretic peptide improves cardiovascular and cerebrovascular risk prediction in the population: the Rotterdam study. Hypertension. 2010;55:785–91. [DOI] [PubMed] [Google Scholar]
- 16.Liu C-Y, Heckbert SR, Lai S, Ambale-Venkatesh B, Ostovaneh MR, McClelland RL, Lima JA and Bluemke DA JJotACoC. Association of elevated NT-proBNP with myocardial fibrosis in the Multi-Ethnic Study of Atherosclerosis (MESA). 2017;70:3102–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zonneveld HI, Ikram MA, Hofman A, Niessen WJ, van der Lugt A, Krestin GP, Franco OH and Vernooij MW. N-Terminal Pro-B-Type Natriuretic Peptide and Subclinical Brain Damage in the General Population. Radiology. 2017;283:205–214. [DOI] [PubMed] [Google Scholar]
- 18.Kerola T, Nieminen T, Hartikainen S, Sulkava R, Vuolteenaho O and Kettunen R. B-type natriuretic peptide as a predictor of declining cognitive function and dementia--a cohort study of an elderly general population with a 5-year follow-up. Ann Med. 2010;42:207–15. [DOI] [PubMed] [Google Scholar]
- 19.Tynkkynen J, Laatikainen T, Salomaa V, Havulinna AS, Blankenberg S, Zeller T and Hernesniemi JA. NT-proBNP and the risk of dementia: a prospective cohort study with 14 years of follow-up. J Alzheimers Dis. 2015;44:1007–13. [DOI] [PubMed] [Google Scholar]
- 20.Mirza SS, de Bruijn RF, Koudstaal PJ, van den Meiracker AH, Franco OH, Hofman A, Tiemeier H and Ikram MA. The N-terminal pro B-type natriuretic peptide, and risk of dementia and cognitive decline: a 10-year follow-up study in the general population. J Neurol Neurosurg Psychiatry. 2016;87:356–62. [DOI] [PubMed] [Google Scholar]
- 21.Daniels LB, Laughlin GA, Kritz-Silverstein D, Clopton P, Chen W-C, Maisel AS and Barrett-Connor E JTAjom. Elevated natriuretic peptide levels and cognitive function in community-dwelling older adults. 2011;124:670. e1–670. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez OA, Duprez DA, Bahrami H, Daniels LB, Folsom AR, Lima JA, Maisel A, Peralta CA and Jacobs DR Jr. The associations between metabolic variables and NT-proBNP are blunted at pathological ranges: the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2014;63:475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggers KM, Venge P and Lind L. Prognostic usefulness of the change in N-terminal pro B-type natriuretic peptide levels to predict mortality in a single community cohort aged >/= 70 years. Am J Cardiol. 2013;111:131–6. [DOI] [PubMed] [Google Scholar]
- 24.Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152:828–34. [DOI] [PubMed] [Google Scholar]
- 25.Ying W, Zhao D, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, Sharma K, Shah SJ, Heckbert SR, Lima JA JTJoCE and Metabolism. Sex Hormones and Change in N-Terminal Pro–B-Type Natriuretic Peptide Levels: The Multi-Ethnic Study of Atherosclerosis. 2018;103:4304–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jhund PS, Anand IS, Komajda M, Claggett BL, McKelvie RS, Zile MR, Carson PE and McMurray JJ. Changes in N-terminal pro-B-type natriuretic peptide levels and outcomes in heart failure with preserved ejection fraction: an analysis of the I-Preserve study. Eur J Heart Fail. 2015;17:809–17. [DOI] [PubMed] [Google Scholar]
- 27.Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, Swedberg K, Desai AS, Gong J, Shi VC and Solomon SD. Prognostic Implications of Changes in N-Terminal Pro-B-Type Natriuretic Peptide in Patients With Heart Failure. J Am Coll Cardiol. 2016;68:2425–2436. [DOI] [PubMed] [Google Scholar]
- 28.Mishra RK, Judson G, Christenson RH, DeFilippi C, Wu AHB and Whooley MA. The Association of Five-Year Changes in the Levels of N-Terminal Fragment of the Prohormone Brain-Type Natriuretic Peptide (NT-proBNP) with Subsequent Heart Failure and Death in Patients with Stable Coronary Artery Disease: The Heart and Soul Study. Cardiology. 2017;137:201–206. [DOI] [PubMed] [Google Scholar]
- 29.Daniels LB, Clopton P, deFilippi CR, Sanchez OA, Bahrami H, Lima JA, Tracy RP, Siscovick D, Bertoni AG, Greenland P, Cushman M, Maisel AS and Criqui MH. Serial measurement of N-terminal pro-B-type natriuretic peptide and cardiac troponin T for cardiovascular disease risk assessment in the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J. 2015;170:1170–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M and Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 31.Ordonez-Llanos J, Collinson PO and Christenson RH. Amino-terminal pro-B-type natriuretic peptide: analytic considerations. Am J Cardiol. 2008;101:9–15. [DOI] [PubMed] [Google Scholar]
- 32.Karl J, Borgya A, Gallusser A, Huber E, Krueger K, Rollinger W and Schenk J. Development of a novel, N-terminal-proBNP (NT-proBNP) assay with a low detection limit. Scand J Clin Lab Invest Suppl. 1999;230:177–81. [PubMed] [Google Scholar]
- 33.Fujiyoshi A, Jacobs DR Jr., Alonso A, Luchsinger JA, Rapp SR and Duprez DA. Validity of Death Certificate and Hospital Discharge ICD Codes for Dementia Diagnosis: The Multi-Ethnic Study of Atherosclerosis. Alzheimer Dis Assoc Disord. 2017;31:168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujiyoshi A, Jacobs DR Jr., Fitzpatrick AL, Alonso A, Duprez DA, Sharrett AR, Seeman T, Blaha MJ, Luchsinger JA and Rapp SR. Coronary Artery Calcium and Risk of Dementia in MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moazzami K, Ostovaneh MR, Ambale Venkatesh B, Habibi M, Yoneyama K, Wu C, Liu K, Pimenta I, Fitzpatrick A, Shea S, McClelland RL, Heckbert S, Gottesman RF, Bluemke DA, Hughes TM and Lima JAC. Left Ventricular Hypertrophy and Remodeling and Risk of Cognitive Impairment and Dementia: MESA (Multi-Ethnic Study of Atherosclerosis). Hypertension. 2018;71:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fine JP. Regression modeling of competing crude failure probabilities. Biostatistics. 2001;2:85–97. [DOI] [PubMed] [Google Scholar]
- 37.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ and Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schou M, Gustafsson F, Nielsen PH, Madsen LH, Kjaer A and Hildebrandt PR. Unexplained week-to-week variation in BNP and NT-proBNP is low in chronic heart failure patients during steady state. Eur J Heart Fail. 2007;9:68–74. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez OA, Duprez DA, Bahrami H, Daniels LB, Folsom AR, Lima JA, Maisel A, Peralta CA and Jacobs DR. The associations between metabolic variables and NT-proBNP are blunted at pathological ranges: The Multi-Ethnic Study of Atherosclerosis. Metabolism. 2014;63:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez OA, Jacobs JD, Bahrami H, Peralta CA, Daniels LB, Lima JA, Maisel A and Duprez DA JJoh. Increasing aminoterminal-pro-B-type natriuretic peptide precedes the development of arterial hypertension: the multiethnic study of atherosclerosis. 2015;33:966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell A, Misialek JR, Folsom AR, Duprez D, Alonso A, Jerosch-Herold M, Sanchez OA, Watson KE, Sallam T and Konety SH. Usefulness of N-terminal Pro–brain Natriuretic Peptide and Myocardial Perfusion in Asymptomatic Adults (from the Multi-Ethnic Study of Atherosclerosis). The American Journal of Cardiology. 2015;115:1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mutlu U, Ikram MA, Hofman A, Jong PT VMd, Klaver CCW and Ikram MK. N-Terminal Pro-B-Type Natriuretic Peptide Is Related to Retinal Microvascular Damage. 2016;36:1698–1702. [DOI] [PubMed] [Google Scholar]
- 43.Sharp TE, Polhemus DJ, Li Z, Spaletra P, Jenkins JS, Reilly JP, White CJ, Kapusta DR, Lefer DJ and Goodchild TT. Renal Denervation Prevents Heart Failure Progression Via Inhibition of the Renin-Angiotensin System. Journal of the American College of Cardiology. 2018;72:2609–2621. [DOI] [PubMed] [Google Scholar]
- 44.del Zoppo GJ, Mabuchi TJ JoCBF and Metabolism. Cerebral microvessel responses to focal ischemia. 2003;23:879–894. [DOI] [PubMed] [Google Scholar]
- 45.Abete P, Della-Morte D, Gargiulo G, Basile C, Langellotto A, Galizia G, Testa G, Canonico V, Bonaduce D and Cacciatore FJ Arr. Cognitive impairment and cardiovascular diseases in the elderly. A heart–brain continuum hypothesis. 2014;18:41–52. [DOI] [PubMed] [Google Scholar]
- 46.Holm H, Nägga K, Nilsson ED, Ricci F, Melander O, Hansson O, Bachus E, Magnusson M and Fedorowski A. Biomarkers of microvascular endothelial dysfunction predict incident dementia: a population-based prospective study. 2017;282:94–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


