Abstract
A 49-year-old woman, previously healthy, presented with recurrent fractures provoked by minimal trauma. She had sustained seven fractures over the previous 2 years. While she was an avid runner, her injuries were determined to be out of proportion to the degree of trauma. Initial evaluation, exploring the more common causes such as low bone density and abnormal vitamin D metabolism, was unremarkable. On repeat of the some of the tests, a low alkaline phosphatase (AP) was noted, which raised suspicion for hypophosphatasia (HPP), a rare cause of recurrent fractures. Subsequent workup revealed a low bone-specific AP and elevated vitamin B6. Subsequently, genetic testing confirmed the diagnosis of adult-onset HPP caused by a heterozygous mutation c.407G>A in the ALPL gene. Asfotase alfa was started; however, the patient developed an allergic reaction leading to the discontinuation of the drug.
Keywords: calcium and bone, sports and exercise medicine
Background
Hypophosphatasia (HPP) is a rare inborn-error-of-metabolism disorder, resulting in defective bone and dental mineralisation. It is caused by loss-of-function mutation(s) of the gene that encodes tissue-non-specific AP (TNSALP), called ALPL.1 It is a rare condition affecting approximately 500–600 individuals in the USA and is part of the differential diagnosis of osteomalacia.2 Apart from odontohypophosphatasia (dental only), HPP is classified into perinatal, infantile, childhood/juvenile and adult forms depending on the age of onset of symptoms.
The diagnosis of HPP requires a high index of suspicion. The adult form of HPP may be the most difficult to diagnose as these patients may be completely asymptomatic or have subtle findings, such as low bone mineral density (BMD) or joint pain, that can be easily confused for more common disorders of the bone such as osteoporosis or osteoarthritis.2 Moreover, a low serum AP on routine testing may be the only abnormality. Once the diagnosis is made, treatment options remain limited in patients with the adult form of HPP.
We report a case of adult-onset HPP, diagnosed after sustaining numerous fractures over the span of 2 years, and the not-so-straightforward workup that ultimately led to the diagnosis. We also discuss available treatment options.
Case presentation
A 49 year-old woman was referred to endocrinology clinic by sports medicine specialist to rule an underlying metabolic bone disease given multiple recurrent stress fractures.
The patient was in her usual state of health until about 2 years prior to presentation when she had a metatarsal stress fracture while running. She had been an avid runner for about 5 years prior to that. Her stress fracture was attributed to high-volume running. However, over the next 24 months, she had six other stress fractures, including multiple metatarsal stress fractures, a left proximal femoral shaft stress fracture (with periosteal oedema) and a rib fracture, all associated with minimal to no trauma. The time of healing varied and depended on the location and severity of the fractures but ranged between 8 and 12 weeks. In all instances, the patient kept a close follow-up with the sports medicine specialist and physical therapist and followed a return to run protocol based on their recommendations. The patient denies having major dental problems, although she did report having numerous cavities as a child. She has never had kidney stones. She denied exposure to steroids. She had regular menstrual cycles.
Patient’s past medical history was positive for anxiety, treated with buspirone. She had no pertinent surgical history. She was not taking any over-the-counter supplements. Family history was negative for bone disorders, but she did report that her son has had major dental problems since childhood. She was a former smoker, having quit smoking at age 42.
On examination, her vital signs were within normal limits. Her body mass index was 23.82 kg/m2. General examination was unremarkable. Oral examination showed no evidence of dental abnormalities, although she had a number of treated cavities with fillings. She did not report any premature tooth loss. Thyroid examination was unremarkable. She did not have any bone or joint deformities or tenderness and did not exhibit muscle weakness. Physical examination was negative for a moon facies, buffalo hump, cervical fullness or purple striae.
Investigations
At the time of referral to endocrinology, the patient had already undergone the basic workup for her history of recurrent fractures. Apart from a bone density scan, which was normal, the initial evaluation included serum calcium, phosphorus, magnesium, creatinine, liver function tests, alkaline phosphatase (AP) and thyroid stimulating hormone, parathyroid hormone (PTH) and vitamin D levels, all of which were unremarkable (table 1).
Table 1.
Relevant laboratory parameters
| Lab test (unit) | Results | Reference range |
| Calcium, serum (mg/dL) | 9.4 | 8.6–10.2 |
| Phosphorus, serum (mg/dL) | 4.4 | 2.5–4.5 |
| Creatinine, serum (mg/dL) | 0.91 | 0.5–1.1 |
| 25-Hydroxy vitamin D (ng/mL) | 49 | 30–100 |
| 1,25-Dihydroxy vitamin D (pg/mL) | 50 | 18–72 |
| TSH (mIU/L) | 1.06 | 0.4–4.5 |
| PTH (pg/mL) | 19 | 14–64 |
| 24-hour urine calcium (mg/24 hours) | 192 | 35–250 |
| AP (U/L) | 31 | 33–115 |
| Bone-specific AP (µg/L) | 3.3 | 5–18.8 |
| Vitamin B6 (ng/mL) | 52 | 2.1–21.7 |
| FGF 23 (RU/mL) | 131 | <180 |
| CTX (pg/mL) | 317 | 40–465 |
| NTX | 27 | 40–64 |
AP, alkaline phosphatase; CTX, C-telopeptide; FGF 23, fibroblast growth factor 23; NTX, N-telopeptide; PEA, phosphoethanolamine; PLP, pyridoxal-5-phosphate; PTH, parathyroid hormone; TSH, thyroid-stimulating hormone.
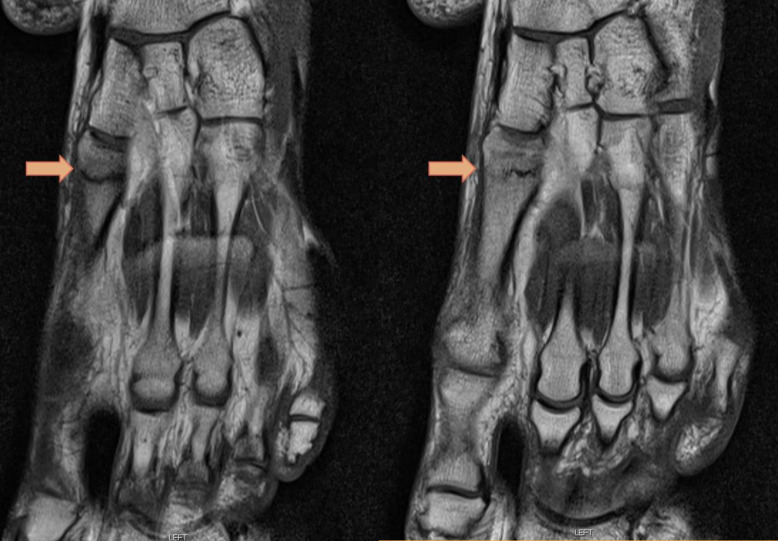
Despite a normal foot X-ray (see figure 1), the patient had several abnormal MRIs showing the various metatarsal stress fractures (figure 2), some of which involved the same metatarsal, although slightly different locations (see table 2 for a list of all recorded fractures)
Figure 1.
Normal X-rays of the left foot: anteroposterior (AP) view (A), oblique view (B).
Figure 2.
T1-weighted axial images of the left foot MRI showing a non-displaced stress fracture in the proximal first metatarsal.
Table 2.
Stress fracture history as reported in patient’s journal
| Date | Location |
| 7/2016 | Left base of second metatarsal |
| 7/2016 | Left cuneiform |
| 1/2017 | Left proximal femoral shaft |
| 5/2017 | Left first metatarsal |
| 8/2017 | Right calcaneal |
| 12/2017 | Rib |
| 3/2018 | Left second metatarsal |
| 3/2018 | Left third metatarsal |
| 10/2018 | Left first metatarsal |
| 10/2018 | Stress reactions: tibia, talus, cuneiform and calcaneus |
| 3/2019 | Left heel |
| 7/2019 | Left femoral stress reaction |
| 12/2019 | Right cuboid |
Given that the general workup completed thus far was non-revealing of a potential cause of the patient’s recurrent fractures, a repeat of the basic workup was done, in addition to a broader evaluation. This included: 24-hour urine collection in order to measure urine electrolytes, which was significant for normal urine calcium but elevated urine phosphorus, and fibroblast growth factor 23 (FGF 23) was 131 RU/mL, which was also within normal limits. Serum and urine protein electrophoresis were obtained to rule out multiple myeloma and were unremarkable. Luteinizing hormone (LH), follicle-stimulating hormone (FSH) and estradiol were also normal (table 1). However, a mild reduction in AP of 31 U/L (33–115) was noted. This prompted further evaluation, which revealed a low bone-specific AP of 3.3 µg/L (5–18.2). Subsequently, serum pyridoxal 5′-phosphate (PLP)/vitamin B6 was done in order to rule out HPP and it was elevated at more than two times the upper limit of normal at 52 ng/mL (2.1–21.7).
Based on the above clinical and laboratory findings, the high suspicion for HPP prompted recommendation for genetic testing. The genetic test revealed a heterozygous mutation in the ALPL gene for a sequence variant defined as c.407G>A which is predicted to result in the amino acid substitution pArg136His. This variant has been reported in patients affected with infantile HPP, odonto HPP and adult HPP, thus confirming the diagnosis of adult-onset HPP.3
Differential diagnosis
Common causes of recurrent, fragility fractures are osteoporosis, idiopathic hypercalciuria or osteomalacia due to vitamin D deficiency. These were ruled out by bone density scan, a 24-hour urine calcium and serum vitamin D level, respectively. Other less common causes of fractures include tumor-induced osteomalacia multiple myeloma, and other endocrine disorders such as primary hyperparathyroidism, thyroid disorders and Cushing’s disease/syndrome were also ruled out. Workup for renal tubular acidosis or Fanconi type of syndrome, or other renal phosphate wasting disorders (FGF 23 was normal) was negative. Osteogenesis imperfecta tarda was also considered but was deemed highly unlikely given the age of onset of symptoms, the normal BMD scan as well as the absence of other defining features of the condition, such as blue sclerae or hearing loss. Other causes of low AP, such as zinc or magnesium deficiency, were not evident.
Treatment
Once the diagnosis of HPP was confirmed, the patient was advised to avoid strenuous high-impact exercise such as long-distance running. She was encouraged to continue close follow-up with the sports medicine specialist and physical therapist. Her serum calcium and vitamin D levels were at goal, and so she did not require supplementation. Teriparatide was discussed; however, the patient wanted to investigate other options. The enzyme replacement drug asfotase alfa (Strensiq) was not recommended initially given relatively mild presentation. In addition, the Food and Drug Administration (FDA) had approved this drug for the treatment of perintatal, infantile and juvenile-onset HPP only and not adult type HPP. In follow-up, however given delayed healing of her metatarsal stress fracture, the patient was refered to a specialised centre for a second opinion regarding enzyme-replacement therapy. On further questioning, she reported having a history of several traumatic fractures as a child as well as joint aches and excessive fatigue, which raised concern for juvenile-onset HPP. Asfotase alfa 69 mg daily (~1 mg/kg) for 6 days/week was started.
Outcome and follow-up
The patient developed nausea, vomiting and dizziness followed by syncope, which was concerning for an anaphylactic reaction to asfotase alfa after the 12th dose, and which prompted her to discontinue the medication. Since then, she has had a left femoral stress reaction, a left heel stress fracture and a right cuboid stress fracture. She had to scale back significantly on her running but continues to engage in low-impact exercise, such as cycling and swimming. She is interested in exploring the option of desensitisation.
Patient was referred to genetic counselling where she was evaluated along with several of her family members. Both her father and her son were found to have the same genetic mutation, although neither of them exhibited any obvious clinical signs or symptoms of the disease.
Discussion
HPP is an inherited inborn-error-of-metabolism that features low serum AP and is caused by loss-of-function mutation(s) of the gene that encodes the tissue-non-specific AP (TNSALP).1 This results in impaired skeletal and dental mineralisation. HPP is a rare condition that affects approximately 500–600 known individuals in the USA, although additional affected individuals may be unrecognised or misdiagnosed. Inherited as either an autosomal recessive or autosomal dominant trait, HPP is characterised by marked variability in clinical expression.2
Aside from odontohypophosphatasia, which is defined by dental complications only, HPP is classified into four types depending on the severity of symptoms and age at presentation: perinatal, infantile, childhood/juvenile and adult, with the adult type being the least severe form. In babies and children, HPP can be fatal due to respiratory insufficiency and can cause severe complications such as poor feeding, failure to thrive and motor and cognitive impairment.4 In adults, main clinical involvement includes early loss of primary or secondary teeth, osteoporosis, bone pain, chondrocalcinosis and fractures. Then again, adults with HPP may be asymptomatic and come to clinical attention by finding low serum AP on routine laboratory testing during the course of evaluation for osteoporosis or fractures.2 However, even though most adult patients may not recall childhood symptoms, detailed questioning may reveal a history of childhood delay in crawling or walking, muscle weakness, bone and muscle pain identified as ‘growing childhood pain’ and poor dentition.5
Low serum total ALP activity for age and sex is a hallmark finding of HPP.5 Levels of AP correlate with disease severity.1 It is important here to note that other causes of low AP should be ruled out, such as zinc or magnesium deficiency, hypothyroidism and starvation.6 On the other hand, AP may be falsely elevated or normal in the setting of an acute fracture.
Other diagnostic criteria include elevated serum pyridoxal-5′-phosphate (vitamin B6) and/or elevation of urinary phosphoethanolamine. The finding of a mutation in the ALPL gene provides additional level of confirmation, which can be crucial for understanding inheritance patterns, but is not a requirement for the clinical diagnosis of HPP.1
The bone-targeted enzyme-replacement therapy, asfotase alfa, was approved in 2015. This came after a pivotal trial demonstrated its effectiveness on skeletal mineralisation in life-threatening perinatal and infantile HPP. This result translated into improvement in pulmonary function and developmental milestones.4 Subsequently, the FDA-approved asfotase alfa (Strensiq) for treatment of perinatal, infantile and juvenile-onset HPP.7 In adults, however, data are scarce and trials with Strensiq are limited to adult patients with HPP but who have had symptoms since childhood. Thus, there are no formal guidelines for the treatment of adult-onset HPP, and choice of therapy in this population is based on the overall presentation, goals of therapy and clinical judgment.
A conservative approach is the mainstay of treatment in adult-onset HPP. This includes pain relief, orthopaedic surgery as indicated and treatment of hypercalcemia if present. Unless vitamin D level is low, supplementation is best avoided so as not to exacerbate any hypercalcemia, hypercalciuria or hyperphosphatemia.1 Treatment with bisphosphonates and possibly denosumab may have adverse effects due to their antiresorptive and AP-lowering effects, which was shown to potentially lead to an increase in and/or worsening of fractures in case studies of adults with previously undiagnosed HPP.2 8 Teriparatide (recombinant human PTH 1–34) has been used in a handful of adult patients with HPP with variable results. The first HPP patient treated with teriparatide for a total of 16 weeks demonstrated fracture repair as evidenced by improvement in pain as well as imaging findings. It also showed correction in hyperphosphatemia, improvement in BMD, and an increase in TNSALP and other bone turnover maker responses consistent with enhanced skeletal remodelling.9 Camacho et al treated two female patients with adult HPP, aged 68 and 53, with teriparatide injected daily for 2 years. AP of both patients increased; however, this improvement was not sustained. BMD of one patient remained unchanged but she did not have any fractures over the course of the study period, while the second patient had a significant increase in femoral neck BMD during the course of teriparatide.10 Of note, abaloparatide has not yet been studied in this setting. The antisclerotstin monoclonal antibody BPS804 was studied in adult patients with HPP in an open label, phase II trial. Eight patients were enrolled in the study, received three escalating doses of BPS804 on days 1, 15 and 29, and were followed for 16 weeks after the last dose. Over the study period, there was an increase in AP and bone-specific AP as well as increase in procollagen type-1 N-terminal propeptide, osteocalcin and PTH, and a decrease in the bone resorption turnover marker C-telopeptide). Lumbar spine BMD also increased. However, the positive effects on bone turnover makers, and presumably BMD, were transient. Furthermore, no data on incidence of fractures could be gleaned from the study due to its nature and short duration. In conclusion, the antisclerostin antibody shows promising results as an anabolic agent in the treatment of HPP, but more long-term, phase III studies are necessary to validate its safety and efficacy.11
The FDA-approved indication for asfotase alfa is for patients of any age with perinatal, infantile or juvenile onset of symptoms that are attributed to HPP. In the first randomised clinical trial (RCT) that looked into the safety and efficacy of asfotase alfa in adults and adolescents, 19 patients, aged 13–65 (13 of which were ≥18 years old), were randomised to receive asfotase alfa or no treatment. Ages reported for HPP sign or symptom onset ranged from 0 to 36 years (median 2.0 years); most patients (14/19) had childhood HPP, four had infantile HPP, and only one had adult HPP. The coprimary efficacy measure of change in plasma PLP concentration was met, with significant decreases in plasma PLP observed in treated vs control patients at month 6. However, statistical significance of the coprimary efficacy measure of change in inorganic pyrophosphate (PPi) concentration during the 6-month primary treatment period was not met. Notably, for most patients in the study, asfotase alfa reduced plasma concentrations of PLP and PPi to within the normal range, which was maintained through 5 years of therapy. Importantly, the decreases in circulating PLP and PPi were associated with improved functional measures in treated patients at the end of the 6-month treatment period. A transiliac bone biopsy was performed at baseline and year 1 in order to evaluate the effect of asfotase alfa on bone mineralisation; the only significant change was improvement in mineralisation lag time in the treated group at year 1. There were no clear differences between treated and control patients in BMD measured by dual-energy X-ray absorptiometry. In conclusion, the data indicate efficacy of asfotase alfa in the treatment of this relatively older population of patients with HPP.12
To date, there are no RCTs investigating the efficacy and safety of asfotase alfa in patients with adult-onset HPP. Thus, the indications for using asfotase alfa in adults are not defined. Shapiro et al proposed that patients with adult-onset HPP who meet one of the several criteria be considered for treatment with asfotase alfa. These criteria include musculoskeletal pain requiring opioids, disabling functional impairment, low BMD and delayed fracture healing, among others. An individualised approach is paramount in guiding therapy, taking accounting for the extent of disability, treatment goals and the patient preference.2
In conclusion, HPP is a rare inborn error of metabolism with that can present at any age and in numerous ways. A low AP is a key to identifying patients who potentially suffer from the condition. Genetic testing, while not strictly necessary for diagnosis, is essential to confirm the diagnosis as this would have significant implications on treatment options and lifestyle choices, as well as provide guidance for genetic counselling. Prior to the recent approval of the enzyme replacement therapy asfotase alfa, a symptom-based approach was the cornerstone of therapy. Asfotase alfa is the long-awaited therapy that can alleviate morbidity and mortality in patients with HPP, but with the advent of this treatment, new challenges emerge and more studies are needed such as its use in the cases of adult-onset HPP.
Learning points.
The diagnosis of hypophosphatasia (HPP) in adults requires a high index of suspicion given the rarity of the condition and its subtle clinical presentation. The hint is a low alkaline phosphatase on routine laboratory testing.
Alkaline phosphatase may be transiently elevated during acute episodes of fractures as well as several conditions, which in turn can result in a delayed or missed diagnosis of HPP.
While enzyme replacement therapy with asfotase alfa is now available for infantile or juvenile-onset HPP, there are no approved treatments or guidelines for patients with adult-onset HPP. Several therapy options can be considered depending on the clinical picture and goals of care.
Footnotes
Contributors: Patient was under the care of NF and DB. NF wrote the manuscript that was edited and approved by DB.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Whyte MP. Hypophosphatasia: an overview for 2017. Bone 2017;102:15–25. 10.1016/j.bone.2017.02.011 [DOI] [PubMed] [Google Scholar]
- 2.Shapiro JR, Lewiecki EM. Hypophosphatasia in adults: clinical assessment and treatment considerations. J Bone Miner Res 2017;32:1977–80. 10.1002/jbmr.3226 [DOI] [PubMed] [Google Scholar]
- 3.Tenorio J, Álvarez I, Riancho-Zarrabeitia L, et al. Molecular and clinical analysis of ALPL in a cohort of patients with suspicion of hypophosphatasia. Am J Med Genet A 2017;173:601–10. 10.1002/ajmg.a.37991 [DOI] [PubMed] [Google Scholar]
- 4.Whyte MP, Greenberg CR, Salman NJ, et al. Enzyme-Replacement therapy in life-threatening hypophosphatasia. N Engl J Med 2012;366:904–13. 10.1056/NEJMoa1106173 [DOI] [PubMed] [Google Scholar]
- 5.Vinan-Vega MN, Abate EG. Hypophosphatasia: clinical assessment and management in the adult PATIENT-A narrative review. Endocr Pract 2018;24:1086–92. 10.4158/EP-2018-0194 [DOI] [PubMed] [Google Scholar]
- 6.Khandwala HM, Mumm S, Whyte MP. Low serum alkaline phosphatase activity and pathologic fracture: case report and brief review of hypophosphatasia diagnosed in adulthood. Endocr Pract 2006;12:676–81. 10.4158/EP.12.6.676 [DOI] [PubMed] [Google Scholar]
- 7.FDA Approves Strensiq™ (asfotase alfa) for Treatment of Patients with Perinatal-, Infantile- and Juvenile-Onset Hypophosphatasia (HPP) [Internet]. FDA Approves Strensiq™ (asfotase alfa) for Treatment of Patients with Perinatal-, Infantile- and Juvenile-Onset Hypophosphatasia (HPP) | Alexion Pharmaceuticals, Inc. Available: https://news.alexionpharma.com/press-release/product-news/fda-approves-strensiq-asfotase-alfa-treatment-patients-perinatal-infantil [Accessed cited 2019Aug24].
- 8.Kishnani PS, Rush ET, Arundel P, et al. Monitoring guidance for patients with hypophosphatasia treated with asfotase alfa. Mol Genet Metab 2017;122:4–17. 10.1016/j.ymgme.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 9.Whyte MP, Mumm S, Deal C. Adult hypophosphatasia treated with teriparatide. J Clin Endocrinol Metab 2007;92:1203–8. 10.1210/jc.2006-1902 [DOI] [PubMed] [Google Scholar]
- 10.Camacho PM, Mazhari AM, Wilczynski C, et al. Adult hypophosphatasia treated with teriparatide: report of 2 patients and review of the literature. Endocr Pract 2016;22:941–50. 10.4158/EP15890.OR [DOI] [PubMed] [Google Scholar]
- 11.Seefried L, Baumann J, Hemsley S, et al. Efficacy of anti-sclerostin monoclonal antibody BPS804 in adult patients with hypophosphatasia. J Clin Invest 2017;127:2148–58. 10.1172/JCI83731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishnani PS, Rockman-Greenberg C, Rauch F, et al. Five-Year efficacy and safety of asfotase alfa therapy for adults and adolescents with hypophosphatasia. Bone 2019;121:149–62. 10.1016/j.bone.2018.12.011 [DOI] [PubMed] [Google Scholar]